Abstract
Clinically symptomatic type 1 diabetes (stage 3 type 1 diabetes) is preceded by a pre-symptomatic phase, characterised by progressive loss of functional beta cell mass after the onset of islet autoimmunity, with (stage 2) or without (stage 1) measurable changes in glucose profile during an OGTT. Identifying metabolic tests that can longitudinally track changes in beta cell function is of pivotal importance to track disease progression and measure the effect of disease-modifying interventions. In this review we describe the metabolic changes that occur in the early pre-symptomatic stages of type 1 diabetes with respect to both insulin secretion and insulin sensitivity, as well as the measurable outcomes that can be derived from the available tests. We also discuss the use of metabolic modelling to identify insulin secretion and sensitivity, and the measurable changes during dynamic tests such as the OGTT. Finally, we review the role of risk indices and minimally invasive measures such as those derived from the use of continuous glucose monitoring.
Graphical Abstract
Supplementary Information
The online version contains a slideset of the figures for download available at 10.1007/s00125-023-06011-5.
Keywords: Diabetes stages, Metabolic modelling, Oral glucose tolerance test, Oral minimal model, Review, Stage 1 type 1 diabetes, Stage 2 type 1 diabetes, Type 1 diabetes
Background
Symptomatic type 1 diabetes (stage 3 type 1 diabetes) is preceded by a prolonged pre-symptomatic phase characterised by progressive loss of functional beta cell mass after the onset of islet autoimmunity, with (stage 2) or without (stage 1) dysglycaemia during an OGTT [1]. Even in the absence of a measurable change in glucose profile during fasting or dynamic tests in early type 1 diabetes (stage 1), impairments of insulin secretion [2, 3] and insulin sensitivity [4–6] have been described, suggesting that beta cell impairment largely pre-dates increases in glucose and affects both insulin secretion and insulin action.
The approval of the first disease-modifying drug—the humanised anti-CD3 antibody teplizumab [7–9]—and extensive research on other agents [10] that may impact the trajectory of beta cell function highlight the need to identify effective measures of beta cell function. In this review we examine the metabolic changes that occur in the early stages of type 1 diabetes and the current methods for quantifying beta cell function, and discuss the possibility of longitudinally tracking the trajectory of beta cell function before progression to stage 3 type 1 diabetes.
The importance of both insulin secretion and insulin sensitivity in evaluating beta cell health
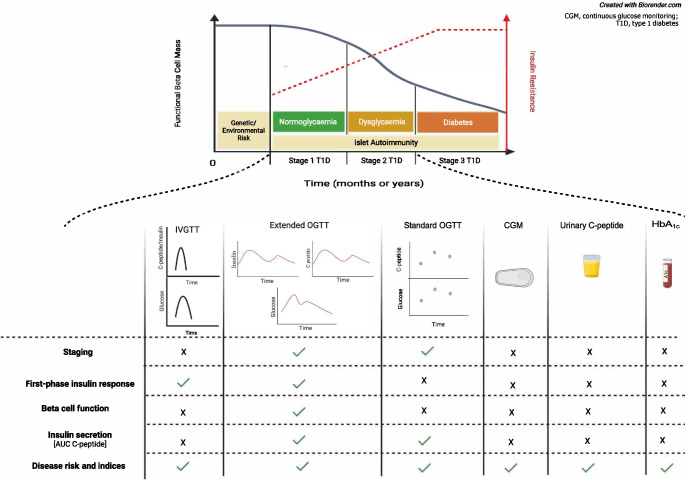
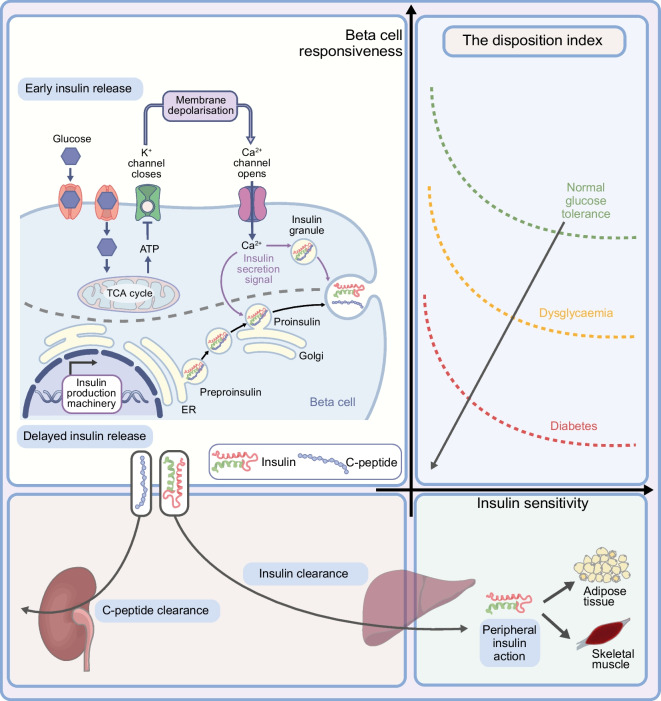
Beta cell function relies on two components, insulin secretion and insulin sensitivity, or, in other terms, on the ability of beta cell functional mass to deliver sufficient insulin to match whole-body requirements and to adequately respond to transient metabolic challenges to maintain normoglycaemia [11]. It is therefore essential to estimate both components to adequately describe beta cell health. The dynamic interaction between insulin secretion and insulin sensitivity is described by a hyperbolic curve that is quantified by the so-called disposition index (DI), namely the capacity of insulin to promote glucose disposal in target organs such as liver and muscle [12, 13]. The healthy beta cell has a substantial insulin secretory reserve that can dynamically match increases in insulin resistance throughout life, thus minimising glucose fluctuations. However, even in the absence of measurable impairment of the glucose profile, changes in insulin secretion and sensitivity have been described in the early stages of type 1 diabetes (Fig. 1) [2, 3, 14–16].
Fig. 1.
