Abstract
Background
The importance of sheep breeding in the Mediterranean part of the eastern Adriatic has a long tradition since its arrival during the Neolithic migrations. Sheep production system is extensive and generally carried out in traditional systems without intensive systematic breeding programmes for high uniform trait production (carcass, wool and milk yield). Therefore, eight indigenous Croatian sheep breeds from eastern Adriatic treated here as metapopulation (EAS), are generally considered as multipurpose breeds (milk, meat and wool), not specialised for a particular type of production, but known for their robustness and resistance to certain environmental conditions. Our objective was to identify genomic regions and genes that exhibit patterns of positive selection signatures, decipher their biological and productive functionality, and provide a "genomic" characterization of EAS adaptation and determine its production type.
Results
We identified positive selection signatures in EAS using several methods based on reduced local variation, linkage disequilibrium and site frequency spectrum (eROHi, iHS, nSL and CLR). Our analyses identified numerous genomic regions and genes (e.g., desmosomal cadherin and desmoglein gene families) associated with environmental adaptation and economically important traits. Most candidate genes were related to meat/production and health/immune response traits, while some of the candidate genes discovered were important for domestication and evolutionary processes (e.g., HOXa gene family and FSIP2). These results were also confirmed by GO and QTL enrichment analysis.
Conclusions
Our results contribute to a better understanding of the unique adaptive genetic architecture of EAS and define its productive type, ultimately providing a new opportunity for future breeding programmes. At the same time, the numerous genes identified will improve our understanding of ruminant (sheep) robustness and resistance in the harsh and specific Mediterranean environment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-023-00936-y.
Keywords: Composite-likelihood ratio, East Adriatic sheep, Extreme ROH islands, Genomic selection signatures, Integrated haplotype score, Number of segregating sites by length
Background
Around 12,000 BP, sheep (Ovis aries) were among the first animals domesticated by humans during the Neolithic Revolution [1–3]. Along with goats, sheep were historically selected and preferred for their milk, meat, and wool production. They thrive outdoors and adapt well to local conditions, which in most cases are not suitable or sufficient for cattle. Sheep production is deeply rooted in the eastern Adriatic and was introduced during the Neolithic migrations [4–6] so all populations have the same non-native origin. Croatian production primarily includes traditional systems without specialized breeding for carcass or milk yield [7] making local breeds such as Istrian sheep, Cres Island sheep, Pag Island sheep, Krk Island sheep, Rab Island sheep, Lika Pramenka sheep, Dalmatian Pramenka sheep, and Dubrovnik Ruda sheep valuable genetic resources. These breeds are bred for milk, meat and to a limited extent wool, but are hardy and resilient in harsh environments. They are informally (also from the breeder’s point of view) considered well suited for meat and dairy production, although they are not favored over international breeds in any of these production directions.
With the development of molecular genetics, the identification of selection signatures reflecting natural or artificial selection has become possible, and numerous methods have been developed [8–11]. Four methods developed to identify selection signatures in livestock populations, all based on within-population approaches: i) extremely high SNP incidence in ROH ("extreme ROH islands" or "eROHi"), ii) integrated haplotype score (iHS), iii) number of segregating sites by length (nSL), and iv) composite likelihood ratio test (CLR), are widely used to identify hard and soft selection signals [12–18]. For example, many previous studies on Mediterranean sheep used statistical methods with medium density genomic data and found candidate genes for coat color, morphology, milk, and wool [19–24].
An analysis by Ciani et al. [25] identified Croatian breeds from the eastern Adriatic as part of the distinct "Balkan sheep group" with considerable genetic variation, which was confirmed by a broader diversity study using Infinium HD SNP arrays [7]. All of these breeds are distributed over an area approximately 1,000 km long and 50 km wide (more information provided in the Additional file 1). Although each breed or population is kept in a small but specific area, such as islands or peninsulas, they still form a unique group with the same origin, breeding environment, and socio-agricultural background. Understanding their genomic variation is critical for sustainable breeding to address environmental and economic challenges, while evidence of positive selection patterns offers insights into domestication, evolution, and post-Neolithic change in Europe.
Using a comprehensive approach involving four selection signature methods, we aimed to uncover adaptive selection signals, profile production types, and elucidate gene functions within selection patterns. These analyses are novel for autosomes in EAS as a member of the "Balkan sheep group" breeds/populations where selection signatures have not been identified previously. We have located selection signature regions, identified candidate genes, and characterized their biological/molecular functions. In general, the knowledge gained in this study improves our understanding of the genetic background of production capacity in EAS, which will help to improve EAS breeding.
Methods
Data and genotyping
The animals included in this study were selected and collected in collaboration with the National Gene Bank of the Croatian Ministry of Agriculture, and all procedures with the animals were performed in accordance with national and European ethical protocols and guidelines. The animals were raised by registered breeders at different locations in Croatia, and information was available on their origin and the exact location of the farm. Sampling of closely related animals (parents with offspring, full or half siblings) was avoided.
A total of 196 animals of East Adriatic sheep breeds [7], were analysed as metapopulation (EAS) in this study, which includes Istrian sheep (n = 25; from 8 flocks), Cres Island sheep (n = 19; from 5 flocks), Pag Island sheep (n = 45; from 16 flocks), Krk Island sheep (n = 19; from 3 flocks), Rab Island sheep (n = 18; from 17 flocks), Lika Pramenka sheep (n = 18; from 18 flocks), Dalmatian Pramenka sheep (n = 25; from 18 flocks), and Dubrovnik Ruda sheep (n = 26; from 17 flocks). Short descriptions of the breeds and pictures can be found in the Additional file 1. While collecting samples, our aim was to collect samples from high number of flocks in order to obtain representative sample and to avoid close genetic relationships, therefore we collected samples from overall 102 flocks. With the exception of the Pag Island sheep, sample sizes were similar. More detailed information on the samples (sex, map locations and coordinates etc.) in this study can be found in Additional file 2. As described in the Background, we decided to analyse EAS as one metapopulation in this study. Our preliminary analysis was performed for each of the breed separately, however, the signals of selection were less clear and more power was obvious in the case of metapopulation due to the higher sample size. Furthermore, we conducted an analysis of the decay of linkage disequilibrium (LD), which we also performed (Additional file 3). This analysis revealed a consistent LD decay pattern across all breeds, providing additional support for the effectiveness of employing a large metapopulation strategy. All animals were genotyped using the Ovine Infinium® HD SNP BeadChip 600 K (606,006 SNPs). Skin tissue samples from the ear were collected as part of the regular sampling of local autochthonous breeds by the National Gene Bank and from the blood, from which DNA was isolated using a commercial kit (DNeasy Blood and Tissue Kits, Qiagene, Germany). Plink 1.9 software [26] was used for quality control and data management. Of the genotypes obtained, we analysed only autosomal SNPs whose chromosomal position was assigned. SNP positions were based on the sheep genome assembly ARS-UI_Ramb_v2.0, and duplicate or misassigned SNPs were excluded from the analysis. SNPs missing more than 5% of genotypes and SNPs with an Illumina GenCall score < 0.7 were excluded from analysis. Sheep missing > 10% of the genotypes were also excluded from further analysis, while the thresholds to filter out all SNPs with a minor allele frequency of < 1% or due to deviations from Hardy–Weinberg equilibrium (HWE) were < 0.01%. Finally, to avoid duplicate and related individuals (first- and second- degree relatives), the degree of common ancestry was calculated for each pair of individuals using a threshold of identity by descent (IBD) > 0.18. After performing these quality control criteria, the final data set consisted of 500,831 SNPs and 195 sheep (98 females and 97 males).
eROHi analysis
ROH analysis was performed with the detectRUNS package in R software [27] and SNP & Variation Suite (v7.6.8 Win64; Golden Helix, Bozeman, MT, USA, www.goldenhelix.com) using the consecutive runs method, a "windowless" method that searches the genome for ROHs regardless of window size. The criteria for detecting runs were determined by recommendations for HD SNP data [28]. Thus, the minimum ROH length was set to 1 Mb, the minimum number of consecutive homozygous SNPs in the called ROH was set to 15, the minimum SNP density for the ROH was set to at least one SNP every 0.1 Mb, the maximum distance (gap) between consecutive homozygous SNPs was set to 1 Mb. In longer runs, one heterozygous SNP was allowed due to the possibility of genotyping or assignment errors. Finally, detected ROHs were categorised based on their length in Mb as follows: 1–2 Mb, 2–4 Mb, 4–8 Mb, 8–16 Mb, and > 16 Mb. To establish a significance threshold, the frequency of ROHs was calculated and normalised by their chromosome means, with the transformed value represented as −log10 (P) from the right tail of the normal distribution for each of the chromosomes separately. In this way, overrepresentation of extreme ROH islands due to chromosome size was avoided. SNPs with −log10 (P value) ≥ 4 were considered outliers, whereas only regions with consecutive outliers were considered as selection signals [29].
iHS and nSL analyses
For the analyses of iHS and nSL, the required haplotypes were phased from SNP data in SHAPEIT2 software [30] with 200 conditioning states and a window size of 2 Mb. The iHS statistic, based on the concept of Extended Haplotype Homozygosity (EHH) [31], was calculated in R software using the rehh package [32]. Two-tailed P values for iHS [33] were calculated as −log10(2Φ(−|iHS|)), where Φ(x) represents the Gaussian cumulative distribution function. Sliding windows of 0.5 Mb with an overlap of 0.01 Mb with adjacent windows were used, and windows with three or more SNPs exceeding the threshold (−log10 (P value) ≥ 4) were considered as selection signatures. Since recombination rates are highly heterogeneous across the genome, we also used the nSL method, which is similar to the iHS method but was proved to be more robust in detecting selection signatures, regardless of the variation in recombination rate [34]. The nSL statistics were calculated with the selscan program [35] using the default parameters. Thus, a gap scale parameter of 0.02 Mb and a maximum allowable distance between two SNPs of 0.2 Mb were used, whereas all SNPs below 0.05 were removed. The results of this analysis were then normalized to 100 frequency bins and to two-tailed P values for each SNP across all chromosomes using the program norm [35]. Using the same approach as the iHS method, selection signatures were determined based on the normalized values.
CLR analysis
The SweeD software, Linux version 3.0 [17], was used to calculate composite likelihood ratios (CLR) to test local reductions in nucleotide diversity along chromosomes. The reimplementation of the SweepFinder CLR test [8] in SweeD provides a robust representation of the genetic hitchhiking process caused by the chromosomal linkage of advantageous alleles to neighbouring polymorphisms that represent signatures of strong directional selection [36]. The SweeD CLR score represents the likelihood of a selective sweep in a tested region based on a denominator (likelihood for a null hypothesis empirically derived across all SNP positions assuming no selection) and a numerator (likelihood of a sweep in a tested region). The grid size determining the number of regions to be tested was set at 10,000 per chromosome. Larger grid sizes were also tested (up to 100,000 per chromosome) without significant impact on the final results. The top 0.1% of hits were interpreted as loci under selection, resulting in a genome-wide threshold of 10.92.
Gene annotation and functional gene analysis
Functional biological interpretation of the discovered selection signatures was performed by conducting GO analyses [37]. Only significant selection signatures (regions whose initial and final positions overlapped) identified by at least two different methods were analysed (Additional file 4). Annotation of candidate genes was performed with the Ensembl [38] tool BioMart using the latest available assembly (ARS-UI_Ramb_v2.0). A total of 19 regions with an average region length of 0.970 Mb met our criteria and were subjected to further analysis (https://www.ensembl.org/biomart/martview). Searching for the associated genes using BioMart yielded 4,984 gene transcripts or 349 genes with unique Gene Stable IDs. The identified genes were further classified and subjected to GO and protein domain (INTERPRO domains) enrichment analysis using publicly available software DAVID [39, 40]. Gene IDs were analysed using the online tool the Database for Annotation, Visualization and Integrated Discovery (DAVID), resulting in 25 functional similarity clusters. The clustering provides useful approach to group a large number of genes which are functionally similar, and therefore make gene annotations more understandable in the context of biology. The clustering algorithm uses Kappa statistics to measure how much genes are shared between two annotations. It also employs fuzzy clustering to categorize similar annotation groups based on these Kappa values. Essentially, if annotations have a greater number of common genes, they are more likely to be grouped together.
