Abstract
This study examined whether children exposed to adversity would exhibit lower epigenetic age acceleration in the context of improved parenting. Children with developmental delays and externalizing behavior problems (N = 62; Mage = 36.26 months; 70.97% boys, 29.03% girls; 71% Latinx, 22.6% Black) were drawn from a larger randomized controlled trial (RCT), which randomized them to receive Internet-delivered parent–child interaction therapy (iPCIT; n = 30) or community referrals as usual (RAU; n = 32). Epigenetic age acceleration was estimated with the pediatric buccal epigenetic clock, using saliva. Adversity was assessed using parent, family, and neighborhood-level cumulative-risk indicators. Adversity interacted with Time 2 (T2) observations of positive and negative-parenting practices to predict epigenetic age acceleration 1.5 years later, regardless of treatment assignment. Children exposed to more adversity displayed lower epigenetic age acceleration when parents evidenced increased positive (b = −0.15, p = .001) and decreased negative (b = −0.12, p = .01) parenting practices.
Keywords: epigenetic age acceleration, parenting, adversity, cumulative risk, developmental delays, young children, early intervention
Early-life adversity, such as poverty, child maltreatment, and community violence, imposes substantial risk for the development of psychopathology and a lifelong risk of physical (Grummitt et al., 2021) and psychological (LeMoult et al., 2020) health problems. Importantly, Latinx and Black children disproportionately experience social, economic, and environmental adversity (Liu et al., 2018), which, in turn, places them at higher risk for developmental, behavioral, or social delays compared to non-Hispanic White children.
Accelerated biological aging may mediate the association between early-life adversity exposure and prospective health problems (Sumner et al., 2023). Epigenetic aging is one such indicator of accelerated biological aging (Horvath, 2013). Epigenetic age is calculated from DNA methylation (DNAm) levels of age-associated CpG sites located on DNA. Then, epigenetic age estimates can be compared to chronological age to determine epigenetic age acceleration. Greater estimates of epigenetic age relative to chronological age indicate acceleration of biological age and predict physical and psychological problems, including all-cause mortality (Horvath & Raj, 2018). Evidence suggests that early-life adversity, including cumulative assessments of risk exposure (Sumner et al., 2019), relates to increases in epigenetic age acceleration (Colich et al., 2020), with prospective evidence documenting risk-associated increases in accelerated aging into adulthood (Copeland et al., 2022). Such findings translate to public health frameworks such as adverse childhood experiences (ACEs; Felitti et al., 1998), which inform policymakers and health-care workers about the importance of early screening and intervention among children exposed to elevated adversity in preventing outcomes such as accelerated biological aging.
Sensitive and responsive caregiving is among the most potent stress-buffering resources available to young children (Masten & Palmer, 2019), and this health-promotive potential may be evident in children’s biological aging. Indeed, parental sensitivity and responsiveness attenuated biological-aging acceleration (assessed using telomere length) among middle-childhood-aged children exposed to elevated stress (Asok et al., 2015; Beijers et al., 2020). The epigenome is particularly sensitive to stress during early childhood (Aristizabal et al., 2020), particularly among preschool-aged children (Lussier et al., 2022), relative to later developmental periods, and this sensitivity may extend to the protective influence of responsive caregiving. For example, recent research suggests that disrupted caregiving (e.g., harsh or inconsistent) is associated with higher young-adult epigenetic age acceleration (Brody et al., 2021), whereas sensitive and responsive caregiving relates to lower young-adult epigenetic age acceleration (Beach et al., 2021). However, extant research on caregiving influences on epigenetic age primarily focuses on adults or older children or uses cross-sectional designs (Wolf et al., 2018). Accordingly, interventions targeting sensitive and responsive caregiving during early childhood may be a powerful way to support caregivers in mitigating biological consequences of stress across development.
Dyadic interventions, such as behavioral parenting interventions, promote early relational health between the child and caregiver through sensitive and responsive caregiving. These treatments are among the most effective methods for promoting child health in the context of adversity exposure (Bourne et al., 2022; Garner & Yogman, 2021), and emerging evidence suggests parenting interventions may attenuate the link between adversity exposure and epigenetic age acceleration. A 7-week parenting intervention implemented in a group of Black adolescents, most of whom lived in the rural southern United States, protected against higher young-adulthood epigenetic age acceleration (Brody et al., 2016) and reduced young-adulthood epigenetic age acceleration through improved self-regulation (Lei et al., 2022). However, only one study has examined parenting-intervention-instigated changes in DNAm in young children (6–21 months; Hoye et al., 2020). One month after a 10-week attachment-based intervention, this pilot study found that DNAm patterns differed across treatment groups, suggesting that time-limited dyadic interventions implemented in young children may alter epigenetic profiles. Accordingly, intervention during this developmental period may be particularly powerful in attenuating the impact of adversity exposure on epigenetic aging, which has not yet been tested.
Statement of Relevance.
Exposure to early-life adversity predicts health problems, and parenting interventions may be one way to promote resilience among children exposed to adversity. Biological aging, measured with epigenetic age acceleration, may offer insight into children at risk of poor health who may benefit from intervention. This study used a sample of mostly Latinx families, many of whom endorsed financial hardship. Some of these families participated in a parenting intervention for children with developmental delay and elevated behavior problems, and some received community referrals. Our findings suggest that increases in positive parenting practices and decreases in negative-parenting practices may protect young children with developmental delay from accelerated epigenetic aging associated with early-life adversity. Results underscore that caregivers dealing with hardship can protect their children from the negative effects of stress, particularly when caregivers are provided access to effective interventions designed to enhance parenting.
The current study recruited from a randomized controlled trial (RCT) of a telehealth-based behavioral parenting intervention (Bagner et al., 2023) to evaluate whether increases in positive parenting (e.g., praise, engagement, warmth) and decreases in negative parenting (e.g., criticism, hostility) buffered the impact of adversity exposure on epigenetic age acceleration among majority Latinx preschoolers with developmental delays. The main outcomes of the primary RCT were that a telehealth-delivered parenting intervention with real-time therapist coaching led to significant and maintained improvements in observed positive and negative-parenting behaviors for this population (Bagner et al., 2023). Thus, we hypothesized that increased levels of observed positive parenting and decreased levels of observed negative parenting (assessed using two time points of observational data) would buffer the association between early-life adversity exposure (i.e., a cumulative risk index inclusive of parent, family, and neighborhood-level factors) and epigenetic age acceleration assessed 1.5 years later in a sample of young children with developmental delays.
