Abstract
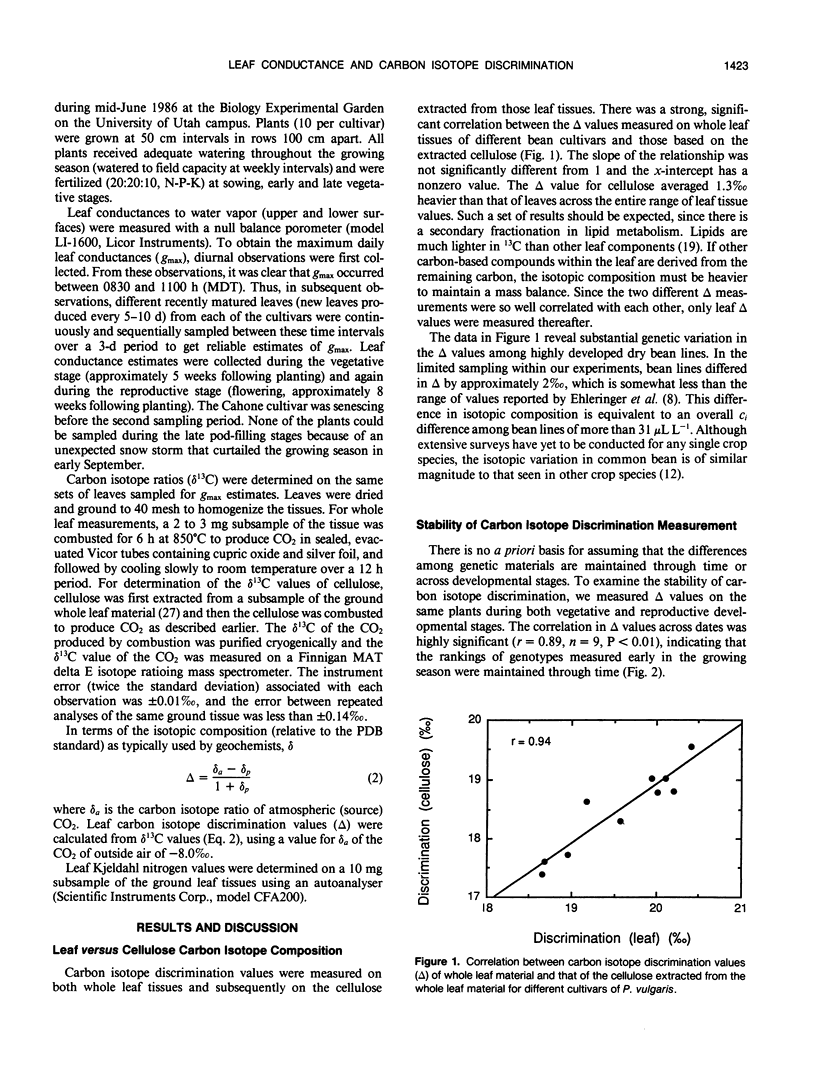
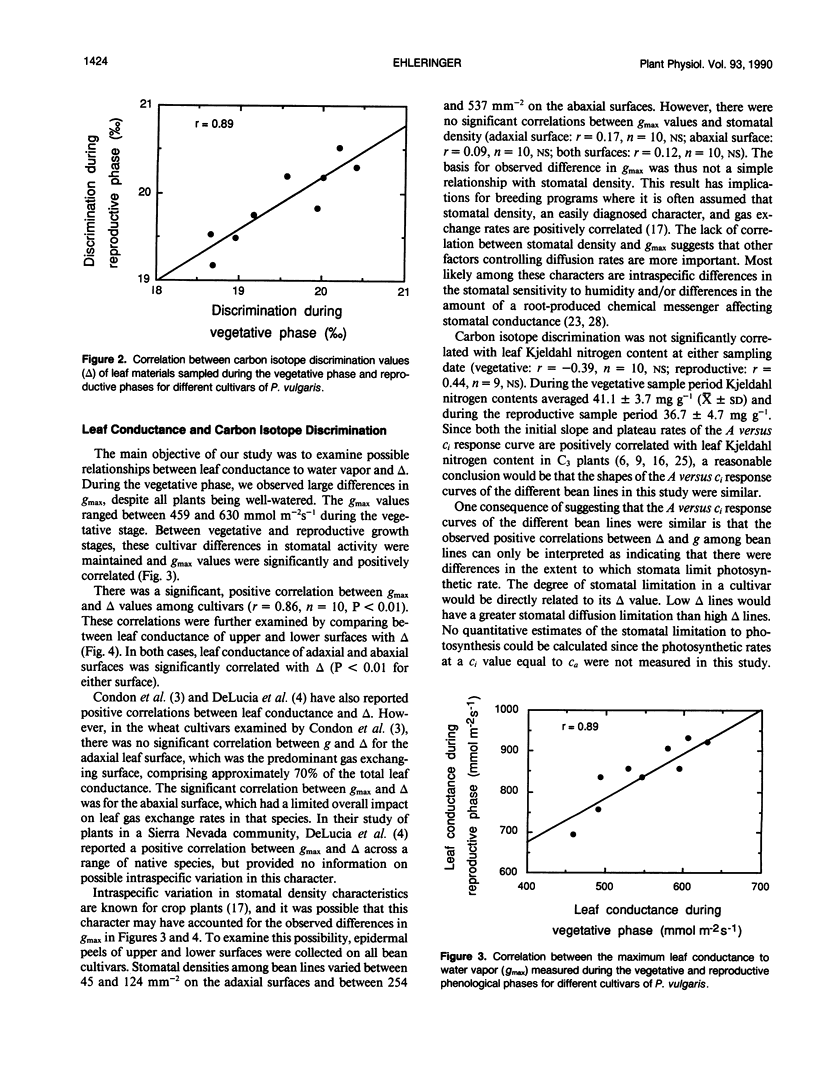
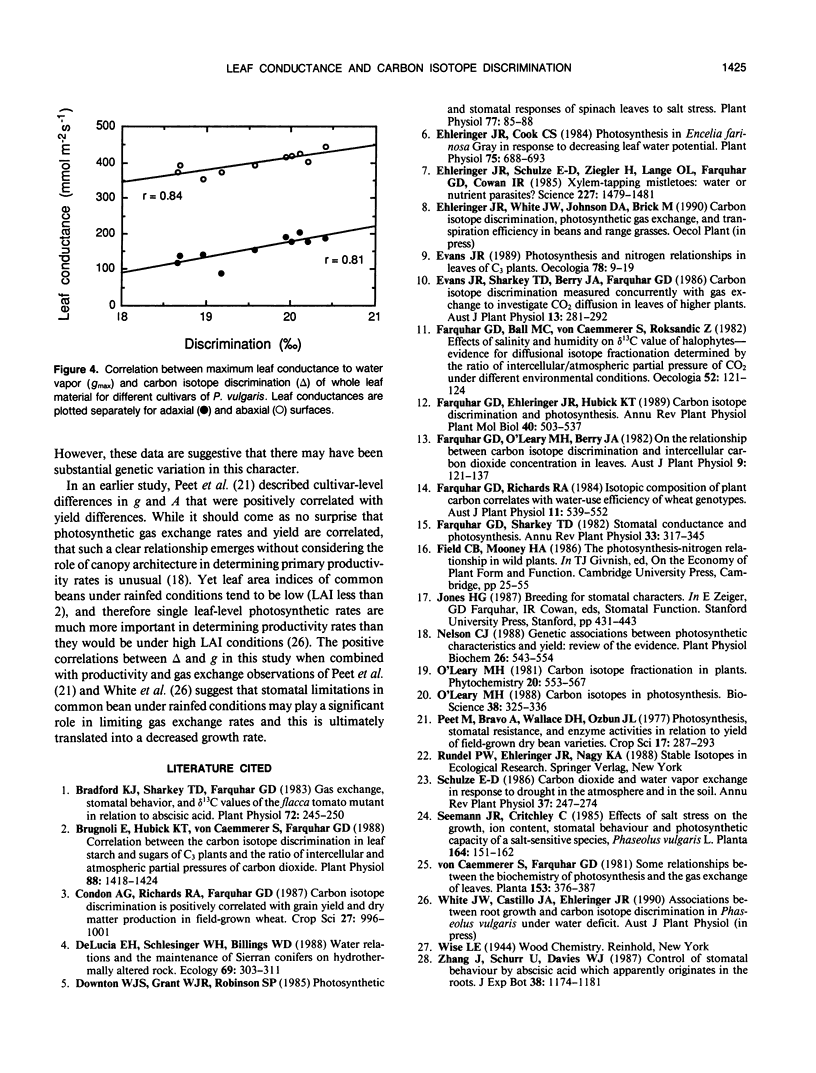
Carbon isotope discrimination (Δ) was measured in the field on 10 cultivars of common bean (Phaseolus vulgaris L.). There was substantial variation (more than 2‰) in leaf Δ values and these differences were maintained between vegetative and reproductive developmental stages. These bean lines also exhibited substantial differences in leaf conductance to water vapor, and again these differences were maintained across developmental stages. The differences in leaf conductance were positively correlated with Δ values, whether conductance was measured as total leaf conductance or as the individual conductances of either upper or lower leaf surfaces. The observed differences in leaf conductance were not associated with differences in stomatal density. There were small differences among bean lines in their leaf Kjeldahl nitrogen contents, which is interpreted as indicating that photosynthetic capacity among bean lines was similar. Thus, because Δ values and leaf conductance were positively correlated, these data suggested that there may have been differences among bean lines in the extent to which stomata limited photosynthetic gas exchange rates.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford K. J., Sharkey T. D., Farquhar G. D. Gas Exchange, Stomatal Behavior, and deltaC Values of the flacca Tomato Mutant in Relation to Abscisic Acid. Plant Physiol. 