Abstract
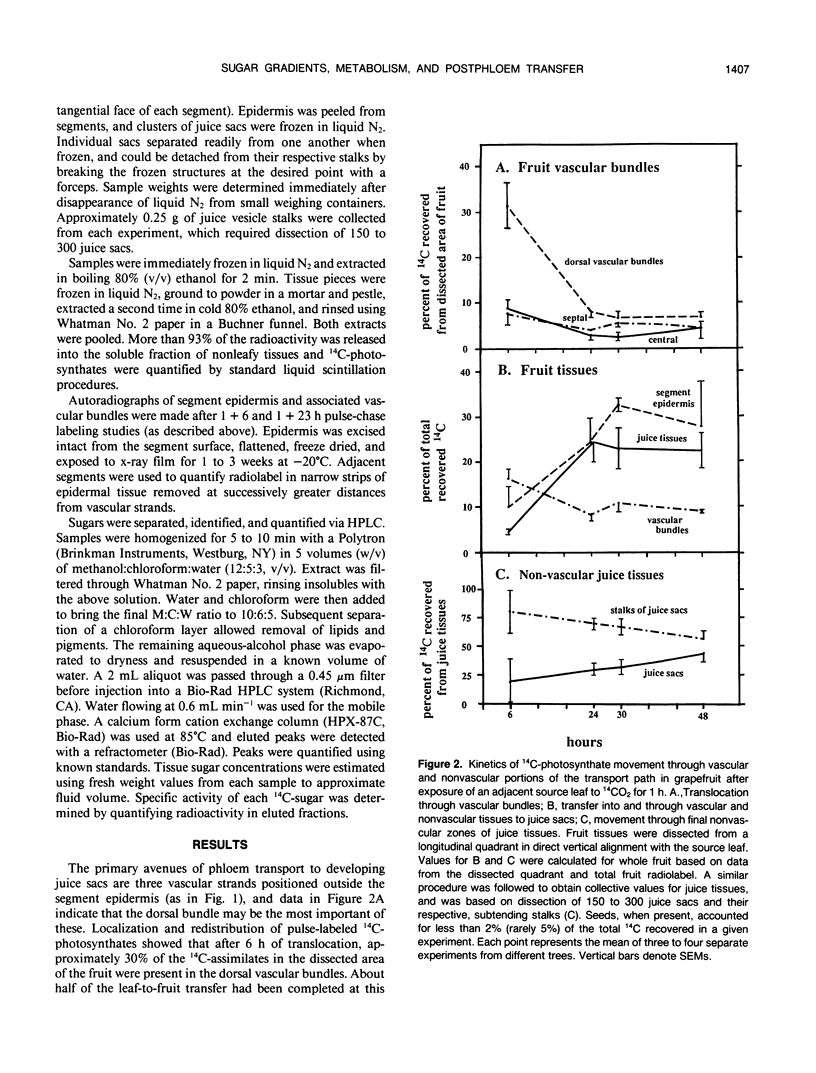
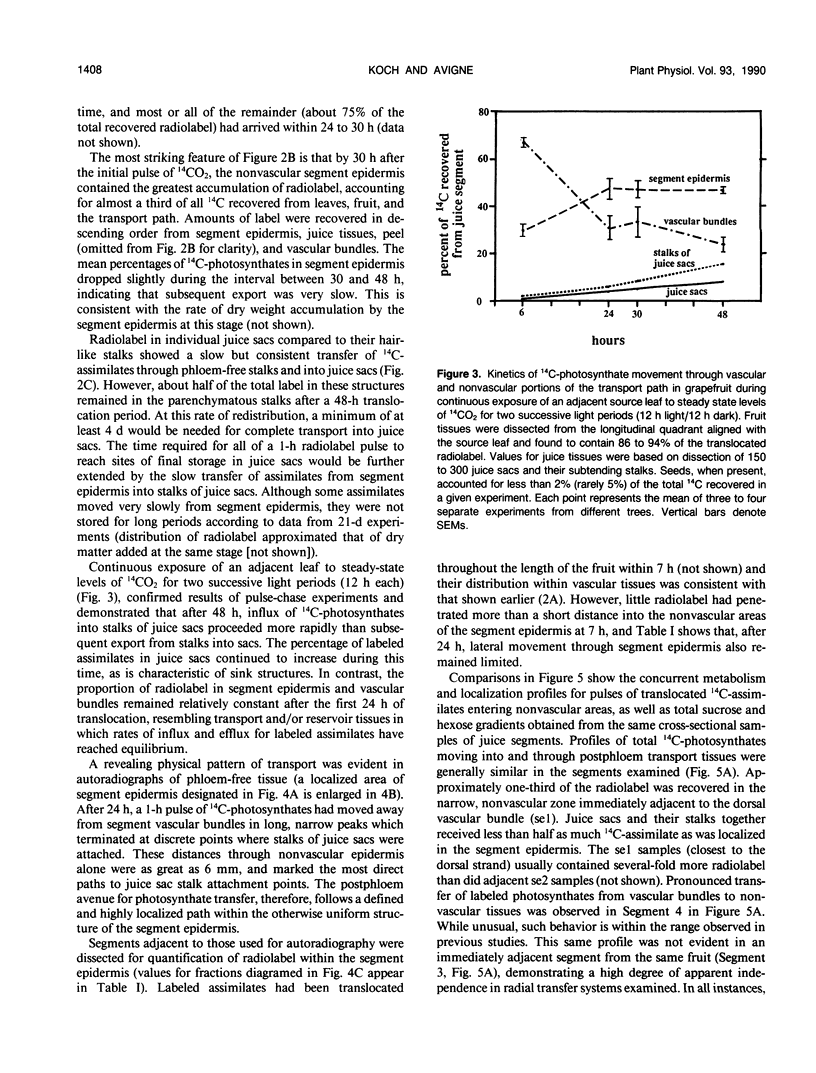
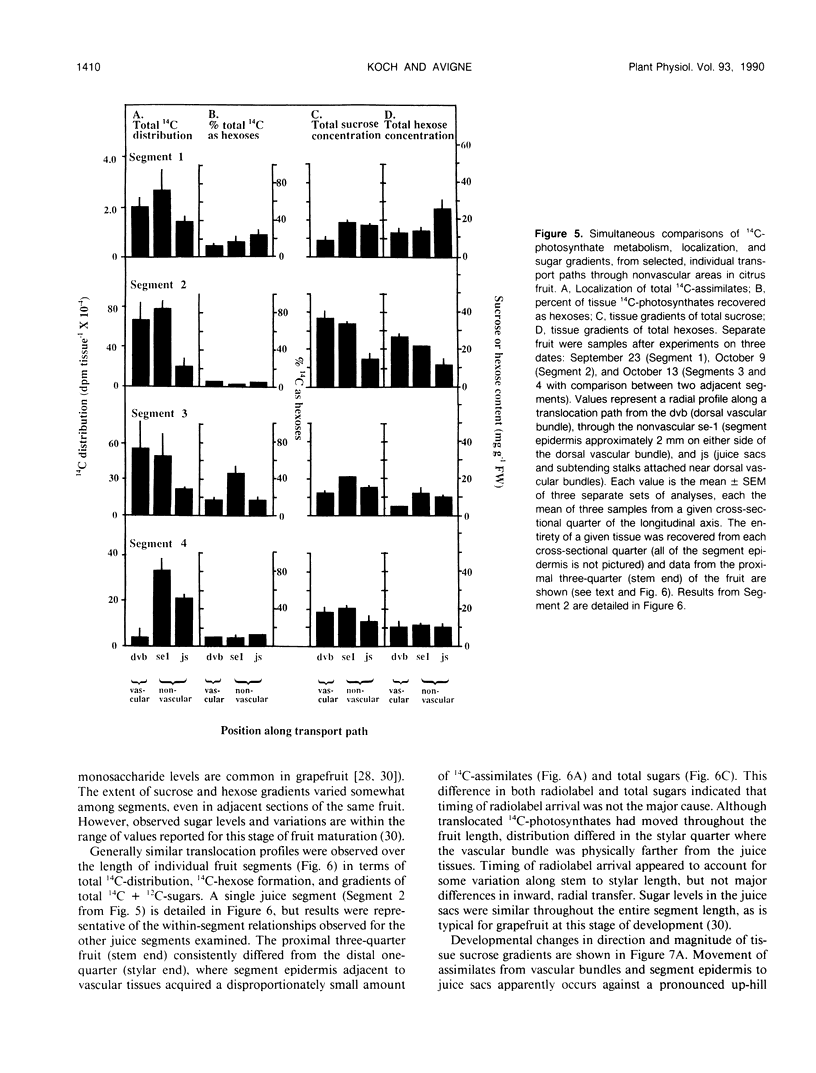
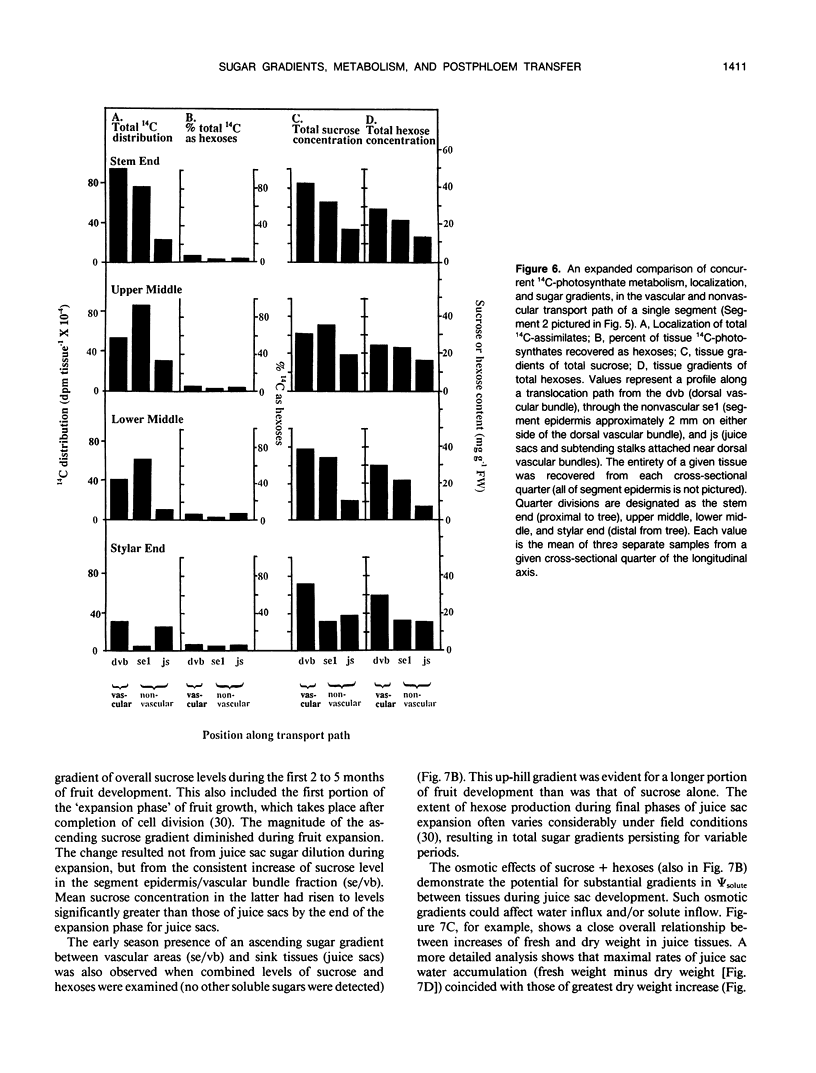
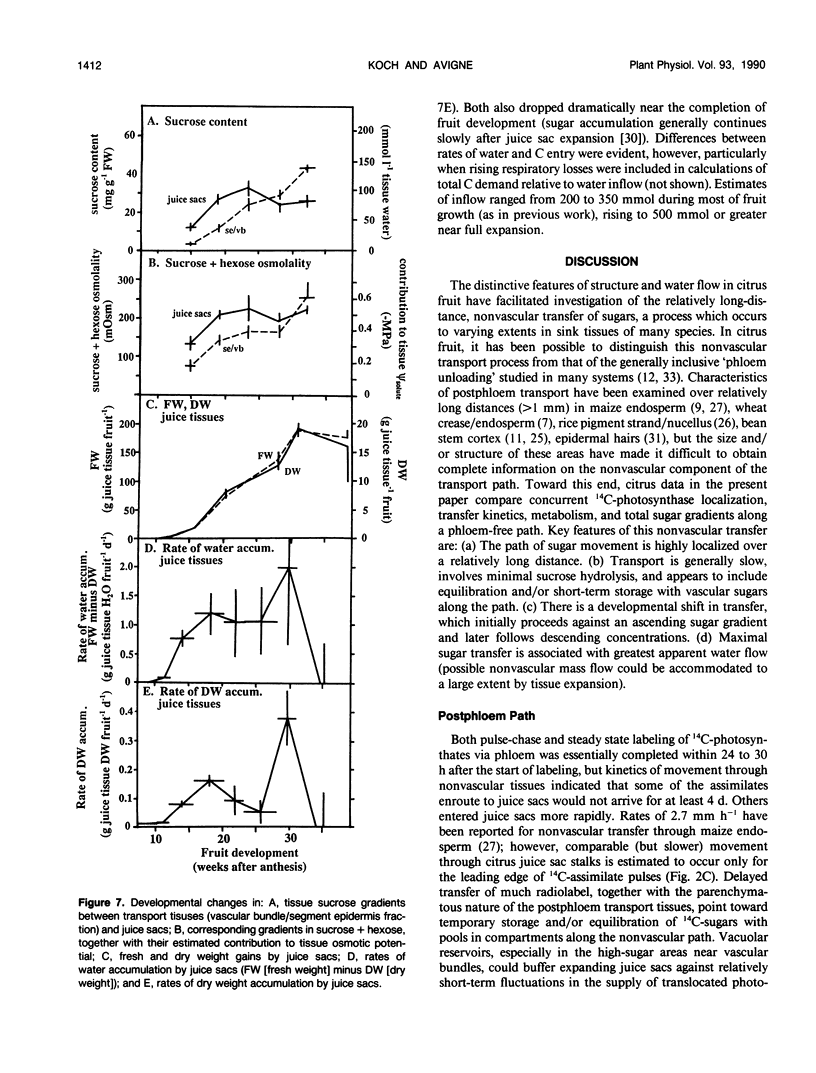
Postphloem, nonvascular assimilate transport occurs over an unusually long area in citrus fruit and thus facilitates investigation of this process relative to sugar entry into many sink structures. Labeled photosynthates moving into juice tissues of grapefruit (Citrus paradisi Macf.) slowed dramatically after entering the postphloem transport path (parenchyma cells, narrow portions of segment epidermis, and hair-like, parenchymatous stalks of juice sacs). Kinetic, metabolic, and compositional data indicated that transfer through the nonvascular area was delayed many hours by temporary storage and/or equilibration with sugars in compartments along the postphloem path. Labeled assimilates were generally recovered as sucrose throughout the path, and extent of hexose formation enroute bore no apparent relationship to the