Abstract
Objectives
Adverse childhood experiences (ACEs) are associated with higher risk of chronic disease, but little is known about the association with late life cognitive decline. We examined the longitudinal association between ACEs and late-life cognitive decline in the Study of Healthy Aging in African Americans (STAR).
Design
Linear mixed models with random intercepts and slope examined the association of individual and composite ACEs with cognitive change adjusting for years from baseline (timescale), baseline age, sex, parental education, childhood socioeconomic status and childhood social support. Participants reported whether they had experienced nine types of ACEs. Executive function and verbal episodic memory were measured up to three times over a 3-year period using the Spanish and English Neuropsychological Assessment Scales.
Settings
Kaiser Permanente Northern California members living in the Bay Area.
Participants
STAR is a cohort study of cognitive ageing launched in 2018 that has enrolled 764 black Americans ages ≥50 years (mean age=67.5; SD=8.5).
Results
Twenty-one per cent of participants reported no ACEs, 24% one ACE, 20% two ACEs, 17% three ACEs and 17% four or more ACEs. Compared with no ACEs, two ACEs (β=0.117; 95% CI 0.052 to 0.182), three ACEs (β=0.075; 95% CI 0.007 to 0.143) and four or more ACEs (β=0.089; 95% CI 0.002 to 0.158) were associated with less decline in executive function. There were no significant associations between number of ACEs and baseline or longitudinal verbal episodic memory or between individual ACEs and executive function or verbal episodic memory.
Conclusion
In this cohort of older black Americans, there was no association between ACEs and baseline cognition or cognitive change in verbal episodic memory; however, experiencing ≥ 2 ACEs was associated with less decline in executive function. These results may indicate that participants who survived to age 50+ and experienced ACEs may have cognitive resilience that warrants further investigation.
Keywords: EPIDEMIOLOGY, PUBLIC HEALTH, Risk Factors, Aging
STRENGTHS AND LIMITATIONS OF THIS STUDY
The impact of adverse childhood experiences (ACEs) on late-life cognition in older black adults is sparse with little research investigating cognitive decline.
The Study of Healthy Aging in African Americans is a well-characterised cohort of older black Americans ages 50 years or older with detailed socioeconomic lifecourse information such as education, region of birth and childhood experiences.
Repeated assessment of cognition in two domains across three waves (approximately 3 years) using the Spanish and English Assessment Scale, a measurement validated in English and Spanish, and in diverse populations.
Linear mixed models allowing for evaluation of ACEs on cognition and cognitive decline adjusting for childhood confounders such as childhood socioeconomic status and childhood support.
ACEs assessments were limited to self-report and there were no questions asked of physical or sexual abuse.
Introduction
Childhood is a sensitive period in the lifecourse for later-life health outcomes,1 2 such that disruptions during this period can have detrimental effects on development and later life health. Adverse childhood experiences (ACEs) are traumatic events in childhood that include abuse, witnessing violence and household dysfunction and have been associated with higher risk of cardiovascular diseases, chronic lung disease and liver disease.1 2 Although ACEs are associated with many of the risk factors for dementia, only a handful of studies have examined the association between ACEs and poor cognitive ageing outcomes in black Americans.3
Cognitive decline and decreased cognitive function are early indicators of Alzheimer’s disease and related dementias (ADRD).4 Signs of these cognitive deficits often include loss of memory and/or loss of the ability to perform high-level mental skills (executive function) such as planning, and management of thoughts and emotions. Therefore, many studies administering cognitive assessments will include some form of memory and executive function assessment.5 6 Some studies indicate that ACEs have negative effects on cognitive functioning later in life.7–10 These studies examining the specific types of ACEs have reported associations of the death of a parent, parental excess alcohol and drug use, mental health problems, physical neglect and emotional abuse experienced during childhood with worse memory later in life. Moreover, two systematic reviews found that abuse and neglect were associated with worse executive function.11 12 Additionally, greater numbers of ACEs are associated with increased risk of developing ADRD.13–15 Despite findings of ACEs being associated with poorer memory and higher risk of ADRD, other studies have shown mixed results, with weak to no association of ACEs with change in cognition over time.16 17 Furthermore, the literature is mixed on the specific impact that ACEs have on late-life cognitive functioning with some ACEs being associated with slower cognitive decline in older black adults but no decline in older white adults.18 19
Two interrelated life course theories serve as a framework for understanding how early life exposures, such as ACEs, may affect later life cognitive outcomes. The cumulative advantage/disadvantage theory posits that structural and institutional processes contribute to differential access to resources or harmful exposures that accumulate in a non-additive way over time.20 21 Individuals who are exposed to more ACEs over time will have an increased risk of negative health outcomes, including poor cognition, later in life. Building on this theory, the cumulative inequality (CI) theory incorporates life course factors that take into consideration the intergenerational, socioeconomic and stress processes important in the environment in which a child grows up.22 23 Both theories recognise that the trajectories established by negative childhood exposures can be altered by positive experiences throughout the life course. ACEs may be a predictor of worse outcomes in later life, but positive experiences such as social support and individual response to adversity may minimise the negative effects on cognitive outcomes. Due to the relationships between both theories, we will consider them together as the CI theory.
