Abstract
Lysosomes play a central role in cellular homeostasis and alterations in this compartment associate with many diseases. The most studied example is that of lysosomal storage disorders (LSDs), a group of 60+ maladies due to genetic mutations affecting lysosomal components, mostly enzymes. This leads to aberrant intracellular storage of macromolecules, altering normal cell function and causing multiorgan syndromes, often fatal within the first years of life. Several treatment modalities are available for a dozen LSDs, mostly consisting of enzyme replacement therapy (ERT) strategies. Yet, poor biodistribution to main targets such as the central nervous system, musculoskeletal tissue, and others, as well as generation of blocking antibodies and adverse effects hinder effective LSD treatment. Drug delivery systems are being studied to surmount these obstacles, including polymeric constructs and nanoparticles that constitute the focus of this article. We provide an overview of the formulations being tested, the diseases they aim to treat, and the results observed from respective in vitro and in vivo studies. We also discuss the advantages and disadvantages of these strategies, the remaining gaps of knowledge regarding their performance, and important items to consider for their clinical translation. Overall, polymeric nanoconstructs hold considerable promise to advance treatment for LSDs.
Keywords: Polymer-based drug delivery systems, nanoparticles, nanoemulsions, lysosomal storage disorders, enzyme replacement therapy, cellular and animal models
Graphical Abstract

1. INTRODUCTION
1.1. Lysosomes and lysosomal storage disorders
Lysosomes are dynamic membrane-bound intracellular compartments, acidic in nature, which correspond to nearly 5% of the total volume of mammalian cells [1]. They are present in animal cells, are heterogeneous in size (0.1 to 1.2 μm) and morphology, yet they are typically smaller than endosomes and clearly distinguishable from them in that lysosomes associate to different Rabs, do not contain mannose-6-phosphate receptors (M6PR), have a more acidic pH, and contain electron-dense deposits and membrane whorls [1,2]. Their main function is that of degrading intracellular materials, both taken up from outside the cell as well as obsolete components of the cell itself (Figure 1). As such, lysosomes represent one of the most common final destinations of endocytic and biosynthetic membrane cargo, affecting many processes like protein turnover in cells, signaling downregulation, plasma membrane repair, and sterol trafficking and homeostasis, among many other functions [3-8]. Therefore, their role is critical in numerous cellular processes linked to homeostasis [1,2].
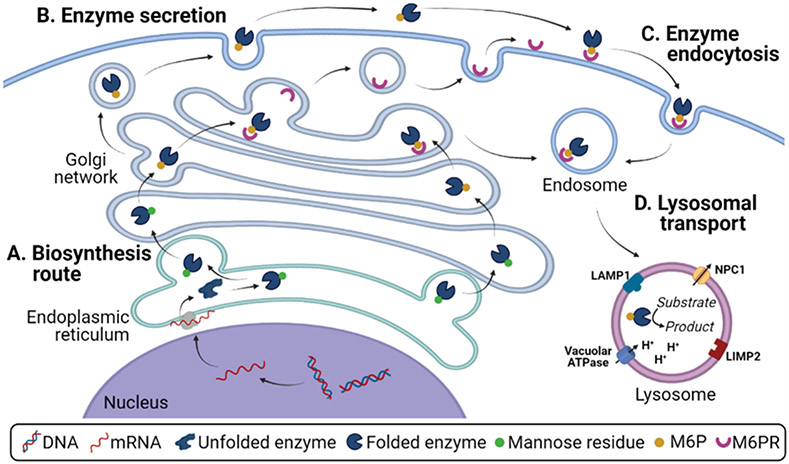
Figure 1. Synthesis, endocytosis, and function of lysosomal components.
(A) Biosynthesis route for lysosomal enzymes, encompassing nuclear transcription, endoplasmic reticulum glycosylation, Golgi apparatus maturation, and transport to endosomes and lysosomes via intracellular mannose-6-phosphate receptors. (B) Secretory route for lysosomal enzymes. (C) Endocytic uptake for extracellular lysosomal enzymes, mediated by cell surface mannose-6-phosphate receptor, for delivery to lysosomes. (D) Lysosomal components, including structural membrane proteins, H+-ATPase pump, membrane enzymes, channels, and transporters, as well as luminal lysosomal enzymes.
Along with mammalian lysosomes, plant and yeast vacuoles are the most acidic compartment in cells and have well-defined lipid and protein compositions that support various functions such as metabolite degradation, storage, and detoxification [9]. Additionally, most of the lysosomal properties are also found in organelles that are cell-type specific and commonly known as “lysosome-related organelles”, such as melanosomes, lytic granules, major histocompatibility complex II compartments, platelet-dense granules, basophil granules, and neutrophil azurophil granules, although they lack the classical degradative role associated with lysosomes [10].
The lysosomal membrane separates luminal acid hydrolases from other cellular constituents (Figure 1). This distinctive membrane is rich in cholesterol and carbohydrates, and presents glycosylated proteins on the luminal leaflet and the unusual phospholipid bis(monoacylglycero)-phosphate (also called lysobisphosphatidicacid), which is exclusive of late endosomes and lysosomes [11,12]. Membrane integral proteins include lysosome associated membrane proteins (LAMPs), lysosomal membrane glycoproteins and lysosomal integral membrane proteins (LIMPs), such as LAMP-1, LAMP-2, tetraspanin CD63 or LAMP-3, LIMP-2, and others [13]. These proteins contribute to lysosomal stability and integrity, and play a role in the transport of soluble metabolites, autophagy, and lysosomal biogenesis [13]. Other transmembrane proteins include proton-pumping vacuolar ATPases, which maintain the lysosomal lumen at around pH 4.5-5.0. This contributes to regulate receptor binding/unbinding interactions with cargo and the activity of lysosomal acidic hydrolases [14].
These lysosomal hydrolases are responsible for the degradation of biomacromolecules such as proteins, nucleic acids, polysaccharides, lipids, as well as those composed of several of these components, such as glycosaminoglycans and glycosphingolipids, among others [1]. In mammalian cells, lysosomal hydrolases are synthesized in the rough endoplasmic reticulum (Figure 1A) and modified with oligosaccharides that contain mannose residues. Subsequent phosphorylation of mannose into mannose-6-phosphate (M6P) in the Golgi complex results in the binding of M6P-modified enzymes to M6PR in the trans-Golgi network [15,16]. From here, transport vesicles bud to deliver these hydrolases to endosomes and then lysosomes, involving fusion and fission cycles, where enzymes dissociate from this receptor as a consequence of the low pH and M6PR can then recycle back to the trans-Golgi network [1,13,15]. In many cell types, a fraction of these enzymes is secreted to the extracellular milieu (Figure 1B) and can then be transported back into nearby cells and lysosomes through clathrin-mediated endocytosis via M6PR at the cell surface (Figure 1C) [16].
Any disorders affecting these processes and lysosomal components alter cellular homeostasis (Figure 1D) and, thus, can impair cell function, leading to disease. In fact, there is a well-recognized group of rare, chronic, and inherited maladies called lysosomal storage disorders (LSDs), which associate to diverse lysosomal dysfunction and affect humans and several animal species [15,17,18]. The term LSD arose in the 60’s, soon after the discovery that a deficiency in α-glucosidase (GAA), a lysosomal enzyme that digests starch into glucose, was the cause of Pompe disease (PD) [19]. Today, this group is known to be comprised of 60+ distinct metabolic diseases with different prevalence, individually considered rare, whose cumulative incidence as a group is about 1 every 2,000-5,000 live births [18,20]. LSDs are mostly caused by dysfunctional lysosomal hydrolyses, but also mutations in genes that encode other components such as integral membrane proteins. This is the case for LAMP-2A alterations in Danon disease and some transporters that export metabolites out of the lysosome, such as Sialin anion transporter in sialic-acid-storage and Salla diseases, cystinosin in Cystinosis disease, and NPC1 or NPC2 in Niemann-Pick type C (NPC) disease associated to cholesterol transport and binding [21-23]. Some other LSDs are caused by defective transport of lysosomal hydrolyses along the ER-Golgi-endosomal pathway during their biosynthesis or subsequent modification and trafficking processes, as is the case for Mucolipidosis types II and III [21]. Yet, as said above, most LSDs derive from direct enzymatic alterations [21]. Regardless the cause, all LSDs are characterized by aberrant accumulation of non-catabolized products due to failure of lysosomal components [21,23]. The specific substrates stored in diseased cells affected by LSDs vary among the different syndromes and are used to classify these pathologies into broad categories [21,23].
LSDs are monogenic disorders and, except from Fabry, Danon, and Mucopolysaccharidosis (MPS) type II diseases, the rest of syndromes in this group are inherited in an autosomal recessive manner [21,23]. Despite being monogenic, there are some specific cases in which the defective enzyme alters the activity of several other enzymes acting downstream, leading to multiple enzyme deficiencies, such as the called multiple sulfatase deficiency [21,23]. LSD severity and age of clinical onset depend on several factors, such as residual enzymatic activity, mutant protein size, location of the mutation with respect to the catalytic site, tissue-specific and cell-specific distribution of substrates, rate of cell turnover and defective protein expression, among other factors [23]. Therefore, oftentimes the same mutation can lead to dissimilar severity in different patients. Furthermore, the clinical variability is evidenced in genotype-phenotype correlations, with environmental factors further impacting the clinical course of these diseases [24].
The onset of clinical manifestation is used to classify LSDs in infantile, juvenile, or adult forms [21]. LSDs affect multiple tissues and organs, leading to a wide range of clinical symptoms and outcomes. Infantile forms of LSDs are the most severe ones and are typically characterized by harsh alterations in the central nervous system (CNS) causing seizures, dementia, and brainstem dysfunction, which lead to death within the first years of life [21]. In adult forms, symptoms develop more slowly and disability often arises from peripheral or visceral impairment, including enlargement of the spleen and liver, as well as heart, lungs and kidney injuries, abnormal bone formation, muscle atrophy, and ocular disease [18,21]. Juvenile forms present with intermediate manifestations to infantile and adult disease forms.
1.2. Lysosomal storage disorder treatments and enzyme replacement therapy
For most of the LSDs no definite treatment is available at present and medical options are limited to disease management rather than therapy. Still, the available treatments for LSDs are generally divided into those that address the symptoms and those that deal with the cause [21,23,25]. Among the many treatments to address symptoms, surgical removal of the spleen (splenectomy) is relatively common in some LSDs, such asGaucher disease (GD), to reduce anemia and thrombocytopenia [25]. Bone-marrow transplantation is another strategy that relies on the production of cells by the transplanted bone-marrow, which secrete normal enzymes to be later internalized by diseased cells [25]. Among the treatments dealing with the cause of the disease, some aim at reducing the biosynthesis of the accumulating substate, called substrate-reduction therapy (SRT), down to levels that are manageable for cells with impaired degradative capacity [25]. Moreover, there are treatments that directly target the defective enzyme, either replacing the defective gene (gene therapy) or the enzymatic protein (enzyme replacement therapy, ERT) with exogenous counterparts [25]. These treatment modalities are implemented either alone or combined with small molecules called chemical chaperones, which stabilize the conformation of endogenous mutant proteins, promoting their folding and enhancing trafficking to the Golgi apparatus and lysosomes, raising residual enzyme activity [25]. Given that LSDs are monogenic disorders and the identity of defective genes is known, gene therapy appears very adequate for their treatment [26-30]. For instance, Libmeldy is an approved therapeutic agent based on ex-vivo gene therapy for the treatment of LSDs, specifically for Metachromatic leukodystrophy [31]. However, gene therapy application is still very limited even though several therapies using viral vectors have already been tested in animal models and many arrived to pre-clinical and clinical phases, such as for MPSIII (Sanfilippo syndrome) and PD, and many similar options are currently under investigation for other LSDs, such as Fabry disease (FD) and other MPS (reference [30] reviews recent studies and clinical developments).
ERT, mentioned above, is to date the most successful available treatment for LSDs, although it is only applied efficiently in non-neurological LSDs [32,33]. This strategy is based on the intravenous (i.v.) infusion to patients of exogenous wild-type recombinant lysosomal enzymes [32]. The rationale behind this strategy comes from the discovery that lysosomal enzymes can be secreted by cells and then they can enter cells by endocytosis and traffic to the lysosome via the M6PR-mediated pathway, as described in the previous section (Figure 1B,C) [34,35]. Importantly, relatively low amounts of the “correcting enzyme” are required to metabolize the accumulated substrates and show a therapeutic effect [36]. ERT is generally well tolerated and capable of reverting substrate accumulated over time, but this therapy is lifelong and its effectiveness is dose dependent. In some cases, higher doses are necessary to ensure that certain organs receive sufficient enzyme for substrate clearance, as for type I GD [37]. Efficiency and selectivity are provided by the interaction of receptors on the cell surface and specific markers on the protein. Because most lysosomal enzymes are glycoproteins, the carbohydrate recognition systems are particularly relevant to their uptake. Although M6PR mediates the uptake of most ERTs, different cell-surface receptors might be needed in some LSDs [29]. In GD, for example, recombinant β-glucosidase is modified to expose core mannose residues that bind to mannose receptors on macrophages cell-surface, which is subsequently delivered to lysosomes to supplement the defective enzyme [38].
Out of these strategies, in addition to symptomatic treatments, the most common in clinical use are SRTs for GD, FD, and Tay-Sach’s diseases, as well as MPS, NPC, and cystinosis, and also the ERTs for Hurler syndrome MPS I), Hunter disease, Maroteaux-Lamy syndrome (MPS VI), Morquio A syndrome (MPS IVA), FD, GD type I, Pompe, lysosomal acid lipase deficiency, and visceral acid sphingomyelinase deficiency (ASMD), among others [39]. For a complete list of clinically approved strategies, please see the article by Dr. Ceccini in this issue [40].
1.3. Current challenges
A key issue pertaining ERTs is the enzyme targeting to defective cells and tissues in need of treatment. ERTs are clinically approved only for LSDs where the main targets are visceral organs and peripheral body sites [32, 40, 41]. However, LSDs often cause CNS pathology, as mentioned above, which is mostly irreversible at the moment of diagnosis [42]. Getting drugs to the brain is an unresolved problem and the blood-brain-barrier (BBB) remains the major obstacle precluding ERTs from being applied to neurological LSDs, despite the fact that recombinant enzymes used for ERT have reasonable pharmacokinetic (PK) profiles [42]. This is the case for neurological ASMD, neuronal ceroid lipofuscinosis (NCL), or GM1 gangliosidosis (GM1G), several MPSs, among many other LSDs [43,44]. Vascular endothelial cells form a continuous lining at the interface between the blood and the CNS, tightly sealed by intercellular protein junctions [45,46]. Additionally, subendothelial cells such as astrocytes, pericytes, microglial cells and neurons, also take part physically and/or biochemically in the establishment and maintenance of this barrier [47]. This type of permeability control applies not only to the BBB but also all interfaces between the vascular endothelium and the CNS [48]. Intracerebral strategies of local administration have succeeded in animal models, for gene therapy and ERT, but their invasive nature and possible neurosurgical complications make these procedures hard to be applied in humans, especially in infants [28,39,49,50]. Other strategies being explored include the use of available transport routes between the circulation and the CNS, mainly vesicular transcytosis [44,51]. Vesicular transcytosis across endothelial cells is a regulatory mechanism that takes place mainly in capillaries and post-capillary venules that irrigate the CNS, including the brain [44,51]. The two major transcytosis pathways are the clathrin- and caveolar-mediated routes, used by most endogenous macromolecules [44,51]. The receptors in charge of trans-BBB transport of transferrin, lipoproteins, insulin, insulin-like growth factors, low density lipoprotein receptor-related protein 1 (LRP-1), and other macromolecules associated to the clathrin-mediated pathway are being investigated to enhance the delivery of lysosomal ERTs to the CNS [39,51-54]. More recently, an alternative transcytosis route was identified by the Muro lab, which is mediated by the cell adhesion molecule (CAM)-mediated pathway [55,56]. All these routes offer additional opportunities to improve CNS treatment, e.g. by using fusion enzymes or enzyme conjugates targeted to respective transcytosis markers, reviewed by Dr. Pardridge in this issue [57], or by loading them within nanodevices able to target them [39]. These routes are further described in Section 2.1 below.
Additionally, several LSDs affect tissues that are challenging to target and treat despite good PK of the used enzymes, such as the case for pathological damage affecting bone and cartilage in patients from MPS IVA and other MPS, as well as GD type I [58-60]. Also in PD, destruction of skeletal, smooth, and cardiac muscles is partly resistant to ERT, as well as tissues characterized by low blood irrigation, such as the case for cartilage, and/or the low number of surface and lysosomal receptors for M6P [15]. In an attempt to overcome these issues, increasing the dose and frequency of ERT applications is the simplest strategy, although not the best solution due to associated side effects and because poor irrigation would still preclude treatment from reaching certain tissues [39]. Therefore, approaches to bypass these caveats may enhance treatment for these patients, for instance by employing implantable depot strategies or by targeting receptors or markers well-expressed in these tissues, which can be achieved using conjugates, fusion proteins, or nanovehicles, as for these and other applications [39,40,57,61-64].
Another complication is the development of hypersensitivity, immunological reactions, and resistance in LSD patients using ERTs, which can alter treatment efficacy and compromise patient safety [65-75]. As ERT involves frequent infusion of exogenous enzymes, oftentimes derived from other species and with sequences that enable their production, this can lead to hypersensitivity reactions such as rash, swelling, abscesses, chills, tachycardia, fever, hypotension, or respiratory symptoms [70]. Some of these symptoms can take place due to anti-enzyme-antibodies present in patients [65,66]. Usually, IgG antibodies are generated, which can be neutralizing or not [71,72]. Neutralizing antibodies bind to the administered enzyme in active sites, either affecting their catalytic site or involved in their interaction with cell receptors [67,68]. Non-neutralizing antibodies bind to non-functional regions, inducing clearance and reducing the available enzyme dose [67,68]. For example, 13% GD patients treated with alglucerase and 50% Hurler’s syndrome patients treated with recombinant α-L-iduronidase (IDUA) developed immune responses against these enzymes early after starting treatment [70]. Similarly, recombinant GAA used for PD and recombinant GLA used for FD have been associated with immune reactions, e.g. anti-enzyme antibodies believed to influence the effectiveness of therapy were detected in 2 out of 3 PD patients, as well as in 88% FD patients in a study involving a 58 patient cohort [65,69]. These reactions tend to diminish with time and the association between treatment efficacy and the presence of anti-enzyme antibodies is not always clear [65-75]. Nevertheless, strategies to avoid these issues could enhance the efficiency and safety of LSD therapies, for instance by masking the enzyme from the immune system prior to reaching lysosomes, by removing enzymes from the circulation into tissue cells, or by using nanomaterials particularly designed to develop tolerance, as shown for other applications [76].
Along with these concerns, the burden to patients, their families and the overall medical system is high regarding current treatments, particularly ERTs, since this requires frequent i.v. infusions, commonly every two weeks, over the course of several hours in specialized medical centers [77]. Finding solutions to lower this burden would greatly benefit all parties. This could be achieved by exploring additional administration strategies, such as oral delivery [78,79], or controlling enzyme release from depot systems located in certain tissues or sustaining intralysosomal enzyme release [61,80], both of which would prolong the therapeutic effect and delay the administration frequency.
Importantly, while the concerns described above may be surmounted by different strategies currently under investigation, there are several additional caveats regarding the progress of LSD treatments. For instance, LSD-associated pathological processes generally initiate before the onset of the symptomatic disease, even at fetal and neonatal stages [81], which indicates that early diagnostics and prompt initiation of therapy are crucial to improve the outcomes. Moreover, the difficulty of carrying clinical trials with sufficient statistics adds further complexity to treatment development. High cost is also a significant obstacle, e.g. ERT and stem-cell therapy, which makes it difficult for health care systems to support patients in many unfavored countries and, even in wealthy ones, this generates challenging ethical dilemmas [82]. Finally, the chronic multimorbidity character of LSDs constitutes an important concern, given that healthcare systems generally tends to focus on single diseases affecting mainly individual organs, which is not the case for LSDs.
In essence, the great majority of available and developing treatments for LSDs may improve the quality of life of patients and prolong their survival, but they would never be total cures. Until gene therapy proves effective in a number of LSDs, ERT should provide a safe and effective means to reverse years of substrate accumulation and to control further deposition. But, as said, the most challenging aspects for ERT include the failure of recombinant enzymes to cross the BBB to treat patients with primary neurological involvement, as well as the complication of achieving enzyme uptake in connective tissue cells, in bone and cartilage tissue [39,58,83]. Importantly, this tissue-accessing problem is not unique to ERT but it also affects both gene therapy and gene editing strategies [84,85]. To avoid problems related to recombinant enzymes not having the targeting residues required, other strategies grounded on glycosylation-independent mechanisms have been explored, including peptide-enzyme fusion proteins and antibody-enzyme conjugates and fusion proteins, reviewed in [39] and [57]. Additionally, some efforts have focused on improving ERTs, lowering side effects associated with cyclodextrin-based cholesterols removal for NPC, and restoring alterations in lysosomal pH characteristic in LSDs using nanomedicine and drug delivery strategies, which are introduced in Section 2 and discussed in details in Section 3.
2. OVERALL DRUG DELIVERY STRATEGIES APPLIED TO LYSOSOMAL STORAGE DISORDERS
2.1. Overview on drug delivery systems
In the past few decades, nanoscience and nanotechnology have influenced the medical and pharmaceutical fields, and offered possible solutions regarding several issues that limit the use of drugs and pharmacological treatments [86-89]. These limiting factors include poor solubility and/or stability of the “naked” drug, rapid degradation or clearance from circulation, systemic toxicity, difficulty in maintaining drug concentrations within therapeutic windows, sustained release, complications to target diseased tissues, organs, or subcellular locations [86-89]. Nanoscale DDS, also referred to as nanocarriers (NCs; Figure 2), are nanosized systems that contribute to enhance the delivery and efficacy of therapeutics, either small molecules or biomacromolecules such as nucleic acids and proteins, by overcoming one or several of the limitations of the naked drug [86-89]. In most cases, NCs have to be modified with different functionalities and many of them are designed to be responsive to certain stimuli, such as an environmental condition inherent of the target destination or an externally applied factor [89]. Currently, there is a strong effort set on engineering precision NCs that, besides improving general drug delivery efficacy, can be tailored to very precise applications, overcome heterogeneous barriers, and be more personalized [90].
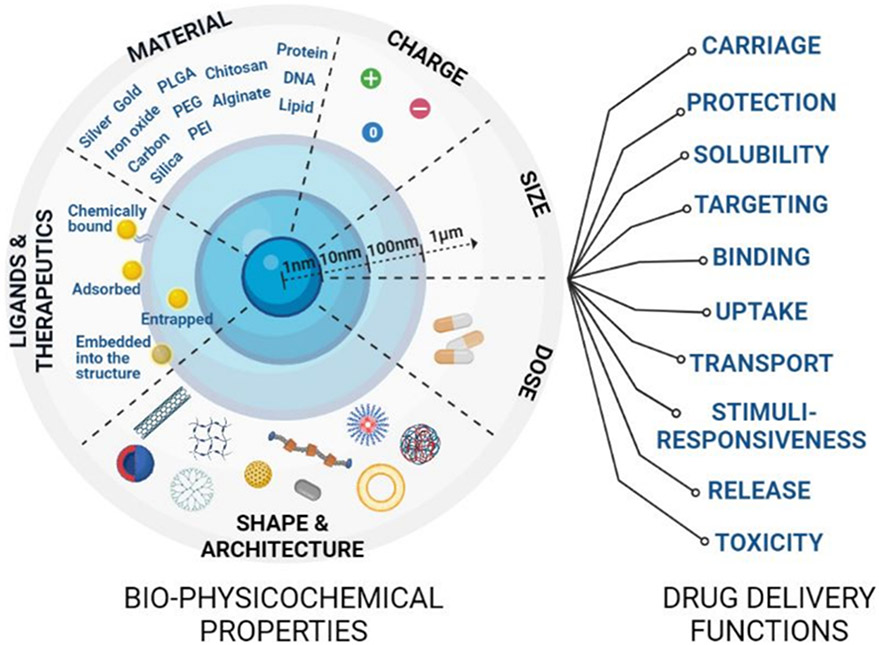
Figure 2. Properties and functions of drug delivery systems.
Drug nanocarriers (NCs) have unique bio-physicochemical properties that can be tuned to modulate drug delivery functions and improve therapeutic outcomes. Tailoring NC composition, dose, drug loading and release mechanisms, size, shape, charge, and affinity molecules can help modulate drug carriage, targeting, protection, solubility, responsiveness to physiological stimuli, and other properties.
NCs of different physicochemical nature have been explored for many applications (Figure 2), for which their design takes into account factors such as the therapeutic molecule, target destination, administration route, treatment duration, among many others parameters [86-89]. One critical issue for DDS is the prevention of their rapid clearance from the circulation when they are administered i.v.. This process is initially mediated by adsorption of opsonins on the NC surface, which facilitates their phagocytosis by cells of the reticuloendothelial system [91]. NC size and surface properties have a direct impact on this factor. For instance, while large NCs (> 200 nm) are mechanically filtered in the spleen and liver [92], smaller NCs (10-100 nm) have a larger surface-to-volume ratio that promotes aggregation, which changes their clearance kinetics [93]. Greater clearance is also observed for hydrophobic or positively charged surfaces [94,95]. Functionalization of the NCs with hydrophilic polymers is a well-known strategy that can help overcome these issues [93,94]. Modification with poly(ethylene glycol) (PEG), also called PEGylation, changes the physical and chemical properties of drug carriers, such as electrostatic binding and hydrophobicity, and results in an improvement in their PK [95]. In general, PEGylation has been proved to decrease immunogenicity and increase stability and blood circulation time of many drugs and NCs [95].
Direct-site targeting can also enhance the efficacy of DDS [88]. Targeting can be achieved in a non-specific manner by common elements, such as negative surface charge of cells or affinity to ubiquitous macromolecules [88]. It can also be pursued by specific means, e.g. following physiological characteristics of a diseased tissue, such as specific physicochemical features or markers overexpressed during pathology [88,96]. Affinity moieties such as peptides, antibodies, proteins, etc. can be coupled to NCs using physical or chemical methods (Figure 2) [88]. In many cases, the targets for therapeutic action are intracellular and this requires targeting a specific pathway to get to the cytosol, to arrive to the nucleus, or to deliver the intended therapeutics within an organelle, such as lysosomes for LSD treatment [21,88,97]. Again, the physicochemical characteristics of NCs, including size, surface charge, responsiveness to external stimuli, etc., along with surface-modification with particular affinity moieties, all play a fundamental role in achieving this goal [88]. Reaching intracellular sites is generally mediated by arriving to the cytosol first and then accessing other organelles [87,89]. The endocytic route is often used for this purpose [87,98], through which objects bound to the cell surface are engulfed into membrane-enveloped vesicles, which can be regulated by different cellular pathways [52,53]. For instance, uptake of particulate ligands via phagocytosis or macropinocytosis is possible for a variety of DDS [52,88,99]. An advantage is that these routes are more permissive regarding the size of drug delivery vehicles being internalized; yet, they more often associate with cells of the immune system, which may prevent access into other cell types in the body [52,88,99]. While this may cause off-target effects for most therapeutic interventions, many LSDs affect immune cells and this property could be an advantage [100]. Alternatively, clathrin- and caveolar-meditated pathways are more restrictive regarding cargo size, but take place more broadly and could be used to target intracellular delivery in virtually any cell type in the body by selecting appropriate receptors associated to them [52,88,99]. Because of the greater knowledge on the regulation and higher number of receptors known for the clathrin-mediated pathway, this has been largely favored for intracellular drug delivery strategies [39,88]. In fact, clathrin-mediated endocytosis often results in endo-lysosomal trafficking, which provides an advantage for lysosomal ERT. Instead, the caveolar route is believed to avoid endo-lysosomal trafficking, for which it may be advantageous regarding delivery of siRNA, plasmids, or gene editing platforms that could also be applied to LSD treatment [52,88,99]. There exist also additional pathways, such as CAM-mediated endocytosis induced by binding to intercellular adhesion molecule 1 (ICAM-1) or platelet-endothelial cell adhesion 1 (PECAM-1) [101]. PECAM-1 is solely expressed on platelets and endothelial cells, for which only LSDs with strong endothelial component could benefit for using this as a target, such as FD [20]. ICAM-1 is expressed on most body cells and is particularly elevated during inflammation, a common hallmark of most LSDs [102]. The CAM-mediated route seems more permissive regarding cargo size compared to clathrin- and caveolar-mediated routes, and has been associated with endo-lysosomal trafficking, an advantage for lysosomal ERT [101,103,104].
Importantly, DDS can also be targeted to pathways associated with transport across cellular barriers, which is essential to achieve sufficient accumulation in certain tissues from the route of administration. To overcome permeability barriers, paracellular and transcellular pathways can be used, yet the former one either involves very minute molecules not often used for LSD treatment or it requires a barrier permeabilization, which may cause further damage [44]. To avoid this, the transcellular route can be used, which involves binding to specific receptors on the apical surface of the cells that form the barrier, followed by endocytosis, trafficking across de the cell body, and exocytosis at the abluminal cell surface, in a process known as transcytosis [44]. This is essential to transport therapeutic cargo, for instance, from the bloodstream into the brain by crossing the BBB, which remains a major hurdle for successful treatment of neuropathic LSDs [44]. As said, this can be induced by biding certain receptors associated with clathrin-, caveolar- or CAM-mediated pathways [54,55, 105,106]. For lysosomal ERT, it is relevant that the receptors targeted at the BBB to induce transcytosis and, thus, penetration into the brain, are also expressed on the surface of cells within this tissue to ensure intra-lysosomal delivery within their interior, such as neurons or glial cells that require therapeutic intervention [44]. Additionally, because most neuropathic LSDs associate with additional effects in peripheral or visceral organs and lysosomes are ubiquitous organelles, broad delivery would be advantageous vs. brain-specific one, an aspect to take into account for the design of DDS aimed to treat these syndromes [44].