The DI results from the hyperbolic interaction of insulin secretion (beta cell responsiveness) and insulin sensitivity. Beta cell health can be quantified through the DI. Schematics representing insulin secretion and insulin sensitivity are shown. Early insulin release represents the response to a rapid glucose rise and is driven by the release of intracellular preformed insulin vesicles following glucose-induced beta cell depolarisation and calcium influx. This is followed by a second, delayed release of insulin in newly formed vesicles. Insulin and C-peptide are released in an equimolar ratio by the beta cell. Insulin is cleared by the first hepatic pass, with ~20% of the secreted insulin reaching peripheral target organs, while C-peptide is cleared by the kidney at a rate that is dependent on the GFR. The graph represents the dynamic interaction between insulin secretion and insulin sensitivity to maintain normoglycaemia. The transition from normal glucose tolerance to diabetes is shown by the failure to increase insulin secretion in response to changes in insulin sensitivity. ER, endoplasmic reticulum; TCA, tricarboxylic acid. This figure is available as part of a downloadable slideset
Beta cell function in the early stages of type 1 diabetes : quantifying insulin secretion
First-phase insulin response
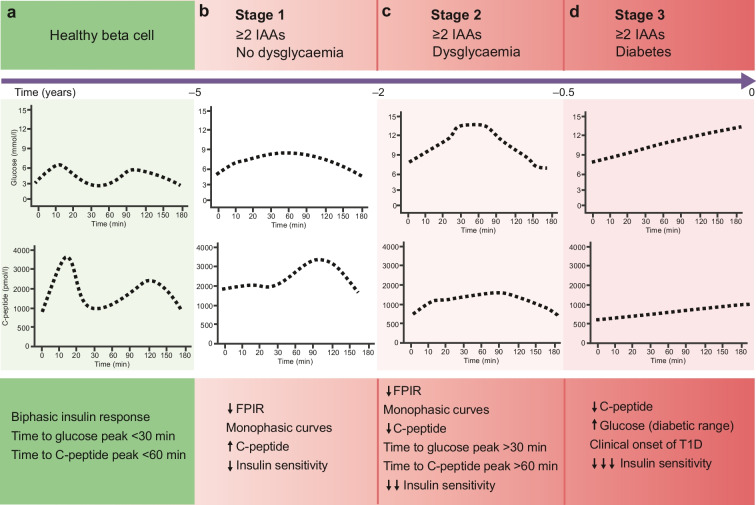
The first-phase insulin response (FPIR) represents the response to a rapid glucose rise and is driven by the release of intracellular preformed insulin vesicles following glucose-induced beta cell depolarisation and calcium influx. The FPIR is classically assessed using an IVGTT, although a frequently sampled OGTT, with early samples taken during the first 30 min after the oral load, may also provide an accurate estimate [17, 18] (Fig. 2a). The loss of the glucose-induced FPIR is the most sensitive and earliest marker of beta cell dysfunction in type 1 diabetes [19–21]. While FPIR loss seems to be a marker of functional beta cell mass, some investigators have suggested that it may be secondary to insulin resistance and mild hyperglycaemia, therefore indicating that insulin resistance—and not secretion—may also be a primary driver for type 1 diabetes; however, this remains controversial [19].
Fig. 2.
Glucose and C-peptide profiles during an OGTT through the progression from healthy beta cells (a) to stage 1 (b), stage 2 (c) and stage 3 (d) type 1 diabetes (T1D). The time (years) to diagnosis of type 1 diabetes is based on the available evidence and is intended as approximative. IAA, islet autoantibody. This figure is available as part of a downloadable slideset
The loss of the FPIR occurs 4 to 6 years before clinically symptomatic type 1 diabetes, accompanying islet autoantibody seroconversion [2, 22], and its measurement has been adopted to stratify diabetes risk in early prevention trials such as the Diabetes Prevention Trial – Type 1 Diabetes (DPT-1) [14] and the European Nicotinamide Diabetes Intervention Trial (ENDIT) study [23].
Late compensatory changes in insulin secretion
The FPIR is physiologically followed by a second, delayed release of insulin in newly formed vesicles, which is represented by the biphasic insulin profile observed during an OGTT in an individual without diabetes [24, 25]. A compensatory increase in C-peptide secretion during this late phase of the OGTT (after 60 min) has been described following the initial loss of the FPIR [3, 26, 27] (Fig. 2b).
Data from the TrialNet Pathway to Prevention (TNPTP) study in relatives with at least two islet autoantibodies of people with type 1 diabetes describe a biphasic trend in AUC C-peptide during an OGTT before the clinical onset of type 1 diabetes, with a prolonged period of apparent stability of C-peptide or, in some studies, a ‘paradoxical’ increase in C-peptide levels [28]. This reaches a critical point ~6 months before the diagnosis of stage 3 type 1 diabetes, when the pattern changes to a rapid decline in C-peptide levels (Fig. 2b–d) [27, 28]. The compensatory increase in the late phase of insulin secretion probably accounts for the early stability or increase in overall AUC C-peptide release, masking a progressive decline in functional beta cell mass (Fig. 2b) [28]. While during stage 1 type 1 diabetes (Fig. 2b) the glucose excursion after an oral load resembles the healthy response, shown by biphasic glucose and C-peptide excursions, during stage 2 the compensatory delayed secretory response results in delayed glucose and C-peptide peaks, with monophasic glucose and C-peptide excursions (Fig. 2c). This is supported by the observation that a time to peak glucose >30 min [29] is associated with a higher risk of metabolic progression. A monophasic glucose curve (with one peak) was also more frequent in progressors to type 1 diabetes than non-progressors among antibody-positive individuals with serial OGTTs in the TNPTP study [30] (Fig. 2c).
Beta cell function in the early stages of type 1 diabetes : quantifying insulin resistance
The role of insulin resistance in type 1 diabetes onset has long been debated. Suggestions range from it being a hypothetical disease accelerator [31] to being the primary determinant of beta cell failure [5, 19]. The exact temporal sequence of insulin secretion and insulin resistance changes remains uncertain. Seroconversion to multiple islet autoantibodies [32] parallels the transient physiological reduced insulin sensitivity described in healthy toddlers and adolescents, which peaks twice: before the age of 2 years, during the so-called mini-puberty, well described in toddlers and featuring hormonal changes comparable to those seen in the actual pubertal transition [33], and after the age of 10 years, during the pubertal transition [13].