Compared with white Americans, black Americans have a higher risk of ADRD and report more ACEs.24–26 However, the relationship between childhood adversity and cognition in later life among black adults remains ambiguous.27–29 The two studies that have examined ACEs and cognitive outcomes in black adults have had mixed results.16 18 One study examining 427 older blacks adults found no associations between ACEs and cognition.16 Another study among 3700 older black adults found that those who reported experiencing food deprivation and having thinner body size than their peers in early life had slower rates of cognitive decline compared with those who did not report food deprivation or being thinner.18
The aim of this paper was to examine the association between total number of ACEs as well as the specific ACEs experienced with cognitive change in a cohort of middle-aged and older black adults. To expand the sparse existing literature, we focus on black individuals and their early-life experiences.3 Based on the CI theory, we hypothesise a dose–response relationship where each additional ACE experienced is associated with faster cognitive decline, and all types of ACEs predict worse cognitive outcomes.
Methods
Study participants and data collection
The Study of Healthy Aging in African Americans (STAR) cohort consists of community-dwelling midlife to older black adults that reside in the San Francisco Bay area of California, primarily the cities of Oakland and Richmond.30 31 STAR aims to evaluate how lifecourse vascular and sociocultural factors influence the trajectory of cognitive ageing and burden of cognitive impairment among black Americans. Individuals eligible for STAR were long-term members of Kaiser Permanente Northern California, an integrated healthcare delivery system, who identified as Black or African American, were aged 50 years or older on 1 January 2018, and had previously participated in Kaiser Permanente multiphasic health check-up exams between 1964 and 1985. Stratified random sampling by age and educational attainment was used with the goal of recruiting approximately equal proportions of participants ages 50–64 and 65 and older. Exclusion criteria included electronic medical record diagnosis of dementia or other neurodegenerative diseases (frontotemporal dementia, Lewy body disease, Pick’s disease, Parkinson’s disease with dementia, Huntington’s disease) and presence of health conditions that would impede participation in study interviews (defined by hospice activity in the past 12 months, history of severe chronic obstructive pulmonary disease in the past 6 months, congestive heart failure hospitalisations in the past 6 months and history of end-stage renal disease or dialysis in the past 12 months). Although most participants of STAR resided in California by the 1960s, more than half of the participants (53%) were born outside of California, and about one-third (36%) of these participants were from the Southern states.
Patient and public involvement
Neither patients nor the public were involved in the design, conduct, reporting or dissemination plans of our research.
Measures
Cognition
Cognitive function was assessed at each STAR wave using the Spanish and English Neuropsychological Assessment Scales (SENAS), a battery of cognitive tests that have undergone extensive development using item response theory methodology for valid comparisons of cognition and cognitive change across racial/ethnic and linguistically diverse groups.32–34 Cognitive domains of executive function and verbal episodic memory were derived from the SENAS. Executive function is a composite constructed from components of category fluency, phonemic/letter fluency and working memory (digit span backward and two list sorting). Verbal episodic memory was derived from two Word List Learning tests. Each domain was z-standardised using to the full baseline sample. Moreover, neither of the cognitive domains is limited by any ceiling or floor effect.32 Details of the administration procedures, development and psychometric characteristics can be found described in-depth elsewhere.32–35 Cognitive trajectories were measured across three waves of data approximately 14 months apart totally over 3 years.
Adverse childhood experiences
STAR fielded a modified version of the ACEs assessment from the REason for Geographic and Racial Disparities in Stroke cohort.36–38 During baseline interviews, participants were asked verbally if they experienced nine separate types of ACEs during childhood from birth to age 16. ACEs included experiences of parents’ divorce or separation, a parent remarrying, witnessing domestic violence, substance abuse by a family member, loss of a job by a parent, a parent going to jail, serious illness of a family member, death of mother and death of father. ACEs were examined individually and as a composite ACE score defined as the sum of ACEs reported and recategorised as 0, 1, 2, 3 or 4 or more ACEs. Summation of ACEs models the cumulative effect that is reflective of the CI theory and cumulative ACEs score from 0 to 4 or more is one of the most commonly used methods for operationalising ACEs. Cumulative ACEs score has been found to have a dose–response association with various health outcomes.2 39–41 Approximately 2% of participants (n=14) had missing ACEs and were excluded from the analyses.
Covariates
As ACEs occurs early in life, we identified potential factors in early life that may cause confounding in the association of ACEs and late-life cognition and cognitive decline.7 10 16–18 We adjusted for five early-life social support factors that may confound the associations of ACEs and later-life cognition. Using a five level Likert-type scale (1=none of the time, 2=a little of the time, 3=some of the time, 4=most of the time and 5=all of the time), participants were asked: ‘How often was there someone in whom you could talk to, trust and confide?,’ ‘How often was there someone who showed you love and affection?,’ ‘How often was there someone who could help you with your homework?,’ ‘How often was there someone who encouraged and pushed you to succeed in school?,’ and ‘How often did you have as much contact as you would like with someone you felt close to, someone in whom you could trust and confide?’ The responses were dichotomised with cut-offs between high frequency (most and all of the time) and low frequency (none, a little and some of the time). A composite score was created for early life factor by summation of the five scores (ranges 0–20) with higher score indicating higher levels of social support.
We additionally adjusted for early life socioeconomic status (SES) by including combined parental education (both parents with less than high school vs at least one parent with high school graduation or more), self-reported childhood family housing status (mortgage or owned home vs rental or others), and how often the participant reported going hungry as a child (never vs ever). Parental education was reported by the participants as highest level of education completed for both maternal and paternal parent. Both parent’s education was combined into one parental education and dichotomised as both parents with less than high school diploma, and either one or both parents with more than high school diploma. Due to the small number of either one or both parents obtaining higher than high school diploma (38%), we operationalise parent education at the high school level cut-off. If both maternal and paternal education was missing, parental education was classified as less than a high school diploma, and these participants were demarcated by including a missing indicator covariate in all models.