NCs can be classified in broad categories according to the material of which they are composed (Figure 2), for instance lipid-based nanoparticles (NPs), inorganic NPs, polymeric NCs, etc. Lipid-based NCs are the most common class of NCs approved by regulatory agencies [107-109]. These can be either vesicles made of synthetic or natural lipids (liposomes) or lipid NPs. They are composed of amphiphilic molecules, which gives them the ability to carry either hydrophobic or hydrophilic molecules [107-109]. Their lipid constituents can also bare different charge, allowing reversible interaction with charged biomolecules, and can be easily functionalized with site-specific targeting moieties and/or hydrophilic polymers that help prolong their circulation time [92,110]. Incorporation of cholesterol can help enhance their stability, and cationic lipids such as 1,2-dioleoyl-3-trime-thylammonium-propane (DOTAP) or lipopeptides derived from cell penetrating peptides can promote their fusion with membranes [107-109,111-113]. Liposomes, the oldest and most studied type of drug delivery system, are vesicles with an aqueous lumen surrounded by a bilayer membrane, whose size ranges from 50 nm to several μm [92,114-116]. They can be made using natural lipids commonly found in cellular membranes, resulting in very low toxicity [114-116]. Additionally, their chemical composition can be varied to tune their physicochemical properties (size, surface charge, mechanical properties, etc.) according to particular applications [114-116]. They have been successfully translated into the clinics, with various applications approved for human use, such as liposomal doxorubicin for cancer (Doxil®, Caelix®, Zolsketil®), liposomal amphotericin for bacterial and fungal infections (Ambisom®), liposomal morphine for pain management (DepoDur™), anti-hepatitis A liposomal vaccine (Epaxal®), and many others, along with additional examples currently in clinical trials [117-119]. As for solid lipid NPs, they have a relatively rigid core composed of hydrophobic lipids that are solid at room and body temperatures, often surrounded by a monolayer of phospholipids or another amphiphile molecule [120,121]. They have been particularly successful regarding the delivery of nucleic acids, such as the case of mRNA in recently approved COVID-19 vaccines developed by Pfizer-BioNTech or Moderna [121]. For a review on these systems and their LSD applications, see the article by Dr. Ventosa in this issue [111]. Apart from liposomes and lipid NPs, biological nanovesicles naturally secreted by cells, called extracellular vesicles (EVs), have also emerged as promising vehicle for the delivery of therapeutics that could overcome issues related to liposomes and other synthetic NCs [122-124]. They are naturally produced by many cell types in the body and are believed to contribute to intercellular communication, which takes place by transporting and delivering diverse biomolecules, including proteins, lipids, RNA, and DNA from producing cells to receiving cells [122-125]. There are currently about 40 clinical trials ongoing for EV-based strategies for treatment and drug delivery, including examples aimed to modify mesenchymal stem cells, treat colon cancer, or ameliorate irritable bowel disease [125], among other applications. A review on this topic and lysosomal applications is provided in the article by Dr. Lu in this issue [126].
Regarding inorganic NCs being explored for imaging/diagnostic or therapeutic applications are normally based on gold, iron oxide, or silica [87,127]. They can be engineered in a variety of sizes, shapes and structures, including nanorods, nanospheres, nanostars, or nanocages [87,127]. They have particular physical, magnetic, electrical and/or optical properties that enable their use for imaging [87,127], e.g. some iron oxide NPs have been clinically approved as contrast agents, thermal-based therapeutics, and iron deficiency-based anemia, including Definity, Optison, Ferrlecit, DexFerrum, etc. [128-130]. Several silica NP formulations are also being explored in clinical trials for photothermal ablation, plasmonic resonance, or oral delivery [131-133]. In addition, quantum dots (QDs) are colloidal semiconductor nanocrystals with unique optical, electronic and photophysical properties, that are primarily used for in vitro imaging and tracing purposes, which can help in fundamental research applications [134].
Polymeric NCs are a group of DDS based on synthetic or natural polymers [135]. These include solid NPs, covalently or ionically crosslinked hydrogel NCs, tree-like branched dendrimers, polymersome nanocapsules, and other constructs such as polyelectrolyte-based layer-by-layer capsules, and micelles (Figure 2). In general, these NCs have greater stability in storage and physiological conditions compared to lipid-based ones, being more amenable for controlled release and, thus, providing more prolonged therapeutic activity [90,135]. All these polymeric NCs can be modified with targeting moieties, fluorescent dyes, radiolabels, and other functional groups, which makes them good candidates for drug delivery applications [135,136]. However, one of their main drawbacks is the risk of aggregation or complement activation which must be carefully controlled [130,137]. Polymersomes and micelles are made of amphiphilic block copolymers, for which both can be loaded with hydrophobic and/or hydrophilic drugs [54,138-142]. Polymersomes have vesicular shape, with an aqueous lumen and a polymeric membrane, thus, they are the polymeric homologs for liposomes but have improved stability and retention efficiency compared to them [54,138-140]. Micelles have a hydrophilic shell and a hydrophobic core and are mostly used with small molecules or biomacromolecules that are poorly soluble in aqueous media [141]. They are less stable than polymersomes, although chemical crosslinking can be used to stabilize them [142]. Dendrimers are hyperbranched polymeric constructs with a very defined structure and homogeneous molecular weight [143,144]. They are mainly used to conjugate drugs to their exposed chemical reactive groups, whose location and number are well controlled [143,144]. The most used dendrimers are charged and based on poly(ethyleneimine) and poly(amidoamine), typically used for delivery of small molecules or nucleic acids [143,144]. Charged linear polymers such as poly(ethyleneimine) and natural polymers and derivatives, mostly based on polysaccharides, are additional examples used to encapsulate biomacromolecules of opposite charge (nucleic acids, proteins), forming polyelectrolyte complexes (PECs), which constitute a type of reversible hydrogel [145]. They are biocompatible and inherently responsive, as their charge is generally modulated by pH changes and, therefore, they are good candidates for intracellular delivery [145]. Regarding solid polymeric NCs, also called NPs, these are made of biocompatible and biodegradable polymers and can be fabricated in a variety of sizes (10-1000 nm) [87,90,92,93,130,146-152]. Therapeutic molecules can be dispersed within the polymeric matrix or adsorbed on their surface [87,93]. Common polymers used to fabricate solid NPs are polyanhydrides, polycaprolactone, polylactides, polyglycolides, poly(methyl methacrylate), poly(acrylic acid), etc., as well as derivative copolymers such as poly(lactic-co-glycolic acid) (PLGA), and NPs can be dispersed and stabilized by the presence of other polymers such as poly(vinyl alcohol) or PEG [148-150]. These NCs can be designed to sustain drug release via diffusion, surface erosion, or slow degradation [92,151]. An example of a polymeric NP that has reached the clinics is Eligard for prostate cancer [90], and numerous clinical trials are currently ongoing, including Genexol-PM for head&neck or breast cancer, CriPec for ovarian cancer, AZD2811 for advanced solid tumors, among many others [90,130,146,152]. Protein-based NPs, mostly based on albumin, also belong to this category, such as clinically approved Abraxane for cancer [147]. Yet, by far, polymer-drug conjugates based on PEGylation, have been the most successful example of polymer-based drugs in the market as well as under clinical trials, such as the case for approved PegIntron for hepatitis C, Oncaspar acute lymphoblastic leukemia, or Neulasta for neutropenia [130]. Particular studies on polymer-based therapeutics for LSD treatment are described below.
2.2. General use for lysosomal storage disorders
Therefore, many attempts are being made to develop drug delivery strategies to overcome the problems associated with the use of “naked” recombinant enzymes in the treatment of LSDs, although this is not the only application for which polymer-based NCs are being developed, as described in Section 3 [39,44,88,153-156]. Theoretically, NCs could provide protection to enzyme cargo by posing a steric hindrance to proteolytic attack. Although this has not been shown yet for lysosomal applications, it appears the case for other enzymes and, thus, should be considered for LSDs. For instance, while 3 min incubation with pronase decreased by 4-fold the activity of naked acetylcholinesterase, intact activity was observed when the enzyme was encapsulated in liposomes [157]. Naked phenylalanine ammonia lyase totally lost its activity after 30 min proteolysis in trypsin, while it retained >80% activity upon encapsulation in a biosilica shell [158]. Catalase lost its activity (< 1% the original value) after 1 h incubation in pronase, yet it retained >80% activity at this time when encapsulated in PLGA NPs and 25% activity was still measured after 24 h incubation in this proteolytic condition [159].
In addition, NCs can be designed to reduce phagocytosis by macrophages and antigen presenting cells, which could theoretically help reduce the formation of anti-enzyme antibodies. For example, PEG has long been investigated to reduce phagocytosis by these cell types and prolong the half-life of NCs and conjugates in circulation, as extensively reviewed in [160] and [161]. Illustrating this, PEGylation of PLA NPs reduced their phagocytosis by THP-1 monocytes in culture and increased by 180-fold NP PK after i.v. injection in rats [162]. Clinically-approved Doxil® [163] and Caelyx have 55 h and 80 h half-life in humans, respectively, due to their PEGylation [164,165]. Poly(2-oxazoline), abbreviated POx, is another hydrophilic and biocompatible polymer more recently explored in this context [166-168]. As an example of this function, tobacco mosaic virus nanotubes were used as drug delivery vehicles; their coating with POx derivatives reduced by 6-8-fold their recognition by antibodies against this virus and reduced 3-4-fold their uptake by RAW 264.7 macrophages in culture [169]. Since PEG can reduce specific binding of ligand-targeted NCs to cell-surface receptors, strategies to combine both properties have been investigated, such as for Janus particles used to segregate targeting ligands from PEG on the NC surface, cleavable linkers that can release PEG in certain microenvironments, etc. [170-172]. Other strategy consists of coupling CD47 on the NC surface, since this molecule interacts with a macrophage surface protein called signal regulatory protein alpha (SIRPα) and initiates a signal cascade aborting phagocytosis, as shown in several studies [173,174]. For instance, NPs displaying anti-ICAM-1 and CD47 on their surface targeted ICAM-1 expressing endothelial cells in culture (>120 NPs/cell vs. <10 NPs/cell for non-targeted control) and were internalized (>70% in 1 h), while CD47 reduced by 67% NP uptake by macrophages (3 h) [174]. This strategy reduced liver uptake of i.v. injected NPs by 30-50% and increased targeting to the lungs by 2-fold [174]. Therefore, similar strategies could help in the case of lysosomal ERTs, yet this remains to be investigated.
With regards to modulating immune system response, NCs can be designed to improve tolerance against antigens [76]. For instance, three injections in mice with PEGylated PLA-PLGA NPs loaded with rapamycin lowered by 3000-fold anti-antigen titres [175]. Repeated antigen challenged post-treatment demonstrated this was not due to a delayed immune response but tolerability, and this outcome was improved compared to treatment with methotrexate [175], which is used to reduce immune response in lysosomal ERTs [176]. NPs were observed to induce tolerogenic dendritic cells and regulatory T cells, and to lower the activation of antigen-specific B and T cells, as demonstrated in vivo in mice, rats, and cynomolgus monkeys [175]. Arguably, this type of strategy could help reduce immune response against lysosomal ERTs or even NP ligands used for targeting, but investigation in this direction is largely unavailable currently and should be a focal point in the next years.
Furthermore, NCs can be functionalized for active-targeting or enhanced transport across cellular barriers, and also targeting to cell receptors in various forms of endocytosis [88]. Importantly, some NC formulations can help reach the CNS, overpassing the BBB, of which we describe specific examples in Section 3 below. They can provide glycosylation-independent targeting mechanisms when there is a glycosylation defect on the enzymes and offer a means to control the release of the enzyme in a sustained manner. The use of enzyme-loaded NCs for LSDs is not yet in the clinics and, in fact, the translation of these systems will require significant effort, as discussed in the concluding remarks section. However, current results have demonstrated good potential for these strategies, providing in vitro and in vivo proof-of-principle for enhanced delivery and effects, deserving further attention and optimization. It is important to keep in mind that any progress in the development of enhanced treatment is also limited by the accessibility of disease models for their assessment, which are reviewed in the article by Dr. Ledesma [177] in this issue.
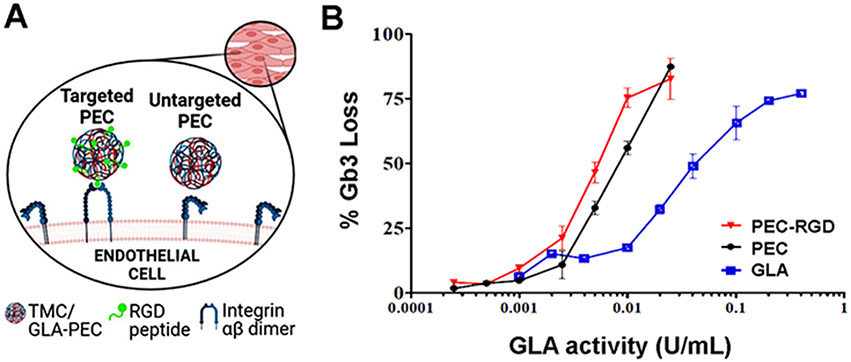
With regards to NCs used for LSDs, those polymer-based ones will be described in details in Section 3 below, while here we will briefly mention efforts using non-polymeric designs. Liposomes were the first to be investigated in this context, back in the 1970-80s. Liposomes were evaluated as potential carriers for several enzymes, including β-fructofuranosidase, α-mannosidase, β-glucuronidase, β-galactosidase, and β-glucosidase [178-183]. The in vivo performance of these liposomes depended strongly on their physicochemical properties, including surface charge or coating. More recently, liposome-based formulations have been explored for lysosomal ERTs, including GD type I, where liposomes modified with lysomotrophic agents and loaded with velaglucerase alfa (VPRIM™) improved the uptake by monocytes and fibroblasts in culture, accumulated in lysosomes, and were active [184-186]. Also, human glucocerebrosidase formulated with nanovesicles composed of Saposin C and DOPS were compared to the naked enzyme [187]. These nanovesicles showed higher stability in serum, were taken up into cells mostly by a mannose receptor-independent pathway, and resulted in higher activity in deficient cells [187]. This formulation also seemed to penetrating through the BBB into the CNS [187]. For FD, liposomes targeted with the RGD peptide could be loaded and could deliver active GLA, reducing the globotriaosylceramide (Gb3) deposits in endothelial cell cultures much more efficiently than naked enzyme [188-190]. These studies demonstrated how the chemical composition of nanovesicles impact the efficiency and activity of treatment in vitro [188-190]. As potential candidates for improved ERT in type B ASMD, liposomes loaded with ASM were prepared and evaluated in vitro [191]. They demonstrated higher efficacy and, importantly, reduced extracellular degradation of sphingomyelin compared to the naked enzyme [191], which had led to a systemic side effect in a clinical trial using the naked enzyme [192]. Improved selectivity to lysosomes was shown when using a guanidinylated neomycin (GNeo) transporter was incorporated in liposomes [193].
Furthermore, EVs are also being explored for LSDs. Though still largely uncharacterized, some studies seem to indicate that some peripheral EVs can cross the BBB and could provide a new approach to treat neurological complications [194]. For instance, macrophage-derived EVs have been investigated for brain delivery of the soluble lysosomal enzyme tripeptidyl peptidase-1, to treat neuronal ceroid lipo-fuscinosis 2 or Batten disease [195]. EVs significantly increased the enzyme stability against protease degradation in vitro, as the enzymatic activity was preserved for at least 25 h in the presence of pronase compared to a 3-fold reduction for the naked enzyme. EVs further provided efficient delivery to target cells in an in vitro model of CLN2 [195]. By fusing human GBA to an exosome-anchoring protein, exosomes were engineered to be directly loaded with this enzyme [196]. In vitro, these exosomes targeted to endocytic compartments exhibited a 40% increase in GBA activity in cell culture compared to control exosomes. The use of GLA-loaded EVs is likewise considered as an alternative to improve ERT in FD [197,198]. Initial studies showed that GLA activity was 10-fold higher when encapsulated in EVs compared to the naked enzyme and this resulted in 4-5 fold improved activity over naked GLA in FD cells. Additionally, i.v. administration in knockout (KO) mice increased brain accumulation of GLA-EVs about 8% resulting in 40% reduction in brain Gb3 while naked GLA did not change Gb3 levels. Other non-liposomal lipid-based formulations have also been considered. Laronidase-functionalized multiple-wall lipid-core nanocapsules showed comparable uptake and enzyme activity in MPSI patient fibroblasts to naked laronidase, although internalization was not mediated by the M6P pathway. However, in vivo studies in KO mice showed higher enzyme activity in serum and peripheral organs for the encapsulated formulation over the naked enzyme, 4 h after i.v. injection, without reaching the brain [199]. Nanostructured lipid carriers were used to encapsulate N-acetylgalactosamine-6-sulfatase for the ERT of MPSIV A, also called Morquio A syndrome, in an attempt to reach the cartilage, which is a limitation of the naked enzyme [200]. Finally, preliminary studies suggested that monoolein-based NPs stabilized by polysorbate 80 could be applied to facilitate CNS delivery of enzymes in LSDs, among other applications, as fluorescently labelled NCs were found in 2-3-fold greater levels in the brain compared to the liver at 3 h and 6 h after intraperitoneal (i.p.) administration in mice [201].
Inorganic NPs have been less explored in the LSD context, but some examples are available. For instance, β-D-glucuronidase and IDUA have been immobilized onto QDs for lysosomal transport, showing sufficient delivery as to restore normal substrate levels in cell models [202]. The use of gold nanorods has been investigated as enhancers of the catalytic activity and stability of lysozyme, used as a model enzyme, which was demonstrated to occur upon adsorption onto these nanostructures [203]. Also, immobilization of lysosomal enzymes on magnetic NPs has been reported [204], and gold NPs decorated with cell-penetrating peptides and lysosomal sorting proteins have tested in cell cultures [205]. However, their poor or inexistant biodegradability limits the use of most inorganic NPs for therapeutic delivery purposes.
3. POLYMER-BASED NANOCARRIERS FOR LYSOSOMAL STORAGE DISORDERS THERAPY
Apart from the non-polymer-based examples described in Section 2.2 above, various polymer-based NCs have been investigated for LSD treatments, which constitute the core of this article and are thoroughly reviewed in this section and summarized in Table 1. By reviewing the literature on this topic, this review aims to: (a) help calling the attention of drug delivery scientists and industry over these diseases, so that a greater effort can be made to advance these pilot ideas, (b) update biologists and doctors about the efforts being made to come up with alternative solutions for LSD treatment, to gain their feedback and guide these efforts, and (c) emphasize concerns and aspects that still remain under-investigated to advance beyond proof-of-principle concepts.
Table 1.
Summary of drug delivery approaches involving polymer-based nanocarriers for LSD treatment.
| LSD | Formulation | Cargo | Targeting & Mechanism | Cell models | Cell tests | Animal model & Administration routes |
In vivo tests | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Lipidoses | Acid Sphingomyelinase deficiency (ASMD), also called Types A and B Niemann-Pick disease | Anti-ICAM-1 or γ3 peptide targeted model PS NPs and PLGA NPs | ASM | Targeting to ICAM-1 & CAM-mediated endocytosis and transcytosis. | Fibroblasts from ASMD patients and cells treated with imipramine, including endothelial HUVECs, HBMECs, astrocytes, pericytes, macrophages, neural SH-SY5Y cells. | Cell binding, uptake and mechanism of endocytosis, intracellular trafficking to lysosomes and transcytosis across BBB models. Enzyme activity delivered, reduction of sphingomyelin substrate, restoration of altered endocytosis, and cytotoxicity. | Wild-type and ASMKO mice; i.v. and i.a. administration. | Circulation and biodistribution of enzyme and NPs. Fluorescence and electron microscopy visualization in mouse organs, intravital and post-mortem. Enzyme activity, substrate levels, lung inflammation in bronchoalveolar lavage fluid, rotarod test. | [55,56,80,104,212,233-240,244,253,254] |
| Fabry disease (FD) | Anti-ICAM-1 targeted model PS NPs | GLA | Targeting to ICAM-1 & cellular transport by CAM-mediated pathway. | Endothelial HUVECs and HBMECs treated with DGJ & Caco-2 cells. | Targeting, internalization, intracellular trafficking to lysosomes, GLA-mediated degradation of Gb3 substrate, and transcytosis. | Wild-type mice; i.v. administration. | Circulation, biodistribution, microscopy and intravital visualization of NPs. | [263,281] | |
| Trimethyl chitosan PECs | GLA | Untargeted. Cell uptake by adsorptive endocytosis. | Endothelial HMEC-1 and MAEC-KO cells. | Cell uptake and lysosomal colocalization. Cell toxicity and in vitro hemocompatibility. NCs enzyme activity (Gb3 degradation). | N/A | N/A | [272,279] | ||
| RGD tagged trimethyl chitosan PECs | GLA | Targeting to αvβ3 integrin. Cell entrance by adsorptive endocytosis. | Same as above. | Same as above. | N/A | N/A | [272,279] | ||
| 30Kc19-albumin nanocapsules | GLA or β-GAL model | Cell uptake suspected by albumin-related caveolar endocytosis and apparent 30Kc19-mediated receptor-independent pathways. | HEK293, cancer HeLa cells, and foreskin fibroblasts from FD patients. | Cell viability, uptake and subcellular localization. Enzyme intracellular delivery and activity. Intracellular Gb3 degradation. | Wild-type mice injected i.p. | Long-term toxicity. Visualization of NCs delivery into tissues. | [260,293,294] | ||
| PEGylated-enzyme (PRX-102®) | GLA | Untargeted | Skin fibroblasts from FD patients. | Cellular uptake, internalization and lysosomal colocalization. | GALKO mice via i.v. injection. | Pharmacokinetics and biodistribution. Quantification of Gb3 levels in tissues after repeated enzyme dosing. Currently being tested in Phase I/II cand a short Phase III clinical trials. | [280] | ||
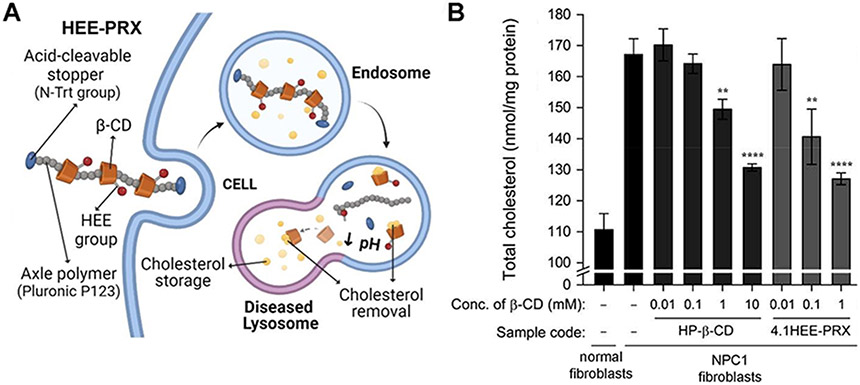
| Niemann-Pick disease type C (NPC) | HE-SS-PRX | β-CD | Untargeted. Internalization by multiple endocytic pathways. | Fibroblasts from NPC1 patients | Cellular binding, uptake and mechanism of internalization. Lysosomal colocalization. Intracellular reduction of cholesterol and cholesterol efflux from the plasmalemma. | Npc1−/− and Wild-type mice via i.p injection or s.c. administration | In vivo fluorescence imaging. Pharmacokinetics and biodistribution. Biochemical and histological analyses of treated mice. Quantification of tissue sterols. | [307,335-340] | |
| HEE-PRXs | β-CD | Same as above. | Fibroblasts from NPC1 and NPC2 patients. | Same as above. | Same as above. | Same as above. | [307,335-340] | ||
| HSPC-DSPE PEG micelles | Unloaded or loaded with HPβCD | Untargeted. Cell entrance by apparent macropinocytosis | Immortalized Npc1−/− and Npc1+/+ mouse embryonic fibroblasts. | Cell internalization, intracellular trafficking, cholesterol efflux and autophagosome maturation in treated cells. | N/A | N/A | [306] | ||
| Gaucher disease (GD) | CTP-, HAp-, and calcium-alginate microspheres | GBA | Untargeted. GBA* endocytosis via mannose-receptors. | MG63 human osteoblasts treated with CBE and type I GD fibroblasts. | Cell adhesion to microspheres. GBA release, cellular uptake, and intracellular enzyme activity. | N/A | N/A | [61,356,371-373] | |
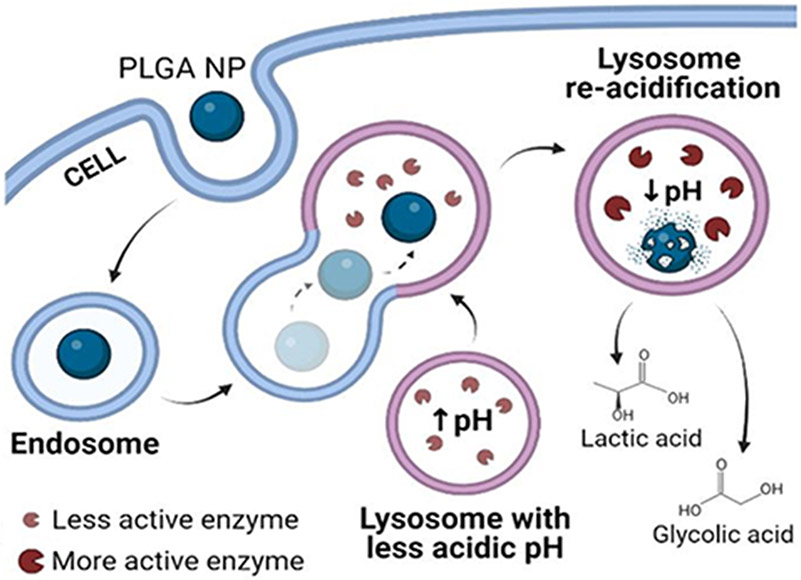
| PLGA NPs | Unloaded. | Untargeted. Endocytosis by apparent non-specific events. | Neuroblastoma BE-M17 cells, GD-derived fibroblasts and GBA-mutant fibroblasts. | Cell viability. Lysosomal trafficking of NPs, pH restoration and integrity by monitoring lysosomal enzymes. | Wild-type mice and mice treated with MPTP. Local administration via stereotaxic injection. | Stereological counting of dopaminergic neurons by immunohistochemistry. | [380] | ||
| Polysorbate-lipid coated PLGA nanoemulsion | Unloaded. | Untargeted. Apparent transcytosis and endocytosis by unspecified mechanisms. | Neuroblastoma BE-M17 cells | Cell viability, lysosomal pH and lysosomal colocalization assays. | Wild-type mice by local stereotaxic injection and systemic administration via retro-orbital injection. | Evaluation of BBB crossing after systemic administration, brain penetration and intracellular distribution. | [381] | ||
| GM1-gangliosidosis (GM1G) | Arginase-responsive DS/PA nanocapsules. | β-GAL | Untargeted. Mechanism of internalization unspecified. | β-GAL deficient SV and R201 mouse fibroblasts. | Cytotoxicity, internalization, intracellular enzyme release and GM1 ganglioside reduction. | N/A | N/A | [389] | |
| Light responsive Gold PSS/PHA nanocapsules. | β-GAL | Untargeted. Mechanism of internalization unspecified. | HeLa cells and L929 WT fibroblasts. | Cell cytotoxicity and internalization assays. | N/A | N/A | [413] | ||
| Neuronal Ceroid Lipofuscinosis-1 (NCL1) | PLGA nanocapsules. | PPT1 CLEAs** | Untargeted. Mechanism of internalization unspecified. | NIH-3T3 mouse embryonic fibroblast and skin fibroblasts from NCL1 patients. | Cytotoxicity and release profile. Enzymatic activity recovery in treated cells. | N/A | N/A | [423] | |
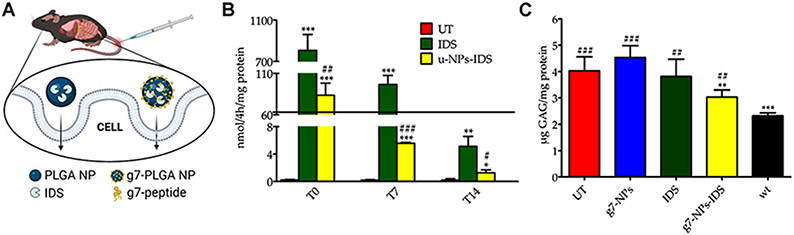
| Krabbe disease (KD) | Ang2-, Tf2-, and g7 targeted PLGA NPs | GALC CLEAs | Targeting to LRP1 (Ang2) or TfR (Tf2). Transport by clathrin-dependent endocytosis and transcytosis. | Fibroblasts from TWI mouse and KD patients. | NPs intracellular delivery and lysosomal colocalization. GALC enzymatic activity in cells. | TWI mouse i.p. injected. | Distribution of NPs and GALC activity assays in mouse tissues. | [426] | |
| Mucopolysaccharidosis (MPS) | MPS1 | g7-targeted PLGA NPs | Unloaded or loaded with albumin model. | Targeting, internalization by clathrin-mediated endocytosis and transcytosis by apparent clathrin vesicles and macropinocytosis. | N/A | N/A | Wild-type or IDUA-KO mice by i.v., i.n., or oral administration. | NP brain levels and quantification of fluorescent-labelled NPs in brain and liver tissue. | [438,498] |
| MPS2 | g7-targeted PLGA NPs | Unloaded, or loaded with albumin model, or IDS. | Targeting, internalization by clathrin-mediated endocytosis and transcytosis by apparent clathrin vesicles and macropinocytosis. | Neurons and glial cells. Fibroblasts from MPSII patients. | Cellular uptake and intracellular enzymatic activity. | Wild-type mice or IDS-KO mice by i.v. administration. | NP brain levels. GAGs levels in tissues and urine. Neuroinflammation by CD68+ microglial cells and GFAP+ astrocytes. | [438,439] | |
| MPS6 | PBCA NPs | ASB | N/A | N/A | N/A | N/A | N/A | [497,547] | |
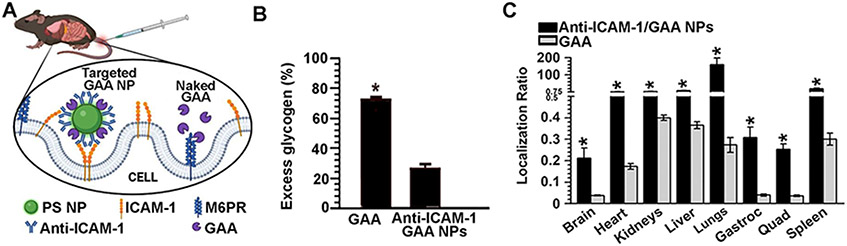
| MPS9 | Anti-ICAM-1 PLGA NPs | HAse | Targeting to ICAM-1 & CAM-mediated endocytosis and transcytosis. | Endothelial HUVECs | Cell binding specificity, uptake, and intracellular trafficking to lysosomes. Intracellular enzymatic activity measured as substrate reduction. | N/A | N/A | [80] | |
| Glycogen storage disease | Pompe disease (PD) | Anti-ICAM-1 Model PS NPs | GAA | ICAM-1 targeting. Cellular transport by CAM-mediated pathway. | Endothelial HUVECs treated with D(+)-turanose and skeletal muscle cells from wild-type mice. | Binding, internalization and lysosomal colocalization of the NPs. Glycogen degradation in treated cells. | Wild-type mice i.v. injected | NCs circulation and organ biodistribution. GAA delivery enhancement compared to non-targeted GAA. | [507] |
| PLGA nanocapsules | GAA | Untargeted. Unspecified uptake mechanism (speculated to be M6P independent). | Fibroblasts from PD patients. | Cellular uptake and lysosomal delivery of internalized enzyme. Intracellular enzyme activity evaluation. | N/A | N/A | [505] |
Ang2: Angiopep-2. ASB: arylsulfatase B. ASM: acid sphingomyelinase. ASMD: acid sphingomyelinase deficiency. BBB: Blood-brain barrier. β-CD: β-cyclodextrin. β-GAL: β-galactosidase.CAM: Cell adhesion molecule. CBE: Conduritol B-epoxide. CLEAs: Cross-linked enzyme aggregates. CTP: Calcium titanium phosphate. DGJ: Deoxygalactonojirimycin hydrochloride. DS/PA: Dextran sulfate/poly-L-arginine. DSPE: Distearyl-hosphatidylethanolamine. GAA: α-glucosidase. GAGs: Glycosaminoglycans. GALC: Galactosylceramidase. Gb3: Globotriaosylceramide. GBA: acid β-glucosidase. GLA: α-galactosidase. HAp: Hydroxyapatite. HAse: hyaluronidase. HBMEC: human brain microvascular endothelial cell. HEE: Acid-labile 2-(2-hydroxyethoxy)ethyl group. HEE-SS: Hydroxyethylated pluronic group. HPβCD: Hydroxypropyl-β-cyclodextrin. HSPC: hydrogenated soy phosphatidylcholine. HUVEC: human umbilical vein endothelial cell. i.a.: Intra-arterial. i.n.: Intranasal. i.p.: Intraperitoneal. i.v.: Intravenous. ICAM-1: Intercellular adhesion molecule-1. IDS: Iduronate-2-sulfatase. IDUA: α-L-iduronidase. KO: Knockout. LRP1: Low-density lipoprotein receptor related protein-1. M6P: Mannose-6-phosphate. MAEC: Mouse aortic endothelial cells. MPTP: 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. N/A: not applicable. NCs: Nanocarriers. NPs: Nanoparticles. PBCA: Poly(butyl cyanoacrylate). PECs: Poly electrolyte complexes. PEG: Poly(ethylene glycol). PLGA: Poly(lactic-co-glycolic acid). PPT1: Palmitoyl-protein thioesterase-1. PS: polystyrene. PSS/PHA: Poly(sodium 4-styrene-sulfonate/Poly(allylamine hydrochloride. R201C: Deficient human β-galactosidase gene-introduced mouse fibroblast (mouse-derived knockout SV fibroblasts). RGD: Arginine-glycine-aspartic acid. s.c.: Subcutaneous. SV: β-galactosidase gene-deficient mouse fibroblasts. Tf2: Transferrin-binding peptide-2. TfR: Transferrin receptor. TWI: Twitcher mouse.