Method
Overview
Participants for the current study were a subsample of parent–child dyads who participated in a primary RCT assessing the efficacy of a telehealth-based behavioral parenting intervention (Bagner et al., 2023) in treating child behavior problems among young children with developmental delays living in a large city in the southeastern United States. Dyads constituting the analytic sample for this article consented to participation in a DNA substudy, completed either the ~20-week parenting intervention or received referrals as usual, and provided a pediatric saliva sample at the most distal assessment, which occurred 12 months after their Time 2 (T2) assessment. The Institutional Review Board at Florida International University approved both the primary RCT study and the DNA substudy, under separate protocols. All parents provided informed consent for participation in both studies, and materials were made available in both English and Spanish.
Participants
Table 1 provides an overview of caregiver-child characteristics (n = 62 whose DNA data passed quality control; child Mage = 36.26 months old). Detailed information regarding the primary RCT sample from which these dyads were drawn is presented in Bagner et al. (2023).
Table 1.
Demographics by Assigned Intervention Group at Time 1
| Substudy sample (N = 62) |
iPCIT (n = 30) |
RAU (n = 32) |
|
|---|---|---|---|
| M (SD) or % (n) | M (SD) or % (n) | M (SD) or % (n) | |
| Child | |||
| Age at Time 1 (months) | 36.26 (.63) | 36.23 (.68) | 36.28 (.58) |
| Child biological sex (%) | |||
| Female | 29.03% (18) | 26.67% (8) | 31.25% (10) |
| Male | 70.97% (44) | 73.33% (22) | 68.75% (22) |
| Race (%) | |||
| White | 72.58% (45) | 83.33% (25) | 62.50% (20) |
| Black/African American | 22.58% (14) | 13.33% (4) | 31.25% (10) |
| Asian | 3.23% (2) | 3.33% (1) | 3.12% (1) |
| Other | 1.61% (1) | — | 3.12% (1) |
| Ethnicity (%) | |||
| Hispanic or Latinx | 70.97% (44) | 83.33% (25) | 59.38% (19) |
| Non-Hispanic/ or Non-Latinx | 27.42% (17) | 16.67% (5) | 37.50% (12) |
| Not reported | 1.61% (1) | — | 3.12% (1) |
| Preferred language (%) | |||
| English | 54.84% (34) | 46.67% (14) | 62.50% (20) |
| Spanish | 43.55% (27) | 53.33% (16) | 34.38% (11) |
| Other language | 1.61% (1) | — | 3.12% (1) |
| Cumulative risk | 2.39 (1.21) | 2.4 (1.25) | 2.38 (1.18) |
| Primary caregiver | |||
| Age (years) | 33.88 (5.67) | 34.21 (6.21) | 33.58 (5.23) |
| Gender (%) | |||
| Male | 6.45% (4) | 3.33% (1) | 9.38% (3) |
| Female | 93.55% (58) | 96.67% (29) | 90.62% (29) |
| Race (%) | |||
| White | 72.58% (45) | 83.33% (25) | 62.50% (20) |
| Black/African American | 24.19% (15) | 13.33% (4) | 34.38% (11) |
| Asian | 3.23% (2) | 3.33% (1) | 3.12% (1) |
| Ethnicity (%) | |||
| Hispanic/Latinx | 67.74% (42) | 80.00% (24) | 56.25% (18) |
| Non-Hispanic/Latinx | 30.65% (19) | 20.00% (6) | 40.62% (13) |
| Not reported | 1.61% (1) | — | 3.12% (1) |
| Relationship to child (%) | |||
| Biological mother | 88.71% (55) | 90.00% (27) | 87.50% (28) |
| Biological father | 6.45% (4) | 6.67% (2) | 6.25% (2) |
| Adoptive mother | 3.23% (2) | — | 6.25% (2) |
| Stepmother | 1.61% (1) | 3.33% (1) | 0.00% |
| Marital status % | |||
| Married/domestic partnership | 64.51% (40) | 60.00% (18) | 68.74% (22) |
| Separated/divorced | 4.84% (3) | 3.33% (1) | 6.24% (2) |
| Single, never married | 30.65% (19) | 36.67% (11) | 25.00% (8) |
| Highest level of education (%) | |||
| Less than high school | 9.68% (6) | 10.00% (3) | 9.37% (3) |
| High school/GED | 11.29% (7) | 20.00% (6) | 3.12% (1) |
| Some college/technical School | 40.32% (25) | 30.00% (9) | 50.00% (16) |
| College degree | 24.19% (15) | 16.67% (5) | 31.25% (10) |
| Some graduate school | 4.84% (3) | 6.67% (2) | 3.12% (1) |
| Graduate/professional degree | 9.68% (6) | 16.67% (5) | 3.12% (1) |
Note: iPCIT = Internet-delivered Parent-Child Interaction Therapy; RAU = referrals as usual.
In the current analytic sample, children were majority Latinx (71%) and White (72.6%) with 22.6% identifying as Black or African American. Children were mostly boys (70.97%), aligning with prevalence rates of behavioral problems in young children (Gershon & Gershon, 2002). Primary caregivers described themselves as White (72.58%), Latina (67.74%), biological mothers (88.71%), and married/partnered (64.51%). Most primary caregivers had completed some college or technical school (40.32%) or possessed a college degree (24.19%). On average, participants reported earning between $20,000 and $40,000 per year, and 69.35% of participants reported earning less than $40,000 per year. Slightly more than half of caregivers indicated that English was the child’s preferred language (54.84%), followed by Spanish (43.55%).
Procedure
Primary RCT
Families were recruited between 2016 and 2019 at three Part C Early Intervention sites in a large city in the southeastern United States. Recruitment occurred during the child’s Early Intervention exit evaluation, which occurred within 3 months of the child’s third birthday. In addition to developmental delay, children considered for inclusion had to have (a) a Child Behavior Checklist externalizing problems T-Score greater than 60 and (b) a primary caregiver who spoke English or Spanish. Children were excluded (a) if they were receiving medication for behavior problems; (b) if the children or their caregiver had deafness or blindness; (c) if the children demonstrated severe social-communication deficits (i.e., caregiver report on Social Responsiveness Scale–Second Edition T-Score > 75); and (d) if the primary caregiver’s standard score was less than 4 on the vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence–Second Edition (for English speakers) or the Escala de Inteligencia Wechsler Para Adultos–Third Edition (for Spanish speakers). For further details, refer to Bagner et al. (2023) and see Figure S1 in the Supplemental Material available online for the primary RCT’s CONSORT diagram.
After providing consent, families completed the Time 1 (T1) assessment. Eligible families (N = 150) were randomized 1:1 (stratified by child sex) to receive up to 20 weeks of either iPCIT (n = 75) or RAUs (n = 75). Major assessments relevant to this analysis, consisting of caregiver reports and observational tasks of family interactions, were conducted in the family’s home at T1 (i.e., before treatment or the RAU period), T2 (following the treatment or the RAU period), and Time 3 (T3; 12 months after intervention or RAU period completion). At T1, groups were the same across demographic variables (e.g., race, ethnicity, caregiver education, primary language) and behavioral variables (e.g., behavioral problems). Families received $100 for each major assessment and a tablet at study completion. All participants completed the treatment phase before the COVID-19 pandemic.