Other covariates included age at baseline interview centred at the mean baseline age, sex (men or women) which was derived from self-report or participant medical records and likely reflected a mixture of sex assigned at birth and gender identity, and self-reported educational attainment (collapsed as less than college degree, some college and college graduate or more) captured during STAR baseline interviews.
Statistical analysis
The distribution of demographics, childhood social support, childhood SES indicators and type of ACEs was estimated overall and stratified by the number of ACEs experienced. Two sets of linear mixed models were used to assess the association of cognition with: (1) composite ACE score and (2) individual ACEs, allowing for random intercept and slope to account for within-person correlation. The models were adjusted for time (as years since baseline) to estimate trajectories across three waves. We sequentially adjusted for covariates in our composite ACEs models by (1) adjusting for baseline mean-centred age and sex, (2) adjusted for childhood SES indicators and (3) adjusted for childhood support. For models with individual ACEs, we sequentially adjusted for covariates by (1) adjusting for baseline mean-centred age, (2) sex and (3) parental education. Interaction terms for time scale with exposure and covariates were added to each model to measure changes in cognition over time.
From a cohort of 764 participants, we excluded 14 participants for missing information on ACEs, 15 participants for missing early life social support and SES covariates, and 16 participants for missing report of sex.
Results
Our analytical sample consisted of 707 participants with a mean age of 68.6 (SD 8.7) years (range 53–95 years) of whom 487 (68.9%) were women compared with 220 (31.1%) men (table 1). About 21% of participants reported no ACE, 23.6% reported one ACE, 20% reported two ACEs, 17.3% reported three ACEs and 16.8% reported four or more ACEs. Seventy-nine per cent of participants had at least one ACE. The most common ACE reported was experiencing parents’ separation or divorce (38.5%), followed by serious family illness (35.4%), and witnessing domestic violence (31.5%). More than one-third (35.5%) of participants had a college degree or higher, and 38% of participants had one or both parents with more than a high school-level education (table 1). Participants in this cohort generally had high levels of support (average composite score of 15.8 and SD=4.7) during childhood with majority of participants reporting someone they could trust (76%), someone to love them (86%), someone to help with homework (66%), someone to motivate or encourage them in school (79%) and someone close to them they could contact (77%) all or most of the time. Most participants self-reported as being well-off or above average financially during childhood (68%), and most participants never experienced childhood hunger (92%). About 83% of our baseline cohort had two waves of cognitive measures, and over 75% of participants had all three waves of cognitive measures.
Table 1.
Baseline characteristics stratified by number of adverse childhood experiences (ACEs), STAR
| Characteristic | Overall sample | 0 ACEs | 1 ACE | 2 ACEs | 3 ACEs | 4+ ACEs |
| N (%) or mean (SD) | ||||||
| No of participants | 707 (100) | 151 (21.4) | 167 (23.6) | 148 (20.0) | 122 (17.3) | 119 (16.8) |
| Baseline age | 68.6 (8.7) | 69.1 (8.2) | 69.0 (8.4) | 68.9 (9.2) | 68.8 (9.6) | 66.7 (8.2) |
| Sex: men | 220 (31.1) | 46 (30.5) | 56 (33.5) | 51 (34.5) | 38 (31.2) | 29 (24.4) |
| College graduate or more | 252 (35.6) | 51 (33.8) | 60 (35.9) | 54 (36.5) | 44 (36.1) | 43 (36.1) |
| Some college | 333 (47.1) | 67 (44.4) | 83 (49.7) | 72 (48.7) | 56 (45.9) | 55 (46.2) |
| High school or less | 122 (17.3) | 33 (21.9) | 24 (14.4) | 22 (14.9) | 22 (18.0) | 21 (17.7) |
| Parent education: more than high school | 267 (37.8) | 63 (41.7) | 59 (35.3) | 54 (36.5) | 43 (35.3) | 48 (40.3) |
| ACEs | N (column % per variables) | |||||
| Parents were separated or divorced | 272 (38.5) | 0 | 30 (18.0) | 63 (42.6) | 73 (59.8) | 106 (89.1) |
| Serious illness of a family member | 250 (35.4) | 0 | 44 (26.4) | 67 (45.3) | 64 (52.5) | 75 (63.0) |
| Witnessed domestic violence | 223 (31.5) | 0 | 37 (22.2) | 41 (27.7) | 60 (49.2) | 85 (71.4) |
| Substance abuse by a family member | 172 (24.3) | 0 | 18 (10.8) | 35 (23.7) | 41 (33.6) | 78 (65.6) |
| Parent remarried | 176 (24.9) | 0 | 2 (1.2) | 39 (26.4) | 58 (47.6) | 77 (64.7) |
| Loss of job by a parent | 106 (15.0) | 0 | 23 (13.8) | 19 (12.8) | 23 (18.9) | 41 (34.5) |
| Death of your father | 70 (9.9) | 0 | 8 (4.8) | 18 (12.2) | 19 (15.6) | 25 (21.0) |
| Parent had to go to jail | 53 (7.5) | 0 | 2 (1.2) | 4 (2.7) | 10 (8.2) | 37 (31.1) |
| Death of your mother | 42 (5.9) | 0 | 3 (1.8) | 10 (6.8) | 18 (14.8) | 11 (9.2) |
| Childhood social support | N (column % per variables) or mean (SD) | |||||
| Composite childhood support (range 0–20) | 15.8 (4.7) | 17.1 (4.2) | 16.4 (4.3) | 15.3 (4.7) | 14.5 (4.8) | 14.9 (5.1) |
| Someone to trust and confide in most to all the times | 537 (75.7) | 125 (82.8) | 136 (81.4) | 104 (70.3) | 87 (71.3) | 83 (69.8) |
| Someone to love most to all the times | 611 (86.4) | 143 (94.7) | 149 (89.2) | 125 (84.5) | 96 (78.7) | 98 (82.4) |
| Someone to help with homework most to all the times | 466 (65.9) | 121 (80.1) | 118 (70.6) | 91 (61.49) | 65 (53.3) | 71 (59.1) |
| Someone to motivate and encourage in school most to all the times | 559 (79.1) | 135 (89.4) | 145 (86.8) | 107 (72.3) | 87 (71.3) | 85 (71.4) |
| Had contact with someone felt close to most or all the times | 547 (77.4) | 126 (83.4) | 138 (82.6) | 112 (75.7) | 87 (71.3) | 84 (70.6) |
| Childhood socioeconomic status | N (column % per variables) | |||||
| Family financially above average or well-off | 483 (68.3) | 125 (82.8) | 120 (71.9) | 101 (68.2) | 70 (57.4) | 67 (56.3) |
| Never hungry during childhood | 650 (91.9) | 146 (96.7) | 157 (94.0) | 131 (88.5) | 110 (90.2) | 106 (89.1) |
| Family had a mortgage or owned a home during childhood | 444 (62.8) | 118 (78.2) | 111 (66.5) | 94 (63.5) | 65 (53.3) | 56 (47.1) |
Childhood social support: composite childhood support derived from individual childhood social support (range 0=no support, 20=most support).
