Abstract
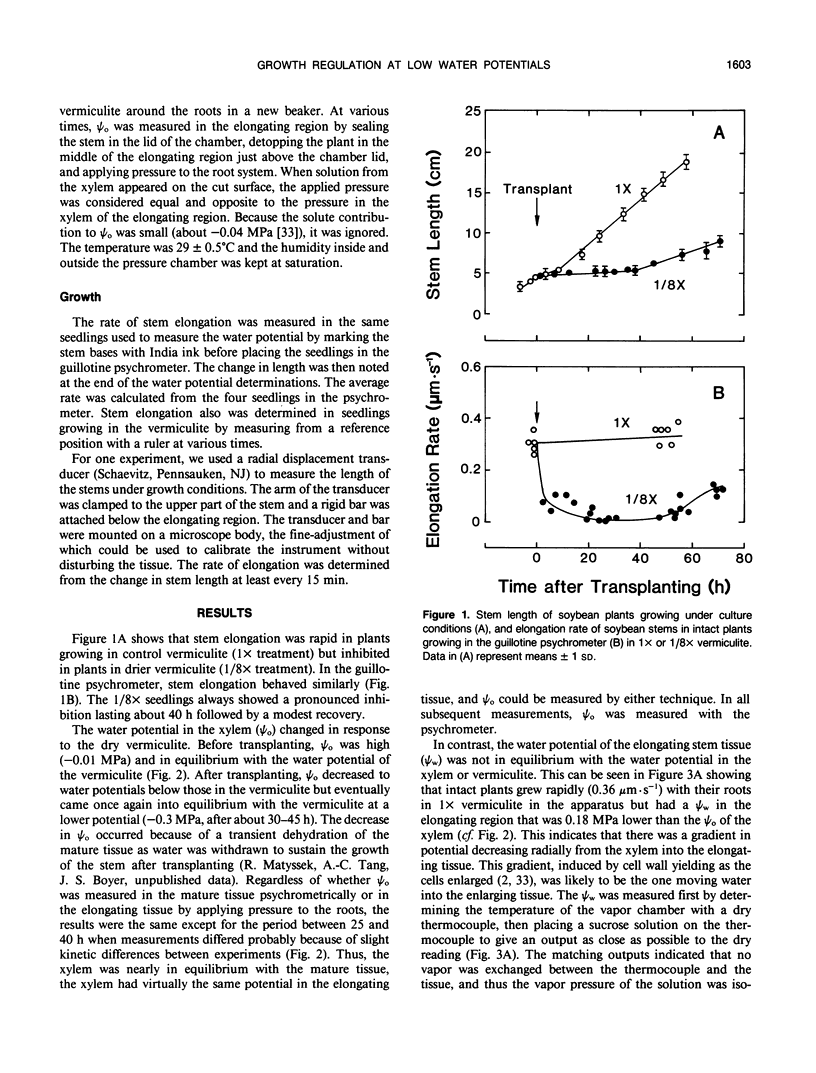
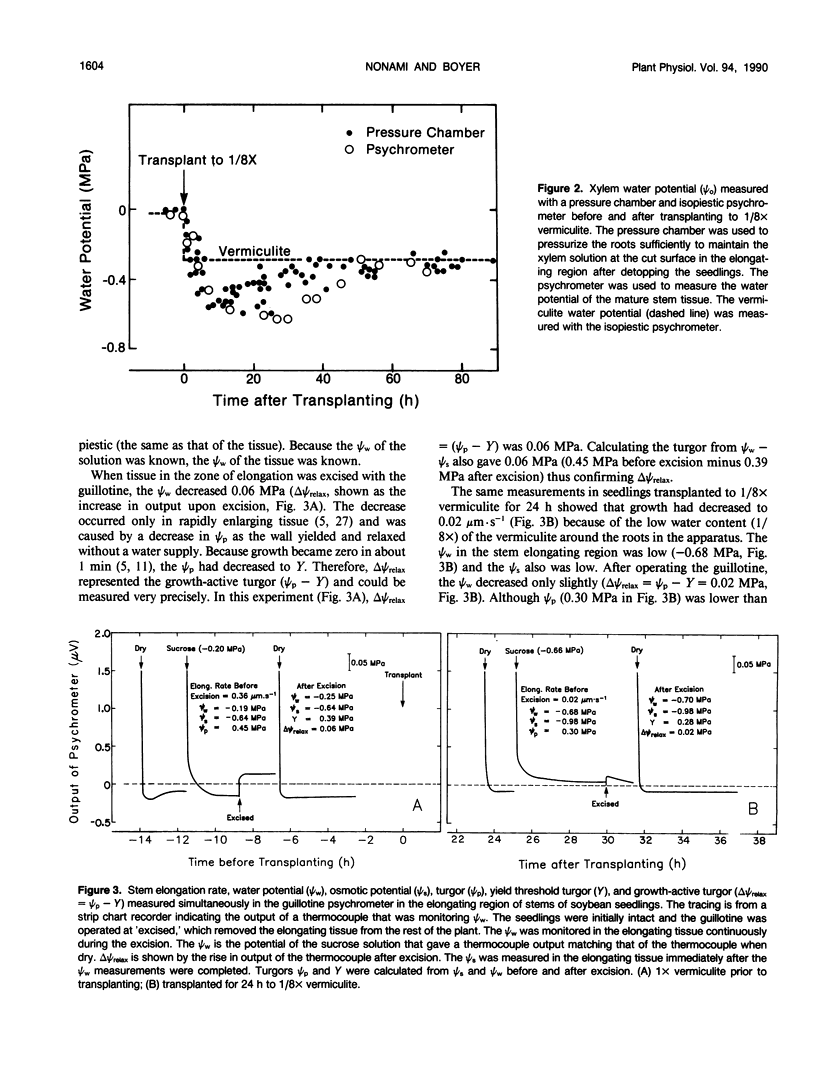
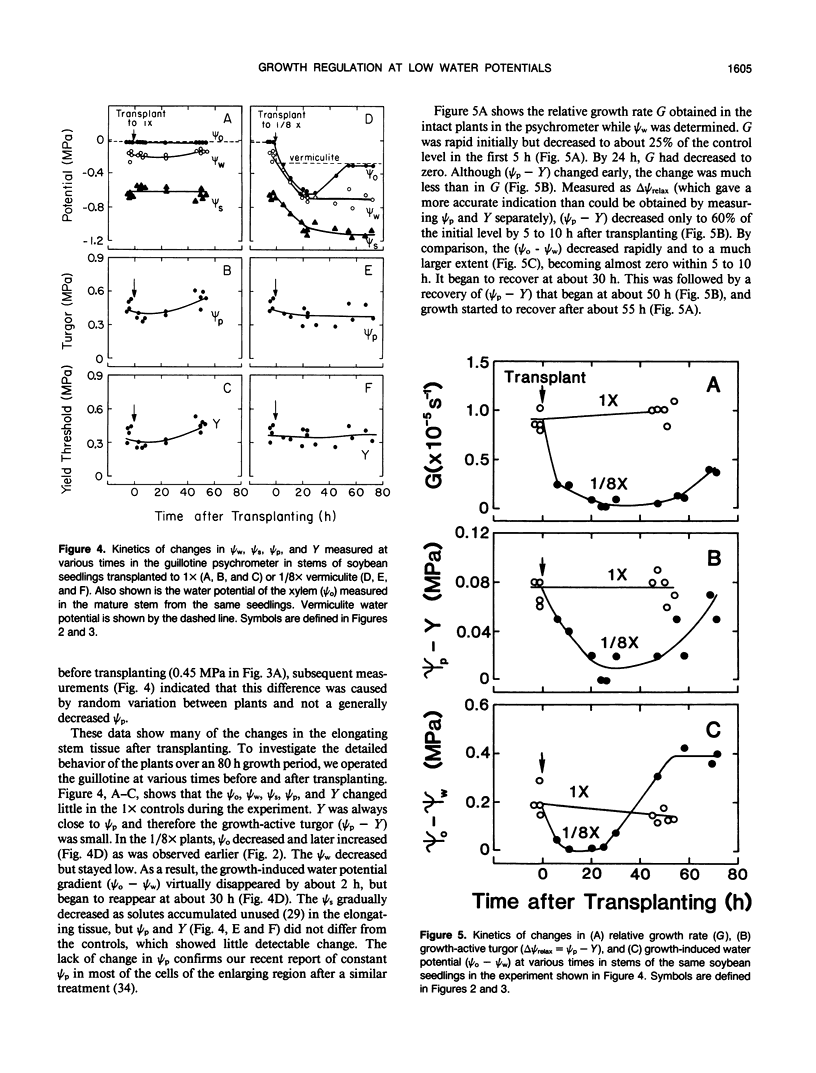
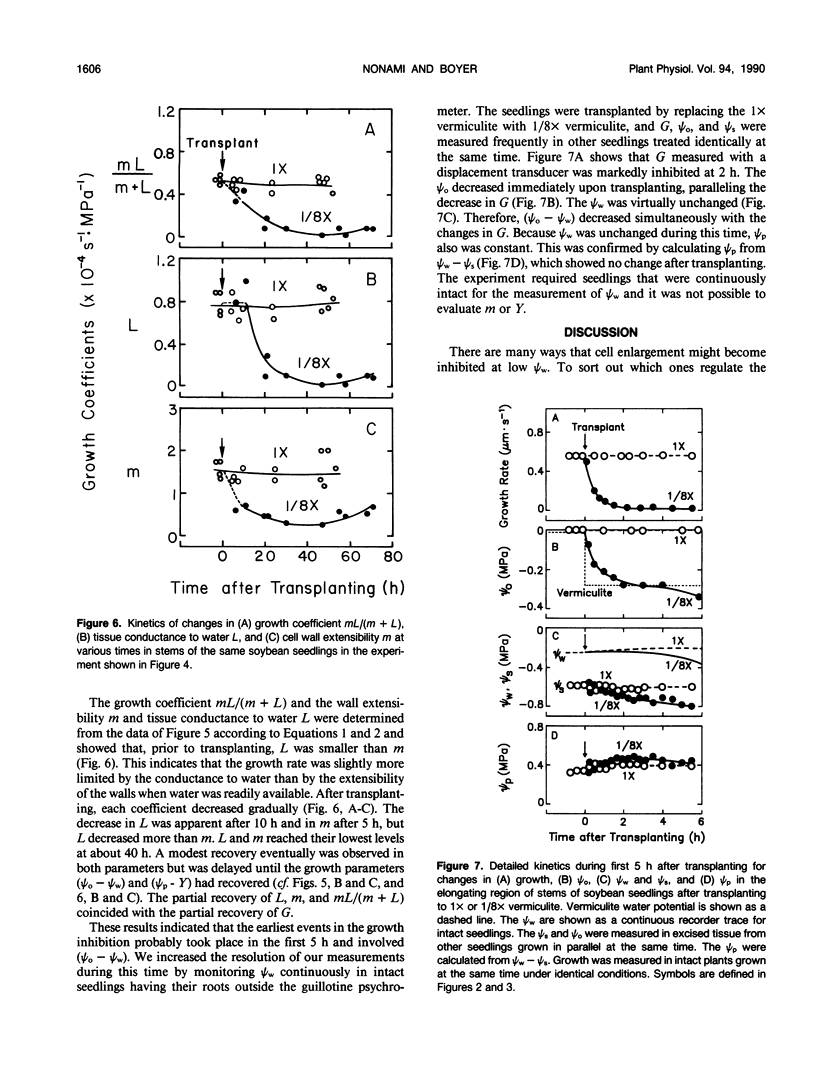
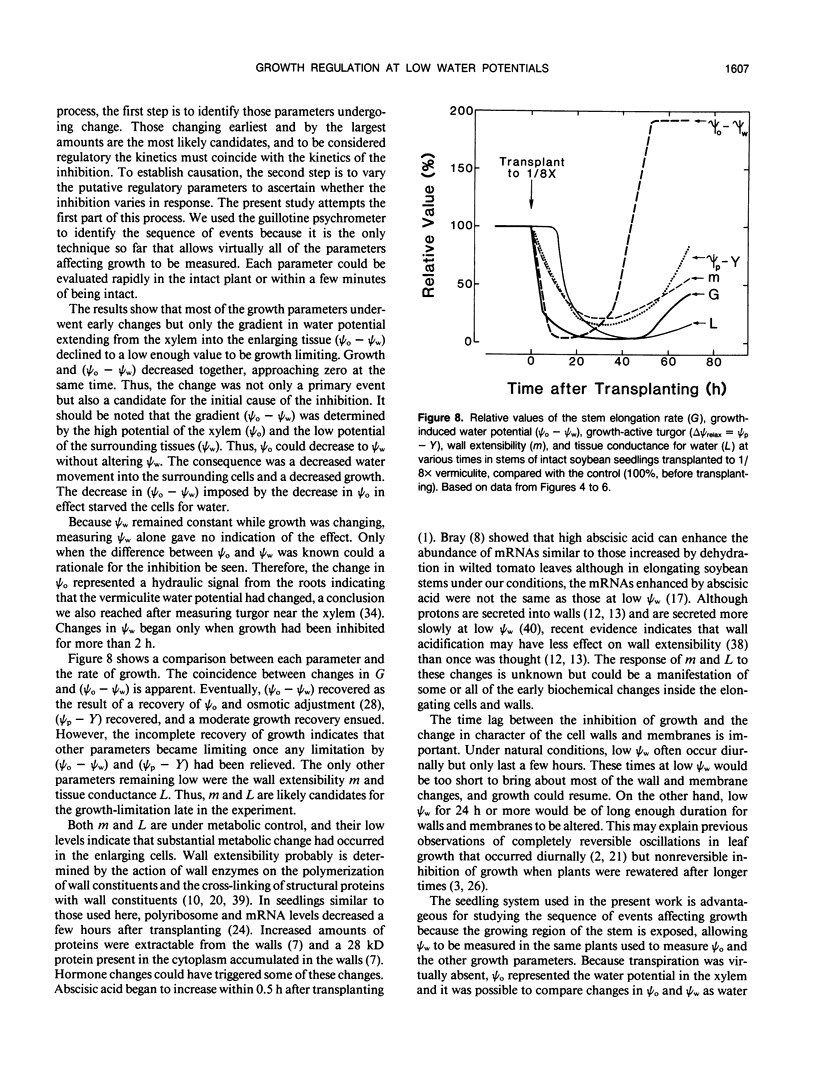
Cell enlargement is inhibited by inadequate water. As a first step toward understanding the mechanism, all the physical parameters affecting enlargement were monitored to identify those that changed first, particularly in coincidence with the inhibition. The osmotic potential, turgor, yield threshold turgor, growth-induced water potential, wall extensibility, and conductance to water were measured in the elongating region, and the water potential was measured in the xylem of stems of dark-grown soybean (Glycine max [L.] Merr.) seedlings. A stepdown in water potential was achieved around the roots by transplanting the seedlings to vermiculite of low water content, and each of the parameters was measured simultaneously in the same plants while intact or within a few minutes of being intact using a newly developed guillotine psychrometer. The gradient of decreasing water potential from the xylem to the enlarging cells (growth-induced water potential) was the first of the parameters to decrease to a growth-limiting level. The kinetics were the same as for the inhibition of growth. The decreased gradient was caused mostly by a decreased water potential of the xylem. This was followed after 