DNA substudy
All participants from the primary RCT were eligible for the DNA substudy. Funding and IRB permission for the DNA substudy became available after the data-collection period for the primary RCT was already under way. Thus, research staff on the primary RCT study team approached participants and interested families on a rolling basis as soon as study IRB permissions were obtained, regardless of the number of sessions they had completed during the intervention phase. Of the 150 families enrolled in the primary RCT, 137 families were approached at T3, and 65 families consented to participation in the DNA substudy at that time and provided a saliva sample. Families did not receive additional compensation for participating in the DNA substudy. Data detailing participants’ rationale for declining participation in the substudy are not available.
Either study staff or a caregiver (under study staff supervision) obtained the children’s saliva samples using a collection kit with a small sponge. For the portion of T3 assessments occurring in the context of COVID-19-instigated safety guidelines, caregivers were provided an at-home saliva-collection kit and detailed video instructions regarding saliva data collection. Of the 65 samples taken at T3, three failed quality-control analyses, resulting in 62 child saliva samples collected at T3 with enough DNA for analysis. Within this group of 62 families, a small pilot group also provided saliva samples at T1 (iPCIT: n = 14; RAU: n = 9).
At T1, the 62 dyads included in the DNA substudy, relative to the 88 dyads who participated only in the RCT, did not significantly differ on the child or caregiver demographics listed in Table 1 or on neighborhood opportunity, child symptoms, or observed parenting (all ps > .05). There was one exception—the substudy sample reported a lower income-to-needs ratio (p = .023), a demographic feature indicative of financial stress that was closely related to some of our adversity indicators (see Measures). This difference indicates that the substudy population may have experienced more financial stress relative to the participants in the primary RCT who did not participate in the substudy.
Parenting intervention
iPCIT uses encrypted videoconferencing technology in which therapists provide live coaching of caregiver-child interactions via webcam and a caregiver-worn earpiece. Like clinic-based parent–child interaction therapy, iPCIT progresses through child-directed interaction (CDI) and parent-directed interaction (PDI) phases. During CDI, caregivers follow their child’s lead in play, learn to use PRIDE skills (Praising child behavior, Reflecting child statements, Imitating child play, Describing child actions, and showing Enjoyment), and avoid questions, commands, and criticisms. They learn to use PRIDE skills in response to appropriate child behaviors and ignore undesirable behaviors. During PDI, caregivers learn to use effective commands and consistently follow through with time-outs to increase child compliance. Families received a tablet (and data plan, for families without Wi-Fi) and a wireless earpiece for treatment. Sessions were conducted weekly by a remote therapist and lasted 1 to 1.5 hours. In the primary RCT, about half of iPCIT-treated families (48%) received treatment in Spanish. A PCIT international global trainer trained postdoctoral or doctoral student therapists. Families randomized to RAU were referred to community-based treatment services.
Measures
Adversity: Cumulative risk
Nine indicators of cumulative risk, including neighborhood-, family-, and parent-level indicators, were assessed at T1. Three neighborhood-level indicators were derived from the Childhood Opportunity Index (Acevedo-Garcia et al., 2014). This index uses family census tracks to operationalize neighborhood-level risk in three domains: educational opportunity (e.g., school poverty, proximity to licensed early childhood educators), health and environmental opportunity (e.g., availability of healthy food, proximity to toxic waste), and social and economic opportunity (e.g., foreclosure rate, proximity to employment). Three family-level socioeconomic-status risks were derived from single-parent status, low income, and primary caregiver’s educational attainment of a high school degree equivalent or fewer years. Given the high cost of living in the geographic area in which this study was conducted, low income was defined as within 250% of the federal poverty line. Three other family-level risks were derived from other self-report measures administered at T1. Specifically, we derived parental depression and anxiety from the Depression, Anxiety, and Stress Scale–21 (DASS-21), using cutoffs indicative of moderate or more severe problems with depression or anxiety (DASS-21 Manual; Crawford et al., 2009), and parenting stress from T1 negative-impact scores from the Family Impact Questionnaire (FIQ; Donenberg & Baker, 1993). This questionnaire assesses the child’s negative impact on the parent’s feelings about parenting (e.g., “my child is more stressful [than other same-age children]”) and the parent’s social life (e.g., “it is more difficult to find a babysitter to stay with [my child]”). Because normative data on the FIQ are not available, parents with mean subscale scores ≥ 1 SD above the sample mean were considered high risk, in line with past cumulative risk research. Numerous studies have operationalized cumulative risk using indicators of parental psychopathology, stress, and conflict (Evans et al., 2013). Across all indicators, risks were coded as present or absent (with any missing risks coded as absent) and combined for a total score with a possible range of 0 to 9; higher scores represented higher cumulative risk.
Observed positive- and negative-parenting behaviors
We assessed parenting using a behavioral coding system with documented reliability and validity: the Dyadic Parent–Child Interaction Coding System–Fourth Edition (DPICS; Eyberg et al., 2014). Parenting assessments were conducted at T1 and T2. Coders masked to treatment condition were trained to 80% agreement with a criterion tape, and 25% of T1 observations were double-coded (mean κ = .83 across codes for the total sample). Positive-parenting behaviors were assessed using “do” skills during child-directed play, which refers to praises, behavior descriptions, and reflections; negative parenting was assessed using “don’t” skills, which refers to questions, commands, and criticisms. Per PCIT theory, all questions are considered “don’t” skills during child-directed play, as questions take control of the conversation, indicate the parent may not be listening to the child, and (particularly when posed one after another) prevent the child from talking. The proportion of caregiver “do” and “don’t” skills was coded to reflect two parenting variables—positive-parenting behaviors versus negative-parenting behaviors—exhibited during a 5-min child-led play task completed at each major assessment.
Child epigenetic age acceleration
At T3, saliva samples were collected from children using Oragene kits (OGR-575) for Assisted Collections (DNA Genotek, Kanata, Ontario, Canada). DNA was extracted and isolated using the DNEasy extraction system (Qiagen, Inc., USA) and assessed for quantity and quality using a Nanodrop spectrophotometer (Smith et al., 2015). Sodium-bisulfite-modified DNA was randomized across 96-well plates on the basis of treatment condition and balancing for sex. DNAm was measured using the Illumina 850K Infinium Methylation EPIC BeadChip (Illumina, San Diego, CA; Pidsley et al., 2016), which interrogates more than 850,000 CpG sites. DNAm was measured in three runs at the University of Minnesota Genomics Center and DNA Genotek. For details on DNAm data processing and quality control, please see the Supplemental Material.