Production and breeding type characterization
We also characterised the production mode of EAS by analysing the properties of positive selection signals ("candidate genes") using the QTL (Quantitative Trait Loci) database for sheep (https://www.animalgenome.org/cgi-bin/QTLdb/OA/index). The QTL database contains sheep QTL/association data from 201 previously published studies and more than 3,000 QTLs. In these analyses, we focused on candidate genes whose significance overlapped with all methods used in this study. QTLs from the QTL database were annotated using the GALLO R package [41] to query the Animal QTLdb (https://www.animalgenome.org/cgi-bin/QTLdb/index) for previously identified QTLs in regions of interest.
Results
Detected signatures of selection
In this study, we used four different intrapopulation methods for highly dispersed genomic information with an average spacing of 5 kb between SNPs, which provided us with a powerful tool to accurately detect positive genomic selection signatures at high resolution. A total of 165 genomic regions with positive selection signals were identified, of which 19 were identified by at least two different methods. Therefore, we conservatively focus our discussion and interpretation only on putative genes located in genomic regions of high confidence. These genes are consequently treated as candidate genes specific to the investigated East Adriatic sheep breeds.
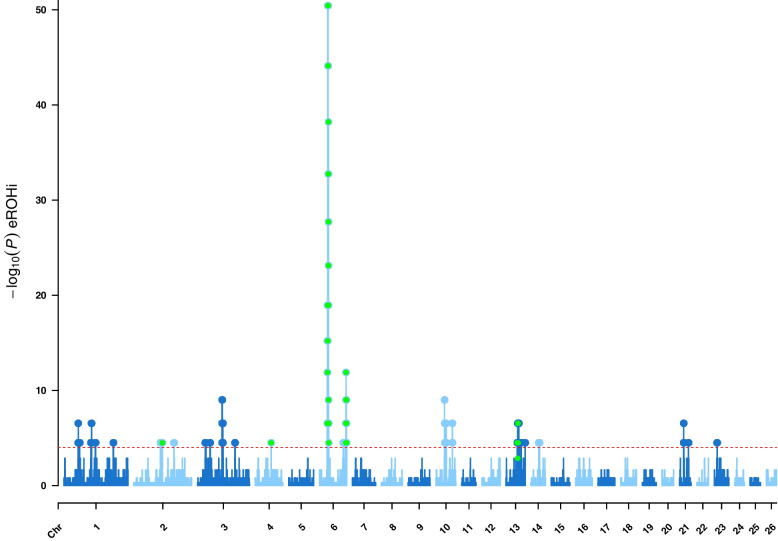
Genomic regions detected as selection signatures by four different methods are shown as Manhattan plots in Fig. 1, 2, 3 and 4 and listed in Table 1. The threshold for detection of selection signals by the eROHi method was set to 4 [−log10 (P value)], and chromosomal regions from 100 kb up and down the genome were highlighted as selection signatures for all detected eROHi-s. In this way, 48 genomic regions were detected as selection signatures only by the eROHi method, while six of them were also confirmed by at least one of the other methods used and were finally highlighted in the Manhattan plots by green colour and vertical lines. More detailed information on the genomic regions detected and the overlap between the methods can be found in Additional file 5.
Fig. 1.
Manhattan plot of genome-wide eROHi analyses on East Adriatic sheep breeds from Croatia. Horizontal red dashed line marks the significance threshold of –log10 (P value) = 4. Chromosomal regions from 100 kb up and down the genome were highlighted as selection signatures for all detected eROHi-s. Regions confirmed by at least one of the other methods were highlighted by green colour
Fig. 2.
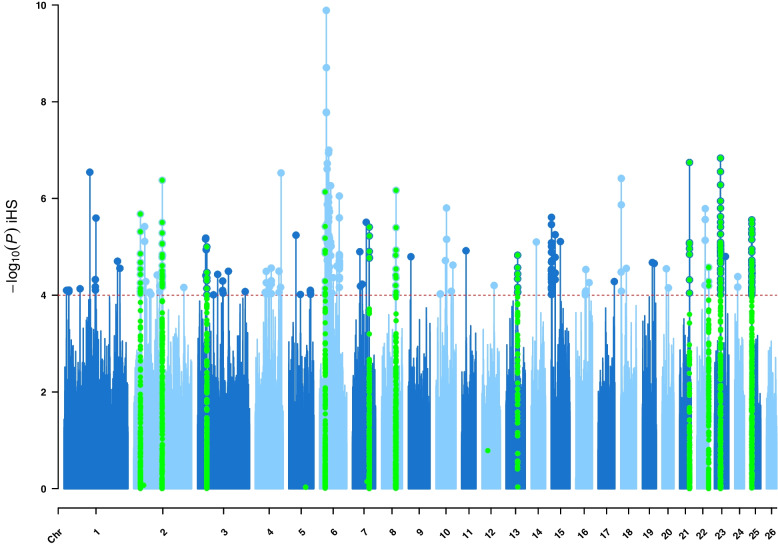
Manhattan plot of genome-wide iHS analyses on East Adriatic sheep breeds from Croatia. Horizontal red dashed line marks the significance threshold of –log10 (P value) = 4, and if the windows with three or more SNPs exceeded this threshold, they were considered as selection signatures. Regions confirmed by at least one of the other methods were highlighted by green colour
Fig. 3.
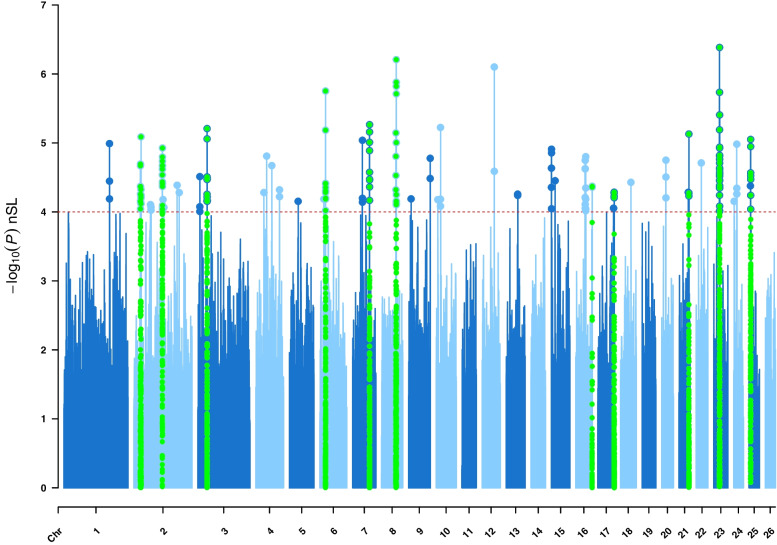
Manhattan plot of genome-wide nSL analyses on East Adriatic sheep breeds from Croatia. Horizontal red dashed line marks the significance threshold of –log10 (P value) = 4, and if the windows with three or more SNPs exceeded this threshold, they were considered as selection signatures. Regions confirmed by at least one of the other methods were highlighted by green colour
Fig. 4.
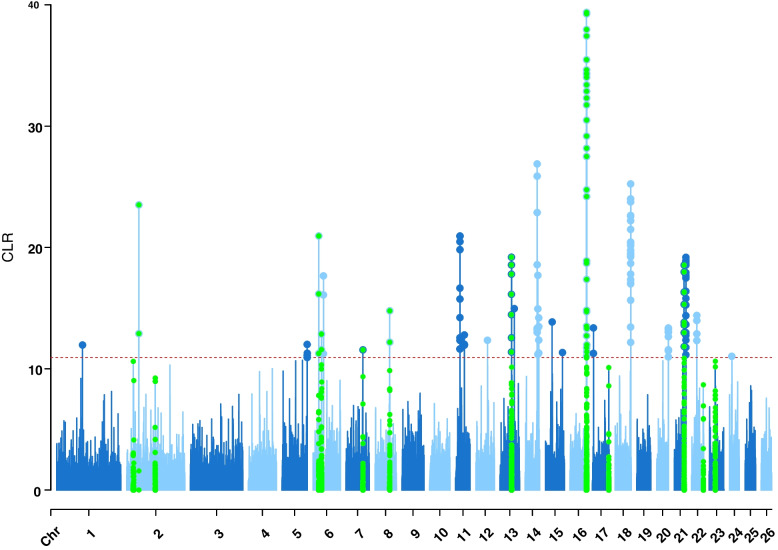
Manhattan plot of genome-wide CLR test on East Adriatic sheep breeds from Croatia. Horizontal red dashed line marks the significant genome wide threshold of 10.92, based on the top 0.1% hits, which were interpreted as selection signatures. Regions confirmed by at least one of the other methods were highlighted by green colour
Table 1.
Candidate regions and genes detected using all methods
| Region | Chr | Position, bpa | Length, Mb | Methods | Genes |
|---|---|---|---|---|---|
| 1 | 2 | 27,846,647–29,059,562 | 1.213 | CLR-IHS-NSL | WNK2, SUSD3, ECM2, ASPN, OMD, OGN, NOL8, W5PEV5 |
| 2 | 2 | 30,200,000–31,190,000 | 0.990 | NSL-CLR | * |
| 3 | 2 | 121,400,000–123,491,237 | 2.091 | ALL | ZSWIM2, FAM171B, ZC3H15, W5Q575, FSIP2 |
| 4 | 3 | 39,840,000–40,930,000 | 1.090 | NSL-IHS | ETAA1 |
| 5 | 4 | 68,150,000–69,110,000 | 0.960 | IHS-ROHS | TAX1BP1, EVX1, W5PII1, HOXA10, HOXA9, W5PJ00, HOXA6, HOXA4, HOXA3, HOXA2, W5PJE3 |
| 6 | 6 | 24,035,512–25,350,000 | 1.314 | CLR-IHS-NSL | EMCN, C6H4orf54 |
| 7 | 6 | 32,870,000–40,660,000 | 7.790 | IHS-ROHS-CLR | CCSER1, TIGD2, FAM13A, PIGY, MED28, FAM184B, LCORL, W5P3G4, KCNIP4 |
| 8 | 6 | 112,846,266–116,882,460 | 4.036 | CLR-ROHS | W5PDR8, SORCS2, W5PQN1, AFAP1, ABLIM2, W5PUD5, GRK4, MFSD10, SH3BP2, FAM193A, RNF4, W5Q0Q8, POLN, W5Q1Y2, TACC3, W5Q3F5, LOC101104663, LOC101105412, GAK, W5Q513, W5Q5J9 |
| 9 | 7 | 71,934,333–73,092,556 | 1.158 | CLR-IHS-NSL | W5QJ23, SGPP1 |
| 10 | 8 | 60,900,000–61,880,000 | 0.980 | NSL-IHS | MTFR2, MAP7 |
| 11 | 8 | 62,319,974–63,470,000 | 1.150 | CLR-IHS-NSL | LOC101113683, W5NRH7, NHSL1 |
| 12 | 13 | 484,42,121–50,598,845 | 2.157 | CLR-IHS-ROHS | RNF24 |
| 13 | 13 | 50,563,961–51,826,354 | 1.262 | CLR-ROHS | ADAM33 |
| 14 | 16 | 68,890,399–71,643,038 | 2.753 | NSL-CLR | W5P6Z2, CEP72, BRD9, W5Q3L2, W5Q3M4, W5Q3S1, W5Q4G2 |
| 15 | 17 | 69,010,000–70,000,000 | 0.990 | NSL-CLR | W5P400, SMTN, RNF185, PIK3IP1, SFI1, PRR14L, DEPDC5 |
| 16 | 21 | 41,901,704–42,960,000 | 1.058 | CLR-IHS-NSL | GPR137, CCDC88B, RPS6KA4, SLC22A11, NRXN2, MAP4K2, W5PK24, MEN1, CDC42BPG, NAALADL1, ZFPL1, TMEM262, FAU, MRPL49, SYVN1, SLC22A20P |
| 17 | 22 | 49,880,000–50,810,000 | 0.930 | IHS-CLR | W5Q425, CFAP46, W5Q8X7, KNDC1, W5QB54, CALY |
| 18 | 23 | 25,350,000–26,730,000 | 1.380 | CLR-IHS-NSL | RNF138, RNF125, DSG2, DSG3, DSG4, DSG1, DSC1, DSC2, LOC101116442 |
| 19 | 25 | 6,540,000–8,070,000 | 1.530 | NSL-IHS | W5NZP4, IRF2BP2, W5NZU5, RBM34 |
aStart and end position of the genomic region
* No candidate genes found
The strongest signals were detected in two regions on Ovis aries chromosome 6 (OAR6), the region between 35.613 and 37.361 kb, where 155 SNPs exceeded the −log10 (P value) of 50, followed by OAR10 and OAR3. The majority of ROH segments detected in the sheep genome were short regions with a length of 1–2 Mb.