Microspheres always remain outside the cells and just the enzyme is analyzed.
Similar NCs were also synthetized using GBA, GALC and GAA as cargo, although no cellular or in vivo tests were performed.
3.1. Lipidoses
3.1.1. Acid sphingomyelinase deficiency
3.1.1.1. Disease, models, and current treatments for acid sphingomyelinase deficiency
The first LSD where polymeric NCs were applied for intracellular delivery [104] was acid sphingomyelinase deficiency (ASMD), historically called types A and B Niemann-Pick disease. ASMD is an autosomal recessive lipid storage disorder caused by mutations in the sphingomyelin phosphodiesterase 1 gene (SMPD1) located on chromosome 11p15.4 [206-209]. Over 180 mutations have been identified affecting this gene, which impair the expression and/or functionality of ASM enzyme (EC 3.1.4.12), leading to aberrant sphingomyelin accumulation within cells and organs throughout the body [206-208]. As a whole, ASMD affects 1 in 250,000 live births, but its prevalence is higher among the Ashkenazi Jewish population, affecting around 1 in 40,000 people [208]. Type A disease (OMIM #257200) is a severe, neurovisceral, infantile ASMD form that effects < 1 patient per 1,000,000 live births (see ORPHA:77292 at the portal for rare diseases and orphan drugs Orpha.net). Patients exhibit hepatosplenomegaly and a rapid CNS involvement, with death occurring between 1.5–3 years of age [207]. Type B disease (OMIM #607616) is a chronic, non-neurologic ASMD form with visceral involvement, which affects 1-9 patients per 1,000,000 individuals (ORPHA:77293). This is characterized by hepatosplenomegaly and lung alterations, where most of the affected children survive until late adulthood [209]. Additionally, patients may present intermediate phenotypes with both peripheral involvement and progressive neurological condition, known as type A/B ASMD [210].
Cellular and animal models are used to study ASMD biology and potential therapeutics. Primary fibroblasts isolated from type A and B patients are widely used cellular models, as they carry patients' mutations [211,212]. However, as primary cultures are not always easy to find for rare disease, ASMD can be pharmacologically mimicked by treating cells with imipramine, a drug that causes proteolytic degradation of cell’s endogenous ASM [104,213,214]. Regarding animal models, ASM enzyme deficiency and respective phenotypic manifestations, such as neurological disease and hepatomegaly, have been observed to occur naturally in several animal species, including dogs, cats, cattle, and raccoons [215-218]. However, none of them have been established as ASMD models, despite the fact that such large animal models are greatly useful because their large size enables: (a) the use of equipment and techniques similar to those employed for human patients; (b) repeated sampling of fluids and tissue for analysis of pathogenesis; (c) greater precision regarding biodistribution, PK/PD measurements; and (d) long-term safety and efficiency studies due to their longer lifespan [216]. Indeed, many canine and feline models have been fundamental for the development of therapeutic strategies for MPSs, NCL, NPC, and many other LSDs [178-182,187]. Instead, only murine models have been established for ASMD [219-232]. The first one was the ASM knockout (ASMKO) mouse, lacking ASM expression [223,224]; another model was created by a transgenic strategy that enabled the establishment of a mouse capable of expressing low levels of lysosomal ASM, but in complete absence of secretory ASM [225]. Regarding the former model, homozygous animals exhibit no ASM activity with progressive storage of sphingomyelin and secondary lipids in reticuloendothelial organs as well as the brain [223,224]. This leads to neurovisceral alterations with a profound inflammatory response, especially in lungs and brain, and defects in ceramide-mediated signal transduction [223,224]. Regarding the latter model, mice have 8-15% residual ASM activity in reticuloendothelial organs without neurological alterations, for which this is a good model for type B patients [225]. More recently, types A and B mouse models were engineered by knocking-in R496L and deltaR608 mutations, respectively [226].
Historically, therapy for ASMD patients focused on symptom amelioration and included procedures such as bone narrow transplantation, liver or spleen size reduction or even splenectomy [207,209,227]. Then, the synthesis of recombinant human ASM produced in Chinese hamster ovary cells (CHO) and its successful application for the treatment of visceral alterations in ASMKO mice encouraged ERT approaches [228]. Thus, a derivative recombinant enzyme called Olipudase alfa was developed and tested in Phase I/II clinical trials to evaluate its safety, tolerability, and efficacy in patients between 1.5 to 17.5 years-old, who showed significant improvements in ASMD visceral pathology (see study results at NCT02292654, ClinicalTrials.gov). Long-term safety and efficacy Phase II/III trials were also conducted in adult patients (NCT02004691 and NCT02004704), leading to recent approval of this drug in Japan (March 28, 2022) for the treatment of non-CNS pathologies in patients with ASMD types A/B and B [229]. From this first approval, Olipudase alfa (commercialized as XENPOZYME®) was also accepted in the European Union (June 24, 2022) [230] and in the United States (August 31, 2022) [231]. Although this drug is effective to treat non-neurological ASMD, it is ineffective for CNC treatment and, also, patients require biweekly infusions of this drug and can present systemic and hypersensitivity reactions, including anaphylaxis [192,207,232]. Therefore, it is important to find new therapeutic approaches for ASMD.
3.1.1.2. Polymeric nanocarriers for acid sphingomyelinase deficiency treatment
As mentioned above, ASMD was the first LSD for which the use of targeted polymeric NCs was investigated, which was examined by the Muro lab, as a tool to improve ERT [104]. Since then, our group has reported numerous studies on the use of these vehicles to enhance the delivery and effects of recombinant ASM in both cellular and animal models [56,80,212,233-240], as summarized below.
3.1.1.2.1. Polystyrene models and poly(lactic-co-glycolic acid) nanoparticles targeted to intercellular adhesion molecule-1
These studies aimed to use NCs addressed to intercellular adhesion molecule 1 (ICAM-1) to enhance ASM delivery to main ASMD target organs, as well as its uptake and lysosomal delivery within key target cells. ICAM-1 is a protein expressed on most cell types in the body, including endothelial cells in capillaries and post-capillary venules, which separate the bloodstream from the surrounding tissue and cells within those tissues [102]. Its expression is low in normal conditions but highly upregulated by inflammatory factors associated with most pathologies, including ASMD and other LSDs [102]. These properties were attractive to improve ERT because delivery of naked ASM to the lungs, though achievable via i.v. administration, rendered important systemic side effects requiring meticulous dose scalation for tolerability and because of the inability of the enzyme to reach the CNS [192,228,241]. In addition, some cells affected by ASMD seemed to present endocytosis alterations, decreasing intracellular ASM delivery. For instance, M6PR-mediated uptake, the receptor most lysosomal enzymes use, was lower in lung macrophages due to enzyme deficiency, lowering intracellular delivery of recombinant ASM [242]. Various alterations affected clathrin-dependent and -independent pathways in ASMD patient fibroblasts and imipramine-treated endothelial cells to mimic ASMD [211,212,236,243]. A study using brain endothelial cells, ASMKO mice, and postmortem brain samples from LSD patients also indicated that ASMD affected markers of different endocytosis routes, reducing transcytosis via transferrin receptor (TfR) or ganglioside GM1 across cellular BBB models and brain targeting in vivo [233]. Though ASMD also lowered CAM-mediated transport, ICAM-1 expression was highly upregulated, compensating said decrease and resulting in the highest transporting potential compared to other formulations [211,233,243].
Therefore, as said, the goal of these studies was to target ICAM-1 using NCs to facilitate ASM transport in ASMD as a proof-of-concept, before focusing on clinically-suitable formulations. As such, most of the original studies on ICAM-1 targeting of ASM were conducted using polystyrene (PS) NP models, where lack of PS biodegradability allowed for easy tracking in mechanistic studies [55,104,211,212,236-239,244]. Yet, most importantly, all in vitro and in vivo data obtained from this PS model have been reproduced using ICAM-1 targeted NPs made of PLGA, including targeting specificity, lung and brain delivery, uptake and mechanism, lysosomal transport, BBB transcytosis, substrate reduction, and other parameters whose details are described below [56,80,233-235,240]. PLGA NPs hold clinical potential since (a) their physicochemical parameters can be manipulated during synthesis to control the delivery and release of cargo molecules [245-248], (b) they are biodegradable into lactic acid and glycolic acid [248], both metabolic byproducts [247], (c) their degradation acidifies the surrounding microenvironment [249], which is an advantage since lysosomal pH is less acidic than normal in lysosomal pathologies [250], and (d) this material is approved for clinical use [251].
Model PS NPs were prepared using commercially available PS beads and coating their surface with both recombinant ASM and anti-ICAM-1 antibodies (Figure 3A), as described below [56,104,235]. PLGA NPs involved 50:50 lactic acid:glycolic acid copolymers ranging between 31 to 68 kDa, either acid or ester terminated [80,234,235]. Nanoprecipitation was used in the case of PLGA NPs aimed for enzyme and antibody coating [80,234,235] mimicking PS models, whereas PLGA NPs aimed to encapsulate enzyme and display antibody alone on the coat were prepared by the double emulsion-solvent evaporation method [80] (Figure 3A). PLGA NPs and PS NPs were coated by surface adsorption [56,80,233-235]. This procedure is similar to antibody adsorption on ELISA plates and has been shown to favor outward orientation of antibodies on the coated surface at high concentrations [252]. Though it is possible that a fraction of antibodies is not properly oriented on this coat, this is no different from chemical linkage where particular amino acids are modified but may reside on different locations on each antibody molecule. Both PS and PLGA formulations showed similar parameters. For instance, model PS NPs from various publications ranged between 190-245 nm diameter, 0.10-0.19 polydispersity index (PDI), and −25 – −39 mV ζ-potential, and their protein content was varied to achieve from 109-233 anti-ICAM-1 molecules and 25-260 ASM molecules per NP [56,211,212,234-236]. PLGA NPs from different publications raged from 230-270 nm, 0.20-0.24 PDI, −29 – −31 mV, 122-233 anti-ICAM-1 molecules and 51-310 enzyme molecules per NP [80,233-235].
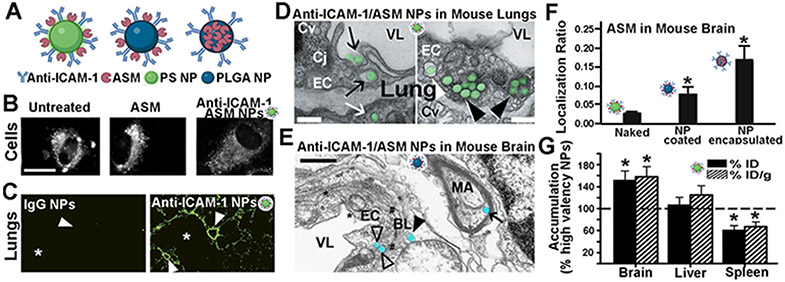
Figure 3. Anti-ICAM NPs for lung and brain enzyme delivery in acid sphingomyelinase deficiency.
(A) Polymer-based nanoparticles (NPs) targeted to intercellular adhesion molecule 1 (ICAM-1) used for acid sphingomyelinase (ASM) delivery, including model polystyrene (PS) NPs and poly(lactic-co-glycolide acid) (PLGA) NPs. (B) Storage level for BODIPY-FLC12-sphingomyelin in fibroblasts from ASM-deficient patients, 3 h after treatment with naked ASM or ASM targeted by anti-ICAM-1 PS NCs. Adapted and reproduced with permission from S. Muro et al. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis, Mol. Ther. 13 (2006) 135–141 [104]. (C) Specific localization of FITC-labeled anti-ICAM-1 PS NPs in mouse lungs, 30 min after intravenous (i.v.) injection vs. control IgG NPs. Small airways = asterisks; vessels = arrowheads. Adapted and reproduced from C. Garnacho et al., Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers, J. Pharmacol. Exp. Ther. 325 (2008) 400–408 [234]. (D) Transmission electron microscopy of wild-type mouse lungs showing anti ICAM-1/ASM PS NPs (green) interacting with endothelial cells (EC) 3 h after i.v. injection. NPs are engulfed by cells (black arrows), within endosomes (white arrowheads) and lysosomes (black arrowheads), and transcytosed into epithelial cells (white arrow). VL, vessel lumen; Cv, caveolar vesicles; Cl, clathrin vesicles; Cj, cell junction. Scale bars, 300 nm. Adapted and reproduced with permission from C. Garnacho et al., Enhanced Delivery and Effects of Acid Sphingomyelinase by ICAM-1-Targeted Nanocarriers in Type B Niemann-Pick Disease Mice, Mol. Ther. 25 (2017) 1686–1696 [235]. (E) Transmission electron microscopy of anti-ICAM-1/ASM PLGA NPs in wild-type mouse brains 3 h after i.v. injection. EC = endothelial cell; BL = basal lamina; MA = myelinated axon; VL = vessel lumen. Open arrowheads = NPs close to the abluminal side of an endothelial cell (EC); closed arrowheads = NP located passed the endothelium and basal lamina; arrow = NP within the myelinated axon of a neuron. * = clathrin-coated pits. # = caveolae-like vesicles. Scale bar = 500 μm. (F) Biodistribution of 125I-ASM after i.v. injection in wild-type mice as naked enzyme or in anti-ICAM-1 PLGA NPs (surface-loaded or encapsulated). The tissue-over-blood localization ratio is shown. (E,F) Adapted and reproduced with permission from E. Muntimadugu et al., Comparison between Nanoparticle Encapsulation and Surface Loading for Lysosomal Enzyme Replacement Therapy. Int J Mol Sci. 2022 Apr 6;23(7):4034 [80]. (G) Biodistribution of anti-ICAM/125I-ASM PS NPs with intermediate targeting valency, 30 min after i.v. injection in wild-type mice, as a percentage of the biodistribution found for high valency NCs. Adapted and reproduced with permission from R.L. Manthe et al., Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme, J. Control. Release. 324 (2020) 181–193 [56].
These PS and PLGA formulations have been studied in various cell culture models, including endothelial cells, fibroblasts, macrophages, astrocytes, pericytes, and neural-derived cells [55,104,174,211,233,244]. Some of them where either available from ASMD patients, treated with imipramine to degrade endogenous ASM, and/or treated with tumor necrosis factor alpha (TNFα) to mimic inflammation, a hallmark of ASMD and most other LSDs [55,104,174,211,233,244]. Flow cytometry, fluorescence microscopy, and/or Western blotting showed that all these cell types had minimal ICAM-1 expression in normal conditions vs. high expression in conditions mimicking disease, e.g. 50-300-fold increase for ASM-deficient endothelial cells under inflammation vs. a matching control [104,233]. All ICAM-1 targeted NPs bound specifically to ICAM-1, e.g. ≥20-fold increase comparing overexpressing cells vs. control cells, ≥ 20-fold increase comparing targeted vs. non-targeted NPs in high expression cells [56,104]. Binding was outcompeted by blocking ICAM-1 on the cell surface but not M6PR, thus bypassing the natural enzyme receptor [104]. This was also the case regarding internalization of recombinant ASM carried by anti-ICAM NPs vs. naked enzyme, e.g. internalization of the former formulation was inhibited by drugs that affect CAM-mediated endocytosis but not clathrin-coated pits, while naked ASM uptake responded contrarily, as expected for an M6PR-mediated event [104,233,240]. As a result, the total amount of recombinant ASM interacting with cells in culture was increased by ≥10-fold within 1 h incubation when delivered via anti-ICAM NPs vs. naked enzyme [104,212]. NPs trafficked efficiently to lysosomes (>80% of all cell-interacting NPs by 5 h), and by 3 h reduced 89% intracellular storage of sphingomyelin in ASMD, restoring wild-type values vs. 35% reduction for naked ASM [104,212] (Figure 3B). Secondary cholesterol storage was also reduced, e.g. 65% for NP-delivered ASM after 3 h vs. 4% for the same dose of naked ASM [211]. These changes improved or restored particular endocytosis-related alterations observed in ASMD cells, such as clathrin-mediated endocytosis of transferrin, caveolar-mediated endocytosis of cholera toxin B, or dextran pinocytosis [211,212,236].
In addition, the ability of anti-ICAM NPs to transcytose across cellular barriers was studied using confluent brain endothelial cell monolayers growing on porous transwell filters, in the presence or absence of subjacent monolayers of astrocytes or pericytes, to mimic the BBB [55,56,233]. These models showed formation of endothelial cell junctions that prevented free leakage of dextran, proteins, and NPs from the apical chamber over the cells (simulating the blood side) to the basolateral chamber underneath the cells (simulating the brain side) [55,56,233]. Both PS and PLGA NPs targeted to ICAM-1 were transported across these cellular mono- or bi-layers in disease-simulating conditions, e.g. 2,000-7,000 NPs/cell depending on the model, equivalent to millions NPs/mm2, within 24 h [55,56,233]. This is quite promising taking into account the enzyme dose each NP can carry (described above) and the total surface area of the BBB. Anti-ICAM-1 NPs surpassed similar formulations targeted to other pathways, such as clathrin-targeted anti-transferrin NPs and caveolar-targeted anti-ganglioside GM1 NPs, which seemed due to the high level of ICAM-1 expression compared to these other markers [233]. Curiously, a fraction of anti-ICAM-1 NPs was also detected residing in lysosomes within brain endothelial cells [56]. Apparently, the targeting valency (number of targeting antibodies per NP) regulated this property, where high valency NPs were more difficult to detach from cells after transcytosis, leading to re-uptake and higher entrapment by barrier cells [56]. Instead, lower valency NPs were the slowest at inducing uptake and transcytosis and intermediate valency NPs transcytosed best as they were less limited on each of these steps [56]. Therefore, this NP parameter could be exploited to regulate the balance between cargo delivery to endothelial lysosomes, also in need of treatment, and trans-endothelial transcytosis to deliver enzyme beyond this barrier into the brain (which was verified in vivo, as described below). Importantly, anti-ICAM-1 NPs have also been observed to efficiently enter both glial cells and neural-derived cells in culture, where they trafficked to lysosomal compartments, in the latter case, within both the cell body and neurites [55,244].
In addition to cellular studies, ICAM-1 targeted NPs have been investigated in vivo using ASMKO and wild-type mice (Figure 3C-G) [56,80,233-235,238,239]. Our studies showed that both PS models and PLGA NPs disappeared rapidly from the circulation after i.v. administration [56,80,233-235,238,239], e.g. ≤ 10% NP injected dose remained in circulation at 30 min, a 3-15-fold decrease compared to naked ASM [80]. However, this was due to rapid NP targeting and accumulation in organs, e.g. 60-85% NP injected dose resided within organs throughout the body, including the lungs, brain, and most visceral organs affected by ASMD, while only 30% naked ASM reached organs by this time [80]. In particular, the anti-ICAM-1 PLGA NPs increased ASM delivery to all organs, from 3–7-fold in the brain to 23-200-fold in lungs after a single i.v. injection, depending on the formulation and animal model [56,80,233-235,238,239]. As expected from in vitro data, intermediate valency NPs increased (1.5-fold) ASM accumulation in the brain compared to high valency NPs, while this did not happen in organs where transcytosis is not required for NP accumulation, such as the liver and spleen [56] (Figure 3G). Also in agreement with in vitro results, NPs were observed both in endothelial lysosomes and beyond the endothelium in the lungs (Figure 3D) and brain 3 h after i.v. injection (Figure 3E), including alveolar epithelial cells and neurons, respectively [56,80,234,235]. This occurred without apparent opening of endothelial cell junctions or protein leakage from the bloodstream into these organs, but rather through the endothelial cell body as expected for transcytosis [56,235]. PLGA NPs encapsulating ASM outperformed formulations where ASM was coated on NPs [56,80,235], e.g. by 6-fold and 2-fold in the lungs and brain, respectively (Figure 3F). NPs increased ASM activity in ASMKO mice above values observed for naked ASM, e.g. 8-fold in lungs [235] after 6 injections and 5-fold in the brain after 20 injections (S. Muro-University of Maryland and E. Schuchman-Mount Sinai Medical School, unpublished results in article under preparation). This reduced 98% excess sphingomyelin in the lungs [235] and 50% in the brain (S. Muro and E. Schuchman, unpublished results), which decreased ASMD lung , inflammation (29% reduction in total alveolar leukocytes and 36% in alveolar macrophages [235]) and stopped neuromotor decline measured by rotarod performance after 8 injections compared to untreated mice (S. Muro and E. Schuchman, unpublished results). Brain data are particularly encouraging since naked ASM has never rendered measurable improvement in this organ [228] and we used a 3-fold lower dose compared to clinical ASM dose for visceral treatment. Importantly, none of the animals suffered any apparent gross side-effects after weekly administrations for 20 weeks, e.g. no deaths and no changes in body weight, grooming, feeding, socializing, activity, light/dark behaviour were observed for this group vs. the untreated one. Yet, samples are being currently analyzed for hematological, biochemical, and renal/liver changes. Interestingly, administration of anti-ICAM-1 targeted systems via the carotid artery has been shown to increase by 100-fold brain delivery [253] and, additionally, brain inflammation appears to induce mobilization of pulmonary macrophages carrying anti-ICAM-1 NPs into the brain [254], strategies which could be used to further improve ASM brain delivery. Noticeably, enzyme delivery to visceral organs beside the brain is a benefit for this and other LSDs, since lysosomal enzymes are ubiquitous and most tissues are affected to some degree [59,207,209]. These combined strategies could further improve the potential of this approach
Nevertheless, translation of this strategy will require further investigation pertaining long-term therapeutic outcomes and side effects. Questions such as precise distribution of NPs and their ASM cargo within the brain and other organs, NP half-life and degradation within cells, maximum tolerated dose, ASM activity half-life in vivo, possible induction of inflammatory or immune cascades, outcomes in larger animal models, etc. will help understand respective therapeutic/risk balance, fine tune design of these formulations, and the establishment of suitable dosages and administration regimes.
3.1.2. Fabry disease
3.1.2.1. Disease, models, and current treatments for Fabry disease
After ASMD, Fabry disease (FD, OMIM #301500) is the next lipidosis where polymeric NCs were investigated for ERT. FD is an X-linked disorder caused by > 900 mutations in the GLA gene located in chromosome Xq22.1 [255,256]. As this gene encodes for α-galactosidase A (GLA, EC 3.2.1.22), the enzyme that hydrolyzes terminal non-reducing α-D-galactose residues in α-D-galactosides, these mutations lead to accumulation of glycosphingolipids with terminal α-galactosyl moieties, predominantly globotriaosylceramide (Gb3) [257]. This abnormal storage mainly affects body fluids as well as endothelial cells and smooth muscle cells in blood vessels, ganglia, heart, kidneys, eyes, and other tissues [256,257]. This causes multi-systemic alterations, including progressive cardiac and renal impairment leading to early death in patients [256,257]. FD is estimated to affect 1 in 40,000 males and 1 in 20,000 females, with an approximate prevalence of 1-5 patients in 10,000 live births (ORPHA:324) [257]. According to its severity, this malady is classified into severe-classical and milder-nonclassical phenotypes [255,257]. The severe-classical phenotype associates with childhood or adolescence onset, affecting mostly hemizygous males with very low to no enzyme activity [257-259]. This phenotype usually leads to severe pain crises in extremities, appearance of angiokeratomas in skin and mucous membranes, corneal and lenticular opacities, sweating abnormalities, proteinuria, and gradual renal deterioration [257-259]. This alterations can lead to end-stage renal disease and morbidity caused by cardiac and/or cerebrovascular impairment [257-259]. In contrast, males with the milder-nonclassical phenotype have higher GLA residual activity and an adult-onset. They may present a cardiac phenotype variant with left ventricular hypertrophy, cardiomyopathy, arrhythmia and proteinuria, but no end-stage renal disease; a renal variant with end-stage renal disease but without skin alterations or chronic pain; or only a cerebrovascular disease [257-259]. On the other hand, symptoms associated to heterozygous females are generally milder and manifest as later onset than males; yet, sometimes they can be asymptomatic or rather present severe symptoms as the ones observed in men with the classic phenotype [257,258].
The advantages and disadvantages associated with FD models have been deeply reviewed by Castelli et al. [256] and, as for other LSDs, most used FD cell models are primary fibroblasts from patients [260,261]. Additionally, a pharmacologically induced FD cell model can be obtained by treating cells with deoxygalactonojirimycin hydrochloride, a specific and potent inhibitor of lysosomal GLA activity due to degradation, which leads to aberrant Gb3 storage [262,263]. In addition, models derived from induced pluripotent stem cells (iPSCs) with FD phenotype have been also established [264-266], e.g. Kawagoe et al. generated iPSCs from human dermal fibroblasts of FD by using a Sendai virus vector loaded with four different reprogramming factors [265]. Resulting iPSCs presented the genetic and phenotypical characteristics of FD primary fibroblasts; yet, unfortunately, these cells could not be easily differentiated up to mature cell types due to intracellular damages produced by the massive accumulation of membranous bodies in lysosomes [265]. On the other hand, mimicking FD symptoms in animal models has also been challenging. Ohshima et al. described the first FD model, a GLAKO mouse (Glatm1Kul) constructed by using a targeting vector containing GLA mouse gene with a 1 kb deletion spanning part of exon III and intron III [267]. Even though this model lacked GLA expression and presented microscopic and biochemical evidence of glycosphingolipids storage, these mice did not develop human FD symptoms, probably due to differences in Gb3 accumulation profiles between mice and humans [259,266,268]. Other attempts to reproduce human symptoms in KO mouse models have been similarly unfortunate [259], for which a new rat GLAKO model was established by CRISPR/Cas9 GLA deletion [269-271]. These animals showed a complete lack of GLA activity, with appropriate accumulation of Gb3 in all tissues, and developed human-like symptomatic FD manifestations [269-271]. However, as this model is more recent, GLAKO mice have been the principal FD model used for in vivo studies [153,197], as well as a source for GLA-deficient primary cells [153,197,272].
FD treatment mostly consists of pharmacologically reduction of symptoms, such as the use of anticonvulsants to reduce pain, angiotensin-converting enzyme inhibitors to reduce proteinuria, or chronic hemodialysis and/or renal transplantation for end-stage renal disease patients [258]. Apart from this, long-term ERT is recommended to prevent renal, cardiac, and vasculo-endothelial manifestations [258]. This is based on the administration of α-agalsidase (Replagal®) or β-agalsidase (Fabrazyme®) [256-259]. The first drug is produced in human cell lines, while the second is produced in CHO cells and both are clinically approved [273-276]. These products have been demonstrated to be safe and effective in terms of improving renal podocyte alterations, lowering cardiomyopathy, delaying end-stage renal pathology, etc. [258]. Although these therapeutics are certainly effective to reduce Gb3 levels and help patients, they also associate with antibody generation and hypersensitivity reactions [277]. Therefore, other non-ERT therapeutics are also being investigated. For instance, in 2018 the U.S. Food and Drug Administration approved the treatment of FD patients with the chemical chaperone Migalastat (Galafold®), aimed to help fold misfolded enzyme molecules, which is currently ongoing a long-term Phase III clinical trial on pediatric patients >12 years of age [278]. However, this drug has been contraindicated for patients with GLA mutations unrelated to folding defects or those with severe renal failure [256]. In addition, two substrate reduction inhibitors, Lucerastat and Venglustat, are being currently evaluated for their efficiency and safety on FD patients. Lucerastat is under investigation in a Phase III trial as an oral mono therapeutic (NCT03425539) and Venglustat is being specifically tested for female FD patients and is under a Phase II trial (NCT02489344) [256].
3.1.2.2. Polymeric nanocarriers studied for Fabry disease treatment
Different polymer-based approaches have been investigated to improve ERT for FD, including polymeric NPs, polyelectrolyte complexes (PECs), modified albumin nanocapsules, and PEGylated enzyme [153,260,263,272,279,280], which are discussed below.
3.1.2.2.1. Enzyme-loaded model polystyrene nanoparticles targeted to intercellular adhesion molecule 1
Following original investigation of polymeric NPs for ASM delivery described above and due to similar cellular and in vivo results for ICAM-1 targeted PS NPs and PLGA NPs, our group used the former model as a proof-of-principle for increased GLA delivery over that of naked GLA in vascular endothelial cells and mice [263,281]. These model NPs were prepared by adsorbing a mix of 95:5 or 50:50 anti-ICAM-1:GLA mass ratio on the surface of 100 nm PS particles, resulting in anti-ICAM-1/GLA NPs with final diameter between 155-240 nm, 0.12-0.13 PDI, −9 – −12 mV ζ-potential, 81-83 anti-ICAM-1 molecules per NP, and 52-524 GLA molecules per NPs, depending on the formulation [263,281]. NPs were stable under various conditions, including (a) centrifugation followed by resuspension and sonication, which caused < 12% protein release, (b) storage in saline at 4 °C for at least three days tested, which led to < 3% protein release, or (c) incubation in 50% serum at 37 °C, which caused < 15% protein release in three days. However, at lysosomal pH and in the presence of GLA substrate, 28% protein release was observed by day 1, implying that these model NPs were suitable for further in vitro and in vivo tests [263].