Children’s epigenetic age was calculated using McEwen et al.’s (2019) Pediatric-Buccal-Epigenetic (PedBE) clock, which performs well in samples of children relative to other epigenetic clocks (Wang & Zhou, 2021). Children’s epigenetic age acceleration was calculated as the residuals derived from regressing PedBE age onto chronological age (with precision to the day) at the time their saliva was collected. Epigenetic age acceleration was also adjusted for the proportion of leukocytes and epithelial cells present in the saliva, consistent with previous studies (McEwen et al., 2019; Middleton et al., 2021). Details and descriptive statistics regarding epigenetic age-acceleration estimation can be found in the Supplemental Material.
Data-analytic plan
We used the lavaan package (Rosseel, 2012) in R (R Core Team, 2017) to conduct analyses. In our primary analyses, we used a path analytic framework leveraging intent-to-treat and full information maximum likelihood estimation to regress T3 epigenetic age acceleration on T1 cumulative risk, T2 observed parenting, and the interaction between T1 cumulative risk and T2 observed parenting. To account for stability in parenting, we adjusted for T1 observed parenting. Models were also adjusted for child biological sex. Primary analyses collapsed across treatment groups to optimize variance in parenting in the context of the small sample size ((Bell et al., 2018). Collapsing across treatment groups optimizes variance in parenting in the context of small samples (Bell et al., 2018). To facilitate interpretation, we calculated the interaction using mean-centered parenting but not mean-centered cumulative risk. Separate models were run for positive and negative parenting. Simple-slope analyses were used to interpret significant interactions by plotting effects at ±1 SD of observed parenting. We summarize the results from several post hoc sensitivity analyses to assess whether the relation between cumulative risk and epigenetic age acceleration varied on the basis of treatment group assignment.
Results
Table 2 presents means, standard deviations, sample sizes, and correlations among primary study variables, collapsed across treatment conditions. Neither cumulative risk nor epigenetic age acceleration correlated with other constructs at statistically meaningful levels; however, descriptively, as expected, higher cumulative risk was associated with lower positive parenting and higher negative parenting observed at T1. Missingness was low, with one family missing T1 parenting data and two families missing T2 data.
Table 2.
Correlations, Means, and Standard Deviations (SDs) Among Primary Variables
| Variable | M (SD) | n | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| 1. Cumulative risk at Time 1 | 2.39 (1.21) | 62 | ||||||
| 2. Positive parenting at Time 1 | 0.13 (.10) | 61 | −0.15 | |||||
| 3. Positive parenting at Time 2 | 0.18 (.15) | 60 | 0.02 | 0.28* | ||||
| 4. Negative parenting at Time 1 | 0.51 (.16) | 61 | 0.16 | −0.35** | −0.06 | |||
| 5. Negative parenting at Time 2 | 0.39 (.19) | 60 | −0.01 | −0.17 | −0.58*** | 0.21 | ||
| 6. Epigenetic age acceleration at Time 3 | 0.01 (.50) | 62 | −0.13 | 0.15 | 0.01 | −0.02 | −0.04 | |
| 7. Child biological sex | 0.29 (.46) | 62 | 0.03 | 0.01 | −0.16 | −0.06 | −0.02 | 0.28* |
Note: Epigenetic age acceleration corrected for cell-type proportions (0 = boys, 1 = girls). Higher levels of cumulative risk reflect increased exposure to adversity.
p < .05. **p < .01. ***p < .001.
Preliminary analyses
To justify collapsing across treatment groups in the context of our primary analyses, we first used a path analytic framework to assess whether there was a main effect of intervention on epigenetic age acceleration. First, using a pilot sample of children who provided saliva at T1 (n = 23), we determined epigenetic age acceleration at T1 did not differ across treatment groups, t(9.44) = −0.42, p = .69. Then we regressed epigenetic age acceleration at T3, corrected for cell-type proportions, on treatment condition and child sex. Girls’ epigenetic age acceleration at T3 was higher than boys’ (b = 0.30, p = .03). Consistent with study hypotheses emphasizing the importance of cumulative risk, there was not a main effect of treatment condition on epigenetic age acceleration at T3 (b = 0.14, p = .25, with iPCIT coded as 0 and RAU coded as 1).
Primary analyses
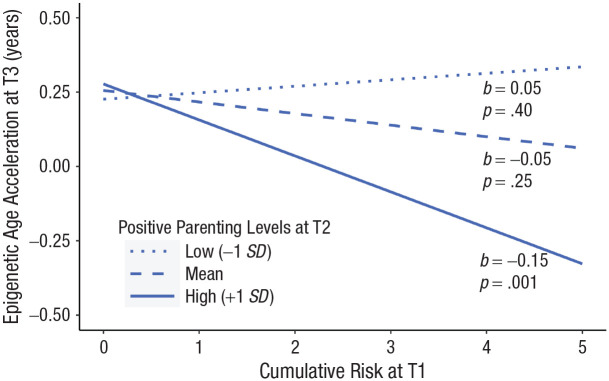
After preliminary analyses, we collapsed the treatment groups to assess the moderating influence of changes in parenting on the association between cumulative risk and child epigenetic age acceleration. Table 3 presents results across models. Model fit was excellent for both positive parenting, χ²(2) = 0.359, p = .81, CFI = 1.00, RMSEA = 0.00, and negative parenting, χ²(2) = 0.359, p = .604, CFI = 1.00, RMSEA = 0.00. Main effects of cumulative risk, positive parenting, and negative parenting on epigenetic age acceleration were not observed in the data; rather, observed parenting modified this association. The interaction between observed positive parenting at T2 and cumulative risk predicted epigenetic age acceleration at T3 (see Table 3 and Fig. 1). Among children whose caregivers displayed more positive T2 parenting behavior (e.g., praising, attending, and rewarding children more, adjusted for T1 levels), children with the highest levels of adversity exposure displayed the lowest levels of T3 epigenetic age acceleration, indicative of slower biological aging for these children. At mean and low levels of positive T2 parenting (i.e., when, descriptively, positive parenting improved slightly, stayed the same, or worsened slightly), cumulative risk was unrelated to epigenetic age acceleration.
Table 3.