assimilate transfer process. Even after 24 hours, radiolabel was restricted to discrete, highly localized areas directly between vascular bundles and juice sacs. Postphloem transfer occurred against an ascending sucrose concentration gradient in young fruit, whereas a descending gradient (favoring diffusion/cytoplasmic streaming) developed only later in maturation. Involvement of a postphloem bulk flow is complicated in the present instance by the extremely limited water loss from juice sacs either via transpiration or fluid backflow. Nonetheless, tissue expansion can account for a collective water inflow of at least 1.0 milliliter per day throughout the majority of juice sac development, thus providing a modest, but potentially important means of nonvascular solution flow. Overall, data indicate postphloem transfer (a) can follow highly localized paths through sizable nonvascular areas (up to 3.0 centimeters total), (b) appears to involve temporary storage and/or equilibration with compartmentalized sugars enroute, (c) can occur either against an overall up-hill sugar gradient (young tissues) or along a descending gradient (near full expansion), and (d) appears to involve at least some contribution by nonvascular mass flow accommodated by tissue expansion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damon S., Hewitt J., Nieder M., Bennett A. B. Sink Metabolism in Tomato Fruit : II. Phloem Unloading and Sugar Uptake. Plant Physiol. 1988 Jul;87(3):731–736. doi: 10.1104/pp.87.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E., Burns J. K. Vacuolar Acid hydrolysis as a physiological mechanism for sucrose breakdown. Plant Physiol. 1989 Jun;90(2):530–533. doi: 10.1104/pp.90.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Gifford R. M. Accumulation and Conversion of Sugars by Developing Wheat Grains : VI. Gradients Along the Transport Pathway from the Peduncle to the Endosperm Cavity during Grain Filling. Plant Physiol. 1986 Dec;82(4):1024–1030. doi: 10.1104/pp.82.