Abstract
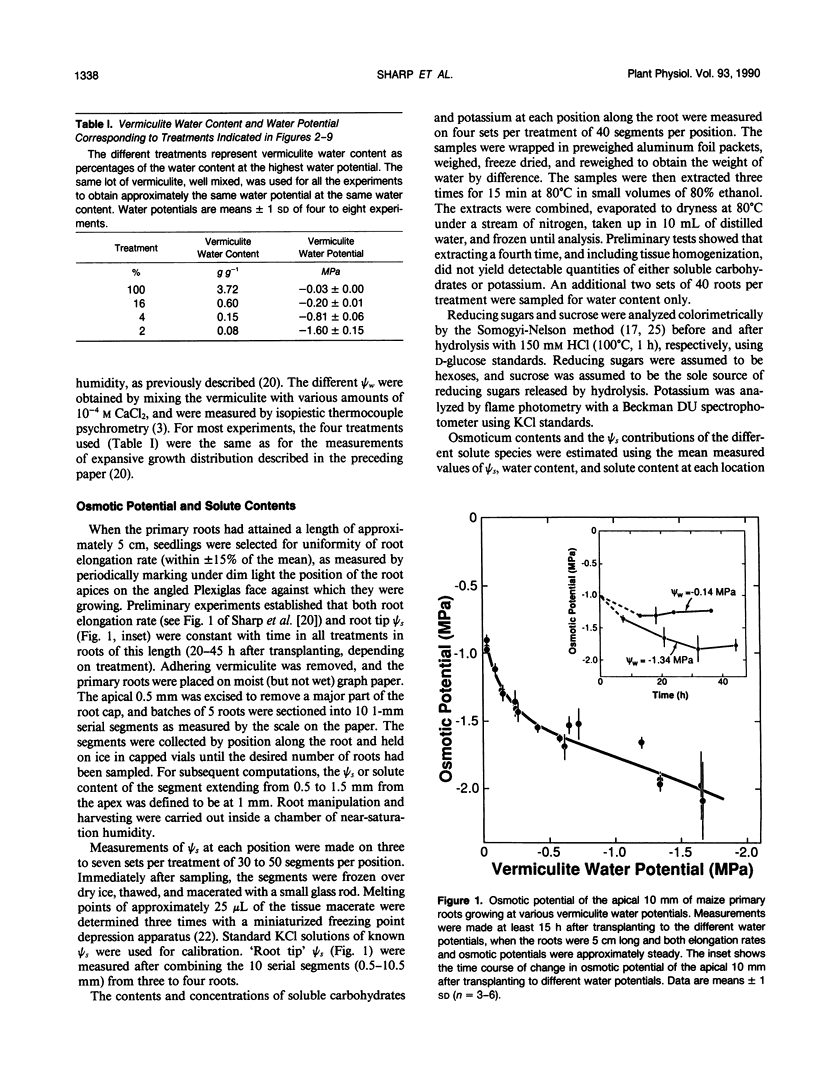
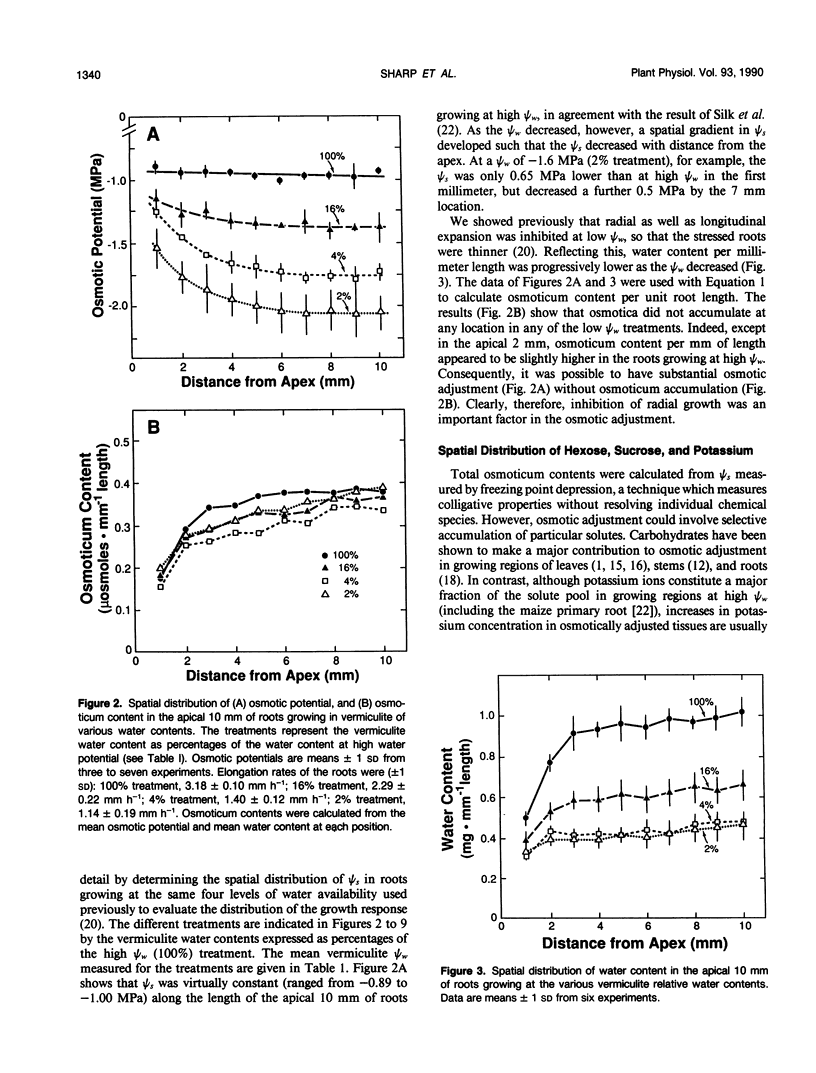
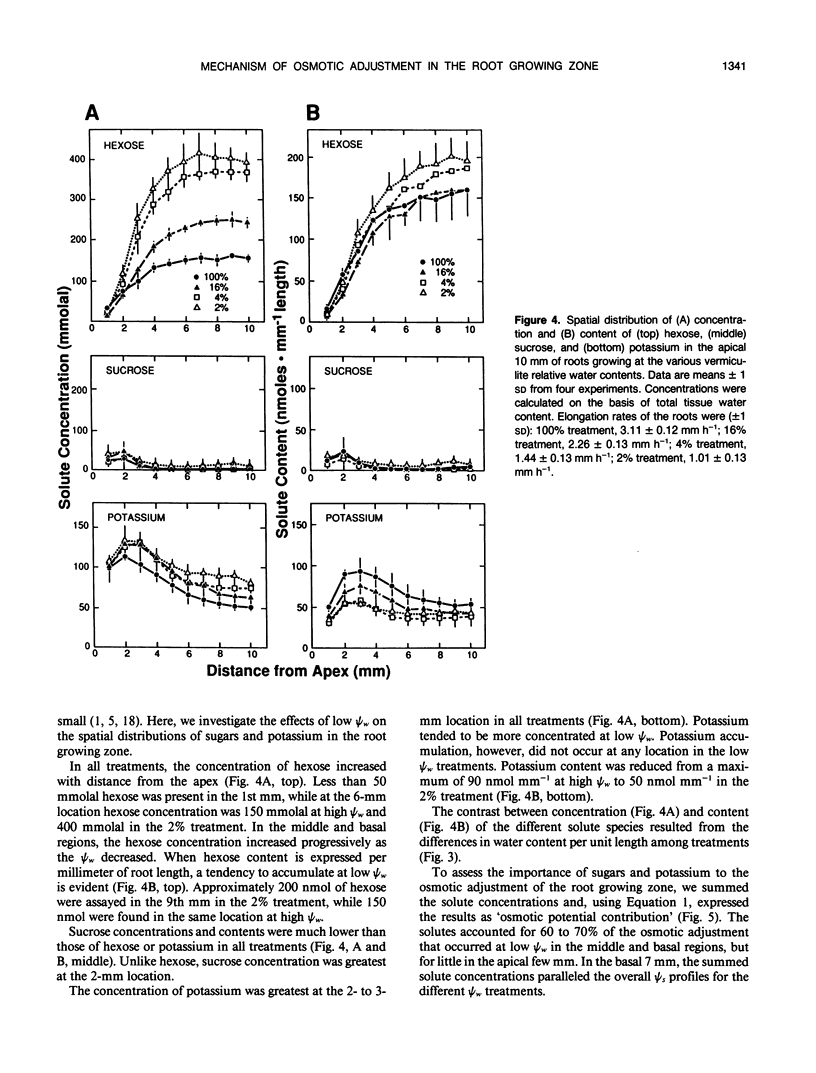
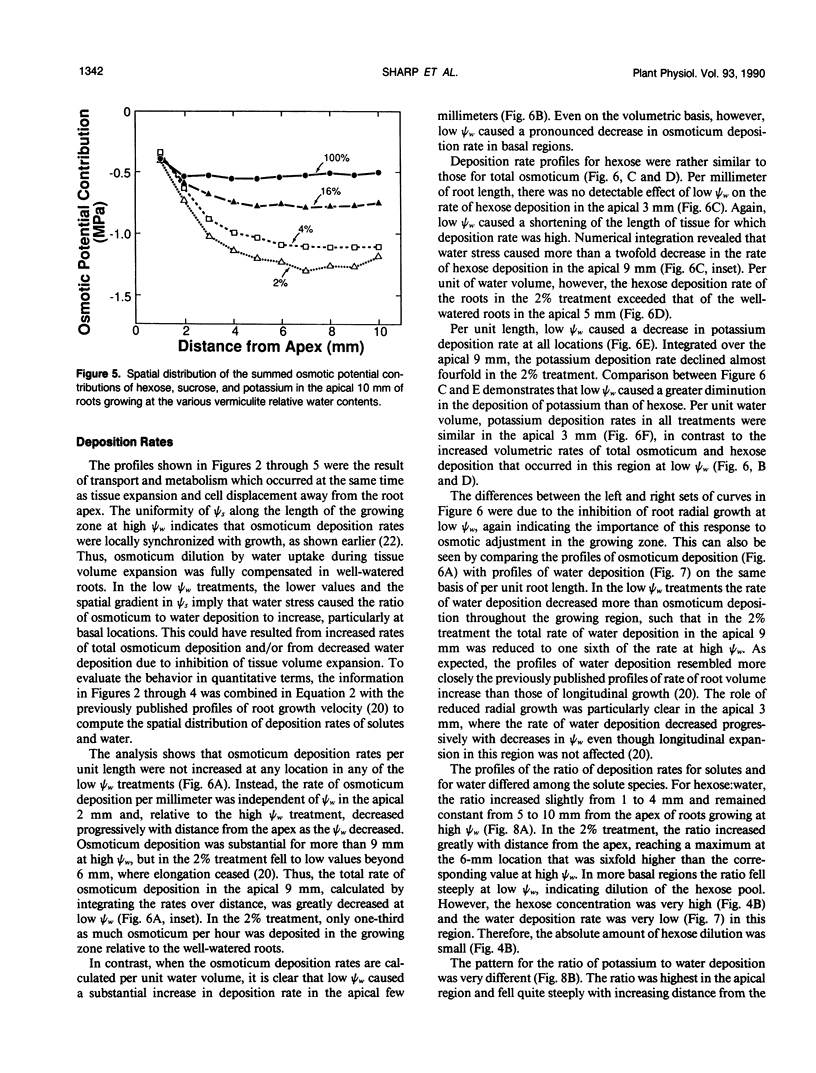
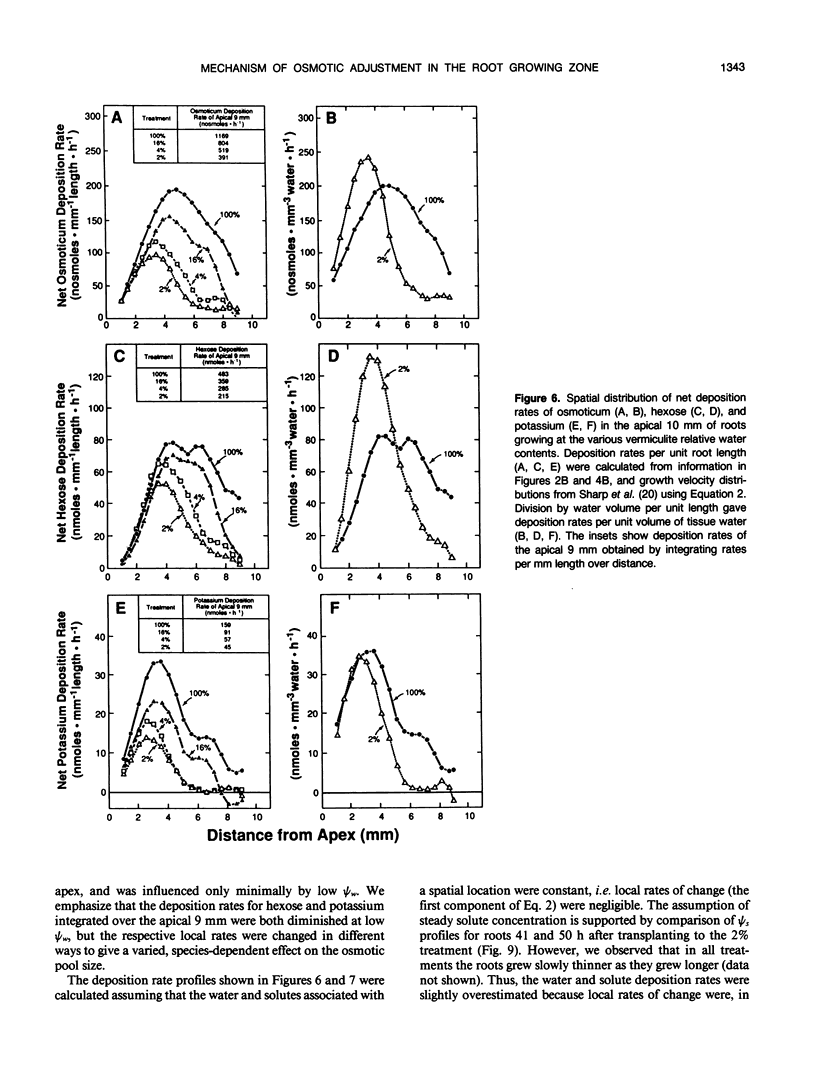
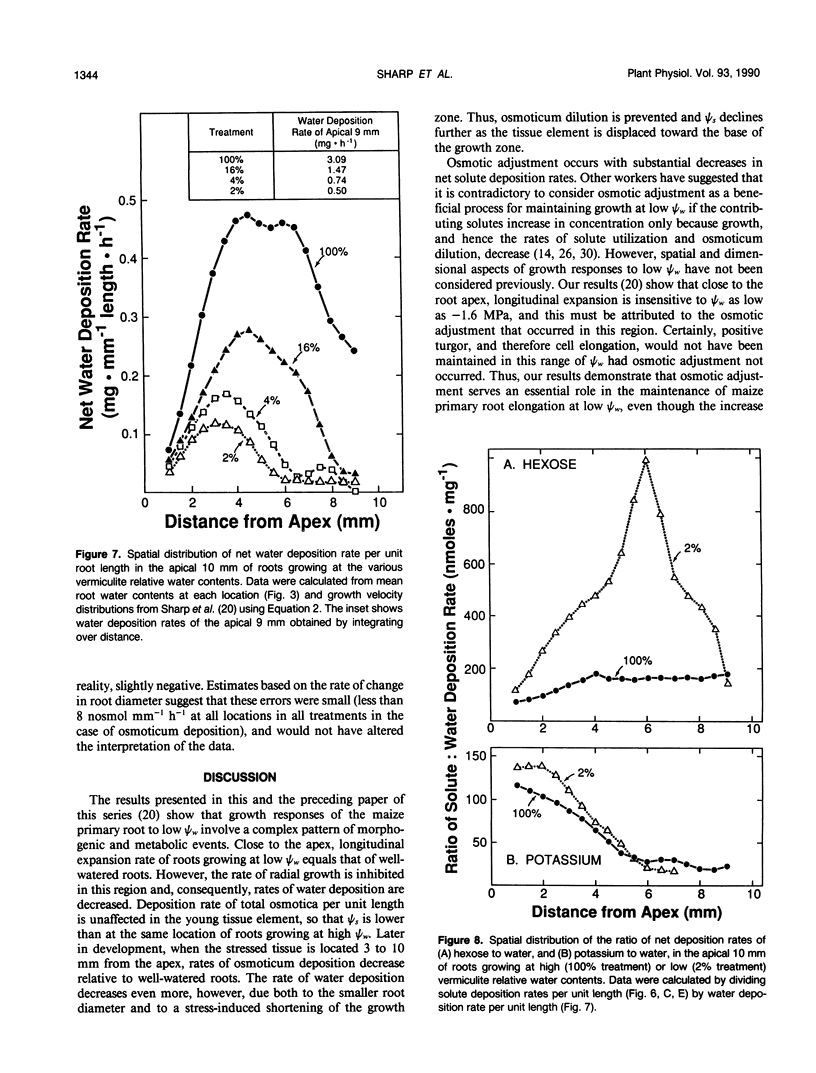
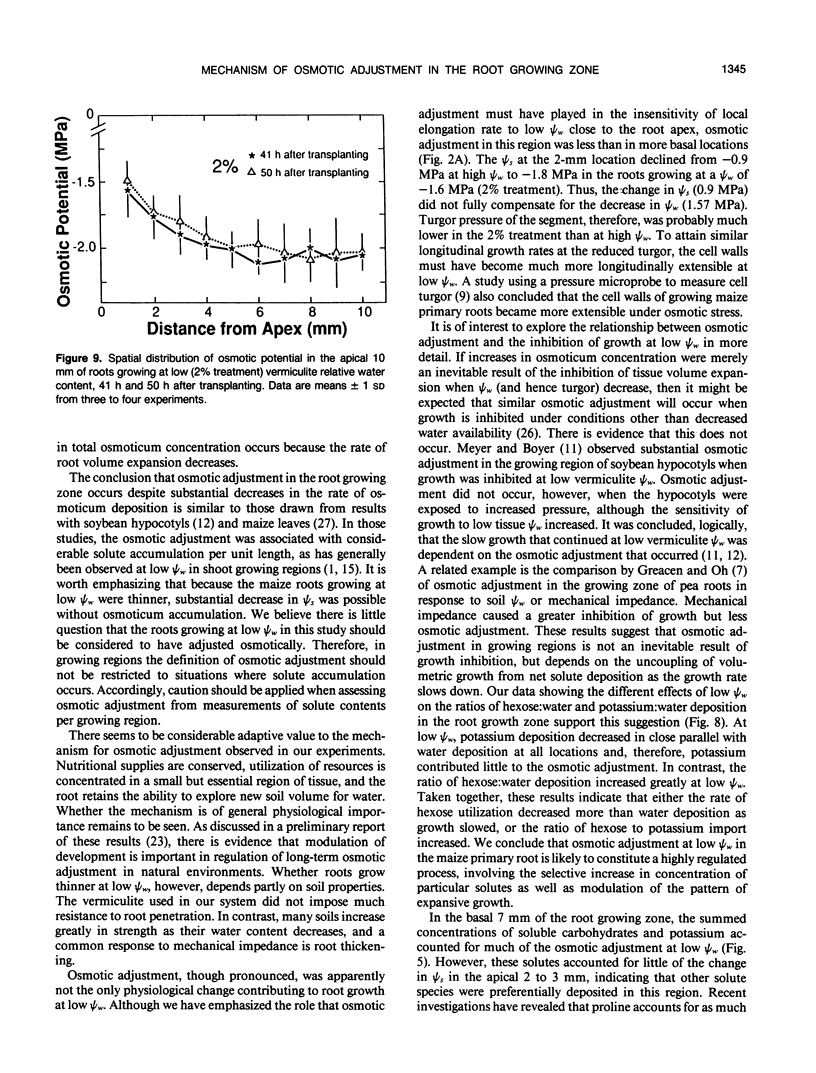
Primary roots of maize (Zea mays L. cv WF9 × Mo17) seedlings growing in vermiculite at various water potentials exhibited substantial osmotic adjustment in the growing region. We have assessed quantitatively whether the osmotic adjustment was attributable to increased net solute deposition rates or to slower rates of water deposition associated with