Cumulative Risk × Parenting Predicting Epigenetic Age Acceleration
| Predictor | B (SE) | 95% CI | p |
|---|---|---|---|
| Observations: Positive-parenting | |||
| Cumulative risk | −0.05 (0.04) | [−0.13, 0.03] | .25 |
| Parenting at Time 2 (mean centered) | 0.26 (0.41) | [−0.55, 1.08] | .53 |
| Parenting at Time 1 | 0.52 (0.64) | [−0.73, 1.77] | .41 |
| Child biological sex | 0.33 (0.14) | [0.05, 0.60] | .02 |
| Risk × Parenting at Time 2 | −0.68 (0.21) | [−1.09, −0.26] | .02 |
| Observations: Negative-parenting | |||
| Cumulative risk | −0.03 (0.04) | [−0.11, 0.04] | .40 |
| Parenting at Time 2 (mean centered) | −0.14 (0.27) | [−0.67, 0.38] | .59 |
| Parenting at Time 1 | −0.08 (0.43) | [−0.92, 0.76] | .86 |
| Child biological sex | 0.27 (0.15) | [−0.02, 0.56] | .06 |
| Risk × Parenting at Time 2 | 0.47 (0.19) | [0.11, 0.84] | .01 |
Note: Betas are standardized (0 = boys, 1 = girls). Epigenetic age acceleration is corrected for cell-type proportions. Higher levels of cumulative risk reflect increased exposure to adversity. CI = confidence interval.
Fig. 1.
Observed positive parenting at Time 2, adjusted for Time 1, moderates the relationship between cumulative risk at Time 1 and epigenetic age acceleration at Time 3.
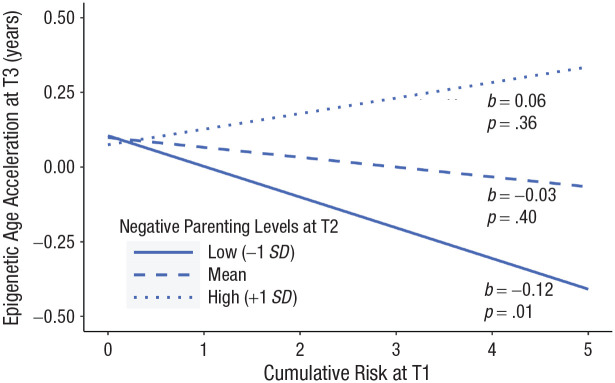
Conceptually paralleling positive-parenting findings, the interaction between observed negative parenting at T2 and cumulative risk predicted T3 epigenetic age acceleration (see Table 3 and Fig. 2). Among children whose caregivers showed less negative-parenting behavior (e.g., criticisms, corrections, and negative statements, adjusted for T1 levels), children with the highest levels of adversity exposure displayed the lowest levels of T3 epigenetic age acceleration, indicative of slower biological aging for these children. At mean and high levels of observed negative T2 parenting (i.e., when, descriptively, negative parenting improved slightly, stayed the same, or worsened slightly), cumulative risk was unrelated to epigenetic age acceleration. Notably, across figures, children whose families endorsed zero risk indicators displayed slightly elevated epigenetic age acceleration.
Fig. 2.
Observed negative parenting at Time 2, adjusted for Time 1, moderates the relationship between cumulative risk at Time 1 and epigenetic age acceleration at Time 3.
Sensitivity analyses
We conducted sensitivity analyses, which are described in detail in the Supplemental Material. Multigroup models, grouped on treatment condition, indicated in the iPCIT condition a significant interaction between T2 positive parenting and T1 cumulative risk, predicting T3 epigenetic age acceleration (b = −0.58, p = .01) in the iPCIT condition, whereas the interaction was nonsignificant in the RAU condition; results from a Wald test indicated differences in the effect of the cumulative Risk × Parenting interaction term on T3 epigenetic age acceleration did not differ across treatment groups (Wald test: p = .95). Thus, differences in parameters across groups should be considered with caution. Using multigroup models, the interaction between cumulative risk and negative parenting was nonsignificant regardless of treatment condition (Wald test: p = .95). Finally, rerunning models using observed parenting change scores instead of T2 levels, adjusted for T1 levels, did not change findings.
Discussion
The present study offers the first controlled evaluation of the relation between adversity exposure, changes in parenting, and epigenetic age acceleration in a sample of predominantly Latinx preschoolers at risk for poor developmental trajectories (i.e., children with developmental delays, children evidencing high levels of behavior problems, or children from low-income families). When positive parenting increased (e.g., via observed praise and behavioral descriptions) and negative parenting decreased (e.g., via observed criticism and commands), children exposed to the highest levels of adversity displayed the lowest epigenetic age acceleration, potentially indicating slower biological aging. Of critical importance, positive and negative-parenting skills constitute the foundational elements of behavioral parenting interventions for young children (Forehand et al., 2014). Caregivers engaging in positive-parenting skills are closely attuned to their child’s behaviors, and they frequently notice and reward behavior that aligns with adaptive social functioning (e.g., sharing, staying focused). Caregivers engaged in fewer negative-parenting behaviors spend less time using criticisms, corrections, and negative statements that have been shown to negatively impact children, caregivers, and their relationship (Forehand et al., 2014).
Notably, cumulative risk was unrelated to epigenetic age acceleration assessed 1.5 years later among families whose parenting practices improved only slightly, stayed the same, or became slightly worse. Indeed, differences in epigenetic age acceleration were small among children reporting low levels of risk exposure, regardless of variability in parenting practices (see Figs. 1 and 2). The strongest biological evidence of buffering was observed in children situated in families in which a parent exhibited positive-parenting practices while coping with high levels of adversity.
Findings align with research indicating that children exposed to relatively high levels of adversity may be most sensitive to improved parenting (Beijers et al., 2020) and that early childhood is a sensitive period for the biological impact of adversity in the epigenome (Aristizabal et al., 2020; Lussier et al., 2022). Further, the direction of the effects of epigenetic age acceleration suggested that increased levels of positive parenting are related to improved health. Research on the relation between epigenetic age acceleration and prospective child health is limited in pediatric samples; however, emerging science suggests that higher levels of epigenetic age acceleration associates with poor health outcomes (e.g., internalizing disorders, Dammering et al., 2021; reduced neonatal brain growth, Gomaa et al., 2022). Our findings suggest that interventions targeting parenting practices may promote resilience in children exposed to adversity. They also show that caregivers dealing with hardship can protect their children from the negative effects of stress, particularly when caregivers are provided access to effective interventions designed to enhance parenting skills.
Importantly, we did not identify a main or moderated effect of intervention assignment on T3 differences in epigenetic age acceleration. However, as reported in Bagner et al. (2023), in the overall sample (n = 150), parents who were randomized to iPCIT evidenced enhanced parenting practices relative to parents in the control condition. In the current analysis, our findings indicated that stress buffering was most evident among children whose caregiver displayed high levels of positive-parenting practices while coping with multiple forms of adversity. Our null findings for intervention on biological aging may be due to diminished sample size (n = 62), rather than a true absence of effect. Testing this model in a larger intervention sample is necessary to better understand the impact of dyadic intervention on epigenetic age acceleration.