Early observations conducted in a subgroup of asymptomatic participants in the DPT-1 trial, exhibiting fasting hyperglycaemia and 2 h glucose values >11.1 mmol/l, demonstrated reduced insulin sensitivity [34]. Similarly, a quantitative assessment of insulin resistance in the DPT-1 cohort based on the model of Mari et al [35] also demonstrated lower insulin sensitivity in those who progressed to clinical diabetes, in the absence of a difference at baseline [5], with a steep decline in insulin sensitivity 1 year before the diagnosis of clinically symptomatic type 1 diabetes [6]. More recently, a lower insulin sensitivity has been described in those with islet autoimmunity in the absence of dysglycaemia (stage 1) compared with their healthy peers [4]. Insulin resistance has also been described as a feature of type 1 diabetes in the absence of traditional risk factors, with the use of a euglycaemic clamp in lean individuals achieving glucose control targets [36–38].
Measuring beta cell health: static vs dynamic tests
Traditionally, measurement of insulin secretion relies on C-peptide plasma concentrations during static (at a single time point) or dynamic (over multiple time points) tests. Up to 80% of insulin secreted in the portal vein is cleared by the liver, with large variability depending on the population studied [39, 40]. On the other hand, C-peptide, secreted in an equimolar ratio with insulin, is not subject to hepatic first-pass clearance and exhibits a longer half-life than insulin (~30 min vs ~4 min) [41], with an almost constant peripheral clearance [41–43]. These characteristics make C-peptide, rather than insulin, the almost ideal analyte for estimating insulin secretion.
C-peptide can be measured in a fasting or non-fasting (random) sample as a simple clinically deployed static test [44]. Dynamic testing, on the other hand, refers to tests such as the OGTT and mixed meal tolerance test (MMTT), which require a glucose challenge (and amino acid challenge in the case of the MMTT) and longitudinal measurement over 2–4 h.
Static tests
Static measures of fasting glucose and C-peptide provide some evidence for actual disease progression in those in the early stages of type 1 diabetes [4, 45]. An early increase in postprandial glucose detectable up to 2 months before seroconversion in paediatric at-risk cohorts has been described [46]. In addition, a longitudinal analysis of The Environmental Determinants of Diabetes in the Young (TEDDY) study and the TNPTP cohorts, including children with islet autoimmunity, demonstrated that a 10% rise in HbA1c in the non-diabetic range was as accurate as 2 h glucose values after an OGTT as a predictor of disease progression to stage 3 type 1 diabetes [47, 48].
The serum proinsulin-to-C-peptide ratio has been demonstrated to be predictive of type 1 diabetes, with higher fasted ratio in progressors ~1 year before clinical onset of type 1 diabetes in the TNPTP cohort [49] and others [50] as a consequence of derailed insulin processing but appeared to change little over time [8]. The urinary C-peptide-to-creatinine ratio may also serve as a surrogate marker of beta cell function [51, 52], with the possibility of capturing insulin resistance, as demonstrated in healthy individuals [53, 54], but has not been studied longitudinally in early-stage type 1 diabetes.
Measurement of random serum C-peptide levels in people with new-onset type 1 diabetes [55, 56] and established type 1 diabetes [57] have confirmed clear differences in disease progression between children and adults. Even among children, those diagnosed with type 1 diabetes at the youngest ages (<7 years) are frequently seen to progress to absolute insulin deficiency more rapidly [55], with many having undetectable levels of C-peptide at diagnosis, which has been shown to mirror the quantifiable beta cell mass in histological studies [55, 57]. As sampling and detection methods have evolved, single measurement of C-peptide levels in blood has become a cheap and easily accessible test, with a recent study demonstrating how transdermal capillary blood collection for C-peptide measurement is a reliable alternative to venous sampling [58].
However, limits of static tests for quantifying the subtle changes in beta cell function in early-stage type 1 diabetes remain. Although these tests are convenient and have some value after diagnosis, they are generally not sufficiently informative to be used alone either in cohort studies or in interventional trials prior to diagnosis.
Dynamic tests
The OGTT remains the gold standard for staging type 1 diabetes according to the current classifications [1, 48, 59] and is the favourite of most screening programmes [30, 59–61], while the MMTT has been mostly used to monitor beta cell function after clinical onset of the disease (early-stage 3 type 1 diabetes) [62, 63]. From a physiology standpoint, while the OGTT measures insulin secretion in response to a single secretagogue, oral glucose, the MMTT also includes amino acids, an additional secretagogue, representing a more physiological test. Surrogate measures of insulin secretion and sensitivity based on glucose and insulin concentrations can be obtained during MMTTs and OGTTs (Table 1). Additionally, several disease risk indices can be estimated from the measurements of glucose, insulin and C-peptide during an OGTT.
Table 1.
Measurable outcomes of dynamic and static metabolic tests in stage 1 and stage 2 type 1 diabetes
| Test | Staging | FPIR | Insulin secretion | Insulin sensitivity | Beta cell function | Disease progression risk | Limitsa |
|---|---|---|---|---|---|---|---|
| Dynamic tests | |||||||
| Standard OGTTb | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ |
• Invasiveness • Age limit ( 7–8 years) |
| Extended OGTTc | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
• Invasiveness • Age limit ( 7–8 years) |
| MMTT | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
• Invasiveness • Age limit ( 7–8 years) |
| IVGTT | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ |
• Invasiveness • Age limit ( 7–8 years) |
| Hyperglycaemic clamp | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
• Invasiveness • Age limit ( 7–8 years) |
| Euglycaemic–hyperinsulinaemic clamp | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ |
• Invasiveness • Age limit ( 7–8 years) |
| Static tests | |||||||
| Urinary C-peptide-to-creatinine ratio | ✗ | ✗ | ? | ✗ | ✗ | ? |
• Invasiveness • No age limit |
| HbA1c/fasting glucose | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
• Invasiveness • No age limit |
| C-peptide capillary dried blood spot test | ✗ | ✗ | ? | ✗ | ✗ | ? |
• Invasiveness • No age limit |
| Risk indices computed during OGTT | |||||||
| DPTRSd | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
• Invasiveness • Age limit ( 7–8 years) |
| Index60e | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ |
• Invasiveness • Age limit ( 7–8 years) |
| Other | |||||||
| CGM | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
• Invasiveness • No age limit |
a relatively low, very low, relatively high, very high
bStandard OGTT: glucose is measured at 0, 1 and 2 h
cExtended OGTT: glucose, insulin and C-peptide are measured at multiple time points (seven or more) and the test can be prolonged up to 240 min. Early sampling (e.g. 10 and 15 min) allows FPIR to be estimated
dDPTRS= (1.57 × log BMI) − (0.06 × age [years]) + (0.81 × glucose summed from 30 to 120 min/100) − (0.85 × C-peptide summed from 30 to 120 min/10) + (0.48 × log C-peptide0)
eIndex60 = 0.36953 × (log C-peptide0 [ng/ml]) + 0.0165 × glucose60 (mg/dl) - 0.3644 × C-peptide60 (ng/ml)
CGM, continuous glucose monitoring
Integrated assessment of beta cell health and the use of modelling
The AUC C-peptide computed during an OGTT or MMTT has been largely adopted as a surrogate marker of beta cell function in most disease prevention trials targeting stage 1 [64] and stage 2 [9] type 1 diabetes, as well as in early-stage 3 type 1 diabetes [65–68]. While a relationship between AUC C-peptide and residual beta cell function has been described, the longitudinal trajectory of such a relationship is still debated [42, 69]. However, as discussed above, the lack of decline in AUC C-peptide until around 6 months before the clinical onset of disease [28] and the evidence for early impairment of insulin sensitivity [4–6] suggest that measures accounting for both C-peptide and glucose profile, as well as insulin action, may be more informative to track the disease trajectory and the efficacy of disease-modifier drugs in early-stage type 1 diabetes.