Genomic regions identified as selection signatures by iHS and nSL methods are shown as Manhattan plots in Fig. 2 and 3. To avoid selecting only SNPs above certain thresholds, we defined a window of 500 kb containing three or more SNPs with −log10 (P values) greater than 4. In this way, 22 genomic regions showing a positive selection pattern were detected by the iHS method, while 15 of them also overlapped with the other methods used (highlighted in green).
Regions exceeding the above conditions for the iHS method were located on OAR2, OAR3, OAR4, OAR6, OAR7, OAR8, OAR13, OAR15, OAR21, OAR22, OAR23 and OAR25. The four strongest signals detected by the iHS method were located at OAR6, OAR23, OAR21 and OAR2, as highlighted in Fig. 2. The average length of the identified genomic regions was 1.1 Mb.
The nSL method identified 17 regions that had a positive selection signature pattern, while 13 were confirmed by other methods. The genomic regions identified by the nSL method were located on OAR2, OAR3, OAR6, OAR7, OAR8, OAR13, OAR15, OAR16, OAR17, OAR20, OAR21, OAR23, OAR24 and OAR25. Although these two methods showed high correlation [9] in detecting selection signatures, surprisingly no complete (or at least high) overlaps were found. Of the total 28 selection signatures detected by iHS and nSL, they overlapped only in nine genomic regions, confirming that the difference in recombination rate affects the results of our analyses. The three strongest signals detected by the nSL method were located at OAR23, OAR8, and OAR6, as highlighted in Fig. 3, while the average length of the detected regions was 1 Mb.
Genomic regions identified as selection signatures by the method CLR are shown as a Manhattan plot in Fig. 4. In this study, the method CLR with a defined threshold of 0.1% highest hits identified 78 selection signatures, 21 of which overlapped with other methods. The genomic regions identified by the method CLR, which were also confirmed by other methods, were on OAR2, OAR6, OAR7, OAR8, OAR13, OAR16, OAR17, OAR21, OAR22 and OAR23. The three highest CLR values were found in genomic regions on OAR16, followed by OAR2 and OAR6, which also overlapped with other methods. The average length of the regions found was 0.95 Mb, which is comparable to the average length of the other regions.
GO analyses
GO and enrichment analysis results are presented only for genes found in the overlapping genomic regions by two or more methods because this approach increases the reliability of the identified genes (otherwise, too many genes would have been shown but their interpretation would have been less reliable [39, 40]). The genes that were annotated in 19 overlapping genomic regions in our analyses are listed in Table 1. Of the total 4,984 gene transcripts, 342 genes (Additional file 6) were identified with unique Gene Stable IDs and subjected to GO analysis using the software DAVID. Functional classification analysis of genes based on the functional similarity algorithm [40] identified 10 gene groups (Additional file 7), providing a clearer picture of the extensive gene list from this study.
This analysis allows us to categorize a large number of genes based on their biological background, which facilitates the interpretation of their functions. These 10 groups consisted of 128 functionally related genes (see Table 1) that were assigned according to their chromosomal position and are considered candidate genes in this study. Modified Fisher's exact P value (EASE score), which ranks the biological significance of the gene groups, was above 3.00 and was highest for the first three groups, 3.43 for the first group and 3.39 and 3.37 for the second and third groups, respectively. Functional annotation analysis identified 116 annotation terms and their associated genes in this study (Additional file 8). Finally, functional annotation cluster analysis identified 25 annotation clusters corresponding to biological functions, which are discussed below.
The terms from the first five annotated clusters (Additional file 4) exceeded a significance of P ≤ 0.05 but only the first two exceeded Benjamini–Hochberg correction for multiple hypothesis testing. In the first cluster, the most abundant and enriched GO terms and INTERPRO domains (IPR009122, GO:0030057, IPR009123 and IPR000233) were associated with desmosomal cadherin, desmoglein, and desmocollin, with the following associated genes DSG3, DSC1, DSG4, DSC2, DSG2 and DSG1 on OAR23. All three protein families and their isoforms are dense adhesion complexes required for tissues to withstand mechanical stresses and are responsible for maintaining tissue integrity and facilitating cell–cell communication [42, 43]. As described above, they are mainly related to immune response and properties of tissue, which is very important in the context of local adaptation in sheep. In the second cluster, the major enriched GO terms and INTERPRO domains (IPR020479, IPR009057, IPR001827, IPR001356 and IPR017970) were linked to homeobox genes (HOXA10, HOXA3, HOXA2, HOXA4, HOXA6 and HOXA9). These are a highly conserved group of genes found not only in animals but also in plants and fungi. They belong to the class of transcription factors that play a key role in developmental processes [44]. As previously shown, several morphological and developmental traits in sheep (e.g., number of vertebrae, inner thigh, tail) were found to be associated with this group of genes, and therefore this analysis confirms their importance in the genetic structure of East Adriatic sheep breeds. The enriched GO terms and INTERPRO domains from the third, fourth, and fifth clusters had significant P values but did not exceed the threshold for multiple comparisons (third cluster—GO:0005509, IPR002048 and IPR011992, all associated with calcium ion binding and hand domain genes; fourth cluster—GO:0061630 and IPR001841: all associated with zinc finger and ubiquitin protein ligase; and fifth cluster—GO:0004842 and IPR000408: all associated with protein transferase activity and regulation of gene expression).
Production type characterisation
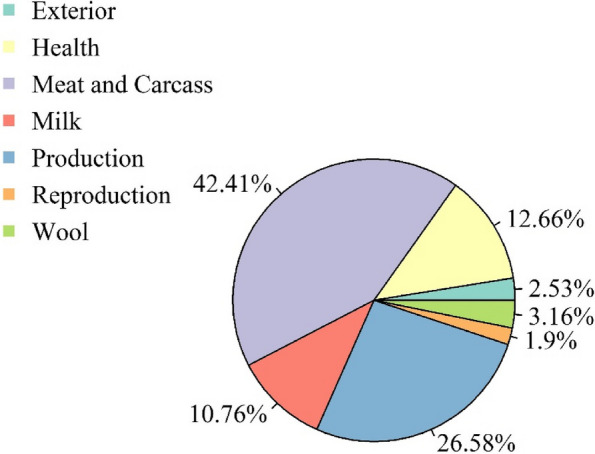
The results of the QTL database analyses are shown in Fig. 5, and Additional file 9 and 10. In Fig. 5, the identified genes are classified based on their previously found associations with traits from the database. Additional file 9 and 10 show the significantly enriched traits/types per chromosome and genome, respectively, as determined by QTL enrichment analysis. The area of the bubbles represents the number of QTLs observed for that class per chromosome, while the colour represents the FDR-adjusted P value as −log10 (P value) (the darker the colour, the smaller the P value). In addition, the x-axis shows the richness factor for each QTL, which is the ratio between the number of observed and expected QTLs.
Fig. 5.
Genes classified based on the Sheep Animal QTL database. QTLs were annotated using the GALLO R package and the Animal QTLdb for the previously identified QTLs in regions of interest. The identified genes are classified based on their associations with traits from the database
Because we have identified numerous and very specific genomic regions in this study using four different methods, we have carefully described the biological functions of the genes in these regions. However, for the breeding context, the genomic background of these candidate genes is usually less clear because of complex mechanisms such as pleiotropy, epistasis or hitchhiking effects [41]. Figure 5 shows the percentage of each QTL type in the identified candidate regions in a pie chart, indicating that EAS breeds are formed primarily for meat and carcass traits, followed by production, health traits, and finally milk.
Discussion
Production type characterisation
A careful review of the old literature and knowledge [45] on sheep breeding in the Croatian Mediterranean region focuses on adaptation to the Mediterranean environment, but without a systematic breeding approach. Historically, this was an area without a sufficient food base for livestock (unlike the eastern and continental areas of Croatia) and with a low standard of living, so the main products were meat, milk, and wool. At the beginning of the nineteenth century, more than 1,000,000 sheep were counted in the eastern Adriatic region of Croatia, which was the highest per capita number of sheep in Europe. All these facts (large sheep population, low standard of living) and local trends in sheep breeding led sheep farmers to focus mainly on their own needs. Therefore, focusing on meat/production, health and milk was a logical breeding decision. In addition, commercial wool production required more specialized breeds with industrial support (e.g., Merino), which was not the case in this breeding area. Since meat production was the main breeding objective, the sheep also had to be well adapted to the harsh environment, so health traits were a very logical breeding objective that was also complemented by natural selection. This is a fairly realistic explanation for our results related to economically important traits or production type characterization for EAS. The overall list of candidate genes identified in this study also supports our conclusion, as most of them are associated with meat/production traits in several species and with health and disease resistance. In addition, the enriched GO terms and INTERPRO domains from the first two gene clusters could be considered the most important in our study, especially since they are consistent with the number of identified individual candidate genes and with the first three trait groups based on the annotated QTLs (meat/production and health traits).
Candidate genes related with meat and carcass traits
On OAR2, SUSD3 (sushi domain containing 3) is a gene found to be associated with intramuscular fat content in pigs [46]. Another two very interesting genes have been discovered on OAR2, the ASPN and the OGN genes, which were precisely mapped and described [47]. Both genes belong to the leucine-rich proteoglycan (SLRP) family and play important roles in various functions such as collagen fibrillogenesis, cell growth, cell differentiation, and migration [48]. This was confirmed by a proteomics and gene expression approach [49], in which thin-tailed sheep had higher ASPN expression levels compared with thick-tailed sheep and was also validated as a protein within the collagen fibril organizing group. In the aforementioned QTL study in pigs [47], ASPN and OGN genes were identified to be significantly associated with carcass traits at a genome-wide level, specifically loin and neck meat weight, shoulder meat weight, and daily gain (110–210 d). On OAR3, gene FAM171B is associated with meat colour in cattle [50], but its function is still unclear. On OAR4, we identified the gene EVX2, which is associated with the limbs and genital buds in sheep [51] and with mammary gland and spinal cord development in pigs [52], confirming its possible function in sex organ development.
The phosphatidylinositol glycan anchor biosynthesis class Y or PIGY gene is a member of the PIG gene family and has an important function in cell–cell interactions. In the study about the copy number variations surrounding this gene in sheep [53], it was found to be associated with growth traits and the type traits of chest girth and cannon bone circumference, suggesting that this gene could be used as a marker for breeding purposes. It has also been associated with meat and carcass quality [54]. Mediator complex subunit 28 (MED28) is a gene with function in regulating smooth muscle cell differentiation and maintaining stem cell population. This gene is also associated with carcass characteristics and bone weight [55]. Two studies found that the SORCS2 gene is involved in intracellular sorting and transport of various neurotrophic factors, transmembrane receptors, and synaptic proteins associated with a variety of cellular processes, including neuronal function, differentiation, and synaptic plasticity in different species [56, 57]. Decreased SORCS2 expression increased oxidative stress and resulted in an enhanced oxidative stress response in primary neurons [57]. The SORCS2 gene was also found to control lipid metabolism in cattle [58]. It is showed that the ABLIM2 gene is required for normal neuronal function in humans, rats, and mice [59], whereas in dogs and pigs [60] it encodes an actin-binding muscle protein. Important GRK4 gene was found in OAR6, near which two ROH islands (ROH100 and ROH101) were found that are unique to Blackhead Persian and Nguni sheep and responsible for chemokine pathways [61]. Another research [62], found a role for the GRK4 as well as the MFSD10 gene in lipid storage, fat cell regulation, and fat tail deposition in Mediterranean sheep. The SMTN gene or smoothelin is expressed in various tissues such as smooth muscle, adipose tissue, cardiac muscle, and skeletal muscle [63], with two main isoforms. It has a function in controlling muscle contraction [64], but no association has been found with economically important traits in livestock.