It is important to highlight that epigenetic age acceleration was elevated in children endorsing exposure to zero risks (see Figs. 1 and 2). All children effectively endorsed at least two risks because of the primary RCT’s aim, which was to evaluate iPCIT in the context of clinically elevated behavior problems and developmental delays. Accordingly, these risks are not a feature of our operationalization of cumulative risk, as they would not lend statistically interpretable variability to our measurement. Importantly, generalizing findings outside of samples of children with behavior problems and developmental delays should be done with caution, both as a function of this study’s small sample size and because of its unique, understudied participant population.
Current study strengths included the longitudinal RCT design in early childhood and in an understudied sample exposed to elevated stress. Further, data were collected using multiple levels of analysis (e.g., self-reported, demographic, geocoded, observational, and biological) from multiple reporters. In the current age of accelerated telehealth adoption (Sullivan et al., 2021), the use of an Internet-based parenting intervention improved the prospective utility and validity of these findings. Telehealth interventions are easier to disseminate to the millions of households for whom it is not feasible to come in person to the clinic, and parenting skills applied via telehealth in each family’s specific home environment, using their own toys in their own space and interacting naturally with other family members, are particularly generalizable (Sullivan et al., 2021). Further, the families who participated in this study represented large and growing portions of the U.S. population—Latinx families (Batalova et al., 2021) and children with developmental delays (Zablotsky et al., 2019); looking forward, it is important to increase efforts to better understand families with these identities, particularly in the context of their current and historical exposure to elevated harm, discrimination, and the resulting risk of poor health (Iruka et al., 2022). Finally, this study used saliva samples to obtain DNA, providing support for the pediatric buccal epigenetic clock as a noninvasive biomarker sensitive to changes in parenting in the context of risk exposure. The current study adds to a growing literature examining how enhancing the family environment may promote resilience and may positively alter stress-related physiological systems among youth, potentially mitigating the risk of prospective development of chronic mental and physical health outcomes.
Findings from this study must be considered in the context of several limitations. First, analyses in this study were from a substudy trial and were underpowered, precluding a primary focus on the role of intervention in driving the parenting and cumulative risk interaction. Only 65 of the 150 families participating in the primary RCT were eligible for the current analyses, and data detailing why families elected not to participate in the substudy are not available. Further, income-to-needs data suggest that participants in the analytic sample of the DNA substudy may experience higher financial stress relative to those families that did not participate. Replication in a larger trial is necessary to conclude that treatment assignment drives the moderating effect of improved parenting on the relation between adversity exposure and accelerated aging. Although we were able to confirm that epigenetic age acceleration was the same across treatment conditions in a pilot sample of participants who provided T1 data, we were unable to control for it across models, and several other key covariates were not assessed, such as preexisting child health conditions that are linked to early mortality. Third, girls were underrepresented in this sample. Because boys are more likely to be seen for behavioral disorders, particularly in this young age group (Gershon & Gershon, 2002), this underrepresentation of girls is expected. Girls evidenced higher levels of epigenetic age acceleration in one model. With so few girls in this study (n = 18), it was difficult to inspect this effect for potential correlates and moderators. Fourth, parenting data largely reflected maternal behaviors. This challenge plagues much of developmental and clinical psychology research, and increased attention to other caregivers (e.g., fathers; Parent et al., 2017) is necessary for future studies.
Future directions
Results from this study suggest that improvements in parenting may attenuate the impact of adversity exposure on child development, specifically on biomarkers of aging such as epigenetic age acceleration. In addition to testing these hypotheses in a larger sample, future research testing both the role of the intervention and the relationship between changes in parenting, adversity exposure, and epigenetic aging is necessary. Future work may also benefit from testing this model with more distal follow-ups to assess the durability of effects. Additionally, aligned with Dammering et al.’s (2021) findings linking epigenetic age and internalizing problems, studies may benefit from testing whether parenting changes influenced the degree to which epigenetic age acceleration is associated with prospective psychological indicators.
Clinical and policy implications
Results may support policy and clinical guidelines. First, they make the compelling case that improvements in parenting, over time, translate to physiological changes in young children. During early childhood, consideration of the caregiver-child relationship is key to promoting a better understanding of development (Garner & Yogman, 2021). Accordingly, promoting positive caregiving practices may also be conceptualized as a primary target of intervention. Presently, at least in the United States, most insurance requires evidence of clinical levels of behavioral health problems before access is granted to parenting interventions. Because accelerated epigenetic age is linked not only to psychological conditions but also to many costly physical health conditions, broadening access to these services may improve child outcomes. Further, many social determinants of health, as well as parental characteristics, precede risk factors for child maladjustment, and such factors fall outside the realm of a childhood behavioral health diagnosis. Therefore, policy changes that reduce adversity (e.g., poverty reduction) may thus also be effective in reducing epigenetic aging acceleration (Troller-Renfree et al., 2022). Reductions in some stressors may also promote better engagement in parenting interventions (Hostinar & Miller, 2019), potentially enhancing the buffering effect of effective parenting practices.
Supplemental Material
Supplemental material, sj-docx-2-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science
Supplemental material, sj-docx-3-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science
Supplemental material, sj-pdf-1-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science
Footnotes
ORCID iD: Alexandra D. W. Sullivan  https://orcid.org/0000-0003-4513-9578
https://orcid.org/0000-0003-4513-9578
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976231194221
Transparency
Action Editor: Daniela Schiller
Editor: Patricia J. Bauer
Author Contribution(s)
Alexandra D. W. Sullivan: Conceptualization; Formal analysis; Funding acquisition; Visualization; Writing – original draft; Writing – review & editing.
Anne K. Bozack: Data curation; Formal analysis; Visualization; Writing – review & editing.
Andres Cardenas: Data curation; Formal analysis; Visualization; Writing – review & editing.
Jonathan S. Comer: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Daniel M. Bagner: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Rex Forehand: Conceptualization; Methodology; Supervision; Writing – review & editing.
Justin Parent: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
J. S. Comer receives textbook royalties from Macmillan Learning and an editorial stipend from the Association for Behavioral and Cognitive Therapies (ABCT) for work unrelated to this research. R. Forehand receives royalties from Guilford Press and McGraw Hill for work related to parenting interventions. A. D. W. Sullivan, A. K. Bozack, A. Cardenas, D. M. Bagner, and J. Parent have no financial or potential conflicts of interest.
Funding: This work was supported by research grants from the National Institute of Child Health and Human Development (to A. D. W. Sullivan, Grant No. F31HD098825; to D. M. Bagner and J. S. Comer, Grant No. R01HD084497) and from the National Center of Minority Health and Health Disparities (to J. Parent, Grant No. R01MD015401).