Individual childhood supports are on Likert scale (1=none of the time, 2=a little of the time, 3=some of the time, 4=most of the time, 5=all of the time).
STAR, Study of Healthy Aging in African Americans.
Composite number of ACEs
In our linear mixed models examining associations of the composite ACEs and baseline executive function (table 2), we observed a negative non-significant associations for one ACE (β=−0.130; 95% CI −0.316 to 0.055), two ACEs (β=−0.039; 95% CI −0.231 to 0.152) and four or more ACEs (β=−0.025; 95% CI −0.228 to 0.178), and a positive non-significant association for three ACEs (β=0.008; 95% CI −0.193 to 0.209) compared with no ACEs. After adjusting for childhood SES, the estimates decreased for one ACE (β=−0.090; 95% CI −0.272 to 0.093), increased for three ACEs (β=0.070; 95% CI −0.132 to 0.271), and changed direction for two ACEs (β=0.008; 95% CI −0.181 to 0.197) and four or more ACEs (β=0.052; 95% CI −0.155 to 0.259) suggesting a non-significant positive association with baseline executive function. The estimates were attenuated after further adjusting for childhood support. We observed a non-significant negative association between composite ACEs and baseline verbal episodic memory for one ACE (β=−0.137; 95% CI −0.321 to 0.048), two ACEs (β=−0.041; 95% CI −0.231 to 0.149) and three ACEs (β=−0.120; 95% CI −0.320 to 0.080), but positive association for four or more ACEs (β=0.105; 95% CI −0.097 to 0.307). After adjusting for childhood SES and childhood support, point estimates for the association between ACEs and baseline verbal episodic memory were attenuated.
Table 2.
Linear mixed models estimate of the association of composite adverse childhood experiences (ACEs) with domain-specific cognition across three waves
| Executive function | Verbal episodic memory | |||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Years from baseline | -0.071 (-0.119 to -0.023) | -0.084 (-0.137 to -0.03) | -0.119 (-0.222 to -0.016) | -0.078 (-0.148 to -0.009) | -0.108 (-0.185 to -0.03) | -0.170 (-0.319 to -0.02) |
| Baseline | ||||||
| ACEs | ||||||
| 0 | ref | ref | ref | ref | ref | ref |
| 1 | -0.130 (−0.316, 0.055) | -0.090 (−0.272, 0.093) | -0.090 (−0.273, 0.092) | -0.137 (−0.321, 0.048) | -0.110 (−0.293, 0.073) | -0.111 (−0.294, 0.072) |
| 2 | -0.039 (−0.231, 0.152) | -0.008 (−0.181, 0.197) | -0.006 (−0.184, 0.196) | -0.041 (−0.231, 0.149) | -0.010 (−0.198, 0.179) | -0.014 (−0.204, 0.176) |
| 3 | 0.008 (−0.193, 0.209) | 0.070 (−0.132, 0.271) | 0.067 (−0.136, 0.271) | -0.120 (−0.320, 0.080) | -0.085 (−0.287, 0.116) | -0.092 (−0.295, 0.111) |
| 4+ | -0.025 (−0.228, 0.178) | 0.052 (−0.155, 0.259) | 0.050 (−0.158, 0.258) | 0.105 (−0.097, 0.307) | 0.156 (−0.051, 0.363) | 0.151 (−0.057, 0.359) |
| Longitudinal | ||||||
| ACEs | ||||||
| 0 | ref | ref | ref | ref | ref | ref |
| 1 | 0.053 (-0.010, 0.116) | 0.056 (-0.007, 0.119) | 0.057 (-0.006, 0.120) | -0.017 (-0.108, 0.075) | -0.020 (-0.112, 0.072) | -0.019 (-0.11, 0.073) |
| 2 | 0.117 (0.052, 0.182) | 0.125 (0.060, 0.191) | 0.128 (0.062, 0.194) | 0.074 (-0.021, 0.169) | 0.077 (-0.019, 0.173) | 0.082 (-0.014, 0.178) |
| 3 | 0.075 (0.007, 0.143) | 0.090 (0.021, 0.159) | 0.094 (0.025, 0.164) | 0.050 (-0.048, 0.148) | 0.050 (-0.05, 0.151) | 0.058 (-0.044, 0.160) |
| 4+ | 0.089 (0.020, 0.158) | 0.108 (0.036, 0.179) | 0.111 (0.039, 0.182) | -0.022 (-0.123, 0.078) | -0.022 (-0.126, 0.082) | -0.017 (-0.121, 0.088) |
Model 1: adjusted for years from baseline, baseline age centred at mean, and sex.