Kinase non-catalytic C-lobe domain containing 1 or KNDC1, plays a key role in many signal transduction pathways that contribute to protein recognition and functional regulation. In chickens, it has been associated with mammillary layer thickness and mammillary density [65], whereas in pigs it has been associated with the fatty acid profile in IMF along with the CALY gene from the same region [66]. In the gene expression study in sheep, KNDC1 was shown to be involved in the regulation of protein phosphorylation and was upregulated in fast-red muscle compared with slow-red muscle [67].
Candidate genes related with growth production traits
The ECM2 gene, which is involved in the extracellular matrix, has been associated with growth in humans [68]. Within the same cluster on OAR2, another member of the same SLRP family, the OMD gene (osteomodulin or osteoadherin), was discovered to be involved in body development processes, such as regulation of the diameter and shape of collagen fibrils and bone formation [69]. The gene ZC3H15 has been associated with skull shape in mice [70]. Another gene on OAR6, LCORL (ligand-dependent nuclear receptor corepressor), is associated with various body size traits in sheep, humans, horses, and cattle [71–82]. As for the POLN gene, it has been reported to have an effect on adult body size in some sheep in the United States [83]. The function of the TACC3 gene on OAR6 has not been fully elucidated, but it is generally suspected that it may be involved in the processes of cell growth and differentiation. Expression of this gene is upregulated in some cancer cell lines and at embryonic d 15 in mice [84]. In OAR16, only one region (2.753 Mb long) was confirmed by two methods (nSL and CLR). In this region two genes, CEP72 and BRD9, play important roles in bovine feed efficiency [85].
Candidate genes related with health traits
The WNK2 gene on OAR2 is associated with tumour suppressive function in humans [86], and when this gene is knocked down, it leads to accelerated tumour growth in mouse models. Another gene on OAR2, ZSWIM2 was associated with perinatal mortality in cattle [87]. At OAR3, we identified an interesting gene, ETAA1 or Ewing tumour-associated antigen 1, that is differentially expressed in fat and short-tailed sheep [88], whereas in humans it is associated with body fat distribution [89]. In cattle, this gene is a strong indicator of mastitis resistance and somatic cell score [90]. Next, on OAR6, the SH3BP2 gene plays an important role in natural killer cell-mediated cytotoxicity, which is likely responsible for the natural immunity/resistance of local sheep breeds [61]. Cyclin G-associated kinase (CAK) is a protein-coding gene on OAR6. Diseases associated with GAK include prostate sealing ring cell adenocarcinoma and Parkinson's disease 15, autosomal recessive early onset. Nance-Horan syndrome-like 1 protein (NHSL1), located on the OAR8 is related to metabolism and has been associated with subclinical ketosis and resistance to gastrointestinal nematodes in adult sheep [91] and tick parasites [92], which is important for better environmental adaptation. The following 4 genes were located on OAR17. The ubiquitin-protein ligase or RNF185 gene has a function in the endoplasmic reticulum (ER) associated degradation pathway (ERAD) and is associated with mitochondrial autophagy processes. The PIK3IP1 gene is involved in the negative regulation of phosphatidylinositol 3-kinase activity and in the negative regulation of phosphatidylinositol 3-kinase signal transduction. These two genes are associated with longevity [93] and disease resistance [94, 95] in cattle, as shown by GWAS.
The SFI1 gene, or centrin-binding protein also on OAR17, has been associated with reproductive traits [96], metabolic digestion [97], and interestingly, cold adaptation, as it affects basal metabolic rate [98]. In addition, in humans, this gene was proved to be associated with glycosylated haemoglobin [99], a marker of average blood glucose levels. The gene Proline Rich 14 Like or PRR14L in this region is mainly studied in humans in the context of blood metabolism (increase in monocytes and decrease in neutrophils) and leukaemia gene expression profiles [100], whereas in sheep was proved to be differentially expressed between certain breeds [101].
On OAR21, the CCDC88B gene encodes a coiled-coil domain-containing protein 88b, a poorly annotated gene specifically expressed in spleen, bone marrow, lymph nodes, and thymus. CCDC88B protein is also abundantly expressed in immune cells, including CD4+ and CD8+ T lymphocytes, and in myeloid cells. Loss of CCDC88B protein expression has pleiotropic effects on T lymphocyte functions, including impaired maturation in vivo, markedly reduced activation, decreased cell division, and impaired cytokine production (IFN-γ and TNF) in response to T cell receptor activation during the course of Plasmodium berghei infection in vivo [102]. Another interesting study [103] was conducted to explore the resistance of different sheep breeds to gastrointestinal nematodes. Analysing the transcriptome of abomasal lymph node tissue, they found a total of 25 significant (P < 0.05) gene interaction networks. The gene interaction network with the largest number of focal molecules (n = 18) including the NAALADL1 gene on OAR21 was assigned to infectious disease, cell-to-cell signalling and interaction, and cell movement. Also on OAR21, FAU or FAU Ubiquitin Like and Ribosomal Protein S30 Fusion is a protein coding gene. In the transcriptome analysis of adipose tissue from two fat-tailed sheep breeds [104], was found the specific gene expression patterns in adipose tissue with 47 common highly expressed genes, of which 28 genes affected FAU gene. Diseases associated with FAU included sarcoma and osteogenic sarcoma [104]. Related pathways included viral mRNA translation and the integrated breast cancer pathway. On the OAR23, important gene family was identified containing the ring finger genes RNF138 and RNF135, both of which belong to the ubiquitin-binding protein family and have strong activities [105, 106]. They play a special role in the regulation of T cells and the immune response. RNF135 was found to be associated with biological pathways that affect human body size [68].
Candidate genes related with milk production
An interesting gene on OAR6 is the endomucin or EMCN gene, which has been associated with growth and carcass traits in sheep [107], with meat marbling in cattle [108], but also with milk fat yield in sheep [109], which is the first described selection signature related to milk production in this study. EMCN is a membrane-bound glycoprotein expressed on the surface of the endothelium in venules and capillaries [110], and its main function is inflammation regulation, so it may be of particular interest in the context of environmental adaptation and disease resistance.
For the FAM13A (family with sequence similarity member 13A) gene located in the second important region on OAR6, a detailed GWAS study found an association with lung disease in humans [111], whereas other studies in sheep confirmed an association with milk yield [112] and body/bone weight in cattle [55, 113]. In the same region on OAR6, MED28 was found to be associated with milk production traits in sheep [112] and expressed in the mammary gland during lactation [114]. The former study also confirmed the association of the FAM184B gene (member B of the family with sequence similarity 184) with somatic cell score and milk production traits. FAM184B is known to be expressed in adipose and skeletal muscle tissue and during skeletal development [55, 113]. DEPDC5 or DEP domain containing 5 is a gene located on OAR17 and involved in stimulus response and associated with growth traits [115]. Whole-genome analyses [116] have also shown that this gene family plays a role in the regulation of lactation in sheep. On OAR22, CFAP46 or cilia and flagella associated protein 46, has been associated to lactose content [117] whereas RBM34 or RNA-binding protein 34 gene on OAR25 has role in nucleic acid binding and nucleotide binding, and it was found by GWAS study on cattle to be associated with milk yield [118].
Candidate genes related with wool traits
The AFAP1 (actin filament associated protein 1) gene on OAR6 plays a role in some characteristics of yearling wool of one-year-old Chinese sheep with fine wool, such as the cleanliness of the fleece [119]. On OAR6, two gene families, desmoglein or DSG protein with four members, DSG1, DSG2, DSG3 and DSG4, and desmocollin protein or DSC with two members, DSC1 and DSC2, were identified in this study. These genes are involved in immune response in sheep [91], but more interestingly, they have been shown to affect fibre properties [120, 121] and hair growth and follicle structure [122]. For this reason, this gene may be particularly important for adaptation to local environmental and climatic conditions and for characterizing production type. On OAR25, IRF2BP2 gene is very interesting and quite important for wool characteristics. It is the Interferon regulatory factor 2 binding protein 2 which acts as a transcriptional coregulator and it was found to be involved in the fleece variation between the hairy/long coat and short/woolly fleece phenotypes [123]. Also, it was confirmed in another study about the functionality of these genes in relation to immune and reproduction response, where high and low gene signalling variants resulted in different hair phenotypes [124].
Candidate genes related to reproduction traits
The coiled-coil serine rich protein 1 or CCSER1 gene on OAR6, which encodes a proline-rich protein, plays an important role in mitosis and cell division and has been linked to several human cancers [125]. SNPs in the intronic region of this gene have been associated with male fertility traits in sheep, such as ejaculate quality [126], and together with the TIGD2 gene with growth and carcass traits in cattle [127], body weight in salmon [128], and meat quality traits in ducks [129]. The TIGD2 gene has also been associated with resistance to disease and bacterial infection in cattle [85], making it an important candidate in our study. The KCNIP gene (potassium voltage-gated channel interacting protein 4) also located on OAR6 has an important function in regulating transmembrane transport of potassium ions. Several GWAS studies confirmed the association of this gene with male fertility [126] and growth traits [55, 130, 131]. On the second important region on OAR6, the RNF4 gene was found to play an important role in litter size in pigs, being involved in a number of reproductive physiological processes [132]. Only one region on OAR7 was detected as a positive selection signal. The SGPP1 gene plays an important role in ovine fertility traits, where increased expression in the endometrium of the non-gravid uterine horn was detected. SGPP1 expression also increased in the placenta late in gestation [84, 133] and in lipid metabolism [134].
Candidate genes related to environmental adaptation
The gene on OAR2, NOL8, which has been associated with circulating fasting glucose levels in mice [135] and may be important for energy expenditure in harsh environments. It has also been identified as a candidate gene for hunting ability in dogs [136] and carcass traits in cattle [137]. The gene FSIP2 also on OAR2, was found to be associated with fecundity in sheep [138]. In the context of domestication, the FSIP2 gene is very interesting because it was previously identified as a gene important for domestication [138, 139], i.e., a gene associated with adaptation rather than production traits. Moreover, the haplotypes of this gene strongly resemble those of Asian mouflon and other wild sheep relatives (snow sheep and argali) but not those of domestic sheep. The HOXa gene family, consisting of six genes (HOXA2, HOXA3, HOXA4, HOXA6, HOXA9 and HOXA10) and possibly important for EAS adaptation, are located on OAR4. The HOXA gene cluster or homeobox A is a group of conserved genes throughout the animal kingdom that encode several transcription factors responsible for nervous system, body, and spinal development [140, 141]. This gene cluster is particularly important in an evolutionary context because a single mutation in this cluster results in drastic body shapes [140, 142]. Several studies have found associations of this region with similar traits related to developmental functions and morphological traits, such as the number of thoracic vertebrae [143] and fat tail development [144] in sheep, inner thigh development [145] in cattle, and body structure traits [146] in pigs. This region has been identified in numerous selection signature studies based on various methods in different breeds of sheep [147–150].
The FAM193A gene on OAR6 was identified as a candidate for adaptation in Moroccan sheep [151]. Two regions were identified on OAR13, each containing only one gene. The gene from the first region (RNF24) is a gene responsible for visual function in sheep and has also been found as a selection signature by other authors [138, 152], and the resulting changes are most likely related to domestication. Vision plays a critical role in animal survival, and many studies have shown that visual acuity is weaker in domestic animals (e.g., chickens, dogs and ducks) compared with their wild ancestors [153–155]. Thus, the functional role of this selection signal with respect to domestication remains to be explored. The second region on OAR13 localized the gene ADAM33, which belongs to the disintegrin and metalloprotease domain family. It is another gene with a possible function in environmental adaptation, as it plays a role in several biological processes, including muscle development and neurogenesis, whereas in humans it is mainly associated with the immune response and allergic asthma [156–158].