Since vascular ECs are main targets for FD treatment, the cellular interactions of anti-ICAM-1/GLA NPs were investigated using micro- and macro-vascular ECs [263]. To mimic FD, cells were treated with both TNFα and deoxygalactonojirimycin hydrochloride to induce an inflammatory phenotype and inhibit endogenous GLA, generating Gb3 storage [263]. NPs were internalized by cells efficiently, e.g. 82-95% of all cell-associated NPs after 1 h incubation, corresponding to > 120 NPs/cell [263]. Just as in previous studies using ASM-loaded formulations, the uptake mechanism for GLA-loaded anti-ICAM-1 NPs associated with CAM-mediated endocytosis but not clathrin-coated pits, bypassing the natural uptake mechanism used by GLA [263]. Also as for anti-ICAM-1/ASM NPs, internalized anti-ICAM-1/GLA NPs trafficked to lysosomes, e.g. 70-75% of all internalized NPs resided in lysosomal compartments after 3 h and reduced ≈ 70% Gb3 storage after 5 h, which represented a significant improvement compared to 32% Gb3 reduction observed in cells treated with the same dose of naked GLA [263].
As for in vivo experiments, similarly to previous observations on anti-ICAM-1/ASM NPs, anti-ICAM-1/GLA NPs disappeared rather fast from the circulation, e.g. < 2-6% the injected dose remained in blood 30 min after i.v. injection compared to 40-60% for naked GLA, depending on the dose used [263,281]. However, just as for ASM formulations, this was due to fast accumulation of anti-ICAM-1/GLA NPs in tissues, e.g. 60-70% the injected dose vs. < 20% for the same dose of naked GLA [263]. ICAM-1 targeted NPs increased GLA accumulation in all body organs, expected because of the known ICAM-1 expression in the vascular endothelium throughout the body and many cell types in tissues. This is an advantage since FD affects the vascular endothelium throughout the body [256,257]. This strategy enhanced GLA specific index in key target organs such as 2-4-fold in the kidneys or 5-17-fold in the heart compared to naked enzyme, depending on the particular formulation [263,281]. This was due to specific ICAM-1 targeting, as control IgG NPs showed lower kidney residence and, instead, higher liver accumulation [263,281].
Furthermore, our group conducted pilot studies to evaluate whether oral administration of these formulations would be possible, which would tremendously benefit ERT by reducing the risk associated with i.v. infusions and allow patients to receive treatment at home. For this purpose, model anti-ICAM-1 NPs were encapsulated within alginate [79], a natural anionic polysaccharide polymer that is biodegradable and has been used to encapsulate vaccines, antibodies, proteins, etc. [282-285]. These capsules were then coated with chitosan, a natural cationic polysaccharide that has mucoadhesive properties [281,284,285]. Capsules were stable for up to 28 days (maximum time tested) stored in CaCl2 at 4 °C and during 2 h incubation in simulated gastric fluid (pH 1.2), preventing digestive degradation by pepsin of the protein content of NPs (<15% degradation) [79]. These capsules burst releasing anti-ICAM-1 NPs upon 1 h incubation in simulated intestinal fluid (pH 7.8), and the released NPs were able to bind specifically to intestinal epithelial cell models in culture (160 NPs/cell vs. < 10 NPs/cell for non-specific IgG NPs) [78,79]. Similarly, encapsulation into chitosan-alginate protected anti-ICAM-1 NPs from degradation when administered by oral gavage in mice (20-40% degradation depending on the gastrointestinal segment, 2-fold protection compared to non-encapsulated NPs) [79,286]. NPs were imaged interacting with and internalized within endocytic compartments in intestinal epithelial cells in vivo [286]. Finally, anti-ICAM-1 NPs transported GLA across monolayers of intestinal epithelial cells in culture at a rate ≈ 40,000 GLA molecules transported per cell in 24 h, which given the large absorptive surface of the intestine (≥ 200 m2) could signify ≥ 10 mg GLA, within the range of GLA clinical dosage (e.g. 14 mg GLA for a 70-kg individual) [78].
Therefore, these studies indicate that ICAM-1 targeting is an interesting option to enhance i.v. delivery of GLA for FD ERT and, perhaps, also explore an oral delivery strategy. Although these studies used PS models, due to similar behavior found both in vitro and in vivo for anti-ICAM-1 PS NPs and their PLGA counterparts, it is expected that this approach could be easily transferable to this more clinically relevant strategy. Nevertheless, the same questions described for anti-ICAM-1/ASM NPs remain to be answered, for which future investigations should focus on long-term therapeutic and side effects.
3.1.2.2.2. Trimethyl chitosan polyelectrolyte complexes
As described earlier, polyelectrolytes are polymers with many ionizable groups, classified in polycations and polyanions depending on whether they are positively or negatively charged, respectively [287]. When they are combined with therapeutic biomacromolecules, such as proteins of opposite charge, colloids known as PECs are formed by self-assembly [279,288]. These PECs can be further ionically crosslinked using small poly-ions to gain better physicochemical properties. Giannotti et al [272] prepared ionically crosslinked PECs based on trimethyl chitosan (TMC), capable of delivering GLA (Figure 4A). Because at pH around 7.3 to 8.0 GLA is negatively charged and TMC is positively charged, their coupling led to stable positively charged complexes with ζ-potentials ranging between 10 and 23 mV [279,289]. In contrast, in acidic environments with 4.5-5.5 pH values, close to GLA’s isoelectric point (pI ~5.7), the complex cannot be formed and, therefore, TMC/GLA PEC can release the enzyme within lysosomes [279]. The resulting NCs also presented good loading efficacy, ranging from 56% to 65%, and size between 50-90 nm diameter. Both characteristics dependent on the TMC/GLA mass ratio used, where a correlation between higher loading or diameter-size was established with higher relative GLA concentration. Nevertheless, acceptable homogeneity was achieved for each NCs population (0.2-0.4 PDI) [279]. Later, this system was improved by adding a peptide ligand containing an arginine-glycine-aspartic acid (RGD) sequence to enhance its targeting [272].
Figure 4. NC formulations for enzyme delivery for Fabry disease.
(A) Trimethyl chitosan (TMC) polyelctrolyte complexes (PEC), targeted by RGD peptide or not, for delivery of GLA to endothelial cells in culture. (B) In vitro activity of PEC-RGD or untargeted PEC vs. naked GLA in MAEC knockout cells, measured as Gb3 reduction (mean±SEM). Adapted and reproduced with permission from M.I. Giannotti et al., Highly Versatile Polyelectrolyte Complexes for Improving the Enzyme Replacement Therapy of Lysosomal Storage Disorders, ACS Appl. Mater. Interfaces. 8 (2016) 25741–25752 [272].
The specific uptake mechanism of TMC/GLA PECs was not determined; yet these formulations were internalized by endothelial cells in culture, e.g. 5-6-fold over their TMC precursor [279]. The internalization process did not occur by membrane disruption since cell viability was not affected after uptake [279], consistent with previous reports indicating that PEC uptake occurred via adsorptive endocytosis [279,290]. When RGD-PECs were used, uptake was slightly enhanced compared with non-targeted PECs, e.g. ≈4 0% cells incubated with fluorescently labeled RGD-PECs were positive after 20 min vs. ≈ 25% for non-targeted PECs [272]. In addition, colocalization studies showed that TMC/GLA PECs fully accumulated within lysosomes after 2 h incubation [279]. Next, the activity of TMC/GLA-PEC, TMC/GLA-PEC-RGD and naked GLA were compared in endothelial cells cultures from GLAKO mice, showing higher efficacy for both PEC formulations: IC50 regarding in vitro Gb3 reduction was 50.3 mU/mL for naked GLA, 7.6 mU/mL for TMC/GLA-PEC, and 5.5 mU/mL for TMC/GLA-PEC-RGD (Figure 4B) [272].
Finally, the in vitro cytotoxicity and hemocompatibility of RGD modified TMC/GLA PECs were examined to evaluate any possible damage on cell membranes due to their interaction with these cationic NCs. Results indicated that both PEC and PEC-RGD showed ≥80% cell viability in cell culture for concentrations below 5.5 μg/mL and <1% hemolysis for all concentrations tested [272].
Therefore, this strategy holds promise; yet, further research is needed to investigate the behavior and potential of these formulations in vivo, which has not been tested thus far, as well as any associated side effects. RGD binds to integrins and could be a more promiscuous targeting element compared to more specific ligands, although perhaps this is not a great concern for LSD treatment due to most tissues being affected. Importantly, inflammation ongoing in regions affected by FD may lead to premature or partial PEC dissociation, with GLA release extracellularly, given the acidic pH at inflammatory sites [291]. Therefore, these items need close examination in future studies.
3.1.2.2.3. Enzyme delivery by 30Kc19-albumin nanocapsules
Among protein NCs, those fabricated with human serum albumin are the most common ones [292]. Lee et al. produced NPs using this protein along with 30Kc19 [260,293], a protein from silkworm hemolymph that has previously been linked to cell penetration and enzyme stabilization effects [294]. This group first tested 30Kc19-albumin NPs loaded with β-galactosidase as a model enzyme [293] and, subsequently, used a similar formulation to deliver GLA in FD cell models [260]. In their studies, authors described a trend for NPs increased diameter from 170 to 350 nm as the weight percentage (wt%) of 30Kc19 component increased. When 0 to 50 wt% was used, uniform and stiff spherical NPs were formed, but when 30Kc19 wt% was >50%, the NP shape was distorted and they agglomerated [293]. A correlation was also detected for a high 30Kc19 wt% and a lower NP homogeneity, e.g. without or with 10 wt% 30Kc19, PDI was ≈0.1, whereas 90 wt% lead to PDI ≈ 0.5 [293]. Moreover, all 30Kc19-albumin formulations tested showed stable dispersions according to their ζ-potential values, rangeing between −20 to −30 mV [293]. Regardless of the enzyme loaded, 80-95% loading efficiency was found, which tended to be higher at higher 30Kc19 wt% [260,293]. Enzyme activity and release were also improved by increasing 30Kc19 wt% up to 50% [260,293].
As for their cellular internalization, different routes were proposed for these formulations in different cell cultures. For instance, authors suggested that the presence of albumin in NPs could favor caveolae-mediated endocytosis by interacting with gpG0 receptor in HeLa cells [295]. Although the influence of 30Kc19 on NP uptake remains uncharacterized [293], this protein seemed to enable NP uptake via receptor-independent pathways [294]. This was postulated to increase the chances of uptake of particles containing 30Kc19 by those cells with low caveolae expression, which was associated with the higher uptake observed in several cell cultures for 30Kc10-albumin NPs compared to NPs made of albumin alone [293]. In FD fibroblasts, GLA loaded 30Kc19-albumin NPs had 1.5-fold increased uptake compared to albumin NPs, and it co-localized with lysosomes [260]. Formulations containing 50 wt% 30Kc19 demonstrated significantly enhanced GLA activity to FD fibroblasts, e.g. degradation of 47% intracellular Gb3 storage after 24 h incubation, which represents a 3.8-fold and 1.6-fold higher activity compared to NPs without 30Kc19 and naked GLA, respectively [260]. These NP formulations showed no significant cellular toxicity in several cell lines, including FD fibroblasts [260,293,294].
Finally, preliminary biodistribution and toxicity studies were conducted using i.p. injections of 30Kc19 alone or conjugated to green fluorescent protein GFP, as a model cargo protein, in mice. In these studies, Park et al. showed that 30Kc19 delivered this protein to several organ, such as the liver and kidney, where 30K19-protein remained up to 12 h after injection [294]. To evaluate in vivo toxicity, mice were injected daily with 4 or 40 mg/kg 30Kc19 for 14 days, after which blood levels of urea nitrogen, creatinine, aspartate aminotransferase, and alanine aminotransferase were measured, showing no changes compared to untreated mice and demonstrating safety [294].
Nevertheless, as for most other formulations described above, in vivo therapeutic and side effects of 30Kc19-albumin NPs remain undetermined and further studies should aim to respond these questions. Although albumin NPs have been approved for clinical applications [147], whether the combined presence of 30Kc19 would induce immune responses or other side effects need to be tested, particular in the context of lysosomal ERT requiring chronic administration.
3.1.2.2.4. Poly(ethylene glycol)-modified enzyme
Although not in a nano- particle, capsule, or vesicle form, polymer-based conjugates have also been approached in the context of LSD treatment. For instance, the two clinically approved products for FD ERT mentioned above have relatively fast PK, with circulation half-life around 2 h [296,297]. A strategy to try to improve this aspect and, with it, the biodistribution of recombinant GLA to delay its administration frequency and alleviate patient burden and side effects, was based on enzyme PEGylation [280]. This approached was developed by the Shaaltiel lab, who engineered a new enzyme design consisting of two enzyme molecules conjugated to 2,000 kDa homo-bifunctional PEG molecules [280], generating a homodimer product. The enzyme counterpart was produced in Tobacco cells and then chemically dimerized with PEG, as said [280]. Thereafter, additional PEG molecules were linked on the surface of the enzyme dimer using lysine residues [280]. Kizhner et al. characterized this product, called α-pegunigalsidase or PRX-102, both in vitro and in vivo. This design significantly stabilized the enzyme, e.g. the product retained 30% activity after 1 h incubation in plasma, while naked enzymes using commercially were fully inactivated during this time [280]. In addition, when lysosomal conditions were simulated in vitro, this PEGylated enzyme maintained 80% activity after 10 days while naked enzymes lost their activity after 2 days [280]. When injected i.v. in GLAKO mice, α-pegunigalsidase increased the half-life in circulation compared to commercial enzymes, i.e. from 13 min to 581 min. PEGylated GLA was also internalized by FD patient fibroblasts in culture, where it co-localized with lysosomal markers [280]. Gb3 levels in GLAKO mice were reduced by 64% in kidneys, 23% in the heart, and down to control levels in the liver after 4 injections every other week [280].
Following these encouraging results, α-pegunigalsidase is being tested in Phase I/II cand a short Phase III clinical trials (NCT03180840, NCT03018730, NCT01678898), which have shown remarkable plasma half-life in human from 53-121 h and 84% reduction in Gb3 content in renal peritubular capillaries, a main target for FD [298]. Some patients developed anti-enzyme antibodies despite the presence of PEG in this product and whether anti-PEG antibodies observed in some individuals in the general population would affect long-term treatment with this drug remains unknown [299]. Most patients reported some kind of adverse effects, including migraine or bronchospasm, although side effects were mild in most cases, such as fatigue, nausea, dizziness, etc [298]. Therefore, this is highly promising strategy which is likely to result into a translated drug in the near future.
3.1.3. Niemann-Pick disease type C
3.1.3.1. Disease, models, and current treatments for Niemann-Pick disease type C
Niemann-Pick type C disease (NPC) was the next lipidoses where polymeric NCs were investigated. This is an atypical autosomal recessive storage disorder affecting around 1 patient in 45,000 to 286,000 live births (ORPHA:646). It can be produced by several mutations in two different genes: the NPC1 on chromosome 18q11.2, which encodes for a membrane glycoprotein called NPC intracellular cholesterol transporter 1 (NPC1, UniProt O15118), or the NPC2 gene located at 14q24.3 locus, which encodes for the NPC intracellular cholesterol transporter 2 (NPC2, UniProt P61916), a soluble lysosomal protein that binds cholesterol [300-302]. Their respective loss of function blocks cholesterol efflux from lysosomes, leading to its impaired processing, utilization and trafficking, which leads to the accumulation of un-esterified cholesterol, glucosylceramide, and gangliosides inside late endo/-lysosomal compartments in visceral organs and neural tissues [208,209]. NPC is further classified as type C1 or type C2, based on the pathogenic mutations affecting NPC1 or NPC2, respectively. Type C1 NPC (NPC-1, OMIM #257220), is the most predominant subtype of the disease, affecting 95% of the patients and presenting > 200 pathogenic variants, while the remaining 5% corresponds to type C2 NPC (NPC-2, OMIM #607625), from which only 5 pathogenic variants have been reported [303]. Both NPC types lead to extremely heterogeneous phenotypic manifestations appearing from perinatal age to adulthood, where visceral symptoms such as hepatosplenomegaly or isolated splenomegaly always precede neuropathological fatal pathologies, which mainly consist of cerebellar ataxia, dysarthria, dysphagia, and progressive dementia [208,209,304].
Many different experimental models including several cell types and animals, both mammals and non-mammals, are available to study NPC-1 and NPC-2, which have been thoroughly reviewed by Pallottini et al. [305]. Most in vitro models used for therapy development are immortalized Npc1−/− embryonic fibroblasts [305,306], primary skin fibroblasts from NPC patients [211,305,307], mutant CHO lines [308], and human iPSC [266,309-311]. In case of in vivo studies, mouse models are the preferred workhorse for NPC, where the first models carried spontaneous mutations in the NPC1 gene, knocking it out and triggering an early disease onset with symptoms including hepatomegaly, weight loss, disturbed motor coordination, ataxia, and premature death [312-315]. These NPC1KO mice were caused either by the insertion of a retroposon (Nih allele, Npc1nih) [312,313] or by a 43 base-pair insertion (spm allele, Npc1spm) [314,315] in BALB/c and C57BL/6J colonies, respectively. Although these models have been widely used, many other mouse models for NPC-1 have been generated by multiple methods (see [305]). For NPC-2, many less mouse models have been developed. The first one was achieved by gene targeting, resulting in mice with just 4% normal NPC2 protein levels [305,316-319]. Feline NPC models are also available, such as the case of affected domestic cats (Felis catus) that developed neurologic symptoms similar to humans at juvenile age, which are caused by distinct mutant alleles for NPC-1 and NPC-2 [320-324].
Nowadays, NPC treatment is symptomatic, requiring multidisciplinary control by health care professionals [325]. In addition to symptoms management, the only licensed disease modifying drug available in the European Union is Miglustat (Zavesca®), a glucosylceramide synthase inhibitor originally created for SRT for GD (see section 3.1.4.1 below), which can decelerate disease progression in some patients [325-328]. However, Miglustat is not effective for all patients and is not recommended for those with profound neurological disease, as it has been difficult to assess any therapy-related improvements [305,325]. Therapeutics being currently investigated are based mostly on cyclodextrins (CDs) because they can form inclusion complexes with cholesterol, removing it [329]. For instance, hydroxypropyl-β-CD (HPβCD: also called VTS-270) is a cholesterol scavenger that promotes the excretion of this lipid out of cells [304,330]. This product has completed two Phase I/II clinical trials and another one is still ongoing (NCT02912793, NCT03887533, NCT03471143) for NPC-1 treatment [304,330]. A short-term Phase III trial (NCT04860960, still recruiting) has also started, as well as a long-term Phase I study to evaluate its safety and efficacy in NPC-1 patients (NCT03893071) [304,330]. Although this drug is promising, its short half-life in the bloodstream makes it necessary to administer very high HPβCD doses for sufficient therapeutic effect, which leads to ototoxicity, pulmonary toxicity, several autophagy-based cellular defects, and hemolysis [331,332]. In addition, other therapeutic approaches are being studied, e.g. N-acetyl-L-leucine to improve neurologic symptoms in pediatric and adult patients (NCT03759639, NCT05163288) [333], or Arimoclomol to target protein misfolding and improve lysosomal function [334], shown to reduce the annual disease progression by 65% (NCT02612129) [334]. However, more investigation is still needed to establish these strategies as NPC treatments.
3.1.3.2. Polymeric nanocarriers studied for Niemann-Pick type C treatment
The use of different polymeric designs, including structures, such as polyrotaxane conjugates and polymeric micelles, is being investigated to diminish CDs toxicity and improve its use for NPC treatment, which is discussed below.
3.1.3.2.1. β-Cyclodextrin-based polyrotaxanes for cholesterol removal
Polyrotaxanes are molecular composites constituted by macrocycle molecules threaded on a linear macromolecule, a polymer chain usually [335]. They can be built containing β-CDs, to reduce their toxicity and enable their controlled release for treating of NPC [335]. The Thompson group fabricated Pluronic-based and poly(decamethylenephosphate)-based polyrotaxanes for this purpose [335-338] and similar compounds have been studied by Tamura and Yui [307,339,340] (Figure 5A). As an example, biocleavable hydroxyethylated Pluronic/β-CD polyrotaxane (HE-SS-PRX) was fabricated using Pluronic P123 as the axle polymeric chain, bearing terminal disulfide linkages to release β-CD after their intracellular cleavage [339]. For this, hydroxyethyl groups were introduced on β-CD moieties to achieve water-solubility [307,339,340]. To provide these polyrotaxanes the ability to be cleaved within lysosomes in a pH-dependent manner, terminal N-triphenylmethyl groups were introduced to create novel acid-labile 2-(2-hydroxyethoxy)ethyl group-modified polyrotaxanes (HEE-PRXs) [307,340]. Several structures were made varying between 1-8 the number of HEE groups on the polyrotaxane [307,340]. Tamura et al showed that the number of HEE groups did not affect cleavage kinetics, though this factor affected the cholesterol-solubilizing ability of the resulting formulations, where structures with high number of HEE groups were unable to reduce cholesterol [340]. According to these results, researchers established that optimal formulations encompassed 4-5 HEE groups per single β-CD threaded onto the polyrotaxane, which reduced intracellular cholesterol in a concentration-dependent manner [340].
Figure 5. Polyrotaxanes nanoformulations for Niemann-Pick type C disease.
(A) Acid-labile 2-(2-hydroxyethoxy)ethyl group-modified polytrioxanes (HEE-PRX) based for pH-mediated delivery of β-cyclodextrin (β-CD) into lysosomes of NPC-1 cells and remove stored cholesterol, to avoid toxic effects of current hydroxypropyl-β-cyclodextrin (HPβCD). (B) Reduction of total cholesterol levels in normal fibroblasts vs. fibroblasts from NPC-1 patients, after treatment for 24 h with described concentrations of HEE-PRX vs. HP-β-CD (mean±SD). Adapted and reproduced with permission from A. Tamura et al. Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease, Sci. Technol. Adv. Mater. 17 (2016) [340].
Egele et al. showed that β-CD could be released from polyrotaxanes for 30 days, which could provide sustained treatment [336]. Studies by both teams showed that β-CD-based polyrotaxanes could be internalized by cells by endocytosis and colocalized with lysosomal compartments in a time-dependent manner, including fibroblasts from NPC-1 and NPC-2 patients[335,338,339]. In the case of HEE-PRXs, internalization was evaluated in the presence of inhibitors of clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis, showing that the cellular uptake of HEE-PRXs was abrogated regardless the inhibitor, indicating that different pathways may be used for uptake [340]. During the internalization process, the polyrotaxane structure masked the toxic effect of β-CD by perturbing the interaction of its hydrophobic cavity with cholesterol present in membranes, avoiding membrane disruption [307,339]. Importantly, these formulations efficiency decreased unesterified cholesterol that aberrantly accumulates in NPC, which was observed both for NPC-1 and NPC-2 patient fibroblasts [307,335,336,338,339]. Depending on the particular formulation and concentrations used, β-CD-based polyrotaxanes where able to reduce between 20-80% cholesterol storage in these cells within a period from 6 h to 24 h [307,335,336,338,339]. This efficiency ranged from 10-100-fold improvement compared to equivalent concentrations of β-CD (Figure 5B) [307,339].
These compounds were also examined in vivo, including wild-type and Npc1−/− mice [307,337]. Regarding studies by the Thompson group, i.p. injections were used [337]. MRI visualization of polyrotaxanes labelled with gadolinium showed a decrease down to 3% the injected dose at 48 h, while levels increased in organs such as liver and spleen (25% and 2.4% injected dose, respectively, at 24 h), and little amount was found in circulation during this time (around 4% injected dose) [337]. Monomeric β-CD showed lower levels in the liver (< 10% injected dose) but had increased kidney/bladder localization [337]. Regarding cholesterol levels, polyrotaxanes efficiency decreased them in the liver, spleen, and kidneys around 17-, 10-, and 100-fold lower doses than β-CD, demonstrating their good potential [337]. No effect was observed in the lungs or brain, which are also key targets for NPC treatment, for which perhaps targeting of polyrotaxanes to these organs could be helpful [337] .
In the Tamura and Yui study, HEE-PRX exhibited a longer plasma half-life than HPβCD (1.41 h vs. 0.59 h); yet, their levels in tissues were comparable, especially in the brain, liver, and spleen [307]. Treatment with weekly subcutaneous administrations of HEE-PRX required lower doses (500 mg/kg) compared to HPβCD (4,000 mg/kg) to achieve a therapeutic effect. In particular, the use of polyrotaxanes effectively suppressed the accumulation of cholesterol and other sterols in diseased mice without altering their levels in wild-type animals [307]. This resulted in a general improvement in many of the pathological alterations observed in Npc1−/− mice, such as tissue vacuolization, neurodegeneration, and hepatomegaly. In addition, animals treated with HEE-PRX presented an evident delay in disease onset, weight loss (from 42 to 56 days), and the life-span (from 68 to 91 days) compared to animals treated with HPβCD [307].
Taken together, these findings indicate that polyrotaxanes could represent a valuable strategy to improve the β-CD-based treatment for NPC, although sufficient delivery in key organs such as the brain may require additional attention and perhaps strategies for active targeting. Future studies should focus on establishing long-term therapeutic and side effects and the relative efficacy of this therapy depending on age and pathological state at the start of treatment.
3.1.3.2.2. Poly(ethylene glycol)-lipid micelles for cholesterol efflux
Another polymeric design for lysosomal cholesterol clearance was proposed by Brown et al. [306]. Also trying to avoid HPβCD toxicity effects, this group first encapsulated HPβCD in liposomes composed of hydrogenated soy phosphatidylcholine and 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE) conjugated to PEG (DSPE-PEG, a PEG-lipid conjugate) [306]. Different lipid-to-PEG molar ratios were used and, unexpectedly, a mixed population of liposomes and micelles was obtained, where increasing PEG-lipid content favored micelles formation, observed by NC decreased size from 150 nm to 10-15 nm [306]. Authors also observed a gradual decrease in HPβCD encapsulation as the PEG-lipid content increased [306]. Thus, in their study, Brown et al. compared liposomes loaded with HPβCD, empty DSPE-PEG micelles, and DSPE-PEG micelles loaded with different concentrations of HPβCD to evaluate their potential synergistic effect [306]. All these formulations were tested using Npc1−/− cells as in vitro models. Results showed that DSPE-PEG was internalized by cells after 2 h incubation, saturating the endo-lysosomal system within 24 h [306]. Giving this slow uptake rate, macropinocytosis was suggested as the route of internalization; yet, no confirmatory studies were performed. Micelles were demonstrated to colocalize with lysosomal compartments, e.g. 50% and 80% colocalization by 2 h and 24 h, respectively [306].
The ability of these different formulations to remove cholesterol from Npc1−/− cells was also tested. Surprisingly, researchers found that DSPE-PEG2K micelles enabled cholesterol clearance from cellular endo-lysosomes and acted synergically with low doses of HPβCD to induce cholesterol efflux, avoiding its intracellular accumulation [306]. Treatment with DSPE-PEG micelles resulted in a dose-dependent decrease in cholesterol levels within cells, achieving the highest cholesterol reduction (≈ 40%) when 10 μM DSPE-PEG was used [306]. Additionally, 75% cholesterol reduction was obtained after treatment with 0.25 mM of HPβCD combined with 10 μM DSPE-PEG2K, reaching cholesterol levels comparable to wild-type [306].
In conclusion, this study presented different PEG-lipid based NCs capable of delivering bioactive drugs with potential therapeutic effect for the treatment of NPC. However, further studies both in vitro and in vivo assays are needed to assess the multiple aspects, such as the stability of these formulations, their PK and biodistribution, their ability to cross the BBB for CNS effects, and their long-term therapeutic action as well as safety
3.1.4. Gaucher disease
3.1.4.1. Disease, models, and current treatments for Gaucher disease
Gaucher disease (GD), also called glucocerebrosidosis, is a recessive autosomal lipidosis caused by > 700 mutations in the GBA gene on chromosome 1q22, which encodes acid β-glucosidase (GBA; EC 3.2.1.45), also called glucosylceramidase or glucocerebrosidase [341-344]. The enzymatic deficiency resulting from these mutations leads to the accumulation of glucocerebroside (glucosylceramide) in cells, mainly macrophages, in organs such as the liver or spleen, as well as cells of the CNS [341-343]. GD is considered the most common LSD in the general population, effecting 1-9 out of 100,000 live births [345,346]. Although other LSDs have a similar prevalence, the annual incidence of this disease is around 1 in 60,000 live births, 1 in 1,000 among the Ashkenazi Jewish population (ORPHA:355). This malady can manifest as one of three major clinical types, called Type I, II, or III (OMIM #230800, #230900, #231000) or two other phenotypes, i.e. the perinatal-lethal and cardiovascular subtypes [59]. Type I GD is a chronic non-neuropathic form of the disease, with childhood or adulthood-onset, spleen- and hepatomegaly, bone anomalies, and cytopenia [59]. Type II GD is an acute neuropathic infantile form, where neurodegeneration and organomegaly rapidly leads to death around 2 years of age [59]. GD Type III is a subacute neuropathic form with onset before 2 years of age and survival until adulthood, where both progressive encephalopathy and type I-like systemic abnormalities are observed [59,346]. Perinatal-lethal GD (OMIM #608013; ORPHA:85212) is a very rare disease subtype that affects < 5% GD cases. It has a fetal onset, manifested mostly by non-immune hydrops fetalis, neonatal distress, decreased or absent fetal mobility, hepatosplenomegaly, ichthyosis, arthrogryposis, and facial dysmorphia [59,347]. Death usually occurs in utero or between the first 3 months after birth [59,347]. GD cardiovascular subtype (OMIM #610539; ORPHA:309252), also known as atypical GD or GD-like disease, is caused by different heterozygous mutations in the PSAP gene (locus 10q22.1) encoding Saposin C, a crucial GBA activator protein [348]. It is very unusual and mostly characterized by a progressive calcification of the aortic and/or mitral valves, splenomegaly, optical alterations, and possible cardiopulmonary complications [59,348]. Also, new mutations have been recently discovered for this type showing a wide heterogeneity in patient’s symptoms and onset age [59,348].