Supporting this hypothesis, an exploratory analysis conducted in unaffected family members of people with type 1 diabetes demonstrated that lower beta cell function (DI) characterised those progressing to later disease stages in the absence of measurable differences in AUC C-peptide [70]. Similarly, a post-hoc analysis conducted in participants in the TrialNet Abatacept study testing the effect of abatacept on disease progression in those with stage 1 type 1 diabetes [64] demonstrated that Index60—a composite measure of glucose and C-peptide—but not AUC C-peptide was able to show a favourable effect of the treatment after 12 months [71].
An original approach, accounting for both glucose and C-peptide response curves (GCRC) during an OGTT, was proposed in a recent analysis of the TNPTP that allowed the identification of GCRC ‘zones’ on the 2D grid plot in association with demographic, metabolic, autoantibody, HLA and risk data [45]. This approach suggested that a higher C-peptide level was a feature of participants with higher glucose levels, therefore pointing to a role of insulin resistance in disease progression.
Modelling beta cell function during dynamic tests
Although C-peptide levels are a well-established surrogate measure for insulin secretion, it is worth noting that C-peptide metabolic clearance exhibits a certain interindividual variability under controlled experimental conditions. Furthermore, C-peptide levels must be interpreted with caution in renal failure, in which blood levels of C-peptide can be falsely elevated [72, 73] and, owing to its half-life (~30 min), may not reflect rapid fluctuations in insulin secretion shorter than 10 min that occur during a dynamic test such as an OGTT or MMTT. As such, metabolic models based on two or three ideal compartments that include C-peptide have been proven to better describe C-peptide kinetics and, in turn, the relationship between C-peptide plasma concentrations and actual insulin secretion. In a classical model, two-compartment kinetics assumes that C-peptide is distributed in a main compartment (plasma) and a peripheral compartment (extravascular space) in rapid equilibration. Two-compartment kinetics justifies the non-linear changes in peripheral C-peptide during the dynamic increase in insulin secretion measured in vivo [74, 75]. As a practical implication, a more accurate assessment of in vivo insulin secretion based on glucose and C-peptide measurements during a dynamic test such as an OGTT or MMTT is obtained when a two (or more) compartment model is applied, rather than using the raw data derived from the test [76, 77].
Metabolic models are simplified representations of actual physiology that allow the estimation of components of beta cell health based on a minimum dataset from a dynamic test [78–80]. Although several models of glucose, insulin and C-peptide kinetics have been proposed [12, 35, 81, 82], here we focus on Cobelli’s oral minimal model [83] and Mari’s model [82] because of their large validation in different age groups, including paediatric cohorts [5, 84], and wide use over the last few decades [35, 79, 81, 84, 85].
The oral minimal model
This model provides a simplified description of the complex glucose and insulin physiology [86]. It adopts quasi-linear differential equations to estimate insulin secretion and sensitivity as a result of metabolic fluxes among different compartments. Briefly, one of the two equations in the model represents insulin kinetics in plasma and the other describes the effects of insulin and glucose itself on restoration of baseline glucose levels after its ingestion or intravenous administration. The model considers a ‘delay’ in insulin action on the target organs (liver and adipose tissue). The oral minimal model [83] is used to estimate insulin sensitivity (SI), beta cell responsiveness ( total) and beta cell function (DI=SI x total). The three-compartment model has been previously validated against model-independent measurements using multiple tracer meal protocols and euglycaemic and hyperglycaemic clamps [84]. A reduced sampling protocol based on a 2 h OGTT and seven samples has been validated against the widely used 3 h nine-sample protocol, demonstrating accuracy in the estimates of total, SI and DI, thus paving the way to shorter and more suitable tests for screening procedures. The physiological underpinnings of the oral minimal model are outlined in Fig. 1 [24, 79]. Glucose-stimulated insulin secretion is made up of two components: a dynamic component, representing the secretion of readily releasable insulin, which is stimulated by the rate of increase in glucose concentration ( dynamic), and a static component, which measures new insulin production in response to a given increment in glucose above basal concentrations ( static) [77, 83, 86].
A major advantage of metabolic modelling is the possibility of estimating both insulin secretion and insulin sensitivity once serial measurements of glucose, C-peptide and insulin are obtained. The availability of early time points in dynamic testing (10 and 20 min or 15 min) is of pivotal importance in estimating early insulin release ( dynamic) [18]. The major limitation of this metabolic modelling in larger populations is the need for qualified personnel to run the analysis and the requirement to obtain multiple samples during testing.
Glucose sensitivity and the potentiation factor during oral dynamic tests
An alternative model-based strategy has been proposed by Mari and colleagues by introducing beta cell glucose sensitivity and the potentiation factor [81, 87]. Briefly, a first component of the insulin secretion model describes insulin secretion with respect to the glucose concentration during an OGTT/MMTT using a dose–response function. The mean slope of the dose–response curve over the measured glucose range is described as beta cell glucose sensitivity and is independent of insulin sensitivity. The dose–response curve is modulated by the so-called potentiation factor, which accounts for non-glucose stimuli, such as gut-derived incretin secretion, during the test. A second component of the insulin secretion model quantifies the dependence of insulin secretion on the rate of change in glucose concentration. This derivative component is described as the ‘rate sensitivity’ and is related to early insulin release [35, 81].