In a study on cattle [159], it was found that the NRXN2 gene (located on OAR21 in sheep), among several other genes, is associated with adaptation, particularly climate adaptation, such as adaptation to tropical humidity and harsh environments. An interesting study [160] was conducted to investigate the key genes and pathways involved in the response to pain in goats and sheep by transcriptome sequencing. The analysis was performed on the dorsal root ganglion (DRG), which is involved in the transmission of pain to the central nervous system and exhibits various pathophysiological changes in chronic pain. Transcriptome analysis in sheep revealed the higher activity of the gene CDC42BPG, which regulates the activity of small GTPases (which act as molecular switches or timers in many basic cellular processes such as signal transduction, protein biosynthesis, translocation of proteins across membranes, etc.). Also on OAR21, the gene encoding Hmg-CoA reductase 1 or SYVN1 degradation may mediate resistance to diabetic retinopathy, as shown in the study on mice [161]. SYVN1 is an important member of the E3 ligase complex in the ERAD pathway that removes misfolded and non-functional proteins from the ER, keeps the ER stable, and reduces ER stress. SYVN1 also inhibits apoptosis induced by ER stress [162, 163].
Conclusions
In this study, we identified selection signatures of East Adriatic sheep breeds using several methods, including reduced local variation, linkage disequilibrium and frequency spectrum (eROHi, iHS, nSL, and CLR). Analysis of selection signatures identified numerous and specific candidate genomic regions and genes (e.g., desmosomal cadherin and desmoglein gene family, and HOXa gene family) that may be important not only for economically important traits but also for adaptation to specific production and environmental conditions. The majority of candidate genes were related to meat/production and health/immune response traits, which seems to be a realistic historical reflection of breeding practices in the Croatian Adriatic region. This was also confirmed by GO and QTL enrichment analysis. Our results will contribute to a better understanding of the breeding potential of EAS, its unique adaptive genetic architecture and its relationships with other populations, and eventually provide a new opportunity to exploit its genomic background in future sustainable breeding programs. Even though these procedures (incorporating knowledge about selection signatures and population structure) aren't simple and readily executable, we hold the view that incorporating this information in breeding programs should become a mandatory aspect in addressing worldwide shifts and striving for enhanced sustainable production, placing an emphasis on improved adaptation to diverse environmental conditions.
Supplementary Information
Additional file 1: Description S1. Contains short description of the breeds and representative pictures of animals used in this study.
Additional file 2: Table S1. Data Info. Contains basic information (breed and number of samples) about the animals used in this study.
Additional file 3: Fig. S1. LD decay. Contains results from the LD decay analysis.
Additional file 4: Table S2. Gene clusters. Contains results from the functional annotation cluster analysis using DAVID software, with identified annotation clusters corresponding to biological functions.
Additional file 5: Table S3. Regions overlap. Contains information on detected selection signatures and regions whose positions overlapped by at least two different methods.
Additional file 6: Table S4. Identified genes ID. Contains detailed information (Gene ID, chr, start and end positions in bp) about the genes identified within genomic regions detected as selection signatures.
Additional file 7: Table S5. Functional gene groups. Contains results from the functional classification analysis of genes based on the functional similarity algorithm using DAVID software.
Additional file 8: Table S6. Functional terms. Contains results from the functional annotation analysis with annotation terms and their associated genes using DAVID software.
Additional file 9: Fig. S2. Significantly enriched traits per chromosome, as determined by QTL enrichment analysis. The area of the bubbles represents the number of QTLs observed for that class per chromosome, while the colour represents the FDR-adjusted P value as –log10 (P value) (the darker the colour, the smaller the P value). The x-axis shows the richness factor for each QTL, which is the ratio between the number of observed and expected QTLs.
Additional file 10: Fig. S3. Significantly enriched traits per genome, as determined by QTL enrichment analysis. The area of the bubbles represents the number of QTLs observed for that class per genome, while the colour represents the FDR-adjusted P value as –log10 (P value) (the darker the colour, the smaller the P value). The x-axis shows the richness factor for each QTL, which is the ratio between the number of observed and expected QTLs.
Acknowledgements
Publication was supported by the Research Team Fund 1134 of the Faculty of agrobiotechnical sciences Osijek and the Open Access Publication Fund of the University of Zagreb Faculty of Agriculture.
Footnotes
Not applicable.
Abbreviations
- CLR
Composite-likelihood ratio test
- EAS
East Adriatic sheep breeds from Croatia
- EHH
Extended Haplotype Homozygosity
- eROHi
Extreme ROH “islands”
- GO
Gene Ontology
- iHS
Integrated haplotype score
- nSL
Number of Segregating sites by length
- QTL
Quantitative Trait Loci
- ROH
Runs of homozygosity
Authors’ contributions
BL, VC-C and IC conceived and designed the study; VC-C, IC, ID and LV organized sample collection, extraction and genotyping; BL, MS and VG performed the analysis; BL and IC wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was financed and supported by Croatian Science Foundation project IP-2018–01-8708—Application of NGS methods in the assessment of genomic variability in ruminants – “ANAGRAMS”; the EU Operational Program Competitiveness and Cohesion 2014–2020 project KK.01.1.1.04.0058—Potential of microencapsulation in cheese production; and the project No. QK1919156 of the Ministry of Agriculture, Czech Republic.
Availability of data and materials
Genotypic data representing eight Croatian sheep breeds are deposited and publicly available at https://doi.org/10.5061/dryad.pg4f4qrsn.
Declarations
Ethics approval and consent to participate
The material used in this study was reviewed and approved by the Bioetic Committee for Animal Welfare and Protection of the University of Zagreb, Faculty of Agriculture. Animal samples were collected within official procedure by National Gene Bank in Croatia, therefore no written statement on informed consent was obtained from the owners.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Boris Lukic, Email: blukic@fazos.hr.
Ino Curik, Email: icurik@agr.hr.
References
- 1.Zeder MA. Domestication and early agriculture in the Mediterranean basin: brigins, diffusion, and impact. Proc Natl Acad Sci USA. 2008;105:11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chessa B, Pereira F, Arnaud F, Amorim A, Goyache F, Mainland I, et al. Revealing the history of sheep domestication using retrovirus integrations. Science. 2009;324:532. doi: 10.1126/science.1170587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv FH, Cao YH, Liu GJ, Luo LY, Lu R, Liu MJ, et al. Whole-genome resequencing of worldwide wild and domestic sheep elucidates genetic diversity, introgression, and agronomically important loci. Mol Biol Evol. 2022;39:2. doi: 10.1093/molbev/msab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forenbaher S, Miracle PT. The spread of farming in the Eastern Adriatic. Doc Praehist. 2006;33:89–100. doi: 10.4312/dp.33.10. [DOI] [Google Scholar]
- 5.Forenbaher S, Kaiser T, Miracle PT. Dating the East Adriatic Neolithic. Eur J Archaeol. 2013;16:589–609. doi: 10.1179/1461957113Y.0000000038. [DOI] [Google Scholar]
- 6.McClure SB, Podrug E, Jović J, Monroe S, Radde HD, Triozzi N, et al. The zooarchaeology of Neolithic farmers: herding and hunting on the Dalmatian coast of Croatia. Quat Int. 2022;634:27–37. doi: 10.1016/j.quaint.2022.06.013. [DOI] [Google Scholar]
- 7.Drzaic I, Curik I, Lukic B, Shihabi M, Li MH, Kantanen J, et al. High-density genomic characterization of native Croatian sheep breeds. Front Genet. 2022;13:940736. doi: 10.3389/fgene.2022.940736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen R. Molecular signatures of natural selection. Annu Rev Genet. 2005;39:197–218. doi: 10.1146/annurev.genet.39.073003.112420. [DOI] [PubMed] [Google Scholar]
- 9.González-Rodríguez A, Munilla S, Mouresan EF, Cañas-Álvarez JJ, Díaz C, Piedrafita J, et al. On the performance of tests for the detection of signatures of selection: a case study with the Spanish autochthonous beef cattle populations. Genet Sel Evol. 2016;48:81. doi: 10.1186/s12711-016-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Ding X, Qanbari S, Weigend S, Zhang Q, Simianer H. Properties of different selection signature statistics and a new strategy for combining them. Heredity (Edinb) 2015;115:426–436. doi: 10.1038/hdy.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saravanan KA, Panigrahi M, Kumar H, Bhushan B, Dutt T, Mishra BP. Selection signatures in livestock genome: A review of concepts, approaches and applications. Livest Sci. 2020;241:104257. doi: 10.1016/j.livsci.2020.104257. [DOI] [Google Scholar]
- 12.Nothnagel M, Lu TT, Kayser M, Krawczak M. Genomic and geographic distribution of SNP-defined runs of homozygosity in Europeans. Hum Mol Genet. 2010;19:2927–2935. doi: 10.1093/hmg/ddq198. [DOI] [PubMed] [Google Scholar]
- 13.Curik I, Ferenčaković M, Sölkner J. Inbreeding and runs of homozygosity: a possible solution to an old problem. Livest Sci. 2014;166:26–34. doi: 10.1016/j.livsci.2014.05.034. [DOI] [Google Scholar]
- 14.Liu J, Shi L, Li Y, Chen L, Garrick D, Wang L, et al. Estimates of genomic inbreeding and identification of candidate regions that differ between Chinese indigenous sheep breeds. J Anim Sci Biotechnol. 2021;12:95. doi: 10.1186/s40104-021-00608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukic B, Ferenčaković M, Šalamon D, Čačić M, Orehovački V, Iacolina L, et al. Conservation genomic analysis of the Croatian indigenous Black Slavonian and Turopolje pig breeds. Front Genet. 2020;11:261. doi: 10.3389/fgene.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shihabi M, Lukic B, Cubric-Curik V, Brajkovic V, Oršanić M, Ugarković D, et al. Identification of selection signals on the X-chromosome in east Adriatic sheep: a new complementary approach. Front Genet. 2022;13:780. doi: 10.3389/fgene.2022.887582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlidis P, Živković D, Stamatakis A, Alachiotis N. SweeD: likelihood-based detection of selective sweeps in thousands of genomes. Mol Biol Evol. 2013;30:2224–2234. doi: 10.1093/molbev/mst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson SH, Hubisz MJ, Clark AG, Payseur BA, Bustamante CD, Nielsen R. Localizing recent adaptive evolution in the human genome. PLoS Genet. 2007;3:e90. doi: 10.1371/journal.pgen.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeniyi OO, Simon R, Bytyqi H, Kugler W, Mehmeti H, Berisha K, et al. Capturing genetic diversity and selection signatures of the endangered Kosovar Balusha sheep breed. Genes (Basel) 2022;13:866. doi: 10.3390/genes13050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cesarani A, Sechi T, Gaspa G, Usai MG, Sorbolini S, Macciotta NPP, et al. Investigation of genetic diversity and selection signatures between Sarda and Sardinian Ancestral black, two related sheep breeds with evident morphological differences. Small Rumin Res. 2019;177:68–75. doi: 10.1016/j.smallrumres.2019.06.014. [DOI] [Google Scholar]
- 21.Ciani E, Lasagna E, D’Andrea M, Alloggio I, Marroni F, Ceccobelli S, et al. Merino and Merino-derived sheep breeds: A genome-wide intercontinental study. Genet Sel Evol. 2015;47:64. doi: 10.1186/s12711-015-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pariset L, Mariotti M, Gargani M, Joost S, Negrini R, Perez T, et al. Genetic diversity of sheep breeds from Albania, Greece, and Italy assessed by mitochondrial DNA and nuclear polymorphisms (SNPs) Sci World J. 2011;11:1641–1659. doi: 10.1100/2011/186342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serranito B, Cavalazzi M, Vidal P, Taurisson-Mouret D, Ciani E, Bal M, et al. Local adaptations of Mediterranean sheep and goats through an integrative approach. Sci Rep. 2021;11:21363. doi: 10.1038/s41598-021-00682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsartsianidou V, Sánchez-Molano E, Kapsona VV, Basdagianni Z, Chatziplis D, Arsenos G, et al. A comprehensive genome-wide scan detects genomic regions related to local adaptation and climate resilience in Mediterranean domestic sheep. Genet Sel Evol. 2021;53:90. doi: 10.1186/s12711-021-00682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciani E, Mastrangelo S, Da Silva A, Marroni F, Ferenčaković M, Ajmone-Marsan P, et al. On the origin of European sheep as revealed by the diversity of the Balkan breeds and by optimizing population-genetic analysis tools. Genet Sel Evol. 2020;52:25. doi: 10.1186/s12711-020-00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biscarini F, Cozzi P, Gaspa G, Marras G. DetectRUNS: Detect runs of homozygosity and runs of heterozygosity in diploid genomes. The Compr R Arch Network. 2018. https://rdrr.io/cran/detectRUNS/f/vignettes/detectRUNS.vignette.Rmd.