The development of GD models representing disease phenotypes has been challenging. GD patients' fibroblasts have been widely used as cell models [61,211], but they do not accumulate glucocerebroside [349]. Therefore, iPSC lines developed from GD patients' cells are being differentiated into different cell types, e.g., macrophages, which present enzyme deficiency and lipid storage, among other phenotypic characteristics [266,350], or dopaminergic neurons that are extremely useful to explore the link between GD and Parkinson disease [351-353]. This is the case because some GBA alterations represent a common risk factor for juvenile Parkinson disease [354]. GD cell models can be also pharmacologically induced by conduritol B-epoxide, an irreversible GBA inhibitor [355]. This treatment has been reported in studies using different cell cultures, such as MG63 human osteoblasts [356]. The generation of animal models that faithful mimic GD symptoms without their premature death has been even harder [353,357]. The first attempt to generate a GD mouse model involved conduritol B-epoxide administration [358]; however, this strategy has not been widely used. The first genetic model was a KO mouse resulting in < 4% GBA activity [359]. Although these GBAKO mice successfully mimicked severe type II GD phenotype [360] with glucocerebroside storage within lysosomes of cells of the reticuloendothelial system; they had a very short lifespan (mice die within 24 h after birth) and this limited their utility [357,359]. Many other attempts were made to create less severe GD mouse models based on point mutations, such as L444P associated to human GD neuropathic forms [361], or conditional KO models such as the type 1 GD mouse created through the Mx1-Cre-loxP system [362]. However, all these models still presented disadvantages, as they either lacked the corresponding symptomatology or their lifespan was still very short [357]. Non-mouse models have also been described for GD. The first one was an Australian Sydney Silky dog that is no longer available [363]. Then, a sheep GD model naturally generated from spontaneous mutations was discovered [216,364]. However, a GD animal model that faithfully recapitulates human disease symptoms, carrying the mutations in all cell types, with different severity, and a long enough lifespan is still missing [357].
Regarding treatment, besides splenectomy, current GD therapies are also based mostly on ERT [39,365,366]. Through the last decades, products such as Aglucerase (Ceredase®), Imiglucerase (Cerecyme®), Velaglucerase (VPRIV®), and Taliglucerase (Elelyso®) have been developed, consisting on enzymes produced by human placenta, CHO cells, fibrosarcoma cells, and carrot cells, respectively [365]. All these therapeutics have been clinically approved and some of them remain in clinical trials to continue examining their therapeutic value and side effects. For instance, Phase IV trials are currently re-routing patients to evaluate the use of Velaglucerase alfa in type I or III GD patients previously treated with SRT therapeutics (NCT04718779) [367,368] or to assess the efficacy and safety of i.v. administration of 60 U/kg Imiglucerase or Taliglucerase alfa (NCT04656600 and NCT04002830, respectively) in GD type III patients [369]. On the other hand, non-ERT treatments are also available for this malady, e.g., Miglustat (Zavesca®, the first SRT to be clinically approved in 2002) and Eeliglustat (Cerdelga®) are marketed glucosylceramide synthase inhibitors used for SRT [341,365]. A Phase III trial is ongoing to evaluate the efficacy of the use of Eliglustat in combination with Imiglucerase in type I and type III GD patients (NCT03485677). Chaperone stimulator, Arimoclomol, is also being tested in a Phase II trial as a GBA enhancer in GD type I or III patients (NCT03746587). Gene-therapy approaches are currently recruiting type II and type I GD patients, respectively, to perform Phase I/II trials with a single dose PR001A, a baculovirus-produced gene therapy vector aimed to deliver normal GBA1-encoding sequence or transplants of hematopoietic stem cells genetically modified with AVR-RD-02, a lentiviral vector containing a functional GBA mRNA sequence (NCT04411654 and NCT04145037). Importantly, although currently approved therapies are clearly useful and widely used, SRTs are only prescribed for adult GD type I patients due to their side effects and lack of long-term safety data, ERTs associate with immunogenicity problems in some cases and, most importantly, tissues such as bone and CNS are not properly treated by ERT strategies [346,365].
3.1.4.2. Polymeric nanocarriers studied for Gaucher disease treatment
As said, therapeutic delivery remains a challenge for GD treatments due to their low accessibility [59,365], for which new therapeutic approaches involving polymer-based strategies are being investigated, as described below.
3.1.4.2.1. Alginate microspheres for enzyme delivery in Gaucher disease
As described above, alginates are linear biocompatible and biodegradable polysaccharides containing different proportions of β-D-mannuronic and α-L-guluronic acid residues [370]. Polyvalent cations such as calcium, zinc, or barium, can induce alginate cross-linking, forming stable and reversible alginate gels [370]. This property has been used to produce ions-alginate particles stabilized by calcium, due to its clinical safety and accessibility [370]. These particles were used in a context of poor bone accessibility, to facilitate the delivery of therapeutics enzymes to bone, including GBA [61,356,371-373]. First, several articles from the Barbosa group described the preparation of calcium titanium phosphate-alginate (CTP-alginate) and hydroxyapatite-alginate (HAp-alginate) microspheres, where the bioactive ceramics CTP and HAp were used to immobilize GBA within their matrices [371-373]. After testing different polymer concentrations, α-L-guluronic acid content, and ceramic-to-polymer ratios, authors reported the formation of homogenous spherical-shaped particles with diameters ranging from 450 μm to 796 μm for CTP-alginate microspheres and from 412 μm to 749 μm for the HAp-alginate ones, depending on the formulation [371-373]. GBA incorporation was pursued by adsorbing the enzyme on these particles [371-373] or by dispersing them in the polymer-ceramic mix [371-373]. This provided different release profiles, with slower enzyme release in the former case, which was characterized by a significant burst release followed by a slower enzyme diffusion through the matrix [371-373]. Loaded HAp-alginate particles were smoother and had a higher surface area than the CTP-alginate ones, e.g. 76.00 cm2/mg vs. 9.84 cm2/mg [371-373]. However, CTP adsorbed a much higher amount of enzyme per unit surface than HAp microspheres, e.g. 41.3-41.8 ng/cm2 vs. 1.9-4.3 ng/cm2, probably due to favored electrostatics since their ζ-potential were ≈ −30 mV for CTP and ≈ −10 mV for HAp [371-373]. The same group prepared GBA-loaded calcium-alginate microspheres by directly dripping a solution of dispersed GBA in sodium-alginate into a crosslinking bath containing CaCl2 [61]. Authors obtained microspheres around 2.4 mm diameter along with particles around 400 μm [61]. In this case, microsphere-entrapped enzyme retained full biological activity with a pH-dependent activity profile, where stability was enhanced at pH values near neutrality [61].
Although the uptake mechanism for released GBA was not determined in these studies, Lamghari et al. showed that MG63 human osteoblasts were capable of internalizing exogenous recombinant GBA and suggested that this process could be partially mediated by mannose receptors [356]. Further investigation was performed to elucidate whether the use of CTP/HAp-alginate microspheres in addition to modulating the enzyme release kinetics, could improve the attachment of osteoblasts to particle, which had beed previously shown to be poor [374]. As expected, no adhered cells were found on control alginate microspheres, while particles with high ceramic-to-polymer ratio (both CTP and HAp) enabled cell attachment, spread and adoption of cells with typical osteoblastic-like morphology [373]. The intracellular activity of ceramic-alginate GBA microspheres vs. naked GBA was also evaluated using fibroblasts from GD patients [61]. CTB-based microspheres increased intracellular GBA activity by 170% while HAp counterparts only achieved 20% activity, showing that the former formulation was more promising for this parameter [372]. Encouraging results were also obtained using calcium-alginate microspheres to deliver GBA to type I GD fibroblasts, where half the GBA dose was sufficient to render the same enzymatic activity as the naked enzyme [61].
Although these formulations could help delivery GBA to bone, for instance using depot-type implants containing these formulations, further investigation in vitro and in vivo is required to elucidate important aspects such as therapeutic effects, toxicity, dosage, degradation of these implants, etc.
3.1.4.2.2. Poly(lactic-co-glycolic acid)-mediated restoration of acidic lysosomal pH
As we previously discussed, proper lysosome functioning needs the lysosomal pH to be maintained between 4.5 and 5 [14]. Lysosomal alkalinization can lead to cellular toxicity and the decrease of lysosomal enzyme activity, since they have maximal activity within this pH range [375]. Due to abnormal substrate storage within lysosomes and secondary disbalances, LSDs associate with lysosomal pH ranges less acidic than normal [376]. Therefore, the use of polymeric NCs to deliver therapeutics (e.g. enzymes) to lysosomes and simultaneously restore their acidic pH could provide an additional advantage [375]. In this context, PLGA NPs hold significant potential since they can enter cells and traffic to lysosomes, where their degradation releases lactic acid and glycolic acid acidic (Figure 6). This renders lysosomal re-acidification, which has been investigated for several pathologies [249,377-379]. In all cases, PLGA NPs restored normal lysosomal pH in a dose dependent manner, enhancing the activity of lysosomal enzymes and the autophagy flux, indicating that the cellular degradative activity was improved [249,377-379]. Using this strategy, the Dehay group conducted several studies using different types of PLGA NPs to elucidate their role in lysosomal de-alkalinization under different disease contexts [380-382]. For this purpose, researchers used standard PLGA NPs with spherical shape, ≈ 150 nm diameter, and −41.5 mV ζ-potential [380-382] and compared them to PLGA-containing nanoemulsions. These formulations were designed to have characteristics similar to PLGA NPs (spherical shape, 187 nm diameter, and −35.7 mV ζ-potential) but increased PLGA concentration, which was achieved by entrapping this copolymer within an oil core containing medium-chain triglyceride oil and soybean phospholipids, and coating them with a surfactant [381].
Figure 6. Polymer-based nanoparticles to correct lysosomal pH in lysosomal storage disorders.
Poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) can enter cells by endocytosis and traffic to lysosomes, where their degradation into lactic acid and glycolic acid can help restore a normally acidic pH, which is less acidic in lysosomal storage disorders. This property was used by M. Bourdenx et al., [380] to ameliorate lysosomal pH alterations associated with GBA mutantions, which aare characteristic of Gauche disease and associated with Parkinson’s disease.
Although the specific mechanism by which PLGA NPs entered cells was not investigated in these studies, they demonstrated that NPs were endocytosed by human dopaminergic neuroblastoma cells used as cell culture models, via a non-specific event that resulted in trafficking to early endosomes and then late endosomes, ultimately reaching lysosomes within 24 h [380]. In this study authors also demonstrated that PLGA NPs successfully reached lysosomal compartments, where they restored normal lysosomal pH[380]. When fibroblasts derived from Parkinson’s disease patients harboring ATP13A2 mutations (L3292), and control human fibroblasts were incubated with PLGA NP, the maturation process of cathepsin D (the predominant lysosomal protease [383]) increased in L3292 fibroblasts, as indicated by a decrease in enzyme proforms and an increased mature/immature cathepsin D ratio [380]. In addition, the restoration of the lysosomal cathepsin D functionality was confirmed by an increased enzymatic activity into the lysosomal compartments of PLGA NPs-treated L3292 fibroblasts compared to untreated L3292 [380]. Based on these results, Prévot et al. tested a new formulation consisting in PLGA NPs encapsulated into a nanoemulsion (NE) containing polysorbate 80 [381] on M17 neuronal cell line stably depleted of ATP13A2, a cell model that displays abnormally high pH in their lysosomes (pH=5) [384]. As PLGA NPs, PLGA NE restored the lysosomal pH up to normal levels (pH=3) in ATP13A2 depleted cells, without lowering the lysosomal pH of control cells (shScr-1) [381]. Althrouth both studies were mainly performed in cell models of Parkinson’s disease, Bourdenx et at. also corroborated the potential of PLGA NPs to rescue lysosomal activity in GBA-mutant cells (fibroblasts from Parkinson’s disease patients with mutations p.N370S and p. G377S) [380], which have been reported as important genetic risks factor for Parkinson’s disease [385]. Hither, the incubation of GBA-mutant fibroblasts with PLGA NPs slightly decreased the lysosomal pH and increased cathepsin D maturation [380].
Finally, in vivo studies were also performed by stereotaxically injecting PLGA NPs [380,382] and NEs [381] into the substantia nigra of mice, a brain region with lysosomal disfunction and dopaminergic degeneration in Parkinson’s disease [386]. PLGA NPs were found inside lysosomes in cells surrounding the injection site 7 days post-administration, without showing cytotoxicity [380]. Prévot et al. used the PLGA NEs to improve PLGA brain-diffusion, where mice showed a greater NP dispersion in the neuronal environment, with good diffusion through the pars compacta of the substantia nigra 5 days after administration [381]. Then, assays were conducted to evaluate the in vivo bioavailability of PLGA NEs after i.v. administration, where 4 injections for 4 consecutive days rendered 0.27 PLGA NE/mm2 compared to 0.10 PLGA NEs/mm2 after a single injection, as observed in mouse brain slices containing cortex, hippocampus, striatum, and midbrain [381]. Authors showed that PLGA NEs were successfully delivered to the lysosomes of cells within the brain parenchyma suggesting their potential to rescue pathological lysosomes deficits [381].
While PLGA NCs did not carry recombinant GBA in these experiments, it is expected that enzyme encapsulation in these formulations would be readily amenable, as for other ERT examples described in this review. This could provide a dual benefit to GBA-deficient patients, both regarding GD and perhaps Parkinson’s patients, derived from improving lysosomal pH and GBA activity in the CNS. However, this remains to be investigated, as well as the possible side effects derived from this approach.
3.1.5. GM1-gangliosidosis
3.1.5.1. Disease, models, and current treatments for GM1-gangliosidosis
Possible therapeutic agents based on polymeric NPs are also studied for GM1-gangliosidosis (GM1G). This malady is a rare, progressive and neurosomatic LSD, inherited in an autosomal recessive manner [343,387,388]. Presenting > 200 pathogenic variants, GMG1 is caused by mutations in the GLB1 gene located on chromosome 3p22.3, which encodes for the enzyme β-galactosidase (β-GAL; EC 3.2.1.23). As this lysosomal enzyme cleaves the terminal non-reducing β-D-galactose residues of β-D-galactosides, its malfunction renders abnormal accumulation of GM1 and GA1 gangliosides, sphingolipids, and cholesterol in brain and spinal cord cells, causing general neurodegeneration and skeletal abnormalities in patients [343,387,388]. Although the real prevalence of this malady is unknown, it is estimated to be approximately 1:100,000 to 200,000 live births (ORPHA:354), where cases can be classified in different disease types according to the onset age. The infantile-onset form, known as GM1G type I (GM1G1; OMIM #230500), is the most severe and progressive form of the disease, whose clinical manifestations includes psychomotor regression, hepatosplenomegaly, and skeletal disease, among other alterations, all appearing before 6 months of age and culminating in early death [387,388]. The late infantile/juvenile form, or GM1G type II (GM1G2; OMIM #230600), appears between 7 months and 3 years of age with characteristic delay of motor and cognitive development [387,388]. In both cases, severe neurocognitive decline is a characteristic feature associated with premature mortality [387,388]. GM1G type III (GM1G3; OMIM #230650) is an adult/chronic form of the disease whose late onset occurs between the third and 30 years of age, mostly presenting slowly progressive dementia, ataxia, and generalized dystonia [387,388].
GM1G cell models are based on GM1G patient fibroblasts or β-GAL-deficient fibroblasts from KO or knock-in mouse models [389]. Also, human-like neurons with GM1G phenotype have been generated using iPSC techniques [266,390,391]. Additionally, different animal models have been established. So far, five different mouse models have been engineered for GM1G [387]. The first model generated was the GLB1KO mouse (β-gal−/−) generated by disruption of the GLB1 gene [392-394], while more recent models have implemented CRISPR/Cas9 technology: e.g. β-GAL deficient mice have been created either by introducing a deletion in exon 8 of GLB1 [395], or by targeting exon 15 with TALENs [396]. On the other hand, a knock-in model was engineered by inserting a human missense mutation into GLB1’s exon 14 [397]. In addition, large animal models have been established for this malady [398]: e.g., the first feline model described, resulting from a G-to-C substitution at position 1448 on the GLB1 gene [399,400] was used as a model for late infantile disease [401], while several mutations were detected in different canine models [402-404], although they have not been established [216].
Currently, only symptomatic and supportive therapy is available for GM1G treatment, including hematopoietic stem cell transplantation. However, different experimental therapies based on SRT, ERT, chaperone therapy (also called enzyme enhancement therapy), and gene therapy have been tested [405]. For instance, as Miglustat can cross the BBB, it was tested as an SRT to treat GM1G mice with encouraging results, as it reduced GM1 gangliosides in mouse CNS, leading to functional improvement [406-408]. Therefore, a Phase IV trial (NCT02030015) was performed to test this SRT in combination with a ketogenic diet in pediatric GM1G patients, where the study showed encouraging results, e.g. patients’ lifespan was prolonged and some neurodevelopmental abilities were improved [387,409]. However, this trial had a small sample size and patients presented great variability in their palliative care, making it difficult to draw reliable conclusions [387,409]. On the other hand, in vitro chaperone therapy with N-octyl-4-epi-β-valienamine [410] and ERT with purified [411] or recombinant [412] β-GAL were tested, but still more investigation is needed to start human clinical trials [387]. Finally, three trials are currently in the recruiting phase to assess β-GAL restoration by gene therapy (NCT03952637, NCT04273269, NCT04713475).
3.1.5.2. Polymeric nanocarriers studied for GM1-gangliosidosis treatment
As for other LSDs, GM1G principally affects the CNS and BBB crossing is a major challenge for treating this malady [387]. Therefore, different polymeric capsules have been investigated to try to overcome this obstacle.
3.1.5.2.1. Arginase-responsive dextran sulfate/poly-L-arginine nanocapsules
Adsorption of oppositely charged polyelectrolytes on enzyme-loaded templates allows a layer-by-layer assembly of nanocapsules capable of delivering enzymes for ERT. This design approach was used by Gupta et al. to create arginase-responsive nanocapsules assembled on β-GAL-loaded calcium carbonate templates, covered by a total of 8 layers with alternating dextran sulfate (an anionic polyelectrolyte) and poly-L-arginine (a cationic poly-amino acid) [389]. Once the layers were ready, the calcium carbonate template was removed and the resulting capsules, hereinafter referred to as arginase-responsive dextran sulfate/poly-L-arginine capsules, were structurally stable, prevented leakage of the loaded enzyme, and maintaining its activity [389]. In addition, these nanocapsules were porous with a rough surface and a large size ranging between 1–3 μm [389].
Although the mechanism of cellular uptake of these NCs was not elucidated, Gupta et al. demonstrated that these arginase-responsive capsules were effectively internalized after 12 h incubation by both β-GAL-deficient mouse fibroblasts and control wild-type cells, with no cytotoxic effects [389]. In vitro studies showed a maximum 77% enzyme release from the capsules within HeLa cells after 12 h incubation, and cell treatment with capsules reduced GM1 ganglioside levels in a time-dependent manner. For instance, after 24 h treatment, GM1 gangliosides were reduced between 1.8- and 3.4-fold depending on the cell type used, while cells treated with naked β-GAL at equivalent concentrations showed no change in GM1 ganglioside levels [389].
These results are encouraging; yet, additional in vivo research is needed to test PK, biodistribution, therapeutic and side effects of these formulations, to better assess their therapeutic potential. The size of these NCs was sufficient large as to predict a poor transport from the circulation into the CNS where their action is needed and their stability in vivo must be tested; thus, efforts should be made to provide results in these scenarios.
3.1.5.2.2. Light responsive gold-polymer hybrid nanocapsules
Gupta et al. also used layer-by-layer assembly to design light-responsive NCs constructed by adsorbing a total of 8 layers of anionic poly(sodium 4-styrene-sulfonate) (PSS) and cationic poly(allylamine hydrochloride) (PHA) on a β-GAL-loaded silica templates, subsequently replaced by a gold NP [389,413]. The resulting formulation had ≈ 500 nm diameter and an enzyme loading efficiency ≈ 25% [413].
Authors did not evaluate the mechanisms of cell uptake of these nanocapsules, but they showed intracellular staining by confocal microscopy in human cell lines and mouse fibroblasts after 12 h incubation, with no cytotoxicity up to 150 capsules per cell [413]. They also demonstrated that these NCs were light-responsive, as their breaking could be triggered by near infrared (980 nm) exposure for different time intervals. For instance, irradiation for 10, 15 or 20 min led to 1.5-, 1.8- and 2.27-fold reduction in the number of capsules and 2.7, 3.6 and 6.6% enzyme release, respectively [413].
Nevertheless, no assays with such capsules have been carried out in GM1G cells or animal models and the logics for using a light-responsive strategy is unclear for this application since the CNS is affected. As for the previous formulation, the size of these NCs seems too large for accessing the brain unless local delivery is used, for which much more investigation is still needed to assess the potential of this strategy.
3.1.6. Neuronal ceroid lipofuscinosis 1
3.1.6.1. Disease, models, and current treatments for neuronal ceroid lipofuscinosis 1
After the studies in GM1G, research for potential polymeric therapeutics against Neuronal Ceroid Lipofuscinosis 1 (NCL1; OMIM #256730) followed. NCL1 is an autosomal recessive LSD caused by > 71 mutations in the PPT1 gene on the 1p34.2 locus, which encodes for palmitoyl-protein thioesterase-1 (PPT1; EC 3.1.2.22) [414]. It is a part of a clinically and genetically heterogeneous group of monogenic neurodegenerative and fatal disorders known as Batten disease [414]. PPT1 catalyzes the hydrolysis of palmitate groups from cysteine residues in lipid-modified proteins and its deficiency leads to lysosomal accumulation of lipofuscin in neurons, an auto fluorescent lipopigment, giving rise to an ultra-structural pattern known as granular osmiophilic deposits and leading to profound and widespread neuronal loss [414,415]. NCL1 is a very rare disease manifested between the 6th and 12th month of age, whose prevalence is below 1 in 1,000,000 live births in general and 1 in 20,000 live births in Finland (ORPHA:79263). Even though infantile-onset NCL1 is the most common and severe form of the disease, late-infantile, juvenile, and adult onset forms have been reported too, appearing from 1.5-4 , 5 to 7 , and >16 years of age, respectively [414]. Regardless of the form of the disease, all NCL types lead to similar clinical features, including at least two of the following: progressive cognitive abilities declination with dementia, epilepsy, motor deterioration, and visual failure [414].
As NCL1 is such a rare disease, finding appropriate cell models has been challenging. Although primary fibroblast derived from NCL1 patients are widely used, they do not represent the neuronal tissue, which is the most affected one [414]. However, the use of iPSC technology has enabled the generation of different neuronal cell models from primary cells obtained from the skin or blood of NCL1 patients [416,417]. However, these patient-derived cells are genetically diverse and, therefore, CRISP-Cas9 technology has been used to introduce specific genetic changes into prenatal pluripotent cells to produce isogenic lines with selected mutations [414,418]. Mice are the only animals used as NCL1 models so far. There are different types, i.e. PPT1KO mice [419] and transgenic mice homozygous for the R151X mutation [420]. Both recapitulate the NCL1 human phenotype with features such as seizures, progressive motor abnormalities, storage of auto-fluorescent material throughout the brain, neuronal loss, and prominent apoptosis [419,420]. Although a canine model of NCL1 has been described [421,422], unluckily, a colony has not been established [216].
Unfortunately, at present, all forms of NCLs are fatal and no curative treatments are available. Current management consists in palliative care including the administration of antiepileptic and muscle relaxant drugs [414]. In addition, although some clinical trials have been conducted in search for treatment, including custeamine-mediated depletion of intracellular cereoid storage or the use of human CNS stem cells to produce enzyme inside the brain (see NCT00028262, NCT00337636, NCT01238315), they have shown little, if any, clinical benefit [414]. Therefore, the search for possible treatments for this disease is urgent.
3.1.6.2. New polymeric nanocarriers for neuronal ceroid lipofuscinosis 1 treatment
Although no therapeutics for NCL1 are available, Galliani et al. have recently presented a polymer-based design for enzyme delivery into NCL1 fibroblasts.
3.1.6.2.1. Poly(lactic-co-glycolic acid) nanocapsules for delivering cross-linked enzyme aggregates
An innovative synthesis method for a new enzyme delivery platform based on cross-linked enzyme aggregates (CLEAs) encapsulated into PLGA NPs has been recently presented [423]. CLEAs are produced by precipitation of the enzyme and crosslinking of respective physical aggregates, giving higher stability in organic solvents and showing unaltered enzyme activity [423]. In their study, Galliani et al. synthetized different CLEAs and subsequently encapsulated them in biodegradable PLGA NPs. Even though the in vitro assays described in this study were done in NCL1 models with PPT1-loaded formulations, authors synthetized several formulations containing different enzymes, including galactosylceramidase deficient in Krabbe disease and acid α-glucosidase deficient in PD (for more information about last two diseases see Sections 3.1.7 and Section 3.3.1). In addition to testing different enzymatic cargos, they compared PLGA NPs synthetized through the traditional double emulsion method to those loaded with non-crosslinked enzyme aggregates and those loaded with CLEAs. All formulations presented a hydrodynamic diameter between 130 and 200 nm, and ζ-potential values around −35 mV [423]. The loading efficiency varied significantly among these formulations, e.g. CLEA NPs had 88% loading efficiency, much higher than NPs prepared using the double emulsion method (32%) or enzyme aggregates (0.9%) [423]. As expected, non-encapsulated CLEAs showed lower activity than classical enzyme aggregates due to their crosslinking (6% vs. 54% PPT1 activity yield, respectively), but this was the opposite when encapsulated within PLGA NPs (64% PPT1 activity yield), likely due to enhanced catalytic activity of NP-immobilized enzymes [423].
These NCs were evaluated in vitro using fibroblasts from NCL1 patients, where PPT1 CLEA NPs were internalized and reached lysosomal compartments 6-24 h after treatment, depending on the formulation, without cytotoxicity [423]. A similar outcome was observed when naked enzyme was used, but in this case, the enzyme disappeared after 24-48 h while PPT1 from CLEA NPs remained visible within cells by this time, suggesting that the former formulation was more stable [423]. At doses < 110 U, cell incubation with PPT1 CLEA NPs or naked PPT1 did not show any differences; but at doses > 110 U, cells treated with naked enzyme reached an activity plateau, while in cells treated with PPT1 CLEA NPs activity remained dose dependent, increasing up to the maximum tested dose of 220 U [423].
Despite these positive results, additional studies are necessary to discern whether this strategy holds any potential to cross the BBB and deliver enzyme within the CNS, the main target for NCL1 treatment, along with studies to establish the PK, full body biodistribution, therapeutic, and side effects of these formulations. It is also plausible that enzyme aggregates of this type can enhance hyper-reactivity and immunological side effects, but this remain to be investigated.
3.1.7. Krabbe disease
3.1.7.1. Disease, models, and current treatments for Krabbe disease
Krabbe disease (KD; OMIM #245200), also known as globoid cell leukodystrophy, has more recently been the focus of possible treatments based on polymeric NCs. KD is a pediatric, autosomal recessive, fatal LSD caused by > 250 mutations in the GALC gene on chromosome 14q31.3, which encodes for galactosylceramidase (GALC; EC 3.2.1.46). Since GALC is involved in the catabolism of galactosylceramide, absent or reduced activity of this enzyme leads to galactosylceramide and psychosine (also called galactosylsphingosine) accumulation mostly on the white matter of the central and peripheral nervous systems, giving rise to characteristic globoid cells in these tissues [424]. KD has an estimated worldwide incidence of 1 in 100,000-250,000 live births (ORPHA:487). Most (85-90%) patients present symptoms within the first 6 to 12 months of life, representing an infantile disease characterized by irritability, muscle weakness, feeding problems, stiff posture, and rapid severe neurologic deterioration that leads to death between 8 months and 9 years of life (2 years on average) [424,425]. The rest (10-15%) of patients have a rarer later onset after 12 months of age, with features among those of late-infantile (6 months to 3 years), juvenile (3 to 8 years), and adult-onset forms [424,425]. These clinical manifestations are generally less severe and much more variable with no specific course [424,425].
As is neuro-pathogenic disease, finding accurate cellular models for this disease has been challenging because of poor availability of established neural cells [426,427]. However, KD patient fibroblasts or murine model-derived cells, such as Twitcher mouse fibroblasts, have been widely implemented [426,427]. In addition, screening for KD treatment has been conducted using different neuron, microglia, astrocyte, and oligodendrocyte cell lines [428]. Twitcher mice (TWI, Galctwi) are the main animal model used for this malady [427], which can be heterozygous (TWI+/−) or homozygous (TWI−/−) for a spontaneous nonsense mutation that produces a truncated GALC transcript, leading to the elimination of GALC protein expression and KD phenotype, although this mutation is not found in KD patients [429]. Another mouse model presents a spontaneous mutation in GALC (GALCtwi-5J), matching the E130K missense mutation present in infantile KD [430]. There is also a conditional GALC mouse generated by using a floxed allele of respective gene, whose early induction causes a more severe KD phenotype with higher psychosine levels in mouse neuronal tissues, leading to a shorter lifespan [431]. Finally, large KD animal models have also been established, including canine [432] and simian [433] colonies showing psychosine accumulation in nervous tissues [424,434].
Unfortunately, no cure is currently available for KD. For now, treatment other than supportive care is limited to hematopoietic stem cell transplantation for some patients, for whom treatment is individualized based on their disease burden and manifestations [424,425]. In addition, bone marrow transplantation is the only clinical method applied to delay disease’s progression [424]. However, some Phase I/II clinical trials for KD gene therapy have been initiated, to assess the safety and efficacy of two different adeno-associated viral vectors: FBX-101, a product expressing human GALC cDNA (NCT04693598) and PBKR03, a vector serotype Hu68 carrying the gene encoding for human GALC (NCT04771416).
3.1.7.2. Polymeric nanocarriers studied for Krabbe disease treatment
To our knowledge, there are very few therapeutic options currently under investigation for KD. This involve the use of polymeric NPs aimed to transport therapeutics across the BBB and treat neuronal tissue affected in this pathology, as described below.
3.1.7.2.1. Brain-targeted enzyme-loaded poly(lactic-co-glycolic acid) nanoparticles
Based on their previous results (see Section 3.1.6.2.1, Galliani et al. [423]), the same group recently reported the encapsulation of GALC CLEAs into PLGA NPs (Del Grosso et al. [426]). They modified this formulation by functionalizing NPs with three different brain-targeting peptides to enhance NP brain delivery through the BBB [426]. The selected peptides were angiopep-2 (Ang2), transferrin-binding peptide-2 (Tf2), and a 7-aminoacid glycopeptide that is believe to target opioid receptors, called g7 [426]. Ang2 had been shown to accumulate in brain endotheial cells and cross the BBB by transcytosis in cellular and brain perfusion models [435]. Tf2 had been previously designed to bind transferrin and was observed to promote the endocytosis of gold NPs by transferrin-expressing cells [436], a receptor also expressed at the BBB [106,233]. Additionally, g7 was reported for the first time by Costantino et al. [437], who synthesized the peptide and subsequently anchored it to PLGA NPs as a potential nanotherapeutic for MPS [438,439], described below in Section 3.2.1.3.2.
Del Grosso et al. [426] synthetized and compared Ang2-, Tf2- and g7-targeted GALC CLEA NPs as well as matching non-targeted counterparts. For this purpose, GALC CLEAs were produced by precipitation in acetone, followed by crosslinking; in parallel, each targeting peptide was covalently attached to the terminal carboxylic acid group of PLGA copolymer, and finally GALC CLEAs were loaded in NPs made of the modified PLGA by nanoprecipitation [426]. All NPs had 150-190 nm diameter, ζ-potential ≈ −30 mV, and were differently loaded with GALC, i.e. 39%, 60%, and 74% encapsulation efficiency for Tf2-, Ang2- and g7-targeted NPs, respectively [426]. Despite their lower encapsulation efficiency, the activity yield for Tf2-targeted GALC CLEA NPs, together with that of Ang2 NPs, were the highest, with values around 67 and 61%, respectively, compared to 37% for GALC g7 NPs [426].