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Gifford R. M. Accumulation and Conversion of Sugars by Developing Wheat Grains : VII. Effect of Changes in Sieve Tube and Endosperm Cavity Sap Concentrations on the Grain Filling Rate. Plant Physiol. 1987 Jun;84(2):341–347. doi: 10.1104/pp.84.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. M., Jones R. J., Brenner M. L. In Vitro Sugar Transport in Zea mays L. Kernels : I. Characteristics of Sugar Absorption and Metabolism by Developing Maize Endosperm. Plant Physiol. 1987 Jun;84(2):467–471. doi: 10.1104/pp.84.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M. R. Water potential components in growing citrus fruits. Plant Physiol. 1970 Jul;46(1):145–149. doi: 10.1104/pp.46.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. E., Johnson C. R. Photosynthate partitioning in split-root citrus seedlings with mycorrhizal and nonmycorrhizal root systems. Plant Physiol. 1984 May;75(1):26–30. doi: 10.1104/pp.75.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle S. E. Evidence for the uptake of sucrose intact into sugarcane internodes. Plant Physiol. 1989 May;90(1):6–8. doi: 10.1104/pp.90.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C. A., Tomlinson P. T., Koch K. E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989 Aug;90(4):1394–1402. doi: 10.1104/pp.90.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C., Yelenosky G. Translocation of carbohydrates and proline in young grapefruit trees at low temperatures. Plant Physiol. 1983 Dec;73(4):877–880. doi: 10.1104/pp.73.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig J. G., Hitz W. D. Transport and Metabolism of a Sucrose Analog (1'-Fluorosucrose) into Zea mays L. Endosperm without Invertase Hydrolysis. Plant Physiol. 1987 Dec;85(4):902–905. doi: 10.1104/pp.85.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker J. E., Mauzerall D., Tucker E. B. Symplastic Transport of Carboxyfluorescein in Staminal Hairs of Setcreasea purpurea Is Diffusive and Includes Loss to the Vacuole. Plant Physiol. 1989 Jul;90(3):1143–1147. doi: 10.1104/pp.90.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]