Open Practices: This study was not preregistered. The data have not been made available on a permanent third-party archive because they include sensitive epigenomic and neighborhood-level data; requests for the data can be sent to the corresponding author. Primary analyses code and output are included as Supplemental Material associated with this article.
References
- Acevedo-Garcia D., McArdle N., Hardy E. F., Crisan U. I., Romano B., Norris D., Baek M., Reece J. (2014). The Child Opportunity Index: Improving collaboration between community development and public health. Health Affairs, 33(11), 1948–1957. 10.1377/hlthaff.2014.0679 [DOI] [PubMed] [Google Scholar]
- Aristizabal M. J., Anreiter I., Halldorsdottir T., Odgers C. L., McDade T. W., Goldenberg A., Mostafavi S., Kobor M. S., Binder E. B., Sokolowski M. B., O’Donnell K. J. (2020). Biological embedding of experience: A primer on epigenetics. Proceedings of the National Academy of Sciences, USA, 117(38), 23261–23269. 10.1073/pnas.1820838116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A., Bernard K., Roth T. L., Rosen J. B., Dozier M. (2015). Parental responsiveness moderates the association between early-life stress and reduced telomere length. Developmental Psychopathology, 25(3), 577–585. 10.1017/S0954579413000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagner D. M., Berkovits M. D., Coxe S., Frech N., Garcia D., Golik A., Heflin B. H., Heymann P., Javadi N., Sanchez A. L., Wilson M. K., Comer J. S. (2023). Telehealth treatment of behavior problems in young children with developmental delay: A randomized clinical trial. JAMA Pediatrics. Advance online publication. 10.1001/jamapediatrics.2022.5204 [DOI] [PMC free article] [PubMed]
- Batalova J., Hanna M., Levesque C. (2021). Frequently requested statistics on immigrants and immigration in the United States. Migration Policy Institute. https://www.migrationpolicy.org/article/frequently-requested-statistics-immigrants-and-immigration-united-states2020 [Google Scholar]
- Beach S. R. H., Gibbons F. X., Carter S. E., Ong M. L., Lavner J. A., Lei M.-K., Simons R. L., Gerrard M., Philibert R. A. (2021). Childhood adversity predicts black young adults’ DNA methylation-based accelerated aging: A dual pathway model. Development and Psychopathology. Advance online publication. 10.1017/S0954579421001541 [DOI] [PMC free article] [PubMed]
- Beijers R., Hartman S., Shalev I., Hastings W., Mattern B. C., de Weerth C., Belsky J. (2020). Testing three hypotheses about effects of sensitive-insensitive parenting on telomeres. Developmental Psychology, 56(2), 237–250. 10.1037/dev0000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell Z., Shader T., Webster-Stratton C., Reid M. J., Beauchaine T. P. (2018). Improvements in negative parenting mediate changes in children’s autonomic responding following a preschool intervention for ADHD. Clinical Psychological Science, 6(1), 134–144. 10.1177/2167702617727559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne S. V., Korom M., Dozier M. (2022). Consequences of inadequate caregiving for children’s attachment, neurobiological development, and adaptive functioning. Clinical Child and Family Psychology Review, 25(1), 166–181. 10.1007/s10567-022-00386-4 [DOI] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen E., Beach S. R., Miller G. E. (2016). Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. Journal of Child Psychology and Psychiatry, 57(5), 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen E., Kobor M., Beach S. R. H., Lei M.-K., Barr A., Lin D. T.-S., Miller G. E. (2021). Risky family climates presage increased cellular aging in young adulthood. Psychoneuroendocrinology, 130, Article 105256. 10.1016/j.psyneuen.2021.105256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N. L., Rosen M. L., Williams E. S., McLaughlin K. A. (2020). Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin, 146, 721–764. 10.1037/bul0000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W. E., Shanahan L., McGinnis E. W., Aberg K. A., van den Oord E. J. C. G. (2022). Early adversities accelerate epigenetic aging into adulthood: A 10-year, within-subject analysis. Journal of Child Psychology and Psychiatry, 63(11), 1308–1315. 10.1111/jcpp.13575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. R., Garthwaite P. H., Lawrie C. J., Henry J. D., MacDonald M. A., Sutherland J., Sinha P. (2009). A convenient method of obtaining percentile norms and accompanying interval estimates for self-report mood scales (DASS, DASS-21, HADS, PANAS, and sAD). British Journal of Clinical Psychology, 48(2), 163–180. 10.1348/014466508X377757 [DOI] [PubMed] [Google Scholar]
- Dammering F., Martins J., Dittrich K., Czamara D., Rex-Haffner M., Overfeld J., de Punder K., Buss C., Entringer S., Winter S. M., Binder E. B., Heim C. (2021). The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiology of Stress, 15, Article 100394. 10.1016/j.ynstr.2021.100394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donenberg G., Baker B. L. (1993). The impact of young children with externalizing behaviors on their families. Journal of Abnormal Child Psychology, 21(2), 179–198. 10.1007/BF00911315 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Li D., Whipple S. S. (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Eyberg S., Nelson M., Ginn N., Bhuiyan N., Boggs S. (2014). Dyadic Parent–Child Interaction Coding System (DPICS): Comprehensive manual for research and training, fourth edition. PCIT International. [Google Scholar]
- Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A. M., Edwards V., Koss M. P., Marks J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Forehand R., Lafko N., Parent J., Burt K. B. (2014). Is parenting the mediator of change in behavioral parent training for externalizing problems of youth? Clinical Psychology Review, 34(8), 608–619. 10.1016/j.cpr.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner A., Yogman M. (2021). Preventing childhood toxic stress: Partnering with families and communities to promote relational health. Pediatrics, 148(2), Article e2021052582. 10.1542/peds.2021-052582 [DOI] [PubMed] [Google Scholar]
- Gershon J., Gershon J. (2002). A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders, 5(3), 143–154. 10.1177/108705470200500302 [DOI] [PubMed] [Google Scholar]