Indices of risk for disease progression
Longitudinal OGTTs have long served to derive indices to stratify the risk for disease progression. Such an approach does not necessarily describe the underlying physiology of beta cell changes over time. Indices including dynamic changes in C-peptide or glucose are expected to perform better than those based on single time points or static measures. Combined risk scores including genetic, clinical and immunological characteristics generally outperform metabolic indices [88]; however, development and validation of such risk scores require large cohorts that can capture wider ranges of genetic risk and backgrounds.
Longitudinal studies have demonstrated that composite measures of both glucose and C-peptide are able to identify antibody-positive individuals with previously unrecognised metabolic abnormalities as being at risk of progressing to stage 3 type 1 diabetes. Examples of such composite measures are the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS) and the Index60.
The DPTRS is a risk score derived by stepwise modelling based on univariate proportional hazards models, developed in islet cell autoantibody-positive individuals and validated in the TNPTP study. Designed to capture the increasing glucose concentrations within the normal range that occur years before diagnosis [53, 89] and the differing trends in the latter stages of progression in relation to post-challenge C-peptide and glucose levels, the DPTRS includes fasting C-peptide, summed OGTT C-peptide and glucose values from 30, 60, 90 and 120 min, and age and BMI [90, 91]. The change in DPTRS from baseline to 1 year was highly predictive of type 1 diabetes in participants in the DPT-1 trial [90], while a DPTRS value ≥7.00 was able to identify antibody-positive individuals within the normal glucose range at substantial risk for progression [92].
Index60, a solely metabolic index comprising the log fasting C-peptide, 60 min glucose and 60 min C-peptide values and derived similarly from univariate proportional hazards modelling within the DPT-1 and TrialNet Natural History Study (TNNHS) cohorts, has also demonstrated utility in identifying impending stage 3 type 1 diabetes among autoantibody-positive individuals with normal 2 h glucose values (<7.8 mmol/l) [93].
Risk indices remain a valuable tool for identifying those who will most likely progress to stage 3 type 1 diabetes; however, they do not describe the underlying metabolic changes, which can be measured through metabolic testing and modelling. Therefore, risk indices and metabolic measures can be seen as complementary, non-overlapping tools for the investigation of the early stages of type 1 diabetes [53, 54].
Minimally invasive measures of beta cell health: continuous glucose monitoring
Continuous glucose monitoring (CGM) has shown promise in meeting the challenge of screening across different age groups owing to its minimal invasiveness, low cost and good acceptance level. There is growing evidence that CGM detects abnormalities in glucose control in children with stage 1 type 1 diabetes [94]. In a small study conducted in antibody-positive children [81], the presence of islet autoimmunity increased glycaemic variability and the percentage of time spent with blood glucose >7.8 mmol/l compared with antibody-negative children. In a larger TNPTP cohort, spending ≥5% of the time with blood glucose ≥7.8 mmol/l or ≥8.9 mmol/l resulted in a 2 year risk of progression to type 1 diabetes of 40% and 62%, respectively [95]. However, evidence from the TNPTP cohort has demonstrated that OGTT-derived metrics still have a higher discriminative ability to predict disease progression than CGM [96].
Conclusion
Measures of C-peptide alone provide an incomplete portrait of beta cell function and disease progression during the early stages of type 1 diabetes, as they do not account for changing insulin sensitivity and the non-linear fluctuations in insulin secretion described in stage 1 and stage 2 type 1 diabetes.
While risk indices have proved to be a valuable tool for stratifying the risk of progression of disease, they provide limited quantitative evidence on actual functional beta cell mass and its longitudinal changes. On the other hand, deep metabolic phenotyping tests may require complex and burdensome procedures that may not be feasible across different age groups. Metabolic modelling of the data derived from standard tests such as the OGTT or MMTT provides a more accurate and convenient way to estimate both insulin secretion and insulin sensitivity in early-stage type 1 diabetes. Further validation of such models in larger longitudinal cohorts is needed to confirm the value of this approach for generating a rapidly responsive endpoint that could be used to accelerate therapeutic trials at this stage of the disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- DI
Disposition index
- DPT-1
Diabetes Prevention Trial – Type 1 Diabetes
- DPTRS
Diabetes Prevention Trial–Type 1 Risk Score
- FPIR
First-phase insulin response
- GCPR
Glucose and C-peptide response curves
- MMTT
Mixed meal tolerance test
- TEDDY
The Environmental Determinants of Diabetes in the Young
- TNPTP
TrialNet Pathway to Prevention
Funding
AG’s work is supported by the Juvenile Diabetes Research Foundation (JDRF SRA-2022-1186-S-B).
Authors’ relationships and activities
CD has lectured for or been involved as an advisor to the following companies: Novo Nordisk, Sanofi-Genzyme, Janssen, Servier, Lilly, AstraZeneca, Provention Bio, UCB, MSD, Vielo Bio, Avotres, Worg and Novartis. He also holds a patent jointly with Midatech and Provention Bio/Sanofi. The other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
AG and ALJC drafted the initial manuscript. MM, PT, PS and CD contributed to the identification of literature, data interpretation and discussion. CD critically revised the manuscript and coordinated the working group. All authors approved the final version of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alfonso Galderisi and Alice L. J. Carr contributed equally to this article.