5 to 10 hours by a similar decrease in cell wall extensibility and tissue conductance for water. Later, the growth-induced water potential recovered as a result of osmotic adjustment and a rise in the water potential of the xylem. Still later, moderate growth resumed at a rate apparently determined by the low wall extensibility and tissue conductance for water. The turgor did not change significantly during the experiment. These results indicate that the primary event during the growth inhibition was the change in the growth-induced water potential. Because the growth limitation subsequently shifted to the low wall extensibility and tissue conductance for water, the initial change in potential may have set in motion subsequent metabolic changes that altered the characteristics of the wall and cell membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensen R. J., Boyer J. S., Mullet J. E. Water deficit-induced changes in abscisic Acid, growth, polysomes, and translatable RNA in soybean hypocotyls. Plant Physiol. 1988 Oct;88(2):289–294. doi: 10.1104/pp.88.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970 Aug;46(2):233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A. Drought- and ABA-Induced Changes in Polypeptide and mRNA Accumulation in Tomato Leaves. Plant Physiol. 1988 Dec;88(4):1210–1214. doi: 10.1104/pp.88.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J. Cell wall yield properties of growing tissue : evaluation by in vivo stress relaxation. Plant Physiol. 1985 Jun;78(2):347–356. doi: 10.1104/pp.78.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Van Volkenburgh E., Cleland R. E. Stress relaxation of cell walls and the yield threshold for growth: demonstration and measurement by micro-pressure probe and psychrometer techniques. Planta. 1984;162(1):46–54. doi: 10.1007/BF00397420. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. Wall relaxation in growing stems: comparison of four species and assessment of measurement techniques. Planta. 1987;171:266–278. [PubMed] [Google Scholar]
- Creelman R. A., Mason H. S., Bensen R. J., Boyer J. S., Mullet J. E. Water Deficit and Abscisic Acid Cause Differential Inhibition of Shoot versus Root Growth in Soybean Seedlings : Analysis of Growth, Sugar Accumulation, and Gene Expression. Plant Physiol. 1990 Jan;92(1):205–214. doi: 10.1104/pp.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. M., Steponkus P. L., Wach M. J., Shahan K. W. Dynamic aspects and enhancement of leaf elongation in rice. Plant Physiol. 1980 Jul;66(1):147–152. doi: 10.1104/pp.66.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H., Yamamoto K., Hirohata K., Sagawa H., Leung K. P., Walker C. B. Localization and identification of root canal bacteria in clinically asymptomatic periapical pathosis. J Endod. 1990 Nov;16(11):534–538. doi: 10.1016/S0099-2399(07)80216-0. [DOI] [PubMed] [Google Scholar]