- Gomaa N., Konwar C., Gladish N., Au-Young S. H., Guo T., Sheng M., Merrill S. M., Kelly E., Chau V., Branson H. M., Ly L. G., Duerden E. G., Grunau R. E., Kobor M. S., Miller S. P. (2022). Association of pediatric buccal epigenetic age acceleration with adverse neonatal brain growth and neurodevelopmental outcomes among children born very preterm with a neonatal infection. JAMA Network Open, 5(11), Article e2239796. 10.1001/jamanetworkopen.2022.39796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummitt L. R., Kreski N. T., Kim S. G., Platt J., Keyes K. M., McLaughlin K. A. (2021). Association of childhood adversity with morbidity and mortality in US adults: A systematic review. JAMA Pediatrics, 175(12), 1269–1278. 10.1001/jamapediatrics.2021.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), Article R115. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S., Raj K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), Article 6. 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- Hostinar C. E., Miller G. E. (2019). Protective factors for youth confronting economic hardship: Current challenges and future avenues in resilience research. American Psychologist, 74(6), 641–652. 10.1037/amp0000520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoye J. R., Cheishvili D., Yarger H. A., Roth T. L., Szyf M., Dozier M. (2020). Preliminary indications that the Attachment and Biobehavioral Catch-up Intervention alters DNA methylation in maltreated children. Development and Psychopathology, 32(4), 1486–1494. 10.1017/S0954579419001421 [DOI] [PubMed] [Google Scholar]
- Iruka I. U., Gardner-Neblett N., Telfer N. A., Ibekwe-Okafor N., Curenton S. M., Sims J., Sansbury A. B., Neblett E. W. (2022). Effects of racism on child development: Advancing antiracist developmental science. Annual Review of Developmental Psychology, 4(1), 109–132. 10.1146/annurev-devpsych-121020-031339 [DOI] [Google Scholar]
- Lei M. K., Brody G. H., Beach S. R. (2022). Intervention effects on self-control decrease speed of biological aging mediated by changes in substance use: A longitudinal study of African American youth. Family Process, 61(2), 659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Humphreys K. L., Tracy A., Hoffmeister J.-A., Ip E., Gotlib I. H. (2020). Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 59(7), 842–855. 10.1016/j.jaac.2019.10.011 [DOI] [PubMed] [Google Scholar]
- Liu S. R., Kia-Keating M., Nylund-Gibson K. (2018). Patterns of adversity and pathways to health among White, Black, and Latinx youth. Child Abuse & Neglect, 86, 89–99. 10.1016/j.chiabu.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Lussier A. A., Zhu Y., Smith B. J., Simpkin A. J., Smith A., Suderman M. J., Walton E., Relton C., Ressler K. J., Dunn E. C. (2022). Sensitive periods for the effect of childhood adversity on DNA methylation: Updated results from a prospective, longitudinal study. Biological Psychiatry Global Open Science. Advance online publication. 10.1016/j.bpsgos.2022.04.002 [DOI] [PMC free article] [PubMed]
- Masten A. S., Palmer A. R. (2019). Parenting to promote resilience in children. In Bornstein M. H. (Ed.), Handbook of parenting: The practice of parenting (Vol. 5, 3rd ed., pp. 156–188). Routledge/Taylor & Francis Group. 10.4324/9780429401695-6 [DOI] [Google Scholar]
- McEwen L. M., O’Donnell K. J., McGill M. G., Edgar R. D., Jones M. J., MacIsaac J. L., Lin D. T. S., Ramadori K., Morin A., Gladish N., Garg E., Unternaehrer E., Pokhvisneva I., Karnani N., Kee M. Z. L., Klengel T., Adler N. E., Barr R. G., Letourneau N., . . . Kobor M. S. (2019). The PedBE clock accurately estimates DNA methylation age in pediatric buccal cells. Proceedings of the National Academy of Sciences, USA, 117(38), 23329–23335. 10.1073/pnas.1820843116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton L. Y. M., Dou J., Fisher J., Heiss J. A., Nguyen V. K., Just A. C., Faul J., Ware E. B., Mitchell C., Colacino J. A., Bakulski K. M. (2021). Saliva cell type DNA methylation reference panel for epidemiological studies in children. Epigenetics, 17(2), 161–177. 10.1080/15592294.2021.1890874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent J., Forehand R., Pomerantz H., Peisch V., Seehuus M. (2017). Father participation in child psychopathology research. Journal of Abnormal Child Psychology, 45(7), 1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidsley R., Zotenko E., Peters T. J., Lawrence M. G., Risbridger G. P., Molloy P., Van Djik S., Muhlhausler B., Stirzaker C., Clark S. J. (2016). Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biology, 17(1), Article 208. 10.1186/s13059-016-1066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rosseel Y. (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(2), 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Smith A. K., Kilaru V., Klengel T., Mercer K. B., Bradley B., Conneely K. N., Ressler K. J., Binder E. B. (2015). DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 168(1), 36–44. 10.1002/ajmg.b.32278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan A. D., Forehand R., Acosta J., Parent J., Comer J. S., Loiselle R., Jones D. J. (2021). COVID-19 and the acceleration of behavioral parent training telehealth: Current status and future directions. Cognitive and Behavioral Practice, 28(4), 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J. A., Cleveland S., Chen T., Gradus J. L. (2023). Psychological and biological mechanisms linking trauma with cardiovascular disease risk. Translational Psychiatry, 13(1), Article 1. 10.1038/s41398-023-02330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J. A., Colich N. L., Uddin M., Armstrong D., McLaughlin K. A. (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S. V., Costanzo M. A., Duncan G. J., Magnuson K., Gennetian L. A., Yoshikawa H., Halpern-Meekin S., Fox N. A., Noble K. G. (2022). The impact of a poverty reduction intervention on infant brain activity. Proceedings of the National Academy of Sciences, USA, 119(5), Article e2115649119. 10.1073/pnas.2115649119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou W.-H. (2021). Epigenetic clocks in the pediatric population: When and why they tick? Chinese Medical Journal, 134(24), 2901–2910. 10.1097/CM9.0000000000001723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E. J., Maniates H., Nugent N., Maihofer A. X., Armstrong D., Ratanatharathorn A., Ashley-Koch A. E., Garrett M., Kimbrel N. A., Lori A., VA Mid-Atlantic MIRECC Workgroup, Aiello A. E., Baker D. G., Beckham J. C., Boks M. P., Galea S., Geuze E., Hauser M. A., Kessler R. C., . . . Logue M. W. (2018). Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology, 92, 123–134. 10.1016/j.psyneuen.2017.12.007 [DOI] [PMC free article] [PubMed]
- Zablotsky B., Black L. I., Maenner M. J., Schieve L. A., Danielson M. L., Bitsko R. H., Blumberg S. J., Kogan M. D., Boyle C. A. (2019). Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics, 144(4), Article e20190811. 10.1542/peds.2019-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science
Supplemental material, sj-docx-3-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science
Supplemental material, sj-pdf-1-pss-10.1177_09567976231194221 for Parenting Practices May Buffer the Impact of Adversity on Epigenetic Age Acceleration Among Young Children With Developmental Delays by Alexandra D. W. Sullivan, Anne K. Bozack, Andres Cardenas, Jonathan S. Comer, Daniel M. Bagner, Rex Forehand and Justin Parent in Psychological Science