Change history
11/27/2024
A Correction to this paper has been published: 10.1007/s00125-024-06335-w
References
- 1.Insel R, Dunne J, Atkinson M et al (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38(10):1964–74. 10.2337/dc15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srikanta S, Ganda O, Rabizadeh A, Soeldner J, Eisenbarth G (1985) First-degree relatives of patients with type I diabetes mellitus. Islet-cell antibodies and abnormal insulin secretion. N Engl J Med 313(8):461–4. 10.1056/NEJM198508223130801 [DOI] [PubMed] [Google Scholar]
- 3.Evans-Molina C, Sims E, DiMeglio L et al (2018) β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI insight 3(15):e120877. 10.1172/jci.insight.120877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galderisi A, Moran A, Evans-Molina C et al (2021) Early impairment of insulin sensitivity, β-cell responsiveness, and insulin clearance in youth with stage 1 type 1 diabetes. J Clin Endocrinol Metab. 10.1210/clinem/dgab344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS (2010) Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59:679–685. 10.2337/db09-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrannini E, Mari A, Monaco G, Skyler J, Evans-Molina C (2023) The effect of age on longitudinal measures of beta cell function and insulin sensitivity during the progression of early stage type 1 diabetes. Diabetologia 66(3):508–519. 10.1007/s00125-022-05836-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans-Molina C, Orma R (2023) Teplizumab approval for type 1 diabetes in the USA. Lancet Diabetes Endocrinol 11(2):76–77. 10.1016/S2213-8587(22)00390-4 [DOI] [PubMed] [Google Scholar]
- 8.Sims E, Bundy B, Stier K et al (2021) Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 13(583):eabc8980. 10.1126/scitranslmed.abc8980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herold K, Bundy B, Long S et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381(7):603–613. 10.1056/NEJMoa1902226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen L, Dayan C (2021) Immunotherapy for type 1 diabetes. Br Med Bull 140(1):76–90. 10.1093/bmb/ldab027 [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN, Ader M, Huecking K, Van Citters G (2002) Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 51(Suppl. 1):S212-220. 10.2337/diabetes.51.2007.s212 [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE, Prigeon RL, McCulloch DK et al (1993) Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42(11):1663–1672. 10.2337/diab.42.11.1663 [DOI] [PubMed] [Google Scholar]
- 13.Caprio S, Plewe G, Diamond MP et al (1989) Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr 114(6):963–967. 10.1016/s0022-3476(89)80438-x [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Trial – Type 1 Diabetes Study Group (2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346:1685–1691. 10.1056/NEJMoa012350 [DOI] [PubMed] [Google Scholar]
- 15.Sims E, Mirmira R, Evans-Molina C (2020) The role of beta-cell dysfunction in early type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 27(4):215–224. 10.1097/MED.0000000000000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko J, Skyler J, Beam C et al (2013) Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes 62(12):4179–4183. 10.2337/db13-0656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baidal D, Warnock M, Xu P et al (2022) Oral glucose tolerance test measures of first-phase insulin response and their predictive ability for type 1 diabetes. J Clin Endocrinol Metab 107(8):e3273–e3280. 10.1210/clinem/dgac285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galderisi A, Evans-Molina C, Martino M, Caprio S, Cobelli C, Moran A (2022) Beta cell function and insulin sensitivity in youth with early type 1 diabetes from a two-hour 7-sample OGTT. J Clin Endocrinol Metab 108(6):1376–1386. 10.1210/clinem/dgac740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir GC, Butler PC, Bonner-Weir S (2021) The β-cell glucose toxicity hypothesis: attractive but difficult to prove. Metabolism 124:154870. 10.1016/j.metabol.2021.154870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezza T, Ferraro PM, Di Giuseppe G et al (2021) Pancreaticoduodenectomy model demonstrates a fundamental role of dysfunctional β cells in predicting diabetes. J Clin Invest 131(12):e146788. 10.1172/JCI146788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keskinen P, Korhonen S, Kupila A et al (2002) First-phase insulin response in young healthy children at genetic and immunological risk for type I diabetes. Diabetologia 45(12):1639–48. 10.1007/s00125-002-0981-8 [DOI] [PubMed] [Google Scholar]
- 22.Koskinen M, Mikk M, Laine A et al (2020) Longitudinal pattern of first-phase insulin response is associated with genetic variants outside the class iI HLA region in children with multiple autoantibodies. Diabetes 69(1):12–19. 10.2337/db19-0329 [DOI] [PubMed] [Google Scholar]
- 23.European Nicotinamide Diabetes Intervention Trial (ENDIT) Group, Bingley P, Gale E (2006) Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 49(5):881–90. 10.1007/s00125-006-0160-4 [DOI] [PubMed] [Google Scholar]
- 24.Pedersen MG, Dalla Man C, Cobelli C (2011) Multiscale modeling of insulin secretion. IEEE Trans Biomed Eng 58(10):3020–3. 10.1109/TBME.2011.2164918 [DOI] [PubMed] [Google Scholar]
- 25.Porte D, Pupo A (1969) Insulin responses to glucose: evidence for a two pool system in man. J Clin Investig 48(12):2309–19. 10.1172/JCI106197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sosenko J, Palmer J, Rafkin L et al (2010) Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care 33(3):620–625. 10.2337/dc09-1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogun M, Bundy B, Goland R, Greenbaum C (2020) C-peptide levels in subjects followed longitudinally before and after type 1 diabetes diagnosis in TrialNet. Diabetes Care 43(8):1836–1842. 10.2337/dc19-2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail H, Cuthbertson D, Gitelman S et al (2022) The transition from a compensatory increase to a decrease in C-peptide during the progression to type 1 diabetes and its relation to risk. Diabetes Care 45(10):2264–2270. 10.2337/dc22-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss M, Cuthbertson D, Cleves M et al (2021) Time to peak glucose and peak C-peptide during the progression to type 1 diabetes in the Diabetes Prevention Trial and TrialNet Cohorts. Diabetes Care 44(10):2329–2336. 10.2337/dc21-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail H, Cleves M, Xu P et al (2020) The pathological evolution of glucose response curves during the progression to type 1 diabetes in the TrialNet Pathway to Prevention Study. Diabetes Care 43(11):2668–2674. 10.2337/dc20-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkin TJ (2013) Is autoimmunity or insulin resistance the primary driver of type 1 diabetes? Curr Diabetes Rep 13(5):651–6. 10.1007/s11892-013-0407-7 [DOI] [PubMed] [Google Scholar]