Model 2: model 1+childhood SES.
Model 3: model 2+composite childhood support.
ACEs, adverse childhood experiences; SES, socioeconomic status.
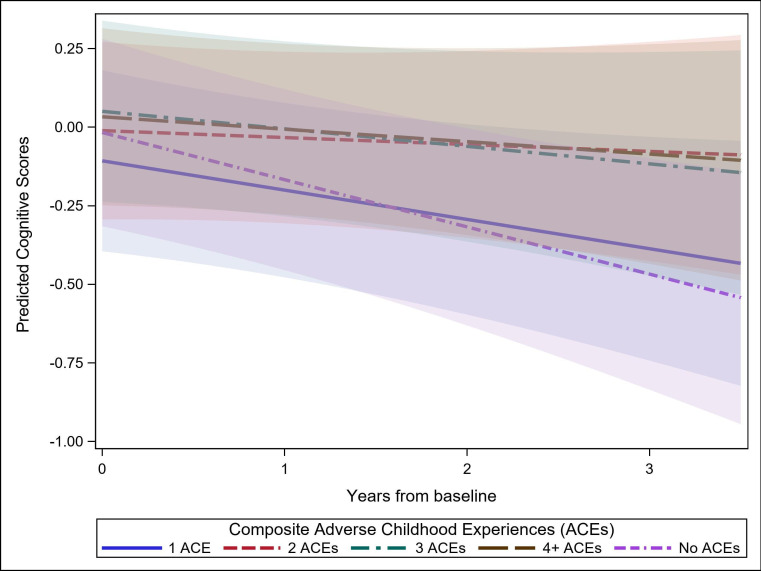
When examined longitudinally, there was significantly slower decline in executive function among those who reported experiencing two ACEs (β=0.117; 95% CI 0.052 to 0.182), three ACEs (β=0.075; 95% CI 0.007 to 0.153) and four or more ACEs (β=0.089; 95% CI 0.002 to 0.158), but not for one ACE (β=0.053; 95% CI −0.010 to 0.116) compared with no ACEs (table 2, figure 1). The estimates and direction of associations remained consistent after adjusting for childhood SES and childhood social support variables (table 2).
Figure 1.
Prediction plot of linear mixed models estimate of the association of composite ACEs with executive function across three waves. Adjusted for years from baseline, baseline age centred at mean, sex, childhood SES, and composite childhood support. SES = socioeconomic status.
There were no significant associations between the composite ACEs score and verbal episodic memory over time (online supplemental figure 1). However, the point estimates were negative for one ACE (β=−0.017; 95% CI −0.108 to 0.075) and four or more ACEs (β=−0.022; 95% CI −0.123 to 0.078), and point estimates were positive for two ACEs (β=0.074; 95% CI −0.021 to 0.159) and three ACEs (β=0.050; 95% CI −0.048 to 0.148) compared with no ACEs (table 2). The longitudinal point estimates changed minimally after adjusting for childhood SES and childhood social support.
bmjopen-2023-072961supp001.pdf (169.9KB, pdf)
Individual ACEs
When evaluating linear mixed models for executive function and verbal episodic memory with individual ACEs as predictors, there were no significant associations with baseline cognition or change of cognition over time (online supplemental table 1). All individual ACEs had non-significant positive associations for executive function, except for death of mother which had non-significant negative association. Longitudinal estimates for verbal episodic memory were mixed with non-significant positive associations for parent separated, parent remarried, serious family illness, death of mother and death of father, while non-significant negative associations were observed for witnessed violence, substance abuse, loss of job and parent jail.
Discussion
In our cohort, ACEs were not significantly associated with baseline executive function or verbal episodic memory. We found that those who experienced multiple ACEs had slower decline in executive function than those who did not experience any ACEs, but we did not see this for verbal episodic memory. Our findings did not align with our hypothesis that exposure to ACEs would be associated with lower baseline cognition and greater cognitive decline. These results are consistent with some prior work in which similar results were observed in the Chicago Health and Aging Project (CHAP) cohort study of over 3700 older black adults (average age 78 years) where those that experienced food deprivation had slower cognitive decline later in life.18 Our study included a younger cohort of black Americans (average age 68 years) compared with CHAP18 on childhood adversity and cognition. In another cross-sectional study, no associations were found between composite and individual ACEs across different ages of childhood with baseline cognition within black older adults when stratified by race.16 There is limited work on early life adversity and late-life cognition in black Americans, and findings in our study, using an all-black cohort, show similar results to previous work in this area.