- Green P. B., Erickson R. O., Buggy J. Metabolic and physical control of cell elongation rate: in vivo studies in nitella. Plant Physiol. 1971 Mar;47(3):423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. An analysis of irreversible plant cell elongation. J Theor Biol. 1965 Mar;8(2):264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E., Boyer J. S. Polysomes, Messenger RNA, and Growth in Soybean Stems during Development and Water Deficit. Plant Physiol. 1988 Mar;86(3):725–733. doi: 10.1104/pp.86.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Riazi A. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiol. 1981 Sep;68(3):571–576. doi: 10.1104/pp.68.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyssek R., Maruyama S., Boyer J. S. Rapid wall relaxation in elongating tissues. Plant Physiol. 1988 Apr;86(4):1163–1167. doi: 10.1104/pp.86.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena V. A., Boyer J. S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982 May;69(5):1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molz F. J. Growth-induced Water Potentials in Plant Cells and Tissues. Plant Physiol. 1978 Sep;62(3):423–429. doi: 10.1104/pp.62.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Origin of growth-induced water potential : solute concentration is low in apoplast of enlarging tissues. Plant Physiol. 1987 Mar;83(3):596–601. doi: 10.1104/pp.83.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S., Steudle E. Pressure probe and isopiestic psychrometer measure similar turgor. Plant Physiol. 1987 Mar;83(3):592–595. doi: 10.1104/pp.83.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Turgor and growth at low water potentials. Plant Physiol. 1989 Mar;89(3):798–804. doi: 10.1104/pp.89.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H., Boyer J. S. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990 Aug;93(4):1610–1619. doi: 10.1104/pp.93.4.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Boyer J. S. Control of Leaf Expansion by Nitrogen Nutrition in Sunflower Plants : ROLE OF HYDRAULIC CONDUCTIVITY AND TURGOR. Plant Physiol. 1982 Apr;69(4):771–775. doi: 10.1104/pp.69.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schopfer P. pH-Dependence of Extension Growth in Avena Coleoptiles and Its Implications for the Mechanism of Auxin Action. Plant Physiol. 1989 May;90(1):202–207. doi: 10.1104/pp.90.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E., Boyer J. S. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985 Jan;77(1):190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate M. E., Boyer J. S. Transpiration- and growth-induced water potentials in maize. Plant Physiol. 1984 Apr;74(4):882–889. doi: 10.1104/pp.74.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]