1983 May;72(1):245–250. doi: 10.1104/pp.72.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoli E., Hubick K. T., von Caemmerer S., Wong S. C., Farquhar G. D. Correlation between the Carbon Isotope Discrimination in Leaf Starch and Sugars of C(3) Plants and the Ratio of Intercellular and Atmospheric Partial Pressures of Carbon Dioxide. Plant Physiol. 1988 Dec;88(4):1418–1424. doi: 10.1104/pp.88.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton W. J., Grant W. J., Robinson S. P. Photosynthetic and stomatal responses of spinach leaves to salt stress. Plant Physiol. 1985 May;78(1):85–88. doi: 10.1104/pp.78.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J. R., Cook C. S. Photosynthesis in Encelia farinosa Gray in Response to Decreasing Leaf Water Potential. Plant Physiol. 1984 Jul;75(3):688–693. doi: 10.1104/pp.75.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J. R., Schulze E. D., Ziegler H., Lange O. L., Farquhar G. D., Cowar I. R. Xylem-tapping mistletoes: water or nutrient parasites? Science. 1985 Mar 22;227(4693):1479–1481. doi: 10.1126/science.227.4693.1479. [DOI] [PubMed] [Google Scholar]
- Ludden T. M., Allen J. P., Valutsky W. A., Vicuna A. V., Nappi J. M., Hoffman S. F., Wallace J. E., Lalka D., McNay J. L. Individualization of phenytoin dosage regimens. Clin Pharmacol Ther. 1977 Mar;21(3):287–293. doi: 10.1002/cpt1977213287. [DOI] [PubMed] [Google Scholar]


