Abstract
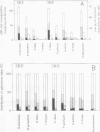
Intact chloroplasts isolated from leaves of eight species of 16:3 and 18:3 plants and chromoplasts isolated from Narcissus pseudonarcissus L. flowers synthesize galactose-labeled mono-, di-, and trigalactosyldiacylglycerol (MGDG, DGDG, and TGDG) when incubated with UDP-[6-3H]galactose. In all plastids, galactolipid synthesis, and especially synthesis of DGDG and TGDG, is reduced by treatment of the organelles with the nonpenetrating protease thermolysin. Envelope membranes isolated from thermolysin-treated chloroplasts of Spinacia oleracea L. (16:3 plant) and Pisum sativum L. (18:3 plant) or membranes isolated from thermolysin-treated chromoplasts are strongly reduced in galactolipid:galactolipid galactosyltransferase activity, but not with regard to UDP-Gal:diacylglycerol galactosyltransferase. For the intact plastids, this indicates that thermolysin treatment specifically blocks DGDG (and TGDG) synthesis, whereas MGDG synthesis is not affected. Neither in chloroplast nor in chromoplast membranes is DGDG synthesis stimulated by UDP-Gal. DGDG synthesis in S. oleracea chloroplasts is not stimulated by nucleoside 5′-diphospho digalactosides. Therefore, galactolipid:galactolipid galactosyltransferase is so far the only detectable enzyme synthesizing DGDG. These results conclusively suggest that the latter enzyme is located in the outer envelope membrane of different types of plastids and has a general function in DGDG synthesis, both in 16:3 and 18:3 plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alban C., Joyard J., Douce R. Preparation and characterization of envelope membranes from nongreen plastids. Plant Physiol. 1988 Nov;88(3):709–717. doi: 10.1104/pp.88.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. I. Electrophoretic and immunochemical analyses. J Biol Chem. 1983 Nov 10;258(21):13273–13280. [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983 Feb;71(2):366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI R. A., BENSON A. A. The path of carbon in photosynthesis of the lipids. Arch Biochem Biophys. 1961 May;93:185–192. doi: 10.1016/0003-9861(61)90248-x. [DOI] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G., Browse J. Glycerolipid labelling kinetics in isolated intact chloroplasts. Biochem J. 1984 Dec 1;224(2):637–643. doi: 10.1042/bj2240637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Schmidt H., Hoch M., Jung K. H., Binder H., Schmidt R. R. Synthesis of different nucleoside 5'-diphospho-sulfoquinovoses and their use for studies on sulfolipid biosynthesis in chloroplasts. Eur J Biochem. 1989 Sep 15;184(2):445–453. doi: 10.1111/j.1432-1033.1989.tb15037.x. [DOI] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman H. A., John J. B. Metabolism of Unsaturated Monogalactosyldiacylglycerol Molecular Species in Arabidopsis thaliana Reveals Different Sites and Substrates for Linolenic Acid Synthesis. Plant Physiol. 1986 Jul;81(3):731–736. doi: 10.1104/pp.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Joyard J., Douce R. Labelling in vivo and in vitro of molecular species of lipids from chloroplast envelopes and thylakoids. Eur J Biochem. 1980;108(1):177–185. doi: 10.1111/j.1432-1033.1980.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Siebertz M., Heinz E. Galactosylation of different monogalactosyldiacylglycerols by cell-free preparations from pea leaves. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):27–34. doi: 10.1515/bchm2.1977.358.1.27. [DOI] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Khan M. U., Leung S. Galactolipid Synthesis in Vicia faba Leaves: I. Galactose, Glycerol, and Fatty Acid Labeling after CO(2) Feeding. Plant Physiol. 1975 Jun;55(6):1038–1042. doi: 10.1104/pp.55.6.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim Biophys Acta. 1978 Apr 28;529(1):44–53. doi: 10.1016/0005-2760(78)90102-9. [DOI] [PubMed] [Google Scholar]