- 32.Pöllänen PM, Ryhänen SJ, Toppari J et al (2020) Dynamics of islet autoantibodies during prospective follow-up from birth to age 15 years. J Clin Endocrinol Metab 105(12):e4638-4651. 10.1210/clinem/dgaa624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannsen TH, Main KM, Ljubicic ML et al (2018) Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab 103(8):3028–3037. 10.1210/jc.2018-00482 [DOI] [PubMed] [Google Scholar]
- 34.Greenbaum C, Cuthbertson D, Krischer J (2001) Type I diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes 50(2):470–6. 10.2337/diabetes.50.2.470 [DOI] [PubMed] [Google Scholar]
- 35.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E (2002) Meal and oral glucose tests for assessment of beta-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 283(6):E1159-66. 10.1152/ajpendo.00093.2002 [DOI] [PubMed] [Google Scholar]
- 36.Donga E, Dekkers O, Corssmit E, Romijn JA (2015) Insulin resistance in patients with type 1 diabetes assessed by glucose clamp studies: systematic review and meta-analysis. Eur J Endocrinol 173(1):101–9. 10.1530/EJE-14-0911 [DOI] [PubMed] [Google Scholar]
- 37.Bergman BC, Howard D, Schauer IE et al (2012) Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab 97(5):1663–72. 10.1210/jc.2011-3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cline GW, Magnusson I, Rothman DL, Petersen KF, Laurent D, Shulman GI (1997) Mechanism of impaired insulin-stimulated muscle glucose metabolism in subjects with insulin-dependent diabetes mellitus. J Clin Invest 99(9):2219–2224. 10.1172/JCI119395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccinini F, Bergman R (2020) The measurement of insulin clearance. Diabetes Care 43(9):2296–2302. 10.2337/dc20-0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Najjar S, Perdomo G (2019) Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda, Md) 34(3):198–215. 10.1152/physiol.00048.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faber O, Hagen C, Binder C et al (1978) Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Investig 62(1):197–203. 10.1172/JCI109106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer J, Fleming G, Greenbaum C et al (2004) C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 53(1):250–64. 10.2337/diabetes.53.1.250 [DOI] [PubMed] [Google Scholar]
- 43.Polonsky K, Frank B, Pugh W et al (1986) The limitations to and valid use of C-peptide as a marker of the secretion of insulin. Diabetes 35(4):379–86. 10.2337/diab.35.4.379 [DOI] [PubMed] [Google Scholar]
- 44.Jones A, Hattersley A (2013) The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabetic Med 30(7):803–17. 10.1111/dme.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sosenko J, Cuthbertson D, Sims E et al (2023) Phenotypes associated with zones defined by area under the curve glucose and C-peptide in a population with islet autoantibodies. Diabetes Care 46(5):1098–1105. 10.2337/dc22-2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warncke K, Weiss A, Achenbach P et al (2022) Elevations in blood glucose before and after the appearance of islet autoantibodies in children. J Clin Invest 132(20):e162123. 10.1172/JCI162123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vehik K, Boulware D, Killian M et al (2022) Rising hemoglobin A1c in the nondiabetic range predicts progression of type 1 diabetes as well as oral glucose tolerance tests. Diabetes Care 45(10):2342–2349. 10.2337/dc22-0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besser R, Bell K, Couper J et al (2022) ISPAD Clinical Practice Consensus Guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatric Diabetes 23(8):1175–1187. 10.1111/pedi.13410 [DOI] [PubMed] [Google Scholar]
- 49.Sims EK, Chaudhry Z, Watkins R et al (2016) Elevations in the fasting serum proinsulin–to–C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 10.2337/dc15-2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Triolo TM, Pyle L, Seligova S et al (2021) Proinsulin:C-peptide ratio trajectories over time in relatives at increased risk of progression to type 1 diabetes. J Transl Autoimmun 4:100089. 10.1016/j.jtauto.2021.100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones A, Besser R, McDonald TJ et al (2011) Urine C-peptide creatinine ratio is an alternative to stimulated serum C-peptide measurement in late-onset, insulin-treated diabetes. Diabetic Med J Br Diabetic Assoc 28(9):1034–8. 10.1111/j.1464-5491.2011.03272.x [DOI] [PubMed] [Google Scholar]
- 52.Oram R, Rawlingson A, Shields B et al (2013) Urine C-peptide creatinine ratio can be used to assess insulin resistance and insulin production in people without diabetes: an observational study. BMJ Open 3(12). 10.1136/bmjopen-2013-003193 [DOI] [PMC free article] [PubMed]
- 53.Nathan B, Redondo M, Ismail H et al (2022) Index60 identifies individuals at appreciable risk for stage 3 among an autoantibody-positive population with normal 2-hour glucose levels: implications for current staging criteria of type 1 diabetes. Diabetes Care 45(2):311–318. 10.2337/dc21-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redondo M, Nathan B, Jacobsen L et al (2021) Index60 as an additional diagnostic criterion for type 1 diabetes. Diabetologia 64(4):836–844. 10.1007/s00125-020-05365-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carr ALJ, Inshaw JRJ, Flaxman CS et al (2022) Circulating C-peptide levels in living children and young people and pancreatic β-cell loss in pancreas donors across type 1 diabetes disease duration. Diabetes 71(7):1591–1596. 10.2337/db22-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis AK, DuBose SN, Haller MJ et al (2015) Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 38(3):476–481. 10.2337/dc14-1952 [DOI] [PubMed] [Google Scholar]
- 57.Harsunen M, Haukka J, Harjutsalo V et al (2023) Residual insulin secretion in individuals with type 1 diabetes in Finland: longitudinal and cross-sectional analyses. Lancet Diabetes Endocrinol 11(7):465–473. 10.1016/S2213-8587(23)00123-7 [DOI] [PubMed] [Google Scholar]
- 58.Besser REJ, Long A, Owen K et al (2022) Transdermal capillary blood collection for C-peptide is a practical, acceptable and reliable alternative to venous sampling children nd adults with type 1 diabetes. Diabetes Technol Therapeut 24(S1):OPO57 [Google Scholar]
- 59.Sims E, Besser R, Dayan C et al (2022) Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes 71(4):610–623. 10.2337/dbi20-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elding Larsson H, Vehik K, Gesualdo P et al (2014) Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes 15(2):118–126. 10.1111/pedi.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teddy Study Group (2007) The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 8(5):286–298. 10.1111/j.1399-5448.2007.00269.x [DOI] [PubMed] [Google Scholar]