- 28.Ferenčaković M, Sölkner J, Curik I. Estimating autozygosity from high-throughput information: effects of SNP density and genotyping errors. Genet Sel Evol. 2013;45:42. doi: 10.1186/1297-9686-45-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32:361–369. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- 30.Delaneau O, Howie B, Cox AJ, Zagury JF, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93:687–696. doi: 10.1016/j.ajhg.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabeti PC, Reich DE, Higgins JM, Levine HZP, Richter DJ, Schaffner SF, et al. Detecting recent positive selection in the human genome from haplotype structure. Nature. 2002;419:832–837. doi: 10.1038/nature01140. [DOI] [PubMed] [Google Scholar]
- 32.Gautier M, Klassmann A, Vitalis R. rehh 2.0: a reimplementation of the R package rehh to detect positive selection from haplotype structure. Mol Ecol Resour. 2017;17:78–90. doi: 10.1111/1755-0998.12634. [DOI] [PubMed] [Google Scholar]
- 33.Gautier M, Naves M. Footprints of selection in the ancestral admixture of a New World Creole cattle breed. Mol Ecol. 2011;20:3128–3143. doi: 10.1111/j.1365-294X.2011.05163.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer-Admetlla A, Liang M, Korneliussen T, Nielsen R. On detecting incomplete soft or hard selective sweeps using haplotype structure. Mol Biol Evol. 2014;31:1275–1291. doi: 10.1093/molbev/msu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szpiech ZA, Hernandez RD. Selscan: An efficient multithreaded program to perform EHH-based scans for positive selection. Mol Biol Evol. 2014;31:2824–2827. doi: 10.1093/molbev/msu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephan W. Genetic hitchhiking versus background selection: The controversy and its implications. Philos Trans R Soc B: Biol Sci. 2010;365:1245–1253. doi: 10.1098/rstb.2009.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Ridwan Amode M, et al. Ensembl 2021. Nucleic Acids Res. 2021;49:884–891. doi: 10.1093/nar/gkaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update) Nucleic Acids Res. 2022;50:216–221. doi: 10.1093/nar/gkac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonseca PAS, Suárez-Vega A, Marras G, Cánovas Á. GALLO: An R package for genomic annotation and integration of multiple data sources in livestock for positional candidate loci. Gigascience. 2020;9:12. doi: 10.1093/gigascience/giaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowndes M, Nelson WJ. Cadherin-Mediated Cell–Cell Adhesion. Encyclopedia of biological chemistry. 2. 2013. pp. 255–60. [Google Scholar]
- 43.Mozaffarian N, Shaw EA, Stevens AM. Maternally mediated neonatal autoimmunity. In: Ohls KR, Maheshwari A, editors. Hematology, immunology and infectious disease: neonatology questions and controversies (second edition). Philadelphia: W.B. Saunders; 2012. p. 129–70.
- 44.Duverger O, Morasso MI. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J Cell Physiol. 2008;216:337–346. doi: 10.1002/jcp.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hrasnica F, Ilančić D, Pavlović S, Rako A, Šmalcelj I. Specijalno stočarstvo. Zagreb: Poljoprivredni nakladni zavod; 1958. [Google Scholar]
- 46.Wang L, Zhou ZY, Zhang T, Zhang L, Hou X, Yan H, et al. IRLnc: a novel functional noncoding RNA contributes to intramuscular fat deposition. BMC Genom. 2021;22:95. doi: 10.1186/s12864-020-07349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stratil A, van Poucke M, Bartenschlager H, Knoll A, Yerle M, Peelman LJ, et al. Porcine OGN and ASPN: Mapping, polymorphisms and use for quantitative trait loci identification for growth and carcass traits in a Meishan x Piétrain intercross. Anim Genet. 2006;37:415–418. doi: 10.1111/j.1365-2052.2006.01480.x. [DOI] [PubMed] [Google Scholar]
- 48.Tasheva ES, Klocke B, Conrad GW. Analysis of transcriptional regulation of the small leucine rich proteoglycans. Mol Vis. 2004;10:758–772. [PubMed] [Google Scholar]
- 49.Han J, Guo T, Yue Y, Lu Z, Liu J, Yuan C, et al. Quantitative proteomic analysis identified differentially expressed proteins with tail/ rump fat deposition in Chinese thin- and fat-tailed lambs. PLoS One. 2021;16(2):e0246279. doi: 10.1371/journal.pone.0246279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bedhane M, van der Werf J, Gondro C, Duijvesteijn N, Lim D, Park B, et al. Genome-wide association study of meat quality traits in Hanwoo beef cattle using imputed whole-genome sequence data. Front Genet. 2019;10:1235. doi: 10.3389/fgene.2019.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren X, Yang GL, Peng WF, Zhao YX, Zhang M, Chen ZH, et al. A genome-wide association study identifies a genomic region for the polycerate phenotype in sheep (Ovis aries) Sci Rep. 2016;6:21111. doi: 10.1038/srep21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verardo LL. Gene networks from genome wide association studies for pig reproductive traits. Vicosa, Minas Gerais: PhD thesis, The Universidade Federal de Vicosa; 2015.
- 53.Feng Z, Li X, Cheng J, Jiang R, Huang R, Wang D, et al. Copy number variation of the pigy gene in sheep and its association analysis with growth traits. Animals. 2020;10:4. doi: 10.3390/ani10040688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naserkheil M, Mehrban H, Lee D, Park MN. Genome-wide association study for carcass primal cut yields using single-step bayesian approach in Hanwoo cattle. Front Genet. 2021;12:752424. doi: 10.3389/fgene.2021.752424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia J, Fan H, Chang T, Xu L, Zhang W, Song Y, et al. Searching for new loci and candidate genes for economically important traits through gene-based association analysis of Simmental cattle. Sci Rep. 2017;7:42048. doi: 10.1038/srep42048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glerup S, Olsen D, Vaegter CB, Gustafsen C, Sjoegaard SS, Hermey G, et al. SorCS2 regulates dopaminergic wiring and is processed into an apoptotic two-chain receptor in peripheral glia. Neuron. 2014;82:1074–1087. doi: 10.1016/j.neuron.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Malik AR, Szydlowska K, Nizinska K, Asaro A, van Vliet EA, Popp O, et al. SORCS2 controls functional expression of amino acid transporter EAAT3 and protects neurons from oxidative stress and epilepsy-induced pathology. Cell Rep. 2019;26:2792–2804. doi: 10.1016/j.celrep.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olivieri BF, Mercadante MEZ, Cyrillo JNDSG, Branco RH, Bonilha SFM, de Albuquerque LG, et al. Genomic regions associated with feed efficiency indicator traits in an experimental Nellore cattle population. PLoS One. 2016;11(10):e0164390. doi: 10.1371/journal.pone.0164390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klimov E, Rud’ko O, Rakhmanaliev E, Sulimova G. Genomic organisation and tissue specific expression of ABLIM2 gene in human, mouse and rat. Bba Gene Struct Expr. 2005;1730:1–9. doi: 10.1016/j.bbaexp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Daza KR, Velez-Irizarry D, Casiró S, Steibel JP, Raney NE, Bates RO, et al. Integrated genome-wide analysis of microRNA expression quantitative trait loci in pig longissimus dorsi muscle. Front Genet. 2021;12:644091. doi: 10.3389/fgene.2021.644091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dzomba EF, Chimonyo M, Pierneef R, Muchadeyi FC. Runs of homozygosity analysis of South African sheep breeds from various production systems investigated using OvineSNP50k data. BMC Genom. 2021;22:7. doi: 10.1186/s12864-020-07314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu SS, Ren X, Yang GL, Xie XL, Zhao YX, Zhang M, et al. Genome-wide association analysis identifies the genetic basis of fat deposition in the tails of sheep (Ovis aries) Anim Genet. 2017;48:560–569. doi: 10.1111/age.12572. [DOI] [PubMed] [Google Scholar]
- 63.van der Loop FTL, Schaart G, Timmer EDJ, Ramaekers FCS, van Eys GJJM. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134:401–411. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murali M, MacDonald JA. Smoothelins and the control of muscle contractility. In: Khali RA, editor. Advances in pharmacology. 2018;81:39–78. [DOI] [PubMed]
- 65.Duan Z, Sun C, Shen MM, Wang K, Yang N, Zheng J, et al. Genetic architecture dissection by genome-wide association analysis reveals avian eggshell ultrastructure traits. Sci Rep. 2016;6:28836. doi: 10.1038/srep28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piazuelo CD. Genomic analysis of fatty acid composition and gut microbiota in pigs. Barcelona: PhD Thesis, Universitat Autònoma De Barcelona; 2018.
- 67.Armstrong E, Iriarte A, Nicolini P, de Los SJ, Ithurralde J, Bielli A, et al. Comparison of transcriptomic landscapes of different lamb muscles using RNA-Seq. PLoS One. 2018;13(7):e0200732. doi: 10.1371/journal.pone.0200732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen HL, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin T, König S. Genome-wide associations and detection of potential candidate genes for direct genetic and maternal genetic effects influencing dairy cattle body weight at different ages. Genet Sel Evol. 2019;51:4. doi: 10.1186/s12711-018-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pallares LFA. Genetic architecture of craniofacial shape in the house mouse: a genetic and morphological perspective. Kiel: PhD thesis, Christian Albrechts University; 2015.