With regards to the ability of these formulations to interact with and enter cells to deliver active enzyme to lysosomes, different assays were conducted. First, all NPs colocalized similarly with lysotracker when observed by confocal microscopy after their incubation with fibroblasts from TWI mice or control mice, and this was also the case for non-targeted NPs [426]. However, whether targeting helped or not on this regard could not be established, likely because samples were observed 24 h after NP incubation and their kinetics could not be appreciated. Importantly, cells recovered GALC activity in a dose-dependent manner, reaching and even surpassing wild-type values (at 3 U and 6 U), and clearly outperforming naked GALC that just reached 75% wild-type activity level [426]. Yet again, no clear advantage of targeting was observed vs. non-targeted NPs [426]. Other in vitro assays using fibroblasts from KD patients showed that GALC activity remained in cells for at least 4 days when delivered by NPs, maximum time investigated, while it significantly decreased for the naked enzyme [426]. Curiously, the activity delivered by g7-targeted formulations remained similarly high for 1 and 4 days after incubation with cells, while those associated with Ang2- or Tf2-targeted NPs were lower after 1 day but increased by day 4 [426]. This may reflect different targeting abilities of these formulations, different levels of receptor expression on these cells, and/or different mechanisms used by respective NPs to enter and traffic into them. Although it is believed that all three formulations target markers associated to clathrin-mediated transport [435,440-443], this was not evaluated and, nevertheless, each marker may associate with a different rate of transport.
Indeed, this seems the case as deducted from an in vivo experiment in TWI mice [426]. Animals were treated with GALC CLEA NPs or naked GALC administered via a single intraperitoneal (i.p.) injection and the enzyme activity was measured after 4 h in different organs/tissues, including the brain, spinal cord, sciatic nerves, liver, and kidneys. Targeted NPs minimally impacted GALC activity in the kidney, sciatic nerve or spinal cord of TWI mice, similarly to non-targeted NPs or naked GALC, indicating that none of the formulations accumulated in these tissues [426]. Contrarily, all formulations raised liver GALC activity similarly, up to levels seen in heterozygous mice, including targeted and non-targeted NPs, as well as naked GALC [426]. This was expected based on the open vasculature of the liver, making it similarly accessible from the circulation. Most importantly, only targeted NPs increased enzyme activity in the brain of TWI mice, up to the level seen for heterozygous animals (≈ 45% activity compared to wild-type), while this was not the case for non-targeted NPs or naked enzyme [426]. Therefore, it would appear from these data that targeted NPs could cross the BBB and enter the brain.
Despite these encouraging results, more investigation is needed. For instance, the PDI and stability of these formulation were not reported. The fact that the NP activity yield was different from what would be expected from their encapsulation efficiency, indicates that the enzyme was differently inactivated. This must be considered for future design of these strategies and the estimation of the associated cost and appropriate dosing. This result is puzzling because all NPs followed a similar preparation method; thus, additional investigation may clarify whether these differences arise from the different peptide-linked copolymers used. Specific NP targeting, cellular uptake mechanism, and their kinetics should be investigated. Additional studies should measure substrate reduction and determine CNS function improvement, as well as toxicity and long-term effects. From the presented in vivo data, it is also difficult to infer which of the targeted formulations performs best, for which cellular experiments in BBB models or PK/PD assays in vivo would help.
3.2. Mucopolysaccharidosis
Mucopolysaccharidosis (MPS) is a family of rare multisystemic LSDs caused by the absence/deficiency of different enzymes involved in the degradation of negatively charged polysaccharide compounds known as glycosaminoglycans (GAGs). This lead to GAGs, mainly heparan sulfate and dermatan sulfate, accumulation in most tissues [444,445]. MPS can be classified into several types according to the affected enzyme [343,444]: MPS type I (MPS1), type II (MPS2) , type III A-D (MPS3), type IV A and B (MPS4), type VI (MPS6), type VII (MPS7), and MPS type IX (MPS9). Although each disease type has its own symptomatology, they all have common pathologies in bones and joints, lungs, heart, liver, spleen, and eyes, while severe MPS, such as type I, II and VII, also impact the CNS [444]. Here, we focus just on those MPS types where polymeric NCs have been investigated: MPS1, MPS2, MPS6, MPS7, and MPS9.
3.2.1. Mucopolysaccharidosis types I, II, VI, VII, and IX
3.2.1.1. Disease characteristics
3.2.1.1.1. Mucopolysaccharidosis type I
MPS1 is a rare autosomal recessive disorder affecting around 1 in 100,000 live births (ORPHA:579) [446]. It is caused by > 100 mutations in the gene encoding α-L-iduronidase (IDUA, EC 3.2.1.76) located on chromosome 4p16.3 [446]. As IDUA hydrolyzes α-L-iduronic acid residues from the non-reducing ends of heparan sulfate and dermatan sulfate, its deficiency leads to the accumulation of these partially degraded subtrates in tissues [446]. Depending on their severity, MPS1 patients can belong to the Hurler (OMIM #607014), Hurler-Scheie (OMIM #607015) or Scheie (OMIM #607016) clinical phenotypes, which respectively correspond to severe, intermediate, and mild forms of the disease, with 57% cases being severe [446]. At birth, Hurler syndrome infants seem normal, but after 1 year they start to show coarse facies, corneal clouding, hernias, progressive skeletal dysplasia and joints atrophy, hepatosplenomegaly and mental retardation, evolving to death in the first 10 years of life [447]. These manifestations are also present in the Hurler-Scheie syndrome, but these patients have little to no intellectual dysfunction, a later onset between 3 to 8 years of age, and they survive to adulthood (see OMIM #607015). Scheie syndrome (earlier known as MPS type V) features are milder, including stiff joints, cornea clouding and little if any intellect impairment, with an onset around 5 years of age and diagnosis between 10 to 20 years of age (see OMIM #607016).
3.2.1.1.2. Mucopolysaccharidosis type II
MPS2 (Hunter syndrome, OMIM #309900), is the only X-linked recessive inherited MPS, affecting about 1-9 patients per 1,000,000 live birth (ORPHA:580) [448,449]. Even though it would not be expected to affect women, few ones have been reported with attenuated MPS2 phenotype. This syndrome is caused by mutations on the gene encoding for iduronate-2-sulfatase (IDS, EC 3.1.6.13) on chromosome Xq28, where > 600 variants have been described [448]. IDS catalyzes the hydrolysis of the 2-sulfate groups of the L-iduronate-2-sulfate units of heparan sulfate, dermatan sulfate, and heparin; therefore, its deficiency leads to the accumulation of these GAGs in almost all cell types. Clinical manifestations include coarse facial features, short stature, skeletal abnormalities, severe airway obstruction, cardiomyopathy, and neurological decline [449]. Patients usually die on their second decade, but less severe ones can survive until their fifth or sixth decade of age [449].
3.2.1.1.3. Mucopolysaccharidosis type VI
MPS6 (Maroteaux–Lamy syndrome, OMIM #253200) is a rare MPS affecting 1-9 patients in 1,000,000 live births (ORPHA:583) [450]. This autosomal recessive LSD is caused by > 130 mutations in the ARSB gene on the 5q14.1 locus, which encodes the lysosomal enzyme arylsulfatase B (ASB, or N-acetylgalactosamine-4-sulfatase; EC 3.1.6.12) [450]. As ASB hydrolyzes 4-sulfate groups from the N-acetyl-D-galactosamine residues of chondroitin sulfate and dermatan sulfate, its deficiency leads to the accumulation of these GAGs in tissues [450]. MPS6 symptoms are varied, but this disease is mostly characterized by osteoarticular alterations such as skeletal dysplasia, with short stature, dysostosis multiplex, and degenerative joint disease [450]. Two different progressive forms have been described: a rapidly progressive form (ORPHA:276212) can present early onset at birth, with severe dysostosis multiplex, elevated urinary GAG (>100μg/mg creatinine), short stature, and death before 20-30 years of age [450]. A slowly progressing form (ORPHA:276223) has a later onset, with mild dysostosis multiplex, moderate content of GAGs in urine (<100μg/mg creatinine), and death between 40 and 50 years of age [450]. Other clinical symptoms may include cardiac valve disease, reduced pulmonary function, hepatosplenomegaly, different ocular, ear, nose and throat manifestations, and some neurological pathologies such as cervical cord compression caused by cervical spinal instability, myelopathy and abnormalities in the ventricular system, white matter and perivascular spaces [450].
3.2.1.1.4. Mucopolysaccharidosis type VII
MPS7 (OMIM #253220), also known as Sly syndrome, is the rarest MPS, having prevalence between 1 in 345,000-5,000,000 live births (ORPHA:584) [451]. This is an autosomal recessive inherited disorder caused by around 50 mutations in the GUSB gene located on the 7q11.21 locus, mostly missense mutations [451]. GUSB encodes β-D-glucuronidase (GUS), a lysosomal enzyme whose deficiency leads to the inability to degrade glucuronic acid-containing GAGs such as heparan sulfate, dermatan sulfate, and chondroitin 4- and 6-sulfates [451]. MPS7 severity ranges between lethal hydrops fetalis to mild forms with survival into adulthood, where the intermediate phenotype shows hepatosplenomegaly, skeletal deformations, short stature, coarse facies, and mental impairment [451].
3.2.1.1.5. Mucopolysaccharidosis type IX
MPS9 (OMIM#601492) is a rare MPS with that affects > 1 in 1,000,000 live births (ORPHA:67041) and is inherited in an autosomal recessive manner [452,453]. It is caused by mutations affecting gene HYAL1, which is located on chromosome 3p21.31 and encodes for hyaluronidase-1 (HYAL1 or HAse), also called hyaluronoglucosaminidase-1 [452,453]. As a consequence, this enzyme deficiency leads to aberrant accumulation of hyaluronic acid (hyaluronan), one of the main GAGs in the extracellular matrix of vertebrates, in various body locations [454]. Onset is during childhood and progresses through life, with clinical manifestations mainly include joint and skeletal abnormalities, including the appearance of periarticular soft-mases associated with swelling, synovitis, and pain in joins such as knee and hip, as well as craniofacial alterations such as cleft palate, flatteted nasal bridge, and other defects, without involvement of visceral organs or CNS [454].
3.2.1.2. Models and current treatments for mucopolysaccharidosis types I, II, VI, VII, and IX
Fibroblasts from MPS patient biopsies are the most used cell model since they recapitulate well GAG storage [448,455,456]. However, human iPSCs are being widely used to study neuropathic phenotypes, because they better represent patients’ cellular pathology [266]. For instance, lower endocytosis rates and lysosomal and autophagic traffic alterations have been documented in iPSC derived MPS2 neuronal progenitor cells [457]. Human iPSCs have been isolated for MPS1 [458,459], MPS2 [457,460-462], and MPS7 [463] patients. In addition, for MPS2, murine neural stem cells have been used to differentiate several neurological cell types, which have facilitated the study of the mechanism leading to neuronal defects in this malady [464].
Regarding animal models, mouse models are the most common. For instance, the first KO mouse for these diseases was generated for MPS2 by replacing part of exon 4 and the entire exon 5 of the IDS gene (Ids−/−) with a neomycin resistance gene [465,466]. This approach was also used to generate two other KO mouse models: a MPS1 model by disrupting the exon 6 of the IDUA gene on a C57BL/6 background (Idua−/−) [467] and another MPS2 model by replacing 14885 bp of exon 2 and 3 of the IDS gene [468]. In addition, two other IDS KO mice were reported, both having the same deletion from exon 2 to exon 5 of the IDS gene [469,470]. All these MPS2 mouse models reflect well the human pathology, with loss of IDS activity and elevated GAGs in urine and organs such as liver, kidney and heart, skeletal deformities, neurological alterations, and vacuolization in many tissues [448]. In case of MPS6, a C57BL/6J mice colony with mutations in the Asb gene, allele m1J (Arsbm1J) was stablished because of it resemblance with MPS6 lysosomal storage of GAGs and phenotypic characteristics, such as stiff joints, dysmorphic facial features and cardiac abnormalities [471]. In addition, a rat model has also been establish established in the Ishibashi hairless (ISH) rat strain. This ASB-deficient rats presented typical pathological features such as facial dysmorphia, increased urinary excretion of GAGs, dysostosis multiplex, and an increased storage of GAGs into the cells of the reticuloendothelial system, cartilage and other connective tissues, but no into the CNS [472]. Lately, for MPS7 many mice models have been described, e.g., two homozygous mutant GUSB mice were naturally generated either by a frameshift mutation (1-bp deletion, c.147delC) in exon 10 (gusmps/mps) [473,474], or by an insertion into intron 8 (gusmps2J/mps2J) of the GUSB gene [475]. Both mice presented severe disease phenotype including dysmorphic features, short life-span, skeletal dysplasia and lysosomal GAGs storage affecting multiple tissues, however, gusmps2J/mps2J mice had <1% normal GUS activity and a milder phenotype than gusmps/mps [451,476]. Transgenic mice expressing inactive human GUS were also engineered [477,478]. These MPS7 mouse presented the same histopathological characteristics of gusmps/mps mice but having immune tolerance against human GUS, becoming useful for preclinical ERT trials [451,476]. The mouse model for MPS9 is a KO model generated by targeted insertion of a neomycin resistance cassette in exon 2 of HYAL1 gene, which rendered no detectable enzyme activity [479]. These mice develop cartilage and articular abnormalities around the third month of life, which progresses through an osteoarthritis phenotype without organomegaly, similar to that of human patients. On the other hand, large animal models have also been stablished for some MPS [216]. For MPS1, MPS6 and MPS7, both canine [480-483] and feline [484-487] spontaneous models have been identified with features that mimic the human conditions, where for MPS1 dogs mimic the Hurler-Scheir syndrome, while cats imitate the Hurler syndrome. Finally, two MPS2 zebrafish (Danio rerio) models were engineered: an IDS knocked-down model was obtained via antisense morpholino oligos [488], and a knocked-out IDS was achieved by CRISPR/Cas9 mediated deletion of 5-pb on IDS exon 2 [489]. Both fish models were used to show IDS gene involvement in developmental processes [488,489].
Besides symptomatic management of the general conditions of the disease, current MPS treatments basically presents two different approaches: (1) hematopoietic stem cell transplantation as a standard procedure with a high risk of severe immunogenic reactions; and (2) ERT with recombinant enzymes produced in CHO cells, as laronidase (Aldurazyme®), idursulfase (Elaprase®), galsulfase (Naglazyme®), and vestronidase-α (Mepsevii™), all clinically approved for MPS1, MPS2, MPS6 and MPS7, respectively. However, a poor penetration of the recombinant enzymes into the BBB as well as the ocular barriers and into poorly vascularized tissues, such as cartilages and bones; infusion-related hypersensitivity reactions; and anti-drug antibodies production in patients treated with some of these enzymes have been detected [60,448]. Even though many other i.v. and intrathecal ERT are being investigated for the different types of MPS, these all still are ongoing clinical trials to elucidate their safety and efficacy. These treatments were well summarized by Safary et al. [444], and the registered trials can be consulted on ClinicalTrials.gov data base e.g., NCT02371226, NCT03053089 and NCT04532047 for MPS1; NCT02262338 and NCT01602601 for MPSII; NCT00214773 for MPS6; and NCT02432144 for MPS7. In addition, a SRT study with Odiparcil in MPS6 patients has recently completed its Phase II trial (NCT03370653); however, results have not been published yet. Gene editing strategies are also being pursued, but still at early research stages, as reviewed by Dr. Matte in this issue [490]. Finally, gene therapy treatments are being widely studied for MPS since this group of diseases have a monogenetic origin and can be potentially treated by systemic cross-correction [448,491]. Currently, several clinical trials using this approach are ongoing e.g., NCT03580083 and NCT03488394 for MPS1; NCT03566043, NCT04571970 and NCT05238324 for MPS2; and NCT03173521 for MPS6.
3.2.1.3. Polymeric nanocarriers studied for mucopolysaccharidosis treatment
Novel treatments based on ERT are under investigation for MPS treatment using polymeric-based NC designs, as described below.
3.2.1.3.1. Poly(butyl cyanoacrylate) nanoparticles
Poly(butyl cyanoacrylate) (PBCA) is a biocompatible and biodegradable homopolymer used in pharmaceutics as a carrier matrix [492]. PBCA NPs have been investigated as carriers for several drugs, including cases where brain delivery was needed [493-496]. The Kreuter group used this type of NPs to carry recombinant ASB, which was achieved by adsorbing the enzyme on the surface of 150 nm PBCA NPs for a pilot proof-of-concept aimed at MPS6 [497]. The resulting formulation presented a homogenous size distribution, having 166 nm diameter and spherical shape, 0.2 PDI, and −4.4 mV ζ-potential [497]. Authors used dextran as a particle stabilizer and varying temperature, pH, and enzyme concentration to optime formulations [497]. Whereas neither temperature nor pH had a very important effect on enzyme adsorption, enzyme concentration had a higher effect, achieving a maximal loading of 64.5 μg ASB/mg NPs at pH 6.3 at 3,000 μg/ml ASB input [497]. Enzyme release from NPs was assessed in vitro in different media at 37 °C. Results showed that 22% of the adsorbed ASB was released immediately after dilution in human serum and, after 60 min incubation, 32% and 30% release was observed in TRIS buffer pH 7.4 and human serum, respectively [497]. Unfortunately, no further studies were conducted, for which many key data are missing for this strategy. This formulation had a ζ-potential close to neutral, which poses aggregation concerns. It is unclear whether the quick enzyme release observed is amenable to withstand the required incubation in cell culture and tissue accumulation in vivo, and whether the released or carried enzyme is active. Additionally, in vitro and in vivo studies in cell cultures and model animals are necessary to evaluate the performance of these NPs in the context of cellular uptake, transcytosis, circulation and biodistribution, as well as any potential therapeutic and side effects.
3.2.1.3.2. Poly(lactic-co-glycolic acid) nanoparticles targeted by g7 glycopeptide
PLGA NPs have also been used as carriers in MPS models by the Tosi group. Aiming at improving the delivery across the BBB, PLGA NPs were modified with the g7 glycopeptide mentioned in Section 3.1.7.2.1 above for brain targeting (Figure 7A) [437-439,498]. These g7-targeted NPs were spherical, with a homogenous size distribution (0.08-0.18 PDI), < 300 nm diameter, and negative ζ-potential (−9 to −34 mV). First, unloaded NPs were tested [437,498]; later, Salvalaio et al. used FITC-albumin as a model cargo with molecular weight similar of that of many lysosomal enzymes [438] and, finally, the group encapsulated IDS as a therapeutic enzyme for MPS2 [439]. The loading efficiency of g7-NPs was around 35% for albumin used as a model protein, and 15% for IDS, around 30-50% lower than the one achieved for non-tagged NPs, probably because loading occurs before the activation and conjugation with peptides, and both process could contribute to possible drug content loss [438,439].
Figure 7. Poly(lactic-co-glycolic acid) nanoparticles for Mucopolysaccharidosis II.
(A) Poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) modified or not with g7 glycopeptide for targeting and uptake in cells after intravenous i.v. injection in mice. NPs were loaded with iduronate-2-sulfatase (IDS) for treatment of Mucopolysaccharididosis type II (MPS2). (B) Fibroblasts from MPS2 patients were left untreated (UT) or treated with naked IDS or IDS-loaded PLGA NPs (u-NPs-IDS), and IDS activity delivered to cells was measured after 0 (T0), 7 (T7) or 14 (T14) days. IDS activity is expressed in nmols of 4-methylumbelliferyl produced in 4 h per mg of protein. (C) GAG content detected in the brain of IDS knockout mice 6 weeks after i.v. injection with g7-targeted PLGA NPs loaded with IDS, compared to non-loaded formulations, naked enzyme, untreated (UT) mice or control wild-type (wt) mice. Data are mean±SD. (B,C) Adapted and reproduced with permission from L. Rigon et al., Targeting brain disease in MPSII: Preclinical evaluation of IDS-loaded PLGA nanoparticles, Int. J. Mol. Sci. 20 (2019) 1–15 [439].
These formulations were studied in vivo. First, Constantino et al. administered these NPs, fluorescently labeled, in rats using femoral perfusion and then examined brain slices obtained at sacrifice after several minutes [437,498]. Microscopy showed fluoresce in different brain regions, including perinuclear regions of cells within the brain, which was more apparent than of animals perfused with non-targeted NPs, although samples were not quantified [437,498]. Then, Salvalaio et al. injected i.v. fluorescenely-labeled albumin-loaded PLGA NPs targeted by g7 in wild-type and IDUAKO mice, and examined brain samples obtained 2 h after administration. In the case of IDUAKO mice, sample quantification revealed around 1,600 NPs per optical field for this targeted formulation, while untargeted counterparts showed 21 NPs/field, demonstrating a great enhancement in brain accumulation for the targeted formulation [437,498]. Compared to wild-type mice, IDUAKO mice accumulated 5-fold increased amount for targeted NPs, which was believed to be due to an altered permeability barrier in the disease mouse model [437,498]. Liver was also observed to accumulate NPs, e.g. around 10,000 NPs/field in the KO model and 7,000 NPs/field in the wild-type control, less different because the open vasculature in this organ does not require targeting. For this reason, it is also expected that significantly larger amount of NPs were found in this organ vs. the brain [437,498]. Just as in the previous study, targeted NPs were visualized in the perinuclear region of cells in the brain [437,498]. Finally, Rigon et al. used a similar formulation loaded with IDS and conducted in vitro and in vivo experiments [439]. MPS2 patient fibroblasts were treated for 7 days with naked IDS or IDS-loaded g7-PLGA NPs, after which they measured significant enzyme activity in cells, twice that activity of cell treated with untargeted NPs, and manyfold above almost undetectable levels of activity in untreated cells [439]. This activity was reduced by almost 10-fold after 7 days, and another 10-fold by day 14 after treatment, although some activity was still measurable (Figure 7B) [439]. Additionally, treatment with IDS-loaded NPs showed significant reduction in GAG storage, down to wild-type levels, which was sustained for 7 days, and this was similar for both targeted and untargeted NPs [439]. Unfortunately, the study did not provide a comparison with naked IDS, to appreciate the gain by NP encapsulation. IDSKO mice were then i.v. injected weekly for 6 weeks with IDS, either naked or encapsulated in g7-PLGA NPs, or with NPs without enzyme, and wild-type mice were used as controls [439]. Results showed GAG reduction in peripheral organs, e.g. 60% in the liver, after IDS-loaded g7-NPs compared to untreated mice, but this reduction was much pronounced (95%) in animals treated with naked IDS [439]. This was believed to be due to the incapacity of naked IDS to cross the BBB, resulting in a greater bioavailability on peripheral organs. However, GAG brain deposits, as well as markers such as LAMP2, CD68, or GFAP, were significantly reduced only in animals treated with IDS-loaded g7-NPs, ≈ 25% reduction compared to untreated mice (Figure 7C), demonstrating the capacity of this formulation to reach the brain tissue and ameliorate disease markers [439].
Therefore, this is a promising strategy for MPS2 treatment and perhaps other LSDs. Questions such as the stability of these formulations, PK, quantitative biodistribution, enzyme release profile, duration of activity, etc. must be investigated, along with additional assessment of the therapeutic outcomes, such as behavioral, cognitive and motor improvement, or potential toxic and side effects of these formulations.
3.2.1.3.3. Poly(lactic-co-glycolic acid) nanoparticles targeted to intercellular adhesion molecule 1
A study performed by our group described in Section 3.1.1.2.1 concerning ASMD also encompassed anti-ICAM-1 PLGA NPs carrying HAse [80]. As for recombinant ASM, HAse was encapsulated in PLGA NPs using the double-emulsion solvent evaporation method. Three different commercial copolymers were first compered, including Resomer, Lactel I and Lactell II, all which contained 50:50 lactic-to-glycolic acid ratio but differed regarding their molecular weight (31, 45, and 68 kDa, respectively) and end termination (acid termination for Resomer vs. ester termination for both Lactel copolymers). Three different surfactants were used to stabilize NPs, including PVA, DMAB, and Pluronic F68. Out of all these formulations, the one using Lactel II copolymer and F68 surfactant presented the best characteristics, e.g. ≈ 210 nm diameter, 0.2 PDI, −18 mV ζ-potential. The protein input was additionally varied by changing the protein-to-copolymer ratio, introducing or not albumin in the encapsulating protein mixture, and varying the enzyme-to-albumin ratio. This rendered 10 different formulations, all with acceptable parameters, e.g. 150–230 nm diameter, ≤ 0.2 PDI, −28 to −36 mV ζ-potential, and their enzyme content varied between 210 and 885 HAse molecules per NP. Anti-ICAM-1 was adsorbed on 140–150 nm NPs, which increase size up to 230-235 nm without impacting PDI much (around 0.21–0.28 after coating) and resulting in120-250 antibody molecules per NP depending on the formulation. These formulations were stable in saline buffer at 4 °C, after lyophilization in 7.5% trehalose and reconstitution, and at least for 48 h at 37 °C in 50% serum (<20% change in size or PDI) [80,234].
Experiments conducted on cellular models revealed that anti-ICAM-1/HAse PLGA NPs bound specifically to ICAM-1 expressing cells (e.g. 118 NPs/cell compared to only 6 NPs/cell for non-targeted NPs, after 30 min incubation), which lead to fast internalization within cells, e.g. 250 NPs/cell by 3 h, and lysosomal colocalization, e.g. 65% after 3 h incubation. Anti-ICAM-1/HAse PLGA NPs increase HAse delivery in cell culture by 18-fold compared to naked enzyme in a 24 h period, and HAse activity was maintained for up to 7 days, decaying by only 30% after 28 days in culture [80,234].
Therefore, this type of formulation also holds value in the context of MPS. Yet, future studies should focus on in vivo models to define circulation, biodistribution, and respective therapeutic/risk balance.
3.3. Glycogen storage diseases
3.3.1. Pompe disease
3.3.1.1. Disease, models, and current treatments for Pompe disease
Pompe disease (PD, OMIM #232300), also called glycogen storage disease type II, is an autosomal recessive muscle disorder caused by > 500 mutations in the GAA gene, located on chromosome 17q25.3 [499]. These alterations can reduce or eliminate the expression or activity of acid α-glucosidase (GAA) also called acid maltase (EC 3.2.1.20), an enzyme that hydrolyzes terminal 1,4-α-glycosidic linkages of oligo- and polysaccharides [499,500]. GAA dysfunction renders glycogen break-down deficiency, leading to accumulation of this polysaccharide within lysosomes of different tissues, mainly cardiac, skeletal and smooth muscle [500]. PD is estimated to affect around 1-9 patients in 100,000 live births (ORPHA:365). Two different forms of the disease can be distinguished: early/infantile vs. the late/adult phenotypes [501,502]. The former is the more severe form, whose symptoms begin in the first month of life, presenting feeding problems, poor weight gain and hypotonia [501]. Then, severe hypertrophic cardiomyopathy, and hepatomegaly appears, leading to death due to cardiorespiratory failure, often complicated by respiratory infections, all before 1 year of age [501]. The late-onset form of the disease generally presents a slower progression, with individuals having the onset before 12 months of age without cardiomyopathy, and those with an onset after 12 months of age, with proximal muscle weakness, respiratory insufficiency, and almost no cardiac involvement [502]. In these patients, the prognosis is dependent upon the extent of respiratory muscle involvement [502].
Primary fibroblasts from PD patients are the most used cell models for in vitro therapeutic efficacy studies [503-505]. However, PD-like models can also be pharmacologically induced, e.g. by overnight incubation with 300-600 μM of D(+)-turanose, a competitive inhibitor of GAA that stimulate glycogen accumulation within cells [505-507]. In addition, cardiomyocytes [508-511] and myocytes [512] expressing different PD phenotypes have been successfully generated from iPSCs. Regarding in vivo studies, animal models are based on GAA-KO mice, which have homozygous disruption of GAA gene (GAA6neo/6neo) and present intra-lysosomal glycogen accumulation in cardiac and skeletal muscle cells, reproducing the typical mobility and strength defects of PD infants [513]. Additionally, Baik et al. recently engineered a new PD mouse model by replacing the GAA gene with the LacZ operon, and exons 2-7 of Cdg3 locus for the orthologous human CD63 exons (Gaa−/− Cd63hu/hu) [514]. The resulting model presents a glycogen storage phenotype equivalent to GAA6neo/6neo mice [514]. Canine [515], bovine [516], and feline [517] animals carrying GAA mutations have been identified, but none of them have been established as models yet [216].
Apart from palliative care such as individualized cardiomyopathy treatment, physical therapy for muscle weakness, feeding and respiratory support [518], PD treatment aims to prevent the primary manifestations of the disease by beginning with ERT as soon as a diagnosis is established [502]. In contrast to the other LSDs, therapeutic enzymes for PD were first developed for the early-onset form of the disease rather than the latter-onset one [519]. Currently, ERT for this malady is based on the administration of Myozyme® or Lumizyme®, both Alglucosidase alfa produced in CHO cells, approved by regulatory agencies for the treatment of infantile-onset and adult-onset PD, respectively [520,521]. Despite their unquestionable value, these treatments presents some drawbacks, such as high risk for patients to develop anti-enzyme antibodies [522-524], as well as the fact that the level of mannose receptor expression on PD skeletal muscle cells is low, needing higher ERT doses to achieve a therapeutic effect [525]. In addition, although an ERT approach combining a novel recombinant GAA (Cipaglucosidase alfa) with an enzyme stabilizer (Miglustat) has been designed to try to increase the effect of the therapeutic enzyme, it appears to have no greater effect than the treatment with Alglucosidase alfa (Phase III trial, NCT03729362). However, long-term safety studies are still pending to determine if the two-component therapy might provide benefits compared with currently available ERTs [526]. On the other hand, gene therapy is being investigated as a potential treatment for this malady. For instance, recombinant adeno-associated virus vectors carrying codon-optimized GAA coding region has been tested in a Phase I clinical trial to evaluate toxicity, biodistribution and potential activity after intramuscular injection in late-onset PD patients (NCT02240407). Although this trial was completed in 2021, its results have not been published yet, and other clinical trials based on similar models are currently in recruiting phase (NCT03533673).
3.3.1.2. Polymeric nanocarriers studied for Pompe disease treatment
Polymer-based NCs under investigation aimed at PD include PS models and PLGA NCs loaded with GAA, either receptor-targeted or not [505-507]. These studies are described below.