Our estimates of the association between individual ACEs and domain-specific baseline cognition and cognitive decline were not statistically significant. The association between composite ACEs and verbal episodic memory were also not statistically significant. However, point estimates and borderline CIs in our study suggest that composite ACEs (two and three ACEs) may be associated with slower verbal episodic memory decline. These findings are consistent with other studies finding that individual household-related ACEs were not associated with cognition.7 9 10 16–19
ACEs were highly prevalent in our cohort with close to 80% experiencing one or more ACEs. We observed that experiencing ACEs was associated with slower decline in executive function, but not verbal episodic memory, indicating possible domain-specificity. A review found that ACEs (emotional and sexual abuse) were associated with better executive function,12 while other studies found that ACEs were associated with worse memory and not executive function.7 8 10 One study examining a Chinese cohort found that experiencing at least two ACEs and three types of ACEs (childhood SES disadvantage, parental trauma, maladaptive parental trauma) were associated with decreased episodic memory,42 43 which was supported by another study that found depressive symptoms during early life to be associated with episodic memory deficit.44 A meta-analysis found that the associations between ACEs and cognition varied by individual ACEs and type of cognitive outcome.19 For example, some studies reported association of ACEs with lower cognitive scores and higher risk of neurocognitive disorder (NCD) diagnosis, while other studies found association of physical or sexual abuse with better cognition, parental death with lower risk of NCD and collective violence with better global cognition.19 Our analysis did not find significant associations of individual ACEs with cognitive decline in any domain.
Although ACEs could influence a child’s development into adulthood through increased toxic stress pathways, these experiences may only partially contribute to cognitive functioning in late life.3 45 Beyond cognition, other studies found that ACEs are associated with higher risk of cardiovascular disease, shortened telomeres and greater functional limitations in black adults.46–48 Environmental, social and behavioural factors throughout a person’s life stand to mediate and even protect against the negative long-term effects of ACEs.9 28 In one study,9 positive childhood environment was found to promote executive functioning. Educational attainment could also be protective for later-life cognitive function through cognitive reserve.10 29 Our cohort was highly educated and reported a high prevalence of childhood support which could explain why ACEs were not associated with lower baseline cognition.
The CI theory provides as a meaningful framework for explaining the observed relationships in our study. One possibility for our findings is that it reflects a pattern of resiliency. Among those who experienced ACEs, many had parents who were separated or divorced (39%), had family members with serious illness (35%) or witnessed domestic violence (32%). CI theory suggests that the detrimental, cumulative impact of experiencing multiple ACEs may have been modified by other factors, such as human agency or social support.23 Most participants reported receiving support during childhood most or all of the time with 76% reported having someone they trust or confide in, 86% having someone show them love, 66% having someone help with homework, 79% having someone to motivate them in school, and 77% having someone close to them that they can contact. In a literature review of black Americans, multiple studies found that lower SES was associated with faster cellular markers of biological ageing and earlier development of memory problems.3 The STAR cohort, on average, has higher SES which may mitigate the impact of ACEs on cognition. Another explanation for our findings is resiliency through selection and survival bias of only the healthiest individuals that chose to participate in the study. Black participants in STAR may be exceptional in that they overcame the negative effects of early childhood adversity, survived long enough and were healthy enough to enrol in a study on cognitive ageing. It is also important to consider that STAR consists of older black individuals who’s early life corresponded with de jure and de facto policies that upheld and endorsed racism in education, access to healthcare, SES and discrimination, which may further affirm that only the most resilient individuals had the opportunity to live into old age.49
Our study had several strengths. First, we used data from a well-characterised cohort of midlife to late-life black participants. By evaluating ACEs in an all-black cohort, we were able to identify early life experiences within this understudied group and assess relationships between ACEs and late-life cognition using a within-group analysis, an approach that is not typically used in studies of minoritised older adults.3 50 Second, we examined cognition using a robust psychometric battery that has specifically been validated for use in black Americans.32–35 By following our cohort over three waves (average 2.3 years of follow-up), we were able to examine changes in cognition over time. Lastly, our ACEs questionnaire was adapted from a robust measure used in other cohort studies with diverse participants.16 37 38
There were several limitations in our study. First, since ACEs occurred early in life, recall bias could influence responses. Older participants were asked to remember potentially traumatic events during childhood, which could lead to underestimation or overestimation of the prevalence of ACEs.41 51 Social desirability bias may also prevent participants from disclosing sensitive and revealing information about their early life.52 Experiences of abuse or neglect were not captured by the ACEs questionnaire, but may reflect other dimensions of childhood adversity with different effects on late-life cognition.53 As a middle-age and older cohort with a relatively shorter follow-up time of approximately 3 years, there could be practice effects impacting cognitive testing. Yet, when we adjusted for practice effects using a first visit indicator in the models, we found estimates to be almost identical.54 Given this short follow-up, it is also possible that participants did not experience substantial decline, and this study cannot examine how ACEs impact long-term cognitive decline yet, but this is a future goal.
Our findings suggest that experiencing ACEs was not associated with worse cognition or cognitive decline in this cohort of older black Americans. Additionally, the accumulation of ACEs may be associated with slower decline in executive function, a finding that needs to be explored further. CI theory posits that early life adversities do not fully determine cognitive trajectories in older adults and resiliency may subsequently develop through midlife and later life. Future studies are needed to understand how resiliency factors such as childhood support, education and financial stability can be protective against ACEs as well as cognitive decline, especially among marginalised and high-risk communities. Specifically, mediation and moderation analyses of these protective factors will be needed to determine their effects on ACEs with late-life cognition and explain potential resiliency observed in black Americans.
Supplementary Material
Footnotes
Twitter: @yi_lor, @PaolaGilsanz
Contributors: YL, KMG, RLP and RAW were involved with study design, data acquisition, data analysis, interpreting the results and drafting the work. PG, CCM, EH-L, LB and DM were involved in critically reviewing the contents. All authors revised, approved final version of the manuscript and agree to accept responsibility of all aspects of the work. YL and RAW are responsible for the overall content as guarantor.
Funding: This work was supported by National Institutes of Health grant number R01AG050782.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Kaiser Permanente Northern California Institutional Review Board (IRB Number: 1121043). Participants gave informed consent to participate in the study before taking part.