- 71.Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson B, Zusmanovich P, et al. Many sequence variants affecting diversity of adult human height. Nat Genet. 2008;40:609–615. doi: 10.1038/ng.122. [DOI] [PubMed] [Google Scholar]
- 72.Signer-Hasler H, Flury C, Haase B, Burger D, Simianer H, Leeb T, et al. A genome-wide association study reveals loci influencing height and other conformation traits in horses. PLoS One. 2012;7(5):e37282. doi: 10.1371/journal.pone.0037282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Mamun HA, Kwan P, Clark SA, Ferdosi MH, Tellam R, Gondro C. Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight. Genet Sel Evol. 2015;47:66. doi: 10.1186/s12711-015-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matika O, Riggio V, Anselme-Moizan M, Law AS, Pong-Wong R, Archibald AL, et al. Genome-wide association reveals QTL for growth, bone and in vivo carcass traits as assessed by computed tomography in Scottish Blackface lambs. Genet Sel Evol. 2016;48:11. doi: 10.1186/s12711-016-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolormaa S, Hayes BJ, van der Werf JHJ, Pethick D, Goddard ME, Daetwyler HD. Detailed phenotyping identifies genes with pleiotropic effects on body composition. BMC Genom. 2016;17:224. doi: 10.1186/s12864-016-2538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rochus CM, Tortereau F, Plisson-Petit F, Restoux G, Moreno-Romieux C, Tosser-Klopp G, et al. Revealing the selection history of adaptive loci using genome-wide scans for selection: an example from domestic sheep. BMC Genom. 2018;19:71. doi: 10.1186/s12864-018-4447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naval-Sanchez M, Nguyen Q, McWilliam S, Porto-Neto LR, Tellam R, Vuocolo T, et al. Sheep genome functional annotation reveals proximal regulatory elements contributed to the evolution of modern breeds. Nat Commun. 2018;9:859. doi: 10.1038/s41467-017-02809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Larrañaga O, Langa J, Rendo F, Manzano C, Iriondo M, Estonba A. Genomic selection signatures in sheep from the Western Pyrenees. Genet Sel Evol. 2018;50:9. doi: 10.1186/s12711-018-0378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Signer-Hasler H, Burren A, Ammann P, Drögemüller C, Flury C. Runs of homozygosity and signatures of selection: a comparison among eight local Swiss sheep breeds. Anim Genet. 2019;50:512–525. doi: 10.1111/age.12828. [DOI] [PubMed] [Google Scholar]
- 81.Saif R, Henkel J, Jagannathan V, Drögemüller C, Flury C, Leeb T. The LCORL locus is under selection in large-sized Pakistani goat breeds. Genes (Basel) 2020;11(2):168. doi: 10.3390/genes11020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graber JK, Signer-Hasler H, Burren A, Drögemüller C. Evaluation of truncating variants in the LCORL gene in relation to body size of goats from Switzerland. Anim Genet. 2022;53:237–239. doi: 10.1111/age.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Posbergh CJ, Huson HJ. All sheeps and sizes: a genetic investigation of mature body size across sheep breeds reveals a polygenic nature. Anim Genet. 2021;52:99–107. doi: 10.1111/age.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunlap KA, Kwak H il, Burghardt RC, Bazer FW, Magness RR, Johnson GA, et al. The sphingosine 1-phosphate (S1P) signaling pathway is regulated during pregnancy in sheep. Biol Reprod. 2010;82:876–87. doi: 10.1095/biolreprod.109.081604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghoreishifar SM, Eriksson S, Johansson AM, Khansefid M, Moghaddaszadeh-Ahrabi S, Parna N, et al. Signatures of selection reveal candidate genes involved in economic traits and cold acclimation in five Swedish cattle breeds. Genet Sel Evol. 2020;52:52. doi: 10.1186/s12711-020-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou SL, Zhou ZJ, Hu ZQ, Song CL, Luo YJ, Luo CB, et al. Genomic sequencing identifies WNK2 as a driver in hepatocellular carcinoma and a risk factor for early recurrence. J Hepatol. 2019;71:1152–63. doi: 10.1016/j.jhep.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Purfield DC, Evans RD, Berry DP. Breed- and trait-specific associations define the genetic architecture of calving performance traits in cattle. J Anim Sci. 2020;98(5):1–18. doi: 10.1093/jas/skaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Zhou G, Xu X, Geng R, Zhou J, Yang Y, et al. Transcriptome profile analysis of adipose tissues from fat and short-tailed sheep. Gene. 2014;549:252–257. doi: 10.1016/j.gene.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 89.Liu CT, Monda KL, Taylor KC, Lange L, Demerath EW, Palmas W, et al. Genome-wide association of body fat distribution in African ancestry populations suggests new loci. PLoS Genet. 2013;9(8):e1003681. doi: 10.1371/journal.pgen.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Ma P, Liu J, Zhang Q, Zhang Y, Ding X, et al. Genome-wide association study in Chinese Holstein cows reveal two candidate genes for somatic cell score as an indicator for mastitis susceptibility. BMC Genet. 2015;16:111. doi: 10.1186/s12863-015-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atlija M, Arranz JJ, Martinez-Valladares M, Gutiérrez-Gil B. Detection and replication of QTL underlying resistance to gastrointestinal nematodes in adult sheep using the ovine 50K SNP array. Genet Sel Evol. 2016;48:4. doi: 10.1186/s12711-016-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahbara AM, Khbou MK, Rhomdhane R, Sassi L, Gharbi M, Haile A, et al. Genome variation in tick infestation and cryptic divergence in Tunisian indigenous sheep. BMC Genom. 2022;23:167. doi: 10.1186/s12864-022-08321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H, Liu A, Wang Y, Luo H, Yan X, Guo X, et al. Genetic parameters and genome-wide association studies of eight longevity traits representing either full or partial lifespan in Chinese Holsteins. Front Genet. 2021;12:634986. doi: 10.3389/fgene.2021.634986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richardson IW. The genetics of Mycobacterium bovis infection in Irish cattle. PhD Thesis. Dublin; Trinity College; 2016.
- 95.Wang P, Li X, Zhu Y, Wei J, Zhang C, Kong Q, et al. Genome-wide association analysis of milk production, somatic cell score, and body conformation traits in Holstein cows. Front Vet Sci. 2022;9:1493. doi: 10.3389/fvets.2022.932034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saravanan KA, Panigrahi M, Kumar H, Bhushan B, Dutt T, Mishra BP. Genome-wide analysis of genetic diversity and selection signatures in three Indian sheep breeds. Livest Sci. 2021;243:104367. doi: 10.1016/j.livsci.2020.104367. [DOI] [Google Scholar]
- 97.Honerlagen H, Reyer H, Oster M, Ponsuksili S, Trakooljul N, Kuhla B, et al. Identification of genomic regions influencing N-metabolism and N-excretion in lactating Holstein-Friesians. Front Genet. 2021;12:699550. doi: 10.3389/fgene.2021.699550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yudin NS, Larkin DM, Ignatieva EV. A compendium and functional characterization of mammalian genes involved in adaptation to Arctic or Antarctic environments. BMC Genet. 2017;18:111. doi: 10.1186/s12863-017-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei FJ, Cai CY, Yu P, Lv J, Ling C, Shi WT, et al. Quantitative candidate gene association studies of metabolic traits in Han Chinese type 2 diabetes patients. Genet Mol Res. 2015;14:15471–15481. doi: 10.4238/2015.November.30.25. [DOI] [PubMed] [Google Scholar]
- 100.Chase A, Pellagatti A, Singh S, Score J, Tapper WJ, Lin F, et al. PRR14L mutations are associated with chromosome 22 acquired uniparental disomy, age-related clonal hematopoiesis and myeloid neoplasia. Leukemia. 2019;33:1184–1194. doi: 10.1038/s41375-018-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hodge MJ, de Las Heras-Saldana S, Rindfleish SJ, Stephen CP, Pant SD. Characterization of breed specific differences in spermatozoal transcriptomes of sheep in Australia. Genes (Basel) 2021;12(2):203. doi: 10.3390/genes12020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kennedy JM, Fodil N, Torre S, Bongfen SE, Olivier JF, Leung V, et al. CCDC88B is a novel regulator of maturation and effector functions of T cells during pathological inflammation. J Exp Med. 2014;211(13):2519–2535. doi: 10.1084/jem.20140455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmed AM, Good B, Hanrahan JP, McGettigan P, Browne J, Keane OM, et al. Variation in the Ovine abomasal lymph node transcriptome between breeds known to differ in resistance to the gastrointestinal nematode. PLoS ONE. 2015;10(5):e0124823. doi: 10.1371/journal.pone.0124823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li B, Qiao L, An L, Wang W, Liu J, Ren Y, et al. Transcriptome analysis of adipose tissues from two fat-tailed sheep breeds reveals key genes involved in fat deposition. BMC Genom. 2018;19:338. doi: 10.1186/s12864-018-4747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giannini AL, Gao Y, Bijlmakers MJ. T-cell regulator RNF125/TRAC-1 belongs to a novel family of ubiquitin ligases with zinc fingers and a ubiquitin-binding domain. Biochem J. 2008;410:101–111. doi: 10.1042/BJ20070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo-Miura J, et al. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) J Biol Chem. 2006;281:20749–20760. doi: 10.1074/jbc.M602089200. [DOI] [PubMed] [Google Scholar]
- 107.Tobar KMC, Álvarez DCL, Franco LÁÁ. Genome-wide association studies in sheep from Latin America. Review Rev Mex Cienc Pecu. 2020;11:659–683. [Google Scholar]
- 108.Carvalho ME, Baldi FS, Alexandre PA, Santana MHA, Ventura RV, Bueno RS, et al. Genomic regions and genes associated with carcass quality in Nelore cattle. Genet Mol Res. 2019;18(1):18226. doi: 10.4238/gmr18226. [DOI] [Google Scholar]
- 109.Sutera AM. Comparison of genome wide association studies for milk production traits in Valle del Belice dairy sheep. Palermo: PhD thesis, University of Palermo; 2018.
- 110.Zahr A, Alcaide P, Yang J, Jones A, Gregory M, dela Paz NG, et al. Endomucin prevents leukocyte-endothelial cell adhesion and has a critical role under resting and inflammatory conditions. Nat Commun. 2016;7:10363. doi: 10.1038/ncomms10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castaldi PJ, Guo F, Qiao D, Du F, Naing ZZC, Li Y, et al. Identification of functional variants in the FAM13A chronic obstructive pulmonary disease genome-wide association study locus by massively parallel reporter assays. Am J Respir Crit Care Med. 2019;199:52–61. doi: 10.1164/rccm.201802-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohammadi H, Farahani AHK, Moradi MH, Mastrangelo S, di Gerlando R, Sardina MT, et al. Weighted single-step genome-wide association study uncovers known and novel candidate genomic regions for milk production traits and somatic cell score in Valle del Belice dairy sheep. Animals. 2022;12(9):1155. doi: 10.3390/ani12091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niu Q, Zhang T, Xu L, Wang T, Wang Z, Zhu B, et al. Integration of selection signatures and multi-trait GWAS reveals polygenic genetic architecture of carcass traits in beef cattle. Genomics. 2021;113:3325–3336. doi: 10.1016/j.ygeno.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 114.Huang CY, Chou YH, Hsieh NT, Chen HH, Lee MF. MED28 regulates MEK1-dependent cellular migration in human breast cancer cells. J Cell Physiol. 2012;227:3820–3827. doi: 10.1002/jcp.24093. [DOI] [PubMed] [Google Scholar]
- 115.Braz CU, Rowan TN, Schnabel RD, Decker JE. Genome-wide association analyses identify genotype-by-environment interactions of growth traits in Simmental cattle. Sci Rep. 2021;11:13335. doi: 10.1038/s41598-021-92455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gai Z, Hu S, Ma J, Wang Y, Gong G, Zhao J. Whole genome-wide analysis of DEP family members in sheep (Ovis aries) reveals their potential roles in regulating lactation. Chem Biol Zechnol Agric. 2022;9:68. doi: 10.1186/s40538-022-00336-w. [DOI] [Google Scholar]
- 117.Awad MAA, Abou-Bakr S, El-Regalaty H, El-Din El-Assal S, Abdel-Shafy H. Determination of potential candidate genes associated with milk lactose in Egyptian buffalo. World's Vet J. 2020;10(1):35–42. [Google Scholar]
- 118.Reynolds EGM, Lopdell T, Wang Y, Tiplady KM, Harland CS, Johnson TJJ, et al. Non-additive QTL mapping of lactation traits in 124,000 cattle reveals novel recessive loci. Genet Sel Evol. 2022;54:5. doi: 10.1186/s12711-021-00694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao H, Zhu S, Guo T, Han M, Chen B, Qiao G, et al. Whole-genome re-sequencing association study on yearling wool traits in Chinese fine-wool sheep. J Anim Sci. 2021;99:9. doi: 10.1093/jas/skab210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ling YH, Xiang H, Zhang G, Ding JP, Zhang ZJ, Zhang YH, et al. Identification of complete linkage disequilibrium in the DSG4 gene and its association with wool length and crimp in Chinese indigenous sheep. Genet Mol Res. 2014;13:5617–5625. doi: 10.4238/2014.July.25.17. [DOI] [PubMed] [Google Scholar]