- 62.Besser R, Shields B, Casas R, Hattersley A, Ludvigsson J (2013) Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care 36(2):195–201. 10.2337/dc12-0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Besser R, Ludvigsson J, Hindmarsh P, Cole P (2022) Exploring C-peptide loss in type 1 diabetes using growth curve analysis. PLOS ONE. 10.1371/journal.pone.0199635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell W, Bundy B, Anderson M et al (2023) Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: a randomized, double-masked, controlled trial. Diabetes Care 46(5):1005–1013. 10.2337/dc22-2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herold K, Gitelman S, Ehlers M et al (2013) Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62(11):3766–74. 10.2337/db13-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haller MJ, Schatz DA, Skyler JS et al (2018) Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA. Diabetes Care 41(9):1917–1925. 10.2337/dc18-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herold K, Gitelman S, Masharani U et al (2005) A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54(6):1763–9. 10.2337/diabetes.54.6.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobsen LM, Bundy BN, Greco MN et al (2020) Comparing beta cell preservation across clinical trials in recent-onset type 1 diabetes. Diabetes Technol Ther 22(12):948–953. 10.1089/dia.2020.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenbaum CJ, Harrison LC (2003) Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes 52(5):1059–1065. 10.2337/diabetes.52.5.1059 [DOI] [PubMed] [Google Scholar]
- 70.Martino M, Carr A, Perazzolo S, Galderisi A, Marcovecchio L, Dayan C (2023) Using disposition index to detect early impairment of beta cell function. 19th IDS Congress – Immunology of Diabetes Society (IDS), Paris, 23–26 May [Poster presentation]. Available from https://www.idsparis2023.com. Accessed 24 Aug 2023
- 71.Sims E, Russell W, Herold K, Sosenko J (2023) The effect of abatacept upon glucose and C-peptide endpoints at one year of treatment and on follow-up. Diabetes 72(Suppl. 1):731-P. 10.2337/db23-731-P [Google Scholar]
- 72.Zavaroni I, Deferrari G, Lugari R et al (1987) Renal metabolism of C-peptide in man. J Clin Endocrinol Metab 65(3):494–8. 10.1210/jcem-65-3-494 [DOI] [PubMed] [Google Scholar]
- 73.Henriksen J, Tronier B, Bülow J (1987) Kinetics of circulating endogenous insulin, C-peptide, and proinsulin in fasting nondiabetic man. Metab Clin Exp 36(5):463–8. 10.1016/0026-0495(87)90044-8 [DOI] [PubMed] [Google Scholar]
- 74.Polonsky K, Licinio-Paixao J, Given B et al (1986) Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Investig 77(1):98–105. 10.1172/JCI112308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherwin R, Kramer K, Tobin J et al (1974) A model of the kinetics of insulin in man. J Clin Investig 53(5):1481–92. 10.1172/JCI107697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J (1980) Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 51(3):520–528. 10.1210/jcem-51-3-520 [DOI] [PubMed] [Google Scholar]
- 77.Van Cauter E, Mestrez F, Sturis J, Polonsky KS (1992) Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41(3):368–377. 10.2337/diab.41.3.368 [DOI] [PubMed] [Google Scholar]
- 78.Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68(6):1456–1467. 10.1172/JCI110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C (2004) Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 287(4):E637-643. 10.1152/ajpendo.00319.2003 [DOI] [PubMed] [Google Scholar]
- 80.Dalla Man C, Campioni M, Polonsky K et al (2005) Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes 54(11):3265–73. 10.2337/diabetes.54.11.3265 [DOI] [PubMed] [Google Scholar]
- 81.Mari A, Tura A, Gastaldelli A, Ferrannini E (2002) Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 51(Suppl 1):S221-6. 10.2337/diabetes.51.2007.s221 [DOI] [PubMed] [Google Scholar]
- 82.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA (2005) Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90(1):493–500. 10.1210/jc.2004-1133 [DOI] [PubMed] [Google Scholar]
- 83.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R (2014) The oral minimal model method. Diabetes 63(4):1203–1213. 10.2337/db13-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sunehag A, Dalla Man C, Toffolo G, Haymond M, Bier D, Cobelli C (2009) Beta-cell function and insulin sensitivity in adolescents from an OGTT. Obesity (Silver Spring, Md) 17(2):233–9. 10.1038/oby.2008.496 [DOI] [PubMed] [Google Scholar]
- 85.Cali AM, Man CD, Cobelli C et al (2009) Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care 32(3):456–461. 10.2337/dc08-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalla Man C, Micheletto F, Sathananthan A, Rizza RA, Vella A, Cobelli C (2010) A model of GLP-1 action on insulin secretion in nondiabetic subjects. Am J Physiol Endocrinol Metab 298(6):E1115-1121. 10.1152/ajpendo.00705.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mari A, Tura A, Natali A et al (2010) Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia 53(4):749–756. 10.1007/s00125-009-1647-6 [DOI] [PubMed] [Google Scholar]
- 88.Ferrat LA, Vehik K, Sharp SA et al (2020) A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 26(8):1247–1255. 10.1038/s41591-020-0930-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sosenko J, Palmer J, Greenbaum C et al (2007) Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care 30(1):38–42. 10.2337/dc06-1615 [DOI] [PubMed] [Google Scholar]
- 90.Sosenko J, Krischer J, Palmer J et al (2008) A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care 31(3):528–33. 10.2337/dc07-1459 [DOI] [PubMed] [Google Scholar]
- 91.Sosenko J, Geyer S, Skyler J et al (2018) The influence of body mass index and age on C-peptide at the diagnosis of type 1 diabetes in children who participated in the diabetes prevention trial-type 1. Pediatric Diabetes 19(3):403–409. 10.1111/pedi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sosenko J, Skyler J, Mahon J et al (2014) Use of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care 37(4):979–984. 10.2337/dc13-2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sosenko J, Skyler J, DiMeglio L et al (2015) A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 38(2):271–6. 10.2337/dc14-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steck A, Dong F, Taki I, Hoffman M, Klingensmith G, Rewers M (2014) Early hyperglycemia detected by continuous glucose monitoring in children at risk for type 1 diabetes. Diabetes Care 37(7):2031–3. 10.2337/dc13-2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson D, Pietropaolo S, Acevedo-Calado M et al (2023) CGM metrics identify dysglycemic states in participants from the TrialNet pathway to prevention study. Diabetes Care 46(3):526–534. 10.2337/dc22-1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ylescupidez A, Speake C, Pietropaolo SL et al (2023) OGTT metrics surpass continuous glucose monitoring data for T1D prediction in multiple-autoantibody-positive individuals. J Clin Endocrinol Metab dgad472. 10.1210/clinem/dgad472 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.