3.3.1.2.1. Polystyrene nanocarrier models targeted to intercellular adhesion molecule 1
As described for ASMD and FD, model PS NCs targeted to ICAM-1 have also been explored by our group as a proof-of-concept to increased GAA delivery (Figure 8A). In this case, a mix of anti-ICAM-1 and GAA of 50:50 or 95:5 antibody:enzyme mass ratio was adsorbed on 100 nm-diameter PS beads for in vitro and in vivo tests, respectively [507]. The resulting NCs had ≈ 180 nm diameter, 0.2 PDI, and carried ≈ 280 GAA molecules per particle, with a loading efficiency > 85%, when co-coated at a 50:50 mass ratio [507]. This coating showed to be stable for the intended experiments. For instance, only 11.6% protein release was observed by repeated pipetting and sonication, and 6% release was observed under storage in buffer at 4°C for 3 days (maximum time tested) [507]. Under simulated physiological conditions (37 °C in serum-containing cell culture medium) 4% and 12% release were measured after 3 and 72 h in neutral pH, suggesting good stability [507]. However, when these conditions were reproduced at pH 4.5 and in presence of glycogen, mimicking PD lysosomes, enzyme release was increased by 3-, 8- and 11.5- fold for 30 min, 3 h and 8 h, respectively, compared to neutral pH, which indicates this is an acceptable model for a proof-of-concept [507].
Figure 8. ICAM-1 targeted nanoparticles for enzyme delivery in Pompe disease.
(A) Enzyme α-glucosidase (GAA), which can bind to the mannose-6-phosphate receptor (M6PR), either naked or loaded in model polystyrene (PS) nanoparticles (NPs) targeted to ICAM-1 (anti-ICAM-1 NPs) for treatment of Pompe disease, which is characterized by abnormal glycogen storage in cells. (B) Cells incubated with turanose to induce glycogen storage as in Pompe disease, were treated for 5 h with naked GAA or anti-CAM-1/GAA NPs. Excess glycogen remaining was determined compared to normal cells. (mean±SEM). (C) Biodistribution of 125I-GAA 30 min after i.v. injection in wild-type mice as either naked enzyme or targeted by anti-ICAM-1 NPs. The tissue-over-blood localization ratio is shown. Adapted and reproduced with permission from J. Hsu et al., Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: comparative performance of a strategy for three distinct lysosomal storage disorders, Nanomedicine. 8 (2012) 731–739 [507].
As for other ICAM-1 targeted NCs, anti-ICAM-1/GAA NPs were internalized by both primary skeletal muscle cells isolated from mice and PD cell models pharmacologically induced with turanose, with around 80 NPs/cell and vs. 2 control IgG NPs and ≈ 80% of all NPs being internalized by 30 min [507]. This occurred via the CAM pathway and rendered lysosomal colocalization, e.g. from 33% after 1 h to 77% after 5 h [507]. As expected, glycogen degradation mediated by anti-ICAM-1/GAA NPs was increased (3-fold) over the same dose of naked GAA, which respectively achieved 75% vs. 26% glycogen reductions compared to untreated cells (Figure 8B) [507].
Finally, in vivo studies were performed in mice treated with an i.v. injection of anti-ICAM-1/GAA NPs [507]. As for other anti-ICAM-1 formulations described above, NPs rapidly disappeared from the circulation, resulting in only 3% the injected dose in blood after 30 min vs. 38% for naked enzyme [507]. Yet, also as in previous examples, this was consistent with fast tissue accumulation of NPs (Figure 8C) [507]. For instance, anti-ICAM-1 NPs increased by the amount of enzyme delivered to heart and two examples of skeletal muscle (gastrocnemius and quadriceps) by 10-fold and 6-7-fold, respectively, along with an increased observed in all other organs compared to naked GAA [507].
It would be relevant to validate these results using ICAM-1 targeted biodegradable NPs, such as the PLGA ones described in Section 3.1.1.2.1 for ASMD, and to extend these studies to evaluate in vivo therapeutic and side effects, just as for the examples described above.
3.3.1.2.2. Poly(lactic-co-glycolic acid) nanocapsules for enzyme delivery
PLGA has been investigated as a tool to avoid immuno-mediated GAA inactivation and enhance its delivery [505]. For this purpose, Tancini et al. synthetized PLGA NPs loaded with Miosyme® (human recombinant GAA), using 15:1 w/w polymer:drug ratio [479]. By modifying NP synthesis conditions, mainly the external phase volume, authors could achieve NP size within 300-360 nm diameter, 0.14-0.19 PDI, and 0-5 mV ζ-potential [505]. These formulations exhibited in vitro enzyme activity, and those prepared with lower external phase volumes resulted in 25-30% higher activity after 120 min incubation with an artificial substrate in vitro than those prepared using higher external volumes [505]. Curiously, activity was greater when an artificial substrate was used, compared to natural glycogen [505], although the causes were not established and comparisons against the naked enzymes were not made.
When these formulations were incubated with patient fibroblasts for 24 h and enzymatic activity was determined 24 h later, formulations prepared with lower external volume resulted in higher activity, up to 30-50% the activity level of wild-type cells, whereas untreated cells had almost no detectable activity [505]. Two particular PLGA formulations delivered similar enzymatic activity to cells although one of them was half as active as the other, suggesting that the latter formulation was more efficiently endocytosed by cells [505]. Furthermore, GAA-loaded PLGA NCs seemed to traffic intracellularly to lysosomes within this time, since Western blotting showed immunodetectable enzyme bands about 110 kDa, the enzyme used and believed to be a precursor, as well as 95 kDa, believed to be an intermediate, and 70 kDa characteristic of the mature form of GAA [505]. Although the activity delivered to cells by naked GAA was only 20% lower than the activity delivered by PLGA NPs at 24 h, the latter formulations resulted in a 7-days sustained activity (maximum time tested) while the former dropped to almost undetectable levels, demonstrating the advantage of the NP strategy [505].
Once again, these encouraging results merit a follow up with research to elucidate whether this formulation is amenable to enter muscle cells that represent the main target in PD, and to determine aspects such as their stability, PK, biodistribution, therapeutic and side effects.
3.4. Other examples of polymer-based nanocarriers aimed at lysosomal storage disorders
In addition to the examples described above, other studies have focused on the development of polymer-based vehicles aimed at LSD treatment, although respective published studies did not use lysosomal enzymes or LSD cellular/animal models since they rather focused on preliminary proof-of-principle for the NC design. This is the case for studies published by the Vicent group, who focused on the so called mask-unmask protein therapy strategy [527,528]. The idea was to conjugate therapeutic proteins such as those used in lysosomal ERTs to biodegradable polymers to mask them until they reach their intended destination, where polymer degradation would unmask the protein free to exert its therapeutic action, a concept first presented by the Duncan group [529]. In particular, a first study by Talelli et al. used poly-L-glutamic acid polymer, which is known to be biodegradable by cathepsin B in lysosomes [530] and used in clinically approved applications [531,532]. This polymer was modified with reduction-sensitive groups to enable protein release in microenvironments such as tumor sites or within endo-lysosomal compartments [527]. Using lysozyme as a model enzyme, authors first thiolated a small number of lysines on this cargo, which only led to about 30% decrease in its catalytic activity. Modified lysozyme was then conjugated to thiolated polymer through the formation of disulfide bonds, which reduced the enzymatic activity by 85-65% (masking effect) [527]. In vitro experiments demonstrated that this bonding was reversible by incubation for 2 h at 37 °C with 20 mM glutathione. In addition, conjugates were incubated with 20 μM, 4 mM, or 10 mM glutathione to mimic the redox states in the bloodstream, lysosomal compartment or tumor environment, respectively [527]. Protein electophoresis showed successful lysozyme release from the polymer after 1 h incubation in the last two conditions vs. the former one (e.g. > 70% release vs. < 10 %, respectively). The activity of the thiolated enzyme was re-gained after its release, back to 65-95% that of masked constructs [527]. Another study by Escalona et al. used a similar strategy, yet it used polyacetals and trypsin as the masking polymer and enzyme cargo, respectively [528]. In this case, the enzyme was conjugated to COOH groups on the polymer chain and masking/unmasking effect was pH responsive, so that the protein cargo could be released in tumor areas or endo-lysosomal compartments [528]. Authors found that polymer-conjugated trypsin was mostly inactive at neutral pH, e.g. 20% the activity of the naked enzyme at this pH. At pH 6.5, the conjugated enzyme gained activity and surpassed that of the naked enzyme at neutral pH, and different polymer-to-protein ratios and linkers influenced this responsiveness [528]. The naked enzyme was mostly inactive at pH 6, which was believed to be due to enzyme autolysis at this pH, as observed by electrophoresis [528]. Thus, these formulations represent interesting strategies for protein release within endosomal and lysosomal compartments.
Future studies should be placed on demonstrating proof-of-concept for these formulations in the realm of lysosomal ERT, by using respective enzymes as well as appropriate cellular and animal models. Lysozyme’s molecular weight is significantly lower (14-15 kDa) compared to most lysosomal enzymes (50-80 kDa) and, thus, whether this masking strategy can feasibly mask/unmask larger cargoes remains to be studied. Whether these conjugates can efficiently distribute within the body to LSD target organs and enter cells to reach lysosomes remains also untested. Because of the inflammatory phenotype associated with most LSDs, premature enzyme unmasking could occur given the reducing environment and acidic pH associated with inflammation. In addition, altered endo-lysosomal acidification in LSDs may also affect the ability of these conjugates to release enzyme within diseased cells.
4. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
As described in the previous sections, diverse polymeric formulations are being explored in the context of treatment for LSDs, showing promise to advance current therapeutic means [39]. Examples described in the literature are still very scarce compared to applications of polymeric NCs for other types of maladies, but growing increasingly popular (for other types of DDS applied to LSD treatment, see articles by Dr. Ceccini and Dr. Ventosa in this issue) [40,111]. This is due to the fact that most polymer-based NCs, as the vast majority of other DDSs, readily traffic to endo-lysosomal compartments upon their uptake by cells (see the articles by Dr. Zhong in this issue [533]), for which this destination represents a logical target [39]. Unlike the great majority of other therapeutic molecules, which can be easily degraded within the lysosomal environment [103,533,534], lysosomal therapeutics such as recombinant enzymes, can naturally withstand the harsh conditions in this compartment [104]. Dissociation and degradation of respective NCs in this intracellular environment is an advantage for LSD-related applications, as it provides cargo release at this destination while avoiding lysosomal buildup of NC-derived materials [80,272]. Importantly, targeting these formulations to cell-surface markers and pathways associated with both transcytosis and endocytosis can provide an interesting means to bypass natural glycosylation-dependent targeting of recombinant enzymes used for lysosomal ERT [56,244,272,437,535]. These systems also can provide alternative routes to cross endothelial barriers separating the circulation from subjacent tissues, such as the case of the BBB [56,244,437,535], and then trafficking to lysosomes within the cells of said tissues [80,244,535]. By masking recombinant enzymes and other cargoes, polymeric NCs could allegedly decrease their recognition by the immune system and avoid premature activity prior to lysosomal delivery, to lower adverse effects associated with both these phenomena, yet this remains to be shown in the context of LSDs [192]. Some of these systems, such as those based on PLGA copolymer, can help restore the pH in lysosomes of deficient cells, which is typically more neutral than in subjects not affected by LSDs, representing an additional therapeutic effect independent from the activity provided by the delivered cargo [249,381]. A breath of encouraging results obtained in cellular [80,202,272,293] and animal models [56,235,426,439] support the idea that this type of approaches should continue being explored and developed, as platforms to enhance the therapeutic effects of existing lysosomal therapeutics, such as current ERTs for peripheral tissues, and as a unique avenue to develop new treatments, for instance for neuropathic LSDs for which no treatment is currently available [39].
It is difficult to speculate which of these polymeric formulations may represent more optimal or promising options for development, since they all differ considerably regarding their possible advantages and disadvantages. Polyrotaxane-cyclodextrin based formulations appear to offer an interesting solution to lower toxicity effects observed in cyclodextrin-mediated treatment of LSDs characterized by cholesterol buildup, such as NPC [307]. Yet, this this strategy cannot help with most other LSDs and whether the strong inflammatory phenotype in NPC may lead to premature hydrolysis and detachment of cyclodextrins from the chain remains to be examined. PS formulations are not biodegradable and hold no clinical interest, although they have been shown to provide a good model for trafficking studies and to reflect well the behavior of PLGA formulations, which do hold translational value [80,235]. Formulations based on QDs also offer valuable proof-of-principle [536], but due to LSDs being chronic diseases and QDs associating to toxic effects, they may not be a suitable option for these treatments [537]. While PLGA is approved for other clinical applications NPs made of this material have shown lower enzyme encapsulation levels compared to other formulations [80], such as albumin-silk nanoparticles or trimethyl-chitosan polyelectrolyte-enzyme complexes [272,293]. Yet, this polymer offers additional advantages such as readily degradation by hydrolysis [538], which may avoid unnecessary buildup of materials in diseased lysosomes, and help restore the typical acidic lysosomal pH, as said, altered in LSDs [250]. Speculatively, smaller sizes observed for trimethyl-chitosan polyelectrolyte-enzyme complexes compared to PLGA NCs may favor transcytosis across cellular barriers and endocytosis into cells, but these formulations have shown a wider PDI range than PLGA NCs [80,272], which may make it more difficult to translate them. Albumin-silk nanoparticles have been associated with a very efficient enzyme loading, but some of these formulations resulted in relatively large sizes and wide PDIs, although this appeared to be easily tunable [293]. Their degradation and disposal of these precise formulations have not been reported, yet other silk-based NCs have been shown to be degraded by proteolysis [539]. Whether their degradation may be efficient or delayed in diseased lysosomes, is to be investigated. However, as said above, cellular and animal studies have shown encouraging results for all these approaches, justifying the continuation of these research efforts. Importantly, PLGA NPs targeted to different markers expressed on the endothelial surface, including anti-ICAM-1-, Ang2-, Tf2-, and g7-targeted formulations, have shown in vivo accumulation of recombinant enzymes in the brain of animal models with encouraging activity results, representing promising strategies for the treatment of neurological LSDs [56,80,235,426,540]. On the other hand, enzyme PEGylation has shown great potential in laboratory models and ongoing clinical trials, representing a readily translatable approach, particularly in cases where CNS delivery is not required and increased PK provided by PEG can lead to enhanced peripheral delivery of therapeutic enzymes [280,298].
Nevertheless, despite this promise, for the most part, knowledge on the translational value of these approaches remains limited and, thus, future advance requires considerable resources. For instance, many of these formulations have been characterized in the test tube and at the cellular level [80,202,272,293], with less examples providing some evaluation in animal models [56,235,307,426,439]. Hence, whether or not these systems behave as expected in terms of their in vivo stability, circulation, and biodistribution to the intended targets and whether they lead to cargo release, activity and therapeutic effects in vivo, remains to be explored. Even at the in vitro level, most often the cellular models used, while valuable for providing initial proof-of-principle, do not adequately reflect the pathology encountered in the disease targets. For instance, HeLa and HEK293 have been frequently used in these assays, but these established cell lines associate with cancer phenotypes and are not representative of the lysosomal and metabolic dysfunction present in cells affected by LSDs [541]. This is relevant because some cancer cell models have been related to a high endocytic activity, while many reports show decreased endocytic uptake and lysosomal trafficking in LSD cells [541]. Therefore, NC performance may greatly vary between these cellular models, which may lead to overestimate NC performance. Even when LSD cell models have been used, most studies have been conducted on skin fibroblasts from patients, which may not reflect either the pathological phenotype of key cellular targets for therapeutic intervention, such neurons, skeletal muscle, bone cells, immune cell, etc. (see the article by Dr. Ledesma in this issue [542]). Therefore, future investigations should verify previous findings using more adequate cellular models, including iPS-derived cells that can be engineered from current patent fibroblasts, thus carrying key mutations, and derived into the most relevant LSD cell targets [543,544]. In addition, the use of more complex organoids and organ-on-a-chip models that can recapitulate the multicellular and structural complexity found in vivo shall increase our level of understanding of these NC systems [545,546]. Similarly, the few studies available on LSD-targeted polymeric NCs in animal models have mostly focused on examining their biodistribution [56,234,263,307,507,547] and even in this case a considerable fraction of these tests were performed in wild-type mice and not animals reflective of LSDs [263,381,498,507]. Furthermore, only very few cases have measured the therapeutic effects derived from NC performance and still these were short-term studies, while LSDs are chronic conditions [235,307,426]. Therefore, there is a need to continue expanding these studies to better appreciate the therapeutic value and optimization of these formulations.
In this line, an important consideration mentioned above is the fact that LSDs are chronic syndromes, thus requiring life-long treatment in the case of most therapeutic options [39], except gene therapy or genome editing [548], discussed in length in the article by Dr. Matte in this issue [490]. In the case of the latter strategies, NCs may help in the delivery of these therapeutic tools without requiring chronic administration. However, this is not the case for the most classical and extended approach for LSD treatment, namely ERT, which must be applied through the life of a patient [39]. Therefore, special attention and care must be place in understanding the degradation and disposal of these formulations, as well as their potential side effects. This is of particular relevance in these diseases, since lysosomes, the main degradative compartment within cells in our bodies, are dysfunctional in LSDs [250]. Hence, data about degradation and disposal of certain nanoformulations available for other cellular and animal models may not necessarily reflect the situation in this case, for which considerable efforts should be placed on this type of research. The fact that lysosomes are one the most relevant intracellular elements mediating cytotoxicity [250], including that pertinent to NCs, reveals the importance of this item. Another concern pertains the potential activation of immune reactions by polymer-based NCs. Those including PEG may be affected, since evidence continues building on the presence of anti-PEG antibodies in humans [549], but this may also be the case for other formulations when administered recurrently in patients. For instance, NC formulations carrying recombinant enzymes could, arguably, facilitate enzyme presentation as a foreign antigen since they enhance transport to the endo-lysosomal system [550]. This could further exacerbate the generation of anti-enzyme antibodies or even induce anti-NC antibodies or reactions. Yet, chronic administration of these formulations could also result in a better tolerance, particularly if paired with strategies to this end, since NPs can be designed to promote tolerance by themselves [551]. These possible yet contrary effects have never been examined and future studies should start focusing on these important parameters to optimize formulations as needed.
Additionally, LSDs are congenital diseases and manifest early after birth or during the first years of life, these treatment strategies concern the pediatric population [39], for which much less is known from previous experience regarding NC dosage, side effects, etc. [552]. In fact, these may represent key regulatory hurdles when moving these strategies toward translation, for which future studies should focus on adequate models and protocols to build these data and early interaction with regulatory agencies to focus on the most valuable items required in this direction. Additional regulatory focus pertains to items relevant to commercial development, including scalability with acceptable reproducibility, which is not always straightforward for polymer-based NCs [90]. Shelf-life of commercial products, and their stability and transport are other important questions to consider, as well as cost imposed by these factors and the loading efficiency of formulations, as mentioned above [90]. Importantly, regulatory agencies often grant orphan drug status to products aimed to treat these maladies, which incentivizes these additional investments [553]. Overall, the effort of surpassing these caveats is outbalanced by the strong medical need, since many LSDs cannot be treated due to inability of current means to efficiently reach their therapeutic targets in the body and/or immune recognition leading to resistance or adverse effects, resulting in a high mortality [39].
ACKNOWLEDGEMENTS
Funding: S.M. was supported by the National Institutes of Health project R01 HL98416, the Spanish Ministry of Science and Innovation project RTI2018–101034-B-I00 (MCIN/AEI/ 10.13039/501100011033 and “ERDF A way of making Europe”), and the Catalan Research Centers CERCA (Generalitat de Catalunya). M.I.G. was supported by the Agency for Management of University and Research Grants (CERCA Program; 2017-SGR-1442 project). M.P was supported by the Predoctoral Fellowship PRE2021-098133, funded by the Spanish Ministry of Science and Innovation (MCIN/AEI/ 10.13039/501100011033 and “ESF Investing in your future”).
Software: Figures 1, 2, 3A, 4A, 5A, 6, 7A, and 8A were created with BioRender.com.
Abbreviations:
- Ang2
angiopep-2
- ASB
arylsulfatase B
- ASM
acid sphingomyelinase
- ASMD
acid sphingomyelinase deficiency
- BBB
blood brain barrier
- β-CD
β-cyclodextrin
- β-GAL
beta galactosidase
- CAM
cell adhesion molecule
- CLEA
cross-linked enzyme aggregate
- CNS
central nervous system
- CTP
calcium titanium phosphate
- DDS
drug delivery systems
- DSPE
distearyl-hosphatidylethanolamine
- ERT
enzyme replacement therapy
- EV
extracellular vesicle
- FD
Fabry disease
- g7
7-aminoacid glycopeptide
- GAA
α-glucosidase
- GAG
glycosaminoglycan
- GALC
galactosylceramidase
- Gb3
globotriaosylceramide
- GBA
acid β-glucosidase
- GD
Gaucher disease
- GLA
α-galactosidase A
- GM1G
GM1-gangliosidosis
- HAp
hydroxyapatite
- HAse
hyaluronidase
- HEE-PRX
2-(2-hydroxyethoxy)ethyl group-modified polyrotaxane
- HPβCD
hydroxypropyl-β-cyclodextrin
- i.v.
intra venous
- ICAM-1
intercellular adhesion molecule 1
- IDS
iduronate-2-sulfatase
- IDUA
α-L-iduronidase
- iPSC
induced pluripotent stem cells
- KD
Krabbe disease
- KO
knockout
- LAMP
lysosome associated membrane protein
- LIMP
lysosomal integral membrane protein
- LSDs
lysosomal storage disorders
- M6P
mannose-6-phosphate; M6PR, mannose-6-phosphate receptor
- MPS
mucopolysaccharidosis
- MW
molecular weight
- NC
nanocarrier
- NCL1
neuronal ceroid lipofuscinosis 1
- NP
nanoparticle
- NPC
Niemann-Pick type C
- PBCA
poly(butyl cyanocrylate)
- PD
Pompe disease
- PDI
polydispersity index
- PEC
polyelectrolyte complex
- PEG
poly(ethylene glycol)
- PK
pharmacokinetics
- PLGA
poly (lactic-co-glycolic acid)
- PPT1
palmitoyl-protein thioesterase-1
- PS
polystyrene
- QD
quantum dot
- RGD
arginine-glycine-aspartic acid peptide
- SRT
substrate-reduction therapy
- Tf2
transferrin-binding peptide-2
- TfR
transferrin receptor
- TMC
trimethyl chitosan
- TWI
twitcher mouse
- wt%
weight percentage
Footnotes
CONFLICT OF INTEREST. The authors declare no conflict of interest.
6 REFERENCES
- [1].Luzio JP, Pryor PR, Bright NA, Lysosomes: fusion and function, Nat. Rev. Mol. Cell Biol 2007 88. 8 (2007) 622–632, 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- [2].Holtzman E, Historical Fragments; Methods; Some Terminology, in: Lysosomes. Cellular Organelles Series Springer; New York, NY, 1989, pp. 1–24. 10.1007/978-1-4899-2540-4.. [DOI] [Google Scholar]
- [3].Mellman I, ENDOCYTOSIS AND MOLECULAR SORTING, Annu. Rev. Cell Dev. Biol 12 (1996) 575–625, 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- [4].Seaman MNJ, Burd CG, Emr SD, Receptor signalling and the regulation of endocytic membrane transport, Curr. Opin. Cell Biol 8 (1996) 549–556, https://doi.org/ 10.1016/S0955-0674(96)80034-2. [DOI] [PubMed] [Google Scholar]
- [5].Andrews NW, Regulated secretion of conventional lysosomes, Trends Cell Biol. 10 (2000) 316–321, 10.1016/S0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- [6].Reddy A, V Caler E, Andrews NW, Plasma Membrane Repair Is Mediated by Ca2+-Regulated Exocytosis of Lysosomes, Cell. 106 (2001) 157–169, 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- [7].Lange Y, Ye J, Steck TL, Circulation of Cholesterol between Lysosomes and the Plasma Membrane, J. Biol. Chem 273 (1998) 18915–18922, 10.1074/jbc.273.30.18915. [DOI] [PubMed] [Google Scholar]
- [8].McNeil PL, Kirchhausen T, An emergency response team for membrane repair, Nat. Rev. Mol. Cell Biol 6 (2005) 499–505, 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- [9].Li SC, Kane PM, The yeast lysosome-like vacuole: Endpoint and crossroads, Biochim. Biophys. Acta - Mol. Cell Res 1793 (2009) 650–663, https://doi.org/ 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS, Lysosome-related organelles, FASEB J. 14 (2000) 1265–1278, https://doi.org/ 10.1096/fasebj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- [11].Showalter MR, Berg AL, Nagourney A, Heil H, Carraway KL, Fiehn O, The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease, Int. J. Mol. Sci . 21 (2020), 10.3390/ijms21218067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lloyd JB, Forster S, The lysosome membrane, Trends Biochem. Sci 11 (1986) 365–368, https://doi.org/ 10.1016/0968-0004(86)90205-7. [DOI] [Google Scholar]
- [13].Eskelinen EL, Tanaka Y, Saftig P, At the acidic edge: emerging functions for lysosomal membrane proteins, Trends Cell Biol. 13 (2003) 137–145, 10.1016/S0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- [14].Mellman I, Fuchs R, Helenius A, ACIDIFICATION OF THE ENDOCYTIC AND EXOCYTIC PATHWAYS, Annu. Rev. Biochem 55 (1986) 663–700, 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- [15].Coutinho MF, Prata MJ, Alves S, Mannose-6-phosphate pathway: A review on its role in lysosomal function and dysfunction, Mol. Genet. Metab 105 (2012) 542–550, https://doi.org/ 10.1016/j.ymgme.2011.12.012. [DOI] [PubMed] [Google Scholar]
- [16].Rosenfeld MG, Kreibich G, Popov D, Kato K, Sabatini DD, Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution , J. Cell Biol 93 (1982) 135–143, 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lübke T, Lobel P, Sleat DE, Proteomics of the lysosome, Biochim. Biophys. Acta - Mol. Cell Res 1793 (2009) 625–635, https://doi.org/ 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meikle PJ, Hopwood JJ, Clague AE, Carey WF, Prevalence of Lysosomal Storage Disorders, JAMA. 281 (1999) 249–254, 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- [19].Hers HG, Inborn Lysosomal Diseases, Gastroenterology. 48 (1965) 625–633, https://doi.org/ 10.1016/S0016-5085(65)80041-5. [DOI] [PubMed] [Google Scholar]
- [20].Fuller M, Meikle PJ, Hopwood JJ, Epidemiology of lysosomal storage diseases: an overview. In: Fabry Disease Perspectives from 5 years of FOS. Oxford PharmaGenesis, Oxford: (2006) Chapter 2. ISBN-10:1-903539-03-X. https://www.ncbi.nlm.nih.gov/books/NBK11603/. [PubMed] [Google Scholar]
- [21].Futerman AH, van Meer G, The cell biology of lysosomal storage disorders, Nat. Rev. Mol. Cell Biol 5 (2004) 554–565, 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- [22].Mancini GMS, Verheijen FW, Lysosomal Transport Disorders. In: eLS (Ed.), 2006. 10.1038/npg.els.0006110. [DOI] [PubMed] [Google Scholar]
- [23].Boustany RM, Lysosomal storage diseases—the horizon expands, Nat. Rev. Neurol 9 (2013) 583–598, 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- [24].Jakóbkiewicz-Banecka J, Gabig-Cimińska M, Banecka-Majkutewicz Z, Banecki B, Węgrzyn A, Węgrzyn G. Factors and processes modulating phenotypes in neuronopathic lysosomal storage diseases. Metab Brain Dis. 29 (2014) 1–8, 10.1007/s11011-013-9455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beck M, Treatment strategies for lysosomal storage disorders, Dev. Med. Child Neurol 60 (2018) 13–18, 10.1111/dmcn.13600. [DOI] [PubMed] [Google Scholar]
- [26].d’Azzo A, Gene Transfer Strategies for Correction of Lysosomal Storage Disorders, Acta Haematol. 110 (2003) 71–85, 10.1159/000072456. [DOI] [PubMed] [Google Scholar]
- [27].Cheng SH, Smith AE, Gene therapy progress and prospects: gene therapy of lysosomal storage disorders, Gene Ther. 10 (2003) 1275–1281, 10.1038/sj.gt.3302092. [DOI] [PubMed] [Google Scholar]
- [28].Sands MS, Davidson BL, Gene therapy for lysosomal storage diseases, Mol. Ther 13 (2006) 839–849, https://doi.org/ 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [29].Platt FM, Emptying the stores: Lysosomal diseases and therapeutic strategies, Nat. Rev. Drug Discov 17 (2018) 133–150, 10.1038/nrd.2017.214. [DOI] [PubMed] [Google Scholar]
- [30].Massaro G, Geard AF, Liu W, Coombe-Tennant O, Waddington SN, Baruteau J, Gissen P, Rahim AA, Gene Therapy for Lysosomal Storage Disorders: Ongoing Studies and Clinical Development, Biomolecules. 11 (2021) 611, 10.3390/biom11040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Libmeldy ∣ European Medicines Agency, (n.d.). https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy (accessed November 28, 2022).