- 121.Xu SS, Gao L, Shen M, Lyu F. Whole-genome selective scans detect genes associated with important phenotypic traits in sheep (Ovis aries) Front Genet. 2021;12:2299. doi: 10.3389/fgene.2021.738879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Y, Zhou G, Zhang R, Guo J, Li C, Martin G, et al. Comparative proteomic analyses using iTRAQ-labeling provides insights into fiber diversity in sheep and goats. J Proteomics. 2018;172:82–88. doi: 10.1016/j.jprot.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 123.Demars J, Cano M, Drouilhet L, Plisson-Petit F, Bardou P, Fabre S, et al. Genome-wide identification of the mutation underlying fleece variation and discriminating ancestral hairy species from modern woolly sheep. Mol Biol Evol. 2017;34:1722. doi: 10.1093/molbev/msx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davies CJ, Fan Z, Morgado KP, Liu Y, Regouski M, Meng Q, et al. Development and characterization of type I interferon receptor knockout sheep: A model for viral immunology and reproductive signaling. Front Genet. 2022;13:2475. doi: 10.3389/fgene.2022.986316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel K, Scrimieri F, Ghosh S, Zhong J, Kim MS, Ren YR, et al. FAM190A deficiency creates a cell division defect. Am J Pathol. 2013;183:296–303. doi: 10.1016/j.ajpath.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Serrano M, Ramón M, Calvo JH, Jiménez M, Freire F, Vázquez JM, et al. Genome-wide association studies for sperm traits in Assaf sheep breed. Animal. 2021;15:2. doi: 10.1016/j.animal.2020.100065. [DOI] [PubMed] [Google Scholar]
- 127.Smith JL, Wilson ML, Nilson SM, Rowan TN, Schnabel RD, Decker JE, et al. Genome-wide association and genotype by environment interactions for growth traits in U.S. Red Angus cattle. BMC Genom. 2022;23:517. doi: 10.1186/s12864-022-08667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshida GM, Lhorente JP, Carvalheiro R, Yáñez JM. Bayesian genome-wide association analysis for body weight in farmed Atlantic salmon (Salmo salar L.) Anim Genet. 2017;48:698–703. doi: 10.1111/age.12621. [DOI] [PubMed] [Google Scholar]
- 129.Guo Q, Huang L, Bai H, Wang Z, Bi Y, Chen G, et al. Genome-wide association study of potential meat quality trait loci in ducks. Genes (Basel) 2022;13(6):986. doi: 10.3390/genes13060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Buzanskas ME, Grossi DA, Ventura RV, Schenkel FS, Sargolzaei M, Meirelles SLC, et al. Genome-wide association for growth traits in Canchim beef cattle. PLoS One. 2014;9(4):e94802. doi: 10.1371/journal.pone.0094802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu X, Feng C, Ma L, Song C, Wang Y, Da Y, et al. Genome-wide association study of body weight in chicken F2 resource population. PLoS One. 2011;6(7):e21872. doi: 10.1371/journal.pone.0021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaishnav S, Chauhan A, Ajay A, Saini BL, Kumar S, Kumar A, et al. Allelic to genome wide perspectives of swine genetic variation to litter size and its component traits. Mol Biol Rep. 2023;50:3705–3721. doi: 10.1007/s11033-022-08168-5. [DOI] [PubMed] [Google Scholar]
- 133.Schneider L, Essmann F, Kletke A, Rio P, Hanenberg H, Wetzel W, et al. The transforming acidic coiled coil 3 protein is essential for spindle-dependent chromosome alignment and mitotic survival. J Biol Chem. 2007;282(40):29273–29283. doi: 10.1074/jbc.M704151200. [DOI] [PubMed] [Google Scholar]
- 134.Zhang W, Xu M, Wang J, Wang S, Wang X, Yang J, et al. Comparative transcriptome analysis of key genes and pathways activated in response to fat deposition in two sheep breeds with distinct tail phenotype. Front Genet. 2021;12:639030. doi: 10.3389/fgene.2021.639030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, Anagnostopoulos A, et al. Mouse genome database (MGD) 2019. Nucleic Acids Res. 2019;47:801–806. doi: 10.1093/nar/gky1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang Q, Chen H, Ye J, Liu C, Wei R, Chen C, et al. Genetic diversity and signatures of selection in 15 Chinese indigenous dog breeds revealed by genome-wide SNPs. Front Genet. 2019;10:1174. doi: 10.3389/fgene.2019.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H. Patterns of genomic variation and whole genome association studies of economically important traits in cattle. Edmonton: PhD thesis, University of Alberta; 2012.
- 138.Chen ZH, Xu YX, Xie XL, Wang DF, Aguilar-Gómez D, Liu GJ, et al. Whole-genome sequence analysis unveils different origins of European and Asiatic mouflon and domestication-related genes in sheep. Commun Biol. 2021;4:1307. doi: 10.1038/s42003-021-02817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao YH, Xu SS, Shen M, Chen ZH, Gao L, Lv FH, et al. Historical introgression from wild relatives enhanced climatic adaptation and resistance to pneumonia in sheep. Mol Biol Evol. 2021;38:838–855. doi: 10.1093/molbev/msaa236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bürglin TR, Affolter M. Homeodomain proteins: an update. Chromosoma. 2016;125:497–521. doi: 10.1007/s00412-015-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Favier B, Dollé P. Developmental functions of mammalian Hox genes. Mol Hum Reprod. 1997;3:115–131. doi: 10.1093/molehr/3.2.115. [DOI] [PubMed] [Google Scholar]
- 142.Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- 143.Cunyuan L, Ming L, Xiaoyue L, Wei N, Yueren X, Rui Y, et al. Whole-genome resequencing reveals loci associated with thoracic vertebrae number in sheep. Front Genet. 2019;10:674. doi: 10.3389/fgene.2019.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan Z, Liu E, Liu Z, Kijas JW, Zhu C, Hu S, et al. Selection signature analysis reveals genes associated with tail type in Chinese indigenous sheep. Anim Genet. 2017;48:55–66. doi: 10.1111/age.12477. [DOI] [PubMed] [Google Scholar]
- 145.Doyle JL, Berry DP, Veerkamp RF, Carthy TR, Evans RD, Walsh SW, et al. Genomic regions associated with muscularity in beef cattle differ in five contrasting cattle breeds. Genet Sel Evol. 2020;52:2. doi: 10.1186/s12711-020-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fan B, Onteru SK, Du ZQ, Garrick DJ, Stalder KJ, Rothschild MF. Genome-wide association study identifies loci for body composition and structural soundness traits in pigs. PLoS One. 2011;6(2):e14726. doi: 10.1371/journal.pone.0014726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fariello MI, Servin B, Tosser-Klopp G, Rupp R, Moreno C, Cristobal MS, et al. Selection signatures in worldwide sheep populations. PLoS One. 2014;9(8):e103813. doi: 10.1371/journal.pone.0103813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Z, Tan X, Wang J, Jin Q, Meng X, Cai Z, et al. Whole genome sequencing of Luxi Black Head sheep for screening selection signatures associated with important traits. Anim Biosci. 2022;35:1340–1350. doi: 10.5713/ab.21.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang W, Zhang X, Zhou X, Zhang Y, La Y, Zhang Y, et al. Deep genome resequencing reveals artificial and natural selection for visual deterioration, plateau adaptability and high prolificacy in Chinese domestic sheep. Front Genet. 2019;10:300. doi: 10.3389/fgene.2019.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yurchenko AA, Daetwyler HD, Yudin N, Schnabel RD, vander Jagt CJ, Soloshenko V, et al. Scans for signatures of selection in Russian cattle breed genomes reveal new candidate genes for environmental adaptation and acclimation. Sci Rep. 2018;8:12984. doi: 10.1038/s41598-018-31304-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Benjelloun B. Diversité des génomes et adaptation locale des petits ruminants d’un pays méditerranéen: le Maroc. Grenoble: PhD Thesis, University of Grenoble Alpes; 2015.
- 152.Zhang DY, Zhang XX, di Li F, Yuan LF, Li XL, Zhang YK, et al. Whole-genome resequencing reveals molecular imprints of anthropogenic and natural selection in wild and domesticated sheep. Zool Res. 2022;43:695–705. doi: 10.24272/j.issn.2095-8137.2022.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Henderson JV, Wathes CM, Nicol CJ, White RP, Lines JA. Threat assessment by domestic ducklings using visual signals: implications for animal-machine interactions. Appl Anim Behav Sci. 2000;69(3):241–53. doi: 10.1016/S0168-1591(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 154.Peichl L. Topography of ganglion cells in the dog and wolf retina. J Comp Neurol. 1992;324(4):603–620. doi: 10.1002/cne.903240412. [DOI] [PubMed] [Google Scholar]
- 155.Wang MS, Zhang RW, Su LY, Li Y, Peng MS, Liu HQ, et al. Positive selection rather than relaxation of functional constraint drives the evolution of vision during chicken domestication. Cell Res. 2016;26:556–573. doi: 10.1038/cr.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Janssen R, Bont L, Siezen CLE, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 157.Ranjbar M, Whetstone CE, Omer H, Power L, Cusack RP, Gauvreau GM. The genetic factors of the airway epithelium associated with the pathology of asthma. Genes (Basel) 2022;13:1870. doi: 10.3390/genes13101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Eerdewegh P, Little RD, Dupuis JE, del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418:426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 159.Liu D, Chen Z, Zhao W, Guo L, Sun H, Zhu K, et al. Genome-wide selection signatures detection in Shanghai Holstein cattle population identified genes related to adaption, health and reproduction traits. BMC Genom. 2021;22:747. doi: 10.1186/s12864-021-08042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng X, Wang D, Wang S, Wang H, Zhou H. Identification of key genes and pathways involved in response to pain in goat and sheep by transcriptome sequencing. Biol Res. 2018;51:25. doi: 10.1186/s40659-018-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang S, He H, Ma QS, Zhang Y, Zhu Y, Wan X, et al. Experimental study of the protective effects of SYVN1 against diabetic retinopathy. Sci Rep. 2015;5:14036. doi: 10.1038/srep14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Amano T, Yamasaki S, Yagishita N, Tsuchimochi K, Shin H, Kawahara KI, et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 2003;17:2436–2449. doi: 10.1101/gad.1096603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaneko M, Ishiguro M, Niinuma Y, Uesugi M, Nomura Y. Human HRD1 protects against ER stress-induced apoptosis through ER-associated degradation 1. FEBS Lett. 2002;532(1–2):147–152. doi: 10.1016/S0014-5793(02)03660-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Description S1. Contains short description of the breeds and representative pictures of animals used in this study.
Additional file 2: Table S1. Data Info. Contains basic information (breed and number of samples) about the animals used in this study.
Additional file 3: Fig. S1. LD decay. Contains results from the LD decay analysis.
Additional file 4: Table S2. Gene clusters. Contains results from the functional annotation cluster analysis using DAVID software, with identified annotation clusters corresponding to biological functions.
Additional file 5: Table S3. Regions overlap. Contains information on detected selection signatures and regions whose positions overlapped by at least two different methods.
Additional file 6: Table S4. Identified genes ID. Contains detailed information (Gene ID, chr, start and end positions in bp) about the genes identified within genomic regions detected as selection signatures.
Additional file 7: Table S5. Functional gene groups. Contains results from the functional classification analysis of genes based on the functional similarity algorithm using DAVID software.
Additional file 8: Table S6. Functional terms. Contains results from the functional annotation analysis with annotation terms and their associated genes using DAVID software.
Additional file 9: Fig. S2. Significantly enriched traits per chromosome, as determined by QTL enrichment analysis. The area of the bubbles represents the number of QTLs observed for that class per chromosome, while the colour represents the FDR-adjusted P value as –log10 (P value) (the darker the colour, the smaller the P value). The x-axis shows the richness factor for each QTL, which is the ratio between the number of observed and expected QTLs.
Additional file 10: Fig. S3. Significantly enriched traits per genome, as determined by QTL enrichment analysis. The area of the bubbles represents the number of QTLs observed for that class per genome, while the colour represents the FDR-adjusted P value as –log10 (P value) (the darker the colour, the smaller the P value). The x-axis shows the richness factor for each QTL, which is the ratio between the number of observed and expected QTLs.
Data Availability Statement
Genotypic data representing eight Croatian sheep breeds are deposited and publicly available at https://doi.org/10.5061/dryad.pg4f4qrsn.