reduced volume expansion. Spatial distributions of total osmotica, soluble carbohydrates, potassium, and water were combined with published growth velocity distributions to calculate deposition rate profiles using the continuity equation. Low water potentials had no effect on the rate of total osmoticum deposition per unit length close to the apex, and caused decreased deposition rates in basal regions. However, rates of water deposition decreased more than osmoticum deposition. Consequently, osmoticum deposition rates per unit water volume were increased near the apex and osmotic potentials were lower throughout the growing region. Because the stressed roots were thinner, osmotic adjustment occurred without osmoticum accumulation per unit length. The effects of low water potential on hexose deposition were similar to those for total osmotica, and hexose made a major contribution to the osmotic adjustment in middle and basal regions. In contrast, potassium deposition decreased at low water potentials in close parallel with water deposition, and increases in potassium concentration were small. The results show that growth of the maize primary root at low water potentials involves a complex pattern of morphogenic and metabolic events. Although osmotic adjustment is largely the result of a greater inhibition of volume expansion and water deposition than solute deposition, the contrasting behavior of hexose and potassium deposition indicates that the adjustment is a highly regulated process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creelman R. A., Mason H. S., Bensen R. J., Boyer J. S., Mullet J. E. Water Deficit and Abscisic Acid Cause Differential Inhibition of Shoot versus Root Growth in Soybean Seedlings : Analysis of Growth, Sugar Accumulation, and Gene Expression. Plant Physiol. 1990 Jan;92(1):205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greacen E. L., Oh J. S. Physics of root growth. Nat New Biol. 1972 Jan 5;235(53):24–25. doi: 10.1038/newbio235024a0. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Riazi A. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiol. 1981 Sep;68(3):571–576. doi: 10.1104/pp.68.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sharp R. E., Silk W. K., Hsiao T. C. Growth of the maize primary root at low water potentials : I. Spatial distribution of expansive growth. Plant Physiol. 1988 May;87(1):50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Erickson R. O. Kinematics of plant growth. J Theor Biol. 1979 Feb 21;76(4):481–501. doi: 10.1016/0022-5193(79)90014-6. [DOI] [PubMed] [Google Scholar]
- Silk W. K., Hsiao T. C., Diedenhofen U., Matson C. Spatial distributions of potassium, solutes, and their deposition rates in the growth zone of the primary corn root. Plant Physiol. 1986 Nov;82(3):853–858. doi: 10.1104/pp.82.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W. K., Walker R. C., Labavitch J. Uronide Deposition Rates in the Primary Root of Zea mays. Plant Physiol. 1984 Mar;74(3):721–726. doi: 10.1104/pp.74.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E., Boyer J. S. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985 Jan;77(1):190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