References
- 1.Felitti VJ. Adverse childhood experiences and adult health. Acad Pediatr 2009;9:131–2. 10.1016/j.acap.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998;14:245–58. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- 3.Graham KL, Paun O, Stillerman A. The impact of adverse childhood experiences on cognition in African American older adults: an integrated literature review. Res Gerontol Nurs 2021;14:265–72. 10.3928/19404921-20210825-04 [DOI] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association . 2021 Alzheimer’s disease facts and figures; Available: https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf
- 5.Cordell CB, Borson S, Boustani M, et al. Alzheimer's association recommendations for operationalizing the detection of cognitive impairment during the Medicare annual wellness visit in a primary care setting. Alzheimers Dement 2013;9:141–50. 10.1016/j.jalz.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord 2012;5(6):349–58. 10.1177/1756285612455733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi LC, Farrell MT, Payne CF, et al. Adverse childhood experiences and domain-specific cognitive function in a population-based study of older adults in rural South Africa. Psychol Aging 2020;35:818–30. 10.1037/pag0000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majer M, Nater UM, Lin J-M, et al. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol 2010;10:61. 10.1186/1471-2377-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie K, Jaussent I, Stewart R, et al. Adverse childhood environment and late-life cognitive functioning. Int J Geriatr Psychiatry 2011;26:503–10. 10.1002/gps.2553 [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Wang Z. Early-life conditions and cognitive function in middle-and old-aged Chinese adults: a longitudinal study. Int J Environ Res Public Health 2020;17:3451. 10.3390/ijerph17103451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund JI, Toombs E, Radford A, et al. Adverse childhood experiences and executive function difficulties in children: a systematic review. Child Abuse Negl 2020;106:104485. 10.1016/j.chiabu.2020.104485 [DOI] [PubMed] [Google Scholar]
- 12.Lund JI, Boles K, Radford A, et al. A systematic review of childhood adversity and executive functions outcomes among adults. Arch Clin Neuropsychol 2022;37:1118–32. 10.1093/arclin/acac013 [DOI] [PubMed] [Google Scholar]
- 13.Donley GAR, Lönnroos E, Tuomainen T-P, et al. Association of childhood stress with late-life dementia and Alzheimer's disease: the KIHD study. Eur J Public Health 2018;28:1069–73. 10.1093/eurpub/cky134 [DOI] [PubMed] [Google Scholar]
- 14.Radford K, Delbaere K, Draper B, et al. Childhood stress and adversity is associated with late-life dementia in aboriginal Australians. Am J Geriatr Psychiatry 2017;25:1097–106. 10.1016/j.jagp.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 15.Tani Y, Fujiwara T, Kondo K. Association between adverse childhood experiences and dementia in older Japanese adults. JAMA Netw Open 2020;3:e1920740. 10.1001/jamanetworkopen.2019.20740 [DOI] [PubMed] [Google Scholar]
- 16.Gold AL, Meza E, Ackley SF, et al. Are adverse childhood experiences associated with late-life cognitive performance across racial/ethnic groups: results from the Kaiser healthy aging and diverse life experiences study baseline. BMJ Open 2021;11:e042125. 10.1136/bmjopen-2020-042125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Shea BQ, Demakakos P, Cadar D, et al. Adverse childhood experiences and rate of memory decline from mid to later life: evidence from the English longitudinal study of ageing. Am J Epidemiol 2021;190:1294–305. 10.1093/aje/kwab019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes LL, Wilson RS, Everson-Rose SA, et al. Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology 2012;79:2321–7. 10.1212/WNL.0b013e318278b607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel P, Oremus M, Heyn PC. The association between adverse childhood experiences and late-life cognition: a systematic review of cross-sectional and case-control studies. Gerontologist 2023;63:1087–103. 10.1093/geront/gnac041 [DOI] [PubMed] [Google Scholar]
- 20.Dannefer D. Cumulative advantage/disadvantage and the life course: cross-fertilizing age and social science theory. J Gerontol B Psychol Sci Soc Sci 2003;58:S327–37. 10.1093/geronb/58.6.s327 [DOI] [PubMed] [Google Scholar]
- 21.Yang MS, Hedeker D. A life-span approach to examining older vulnerable population's subjective well-being: the role of adversity and trauma. Aging Ment Health 2020;24:2043–52. 10.1080/13607863.2019.1652245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraro KF, Schafer MH, Wilkinson LR. Childhood disadvantage and health problems in middle and later life: early imprints on physical health? Am Sociol Rev 2016;81:107–33. 10.1177/0003122415619617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferraro KF, Shippee TP. Aging and cumulative inequality: how does inequality get under the skin? Gerontologist 2009;49:333–43. 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giano Z, Wheeler DL, Hubach RD. The frequencies and disparities of adverse childhood experiences in the U.S. BMC Public Health 2020;20:1327. 10.1186/s12889-020-09411-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire-Jack K, Lanier P, Lombardi B. Investigating racial differences in clusters of adverse childhood experiences. Am J Orthopsychiatry 2020;90:106–14. 10.1037/ort0000405 [DOI] [PubMed] [Google Scholar]
- 26.Merrick MT, Ford DC, Ports KA, et al. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 States. JAMA Pediatr 2018;172:1038–44. 10.1001/jamapediatrics.2018.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayeda ER, Glymour MM, Quesenberry CP, et al. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–24. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson RL, Fain MJ, A Butler E, et al. The role of social and behavioral risk factors in explaining racial disparities in age-related cognitive impairment: a structured narrative review. Aging Neuropsychol Cogn 2020;27:173–96. 10.1080/13825585.2019.1598539 [DOI] [PubMed] [Google Scholar]
- 29.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 2018;29:151–9. 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George KM, Gilsanz P, Peterson RL, et al. Impact of cardiovascular risk factors in adolescence, young adulthood, and midlife on late-life cognition: study of healthy aging in African Americans. J Gerontol 2021;76:1692–8. 10.1093/gerona/glab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitmer RA, Barnes LL, Richards AE, et al. Introducing the study of healthy aging in African Americans (STAR): looking back to move forward. Alzheimer’s & Dementia 2020;16:S10. 10.1002/alz.046614 Available: https://alz-journals.onlinelibrary.wiley.com/toc/15525279/16/S10 [DOI] [Google Scholar]
- 32.Mungas D, Reed BR, Crane PK, et al. Spanish and English neuropsychological assessment scales (SENAS): further development and psychometric characteristics. Psychol Assess 2004;16:347–59. 10.1037/1040-3590.16.4.347 [DOI] [PubMed] [Google Scholar]
- 33.Mungas D, Reed BR, Haan MN, et al. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology 2005;19:466–75. 10.1037/0894-4105.19.4.466 [DOI] [PubMed] [Google Scholar]
- 34.Mungas D, Reed BR, Marshall SC, et al. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology 2000;14:209–23. 10.1037//0894-4105.14.2.209 [DOI] [PubMed] [Google Scholar]
- 35.Mungas D, Widaman KF, Reed BR, et al. Measurement Invariance of neuropsychological tests in diverse older persons. Neuropsychology 2011;25:260–9. 10.1037/a0021090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howard VJ, McClure LA, Glymour MM, et al. Effect of duration and age at exposure to the stroke belt on incident stroke in adulthood. Neurology 2013;80:1655–61. 10.1212/WNL.0b013e3182904d59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen IH, Bennett A, Allen S, et al. Childhood residential mobility and mental and physical health in later life: findings from the reasons for geographic and racial differences in stroke (REGARDS) study. J Appl Gerontol 2023;42:1859–66. 10.1177/07334648231163053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 39.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci 2006;256:174–86. 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell TL. Screening for adverse childhood experiences (aces) in primary care: a cautionary NOTE. JAMA 2020;323:2379–80. 10.1001/jama.2020.4365 [DOI] [PubMed] [Google Scholar]
- 41.Lacey RE, Minnis H. Practitioner review: twenty years of research with adverse childhood experience scores - advantages, disadvantages and applications to practice. J Child Psychol Psychiatry 2020;61:116–30. 10.1111/jcpp.13135 [DOI] [PubMed] [Google Scholar]
- 42.Lin L, Cao B, Chen W, et al. Association of adverse childhood experiences and social isolation with later-life cognitive function among adults in China. JAMA Netw Open 2022;5:e2241714. 10.1001/jamanetworkopen.2022.41714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding R, He P. Associations between childhood adversities and late-life cognitive function: potential mechanisms. Soc Sci Med 2021;291:114478. 10.1016/j.socscimed.2021.114478 [DOI] [PubMed] [Google Scholar]
- 44.Barch DM, Harms MP, Tillman R, et al. Early childhood depression, emotion regulation, episodic memory, and hippocampal development. J Abnorm Psychol 2019;128:81–95. 10.1037/abn0000392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morsy L, Rothstein R. Toxic stress and children’s outcomes. Econ Policy Inst 2019. Available: https://www.epi.org/publication/toxic-stress-and-childrens-outcomes-african-american-children-growing-up-poor-are-at-greater-risk-of-disrupted-physiological-functioning-and-depressed-academic-achievement/ [Google Scholar]
- 46.Kliewer W, Robins JL. Adverse childhood experiences are associated with cardiometabolic risk indicators and telomere length in low-income African-American adolescents. Int J Behav Med 2022;29:131–5. 10.1007/s12529-021-09978-w [DOI] [PubMed] [Google Scholar]
- 47.Islam SJ, Hwan Kim J, Joseph E, et al. Association between early trauma and ideal cardiovascular health among black Americans: results from the morehouse-emory cardiovascular (MECA) center for health equity. Circ Cardiovasc Qual Outcomes 2021;14:e007904. 10.1161/CIRCOUTCOMES.121.007904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauerteig MR, Ferraro KF, Bauldry S. Life course stressors and functional limitations in later life among white, black, and hispanic adults: deleterious, hardening, or benign? J Gerontol B Psychol Sci Soc Sci 2022;77:249–59. 10.1093/geronb/gbab066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 2008;18:223–54. 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- 50.Whitfield KE, Allaire JC, Belue R, et al. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci 2008;63:301–8. 10.1093/geronb/63.5.p301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briggs EC, Amaya-Jackson L, Putnam KT, et al. All adverse childhood experiences are not equal: the contribution of synergy to adverse childhood experience scores. Am Psychol 2021;76:243–52. 10.1037/amp0000768 [DOI] [PubMed] [Google Scholar]
- 52.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc 2016;9:211–7. 10.2147/JMDH.S104807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krinner LM, Warren-Findlow J, Bowling J, et al. The dimensionality of adverse childhood experiences: a scoping review of ACE dimensions measurement. Child Abuse Negl 2021;121:105270. 10.1016/j.chiabu.2021.105270 [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Calmasini C, Swinnerton K, et al. Pragmatic approaches to handling practice effects in longitudinal cognitive aging research. Alzheimers Dement 2023;19:4028–36. 10.1002/alz.13067 Available: https://alz-journals.onlinelibrary.wiley.com/toc/15525279/19/9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072961supp001.pdf (169.9KB, pdf)
Data Availability Statement
Data are available on reasonable request.