- [32].Desnick RJ, Schuchman EH, Enzyme replacement and enhancement therapies: lessons from lysosomal disorders, Nat. Rev. Genet 2002 312. 3 (2002) 954–966, 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- [33].Muro S, New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2 (2010) 189–204, 10.1002/wnan.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Deduve C, From cytases to lysosomes, Fed Proc. 23 (1964) 1045–1049. [PubMed] [Google Scholar]
- [35].Kornfeld S, Lysosomal enzyme targeting, Biochem. Soc. Trans 18 (1990) 367–374, 10.1042/bst0180367. [DOI] [PubMed] [Google Scholar]
- [36].Barton RW, Neufeld EF, The Hurler Corrective Factor: PURIFICATION AND SOME PROPERTIES, J. Biol. Chem 246 (1971) 7773–7779, https://doi.org/ 10.1016/S0021-9258(19)45842-0. [DOI] [PubMed] [Google Scholar]
- [37].Grabowski GA, Leslie N, Wenstrup R, Enzyme therapy for Gaucher disease: the first 5 years, Blood Rev. 12 (1998) 115–133, https://doi.org/ 10.1016/S0268-960X(98)90023-6. [DOI] [PubMed] [Google Scholar]
- [38].Grabowski GA, Hopkin RJ, Enzyme Therapy for Lysosomal Storage Disease: Principles, Practice, and Prospects, Annu. Rev. Genomics Hum. Genet 4 (2003) 403–436, 10.1146/annurev.genom.4.070802.110415. [DOI] [PubMed] [Google Scholar]
- [39].Solomon M, Muro S, Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives, Adv. Drug Deliv. Rev 118 (2017) 109–134, 10.1016/j.addr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Del Grosso A, Parlanti G, Mezzena R, Cecchini M, Current treatment options and novel nanotechnology-driven enzyme replacement strategies for lysosomal storage disorders, Adv. Drug Deliv. Rev 188 (2022) 114464, 10.1016/J.ADDR.2022.114464. [DOI] [PubMed] [Google Scholar]
- [41].Dwek RA, Butters TD, Platt FM, Cox TM, Brady RO, Enzyme replacement therapy: conception, chaos and culmination, Philos. Ser. B Biol. Sci 358 (2003) 915–919, 10.1098/rstb.2003.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mechler K, Mountford WK, Hoffmann GF, Ries M, Pressure for drug development in lysosomal storage disorders – a quantitative analysis thirty years beyond the US orphan drug act, Orphanet J. Rare Dis 10 (2015) 46, 10.1186/s13023-015-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Begley D, Pontikis C, Scarpa M, Lysosomal Storage Diseases and the Blood-Brain Barrier, Curr. Pharm. Des 14 (2008) 1566–1580, 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- [44].Muro S, Strategies for delivery of therapeutics into the central nervous system for treatment of lysosomal storage disorders, Drug Deliv. Transl. Res 2 (2012) 169–186, 10.1007/s13346-012-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dejana E, Endothelial cell–cell junctions: happy together, Nat. Rev. Mol. Cell Biol 5 (2004) 261–270, 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- [46].Zihni C, Mills C, Matter K, Balda MS, Tight junctions: from simple barriers to multifunctional molecular gates, Nat. Rev. Mol. Cell Biol 17 (2016) 564–580, 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- [47].Haseloff RF, Blasig IE, Bauer H-C, Bauer H, In Search of the Astrocytic Factor(s) Modulating Blood–Brain Barrier Functions in Brain Capillary Endothelial Cells In Vitro, Cell. Mol. Neurobiol 25 (2005) 25–39, 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR, Strategies to advance translational research into brain barriers, Lancet Neurol. 7 (2008) 84–96, https://doi.org/ 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- [49].Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P, Souweidane M, Hosain S, Heier L, Ballon D, Dinner M, Wisniewski K, Kaplitt M, Greenwald BM, Howell JD, Strybing K, Dyke J, Voss H, Administration of a Replication-Deficient Adeno-Associated Virus Gene Transfer Vector Expressing the Human CLN2 cDNA to the Brain of Children with Late Infantile Neuronal Ceroid Lipofuscinosis, Hum Gene Ther. 15 (2004) 1131–1154, 10.1089/hum.2004.15.1131 [DOI] [PubMed] [Google Scholar]
- [50].Favret JM, Weinstock NI, Feltri ML, Shin D, Pre-clinical mouse models of neurodegenerative Lysosomal Storage Diseases. Front. Mol. Biosci 7 (2020) 57, 10.3389/fmolb.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pardridge WM, Biopharmaceutical drug targeting to the brain, J. Drug Target 18 (2010) 157–167, 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- [52].Jones AT, Gateways and tools for drug delivery: Endocytic pathways and the cellular dynamics of cell penetrating peptides, Int. J. Pharm 354 (2008) 34–38, https://doi.org/ 10.1016/j.ijpharm.2007.10.046. [DOI] [PubMed] [Google Scholar]
- [53].Muro S, Koval M, Muzykantov V, Endothelial Endocytic Pathways: Gates for Vascular Drug Delivery, Curr. Vasc. Pharmacol 2 (2004) 281–299, 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- [54].Tian X, Nyberg S, Sharp PS, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, Battaglia G, LRP-1-mediated intracellular antibody delivery to the Central Nervous System, Sci. Reports 5 (2015) 11990, 10.1038/srep11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hsu J, Rappaport J, Muro S, Specific Binding, Uptake, and Transport of ICAM-1-Targeted Nanocarriers Across Endothelial and Subendothelial Cell Components of the Blood-Brain Barrier, Pharm. Res 31 (2014) 1855–1866, 10.1007/s11095-013-1289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Manthe RL, Loeck M, Bhowmick T, Solomon M, Muro S, Intertwined mechanisms define transport of anti-ICAM nanocarriers across the endothelium and brain delivery of a therapeutic enzyme, J. Control. Release 324 (2020) 181–193, 10.1016/j.jconrel.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pardridge WM, Blood-brain barrier delivery for lysosomal storage disorders with IgG-lysosomal enzyme fusion proteins, Adv. Drug Deliv. Rev 184 (2022), 114234, 10.1016/J.ADDR.2022.114234. [DOI] [PubMed] [Google Scholar]
- [58].Kishnani PS, Challenges of enzyme replacement therapy: Poor tissue distribution in lysosomal diseases using pompe disease as a model. In: Rosenberg A, Demeule B, (Eds.) Biobetters, AAPS Adv. Pharm. Sci. Ser, Springer, New York, NY, 2015, vol 19, pp. 9–21, 10.1007/978-1-4939-2543-8_2. [DOI] [Google Scholar]
- [59].Pastores GM, Hughes DA, Gaucher Disease. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A (Eds.), GeneReviews® [Internet]. University of Washington, Seattle (WA), 2000. [updated 2018 Jun 21], pp. 1993–2023. ISSN: 2372-0697. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1269/. [Google Scholar]
- [60].Muenzer J, Overview of the mucopolysaccharidoses, Rheumatology. 50 (2011) v4–v12, 10.1093/RHEUMATOLOGY/KER394. [DOI] [PubMed] [Google Scholar]
- [61].Barrias CC, Lamghari M, Granja PL, Sá Miranda MC, Barbosa MA, Biological evaluation of calcium alginate microspheres as a vehicle for the localized delivery of a therapeutic enzyme, J. Biomed. Mater. Res 74A (2005) 545–552, 10.1002/jbm.a.30348. [DOI] [PubMed] [Google Scholar]
- [62].Chen Y, Wu X, Li J, Jiang Y, Xu K, Su J, Bone-Targeted Nanoparticle Drug Delivery System: An Emerging Strategy for Bone-Related Disease, Front. Pharmacol 13 (2022) 909408, 10.3389/FPHAR.2022.909408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rao C, Shi S, Development of Nanomaterials to Target Articular Cartilage for Osteoarthritis Therapy, Front. Mol. Biosci 9 (2022) 900344, 10.3389/FMOLB.2022.900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Young RN, Grynpas MD, Targeting therapeutics to bone by conjugation with bisphosphonates, Curr. Opin. Pharmacol 40 (2018) 87–94, 10.1016/J.COPH.2018.03.010. [DOI] [PubMed] [Google Scholar]
- [65].Messinger YH, Mendelsohn NJ, Rhead W, Dimmock D, Hershkovitz E, Champion M, Jones SA, Olson R, White A, Wells C, Bali D, Case LE, Young SP, Rosenberg AS, Kishnani PS, Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease, Genet. Med 14 (2012) 135–142, 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ghosh S, Alam S, Rathore AS, Khare SK, Stability of Therapeutic Enzymes: Challenges and Recent Advances. In: Labrou N (Ed.) Therapeutic Enzymes: Function and Clinical Implications. Advances in Experimental Medicine and Biology, vol 1148. Springer, Singapore, 2019, pp. 131–150, 10.1007/978-981-13-7709-9_7. [DOI] [PubMed] [Google Scholar]
- [67].de la Fuente M, Lombardero L, Gómez-González A, Solari C, Angulo-barturen I, Acera A, Vecino E, Astigarraga E, Barreda-gómez G, Enzyme Therapy: Current Challenges and Future Perspectives, Int. J. Mol. Sci 22 (2021) 9181, 10.3390/ijms22179181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, McVie-Wylie A, Sullivan JA, Hoganson GE, Phillips JA, Schaefer GB, Charrow J, Ware RE, Bossen EH, Chen Y-T, Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial, Genet. Med 3 (2001) 132–138, 10.1038/gim200127. [DOI] [PubMed] [Google Scholar]
- [69].Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ, Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease, N. Engl. J. Med 345 (2001) 9–16, 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- [70].Brooks DA, Kakavanos R, Hopwood JJ, Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder, Trends Mol. Med 9 (2003) 450–453, 10.1016/j.molmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- [71].Kishnani PS, Dickson PI, Muldowney L, Lee JJ, Rosenberg A, Abichandani R, Bluestone JA, Burton BK, Dewey M, Freitas A, Gavin D, Griebel D, Hogan M, Holland S, Tanpaiboon P, Turka LA, Utz JJ, Wang Y-M, Whitley CB, Kazi ZB, Pariser AR, Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction, Mol. Genet. Metab 117 (2016) 66–83, https://doi.org/ 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [72].Banugaria SG, Patel TT, Mackey J, Das S, Amalfitano A, Rosenberg AS, Charrow J, Chen Y-T, Kishnani PS, Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: Need for agents to target antibody-secreting plasma cells, Mol. Genet. Metab 105 (2012) 677–680, https://doi.org/ 10.1016/j.ymgme.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED, Brooks DA, Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I, Lancet. 361 (2003) 1608–1613, 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- [74].Schweighardt B, Tompkins T, Lau K, Jesaitis L, Qi Y, Musson DG, Farmer P, Haller C, Shaywitz AJ, Yang K, O’Neill CA, Immunogenicity of Elosulfase Alfa, an Enzyme Replacement Therapy in Patients with Morquio A Syndrome: Results from MOR-004, a Phase III Trial, Clin. Ther 37 (2015) 1012–1021.e6, 10.1016/j.clinthera.2014.11.005. [DOI] [PubMed] [Google Scholar]
- [75].Qi Y, Musson DG, Schweighardt B, Tompkins T, Jesaitis L, Shaywitz AJ, Yang K, O’Neill CA, Pharmacokinetic and Pharmacodynamic Evaluation of Elosulfase Alfa, an Enzyme Replacement Therapy in Patients with Morquio A Syndrome, Clin. Pharmacokinet 53 (2014) 1137–1147, 10.1007/s40262-014-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Thorp EB, Boada C, Jarbath C, Luo X, Nanoparticle Platforms for Antigen-Specific Immune Tolerance, Front. Immunol 11 (2020) 945, 10.3389/FIMMU.2020.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Koto Y, Ueki S, Yamakawa M, Sakai N, Experiences of patients with lysosomal storage disorders treated with enzyme replacement therapy: a qualitative systematic review protocol, JBI Evid. Synth 19 (2021) 702–708, 10.11124/JBIES-20-00017. [DOI] [PubMed] [Google Scholar]
- [78].Ghaffarian R, Bhowmick T, Muro S, Transport of nanocarriers across gastrointestinal epithelial cells by a new transcellular route induced by targeting ICAM-1, J. Control. Release 163 (2012) 25–33, 10.1016/j.jconrel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ghaffarian R, Pérez-Herrero E, Oh H, Raghavan SR, Muro S, Chitosan-Alginate Microcapsules Provide Gastric Protection and Intestinal Release of ICAM-1-Targeting Nanocarriers, Enabling GI Targeting in Vivo, Adv. Funct. Mater 26 (2016) 3382–3393, 10.1002/adfm.201600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Muntimadugu E, Silva-Abreu M, Vives G, Loeck M, Pham V, Del Moral M, Solomon M, Muro S, Comparison between Nanoparticle Encapsulation and Surface Loading for Lysosomal Enzyme Replacement Therapy, Int. J. Mol. Sci 23 (2022) 4034, 10.3390/IJMS23074034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lake BD, Young EP, Winchester BG, Prenatal Diagnosis of Lysosomal Storage Diseases, Brain Pathol. 8 (1998) 133–149, https://doi.org/ 10.1111/j.1750-3639.1998.tb00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bailey L, An overview of enzyme replacement therapy for lysosomal storage diseases, OJIN: Online J. Issues Nurs 13 (2008) 3, 10.3912/OJIN.Vol13No01Man03.. [DOI] [Google Scholar]
- [83].Desnick RJ, Schuchman EH, Enzyme Replacement Therapy for Lysosomal Diseases: Lessons from 20 Years of Experience and Remaining Challenges, Annu. Rev. Genomics Hum. Genet 13 (2012) 307–335, 10.1146/annurev-genom-090711-163739. [DOI] [PubMed] [Google Scholar]
- [84].Pardridge WM, Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain, Front. Aging Neurosci 11 (2020) 373, 10.3389/FNAGI.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zou Y, Sun X, Yang Q, Zheng M, Shimoni O, Ruan W, Wang Y, Zhang D, Yin J, Huang X, Tao W, Park JB, Liang XJ, Leong KW, Shi B, Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy, Sci. Adv 8 (2022) 8011, 10.1126/SCIADV.ABM8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Torchilin VP, Multifunctional nanocarriers, Adv. Drug Deliv. Rev 58 (2006) 1532–1555, https://doi.org/ 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- [87].Parveen S, Misra R, Sahoo SK, Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging, Nanomedicine Nanotechnology, Biol. Med 8 (2012) 147–166, https://doi.org/ 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [88].Muro S, Challenges in design and characterization of ligand-targeted drug delivery systems, J. Control. Release 164 (2012) 125–137, 10.1016/J.JCONREL.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Langer R, Drug delivery and targeting, Nature 392 (6679 Suppl) (1998) 5–10. Available from: https://pubmed.ncbi.nlm.nih.gov/9579855/. [PubMed] [Google Scholar]
- [90].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discov 20 (2021) 101–124, 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Moghimi SM, Hunter AC, Murray JC, Long-circulating and target-specific nanoparticles: Theory to practice, Pharmacol. Rev 53 (2001) 283–318. Available from: http://pharmrev.aspetjournals.org/cgi/reprint/53/2/283 [PubMed] [Google Scholar]
- [92].Beija M, Salvayre R, Lauth-de Viguerie N, Marty J-D, Colloidal systems for drug delivery: from design to therapy, Trends Biotechnol. 30 (2012) 485–496, 10.1016/j.tibtech.2012.04.008. [DOI] [PubMed] [Google Scholar]
- [93].Moghimi SM, Hunter AC, Murray JC, Nanomedicine: current status and future prospects, FASEB J. 19 (2005) 311–330, 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- [94].Alexis F, Pridgen E, Molnar LK, Farokhzad OC, Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles, Mol. Pharm 5 (2008) 505–515, 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Amoozgar Z, Yeo Y, Recent advances in stealth coating of nanoparticle drug delivery systems, WIREs Nanomedicine and Nanobiotechnology. 4 (2012) 219–233, https://doi.org/ 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Vasir JK, Labhasetwar V, Targeted Drug Delivery in Cancer Therapy, Technol. Cancer Res. Treat 4 (2005) 363–374, 10.1177/153303460500400405. [DOI] [PubMed] [Google Scholar]
- [97].Schmaljohann D, Thermo- and pH-responsive polymers in drug delivery, Adv. Drug Deliv. Rev 58 (2006) 1655–1670, https://doi.org/ 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- [98].Sahay G, Alakhova DY, V Kabanov A, Endocytosis of nanomedicines, J. Control. Release 145 (2010) 182–195, 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Griffiths G, Gruenberg J, Marsh M, Wohlmann J, Jones AT, Parton RG, Nanoparticle entry into cells; the cell biology weak link, Adv. Drug Deliv. Rev 188 (2022) 114403, 10.1016/J.ADDR.2022.114403. [DOI] [PubMed] [Google Scholar]
- [100].Rigante D, Cipolla C, Basile U, Gulli F, Savastano MC, Overview of immune abnormalities in lysosomal storage disorders, Immunol. Lett 188 (2017) 79–85, 10.1016/J.IMLET.2017.07.004. [DOI] [PubMed] [Google Scholar]
- [101].Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M, A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1, J. Cell Sci 116 (2003) 1599–1609, 10.1242/JCS.00367. [DOI] [PubMed] [Google Scholar]
- [102].Muro S, Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1. In: Aird W (Ed.), Endothelial Biomedicine, Cambridge University Press, Cambridge, 2007, pp. 1058–1070, 10.1017/CBO9780511546198.118. [DOI] [Google Scholar]
- [103].Muro S, Cui X, Gajewski C, Murciano J-C, Muzykantov VR, Koval M, Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress, Am. J. Physiol. Physiol 285 (2003) C1339–C1347, 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- [104].Muro S, Schuchman EH, Muzykantov VR, Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis, Mol. Ther 13 (2006) 135–141, 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- [105].Oh P, Borgström P, Witkiewicz H, Li Y, Borgström BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE, Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung, Nat. Biotechnol 25 (2007) 327–337, 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pardridge WM, Blood–brain barrier delivery, Drug Discov. Today 12 (2007) 54–61, https://doi.org/ 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [107].Anselmo AC, Mitragotri S, Nanoparticles in the clinic, Bioeng. Transl. Med 1 (2016) 10–29, 10.1002/BTM2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R, Advances in Biomaterials for Drug Delivery, Adv. Mater 30 (2018) 1705328, https://doi.org/ 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Grimaldi N, Andrade F, Segovia N, Ferrer-Tasies L, Sala S, Veciana J, Ventosa N, Lipid-based nanovesicles for nanomedicine, Chem. Soc. Rev 45 (2016) 6520–6545, 10.1039/C6CS00409A. [DOI] [PubMed] [Google Scholar]
- [110].Sahoo SK, Parveen S, Panda JJ, The present and future of nanotechnology in human health care, Nanomed. Nanotech. Biol. Med 3 (2007) 20–31, https://doi.org/ 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- [111].Tomsen-Melero J, Merlo-Mas J, Carreño A, Sala S, Córdoba A, Veciana J, González-Mira E, Ventosa N, Liposomal formulations for treating lysosomal storage disorders, Adv. Drug Deliv. Rev 190 (2022) 114531, 10.1016/J.ADDR.2022.114531. [DOI] [PubMed] [Google Scholar]
- [112].Kolašinac R, Kleusch C, Braun T, Merkel R, Csiszár A, Deciphering the Functional Composition of Fusogenic Liposomes, Int. J. Mol. Sci 2018, Vol. 19, Page 346. 19 (2018) 346, 10.3390/IJMS19020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Botet-Carreras A, Marimon MB, Millan-Solsona R, Aubets E, Ciudad CJ, Noé V, Montero MT, Domènech Ò, Borrell JH, On the uptake of cationic liposomes by cells: From changes in elasticity to internalization, Colloids Surf. B: Biointerfaces 221 (2023) 112968, 10.1016/J.COLSURFB.2022.112968. [DOI] [PubMed] [Google Scholar]
- [114].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. Drug Discov 4 (2005) 145–160, 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- [115].Musacchio T, Torchilin VP, Recent developments in lipid-based pharmaceutical nanocarriers, Front. Biosci. (Landmark Ed.) 16 (2011) 1388–1412, 10.2741/3795. [DOI] [PubMed] [Google Scholar]
- [116].Bangham AD, Standish MM, Watkins JC, Diffusion of univalent ions across the lamellae of swollen phospholipids, J. Mol. Biol 13 (1965) 238–252, IN26-IN27, https://doi.org/ 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- [117].Pattni BS, Chupin VV, Torchilin VP, New Developments in Liposomal Drug Delivery, Chem. Rev 115 (2015) 10938–10966, 10.1021/ACS.CHEMREV.5B00046. [DOI] [PubMed] [Google Scholar]
- [118].Bulbake U, Doppalapudi S, Kommineni N, Khan W, Liposomal Formulations in Clinical Use: An Updated Review, Pharmaceutics 9 (2017) 12, 10.3390/PHARMACEUTICS9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Liu P, Chen G, Zhang J, A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives, Mol. 27 (2022) 1372, 10.3390/MOLECULES27041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Siva Kumar N, Vekariya RL, A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems, RSC Adv. 10 (2020) 26777–26791, 10.1039/D0RA03491F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hou X, Zaks T, Langer R, Dong Y, Lipid nanoparticles for mRNA delivery, Nat. Rev. Mater 6 (2021) 1078–1094, 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].de Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, Vader P, Schiffelers RM, Drug Delivery with Extracellular Vesicles: From Imagination to Innovation, Acc. Chem. Res 52 (2019) 1761–1770, 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SEL, Vader P, Extracellular vesicles as drug delivery systems: Why and how?, Adv. Drug Deliv. Rev 159 (2020) 332–343, https://doi.org/ 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- [124].van der Koog L, Gandek TB, Nagelkerke A, Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization, Adv. Healthc. Mater 11 (2022) 2100639, https://doi.org/ 10.1002/adhm.202100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Rezaie J, Feghhi M, Etemadi T, A review on exosomes application in clinical trials: perspective, questions, and challenges, Cell Commun. Signal 20 (2022) 1–13, 10.1186/S12964-022-00959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lu B, Ku J, Flojo R, Olson C, Bengford D, Marriott G, Exosome- and extracellular vesicle-based approaches for the treatment of lysosomal storage disorders, Adv. Drug Deliv. Rev 188 (2022) 114465, 10.1016/j.addr.2022.114465. [DOI] [PubMed] [Google Scholar]
- [127].Yang W, Liang H, Ma S, Wang D, Huang J, Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment, Sustain. Mater. Technol 22 (2019) e00109, 10.1016/j.susmat.2019.e00109. [DOI] [Google Scholar]
- [128].Arias LS, Pessan JP, Vieira AP, Lima TM, Delbem AC, Monteiro DR, Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity, Antibiot. 7 (2018) 46, 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Soetaert F, Korangath P, Serantes D, Fiering S, Ivkov R, Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies, Adv. Drug Deliv. Rev 163–164 (2020) 65–83, 10.1016/J.ADDR.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Anselmo AC, Mitragotri S, Nanoparticles in the clinic: An update, Bioeng. Transl. Med 4 (2019) e10143, 10.1002/BTM2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xu C, Nam J, Hong H, Xu Y, Moon JJ, Positron Emission Tomography-Guided Photodynamic Therapy with Biodegradable Mesoporous Silica Nanoparticles for Personalized Cancer Immunotherapy, ACS Nano. 13 (2019) 12148–12161, 10.1021/acsnano.9b06691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Huang K-W, Hsu F-F, Qiu JT, Chern G-J, Lee Y-A, Chang C-C, Huang Y-T, Sung Y-C, Chiang C-C, Huang R-L, Lin C-C, Dinh TK, Huang H-C, Shih Y-C, Alson D, Lin C-Y, Lin Y-C, Chang P-C, Lin S-Y, Chen Y, Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer, Sci. Adv 6 (2020) eaax5032, 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Janjua TI, Cao Y, Yu C, Popat A, Clinical translation of silica nanoparticles, Nat. Rev. Mater 6 (2021) 1072–1074, 10.1038/s41578-021-00385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wagner AM, Knipe JM, Orive G, Peppas NA, Quantum dots in biomedical applications, Acta Biomater. 94 (2019) 44–63, 10.1016/j.actbio.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Liechty WB, Kryscio DR, Slaughter BV, Peppas NA, Polymers for drug delivery systems, Annu. Rev. Chem. Biomol. Eng 1 (2010) 149–173, 10.1146/ANNUREV-CHEMBIOENG-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Valcourt DM, Dang MN, Scully MA, Day ES, Nanoparticle-Mediated Co-Delivery of Notch-1 Antibodies and ABT-737 as a Potent Treatment Strategy for Triple-Negative Breast Cancer, ACS Nano. 14 (2020) 3378–3388, 10.1021/acsnano.9b09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].La-Beck NM, Islam MR, Markiewski MM, Nanoparticle-Induced Complement Activation: Implications for Cancer Nanomedicine, Front. Immunol 11 (2020) 603039, 10.3389/FIMMU.2020.603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Discher DE, Ahmed F, Polymersomes, Annu. Rev. Biomed. Eng 8 (2006) 323–341, 10.1146/ANNUREV.BIOENG.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- [139].Zhang X, Zhang P, Polymersomes in Nanomedicine - A Review, Curr. Med. Chem 13 (2017) 124–129, 10.2174/1573413712666161018144519. [DOI] [Google Scholar]
- [140].Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K, Liposomes and polymersomes: a comparative review towards cell mimicking, Chem. Soc. Rev 47 (2018) 8572–8610, 10.1039/C8CS00162F. [DOI] [PubMed] [Google Scholar]
- [141].Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantù L, Nicoli S, Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions, J. Control. Release 332 (2021) 312–336, https://doi.org/ 10.1016/j.jconrel.2021.02.031. [DOI] [PubMed] [Google Scholar]
- [142].Lu Y, Zhang E, Yang J, Cao Z, Strategies to improve micelle stability for drug delivery, Nano Res. 11 (2018) 4985–4998, 10.1007/S12274-018-2152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Xu L, Zhang H, Wu Y, Dendrimer Advances for the Central Nervous System Delivery of Therapeutics, ACS Chem. Neurosci 5 (2014) 2–13, 10.1021/cn400182z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Palmerston Mendes L, Pan J, Torchilin VP, Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy, Mol. 22 (2017) 1401, 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Meka VS, Sing MKG, Pichika MR, Nali SR, Kolapalli VRM, Kesharwani P, A comprehensive review on polyelectrolyte complexes, Drug Discov. Today 22 (2017) 1697–1706, 10.1016/J.DRUDIS.2017.06.008. [DOI] [PubMed] [Google Scholar]
- [146].Banik BL, Fattahi P, Brown JL, Polymeric nanoparticles: the future of nanomedicine, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 8 (2016) 271–299, 10.1002/WNAN.1364. [DOI] [PubMed] [Google Scholar]
- [147].Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z, Current approaches of nanomedicines in the market and various stage of clinical translation, Acta Pharm. Sin. B 12 (2022) 3028–3048, 10.1016/J.APSB.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kayser O, Lemke A, Hernandez-Trejo N, The Impact of Nanobiotechnology on the Development of New Drug Delivery Systems, Curr. Pharm. Biotechnol 6 (2005) 3–5, 10.2174/1389201053167158. [DOI] [PubMed] [Google Scholar]
- [149].Ghandehari H, Materials for advanced drug delivery in the 21st century: a focus area for Advanced Drug Delivery Reviews, Adv. Drug Deliv. Rev 60 (2008) 956, 10.1016/j.addr.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Scheirs J, Priddy D, Modern Styrenic Polymers: Polystyrenes and Styrenic Copolymers. In: Wiley Series in Polymer Science, Wiley, Chichester, West Sussex, England, Hoboken, NJ: 2003. 10.1002/0470867213. [DOI] [Google Scholar]
- [151].Panyam J, Labhasetwar V, Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Adv. Drug Deliv. Rev 55 (2003) 329–347, 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- [152].Onoue S, Yamada S, Chan HK, Nanodrugs: pharmacokinetics and safety, Int. J. Nanomedicine 9 (2014) 1025–1037, 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Abasolo I, Seras-Franzoso J, Moltó-Abad M, Díaz-Riascos V, Corchero JL, Pintos-Morell G, Schwartz S, Nanotechnology-based approaches for treating lysosomal storage disorders, a focus on Fabry disease, Wiley Interdiscip. Rev. Nanomedicine, Nanobiotechnology 13 (2021) e1684, 10.1002/wnan.1684. [DOI] [PubMed] [Google Scholar]
- [154].Sato Y, Minami K, Hirato T, Tanizawa K, Sonoda H, Schmidt M. Drug delivery for neuronopathic lysosomal storage diseases: evolving roles of the blood brain barrier and cerebrospinal fluid. Metab Brain Dis. 37 (2022) 1745–1756, 10.1007/s11011-021-00893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Edelmann MJ, Maegawa GHB, CNS-targeting therapies for lysosomal storage diseases: current advances and challenges, Front. Mol. Biosci 7 (2020) 559804, 10.3389/fmolb.2020.559804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Gigliobianco RM, Di Martino P, Deng S, Casadidio C, Censi R, New Advanced Strategies for the Treatment of Lysosomal Diseases Affecting the Central Nervous System, Curr. Pharm. Des 25 (2019) 1933–1950, https://doi.org/ 10.2174/1381612825666190708213159. [DOI] [PubMed] [Google Scholar]
- [157].Nasseau M, Boublik Y, Meier W, Winterhalter M, Fournier D, Substrate-permeable encapsulation of enzymes maintains effective activity, stabilizes against denaturation, and protects against proteolytic degradation, Biotechnol. Bioeng 75 (2001) 615–618, 10.1002/BIT.10074. [DOI] [PubMed] [Google Scholar]
- [158].Cui J, Zhao Y, Feng Y, Lin T, Zhong C, Tan Z, Jia S, Encapsulation of Spherical Cross-Linked Phenylalanine Ammonia Lyase Aggregates in Mesoporous Biosilica, J. Agric. Food Chem 65 (2017) 618–625, 10.1021/ACS.JAFC.6B05003. [DOI] [PubMed] [Google Scholar]
- [159].Dziubla TD, Karim A, Muzykantov VR, Polymer nanocarriers protecting active enzyme cargo against proteolysis, J. Control. Release 102 (2005) 427–439, 10.1016/J.JCONREL.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [160].Owens DE, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int. J. Pharm 307 (2006) 93–102, 10.1016/J.IJPHARM.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [161].Fam SY, Chee CF, Yong CY, Ho KL, Mariatulqabtiah AR, Tan WS, Stealth coating of nanoparticles in drug-delivery systems, Nanomater. 10 (2020) 787, 10.3390/nano10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bazile D, Prud’homme C, Bassoullet M-T, Marlard M, Spenlehauer G, Veillard M, Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system, J. Pharm. Sci 84 (1995) 493–498, 10.1002/JPS.2600840420. [DOI] [PubMed] [Google Scholar]
- [163].Haroon HB, Hunter AC, Farhangrazi ZS, Moghimi SM, A brief history of long circulating nanoparticles, Adv. Drug Deliv. Rev 188 (2022) 114396, 10.1016/J.ADDR.2022.114396. [DOI] [PubMed] [Google Scholar]
- [164].ALZA Pharmaceuticals, Doxill®, (n.d.). https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50718s06lbl.pdf (accessed November 28, 2022).
- [165].EMA, Annex I Summary of Product characteristics, (n.d.). https://www.ema.europa.eu/en/documents/product-information/caelyx-pegylated-liposomal-epar-product-information_en.pdf (accessed November 28, 2022).
- [166].Chapman RG, Ostuni E, Takayama S, Holmlin RE, Yan L, Whitesides GM, Surveying for surfaces that resist the adsorption of proteins, J. Am. Chem. Soc 122 (2000) 8303–8304, 10.1021/JA000774F. [DOI] [Google Scholar]
- [167].Moreadith RW, Viegas TX, Standaert DG, Bentley MD, Fang Z, Dizman B, Yoon K, Weimer R, Harris JM, Ravenscroft P, Johnston TH, Hill M, Brotchie JM, SER-214, A novel polymer-conjugated rotigotine formulation affords greatly extended duration of anti-parkinsonian effect and enhanced plasma exposure following a single administration in rodents and primates. In: 16th International Conference of Parkinson’s Disease and Movement Disorders, Movement Disorder Society, Dublin, Ireland, June 17-21, 2012, Late Breaking Abstract 5. Available from: http://serina.100danish.com/wp-content/uploads/2015/09/Serina-Poster-for-2012-MDS-conference-v3.pdf [Google Scholar]
- [168].Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B, Weimer R, Mero A, Pasut G, Veronese FM, Polyoxazoline: chemistry, properties, and applications in drug delivery, Bioconjug. Chem 22 (2011) 976–986, 10.1021/BC200049D. [DOI] [PubMed] [Google Scholar]
- [169].Bludau H, Czapar AE, Pitek AS, Shukla S, Jordan R, Steinmetz NF, POxylation as an alternative stealth coating for biomedical applications, Eur. Polym. J 88 (2017) 679–688, 10.1016/J.EURPOLYMJ.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sanchez L, Yi Y, Yu Y, Effect of partial PEGylation on particle uptake by macrophages, Nanoscale 9 (2017) 288–297, 10.1039/C6NR07353K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Dong H, Tang M, Li Y, Li Y, Qian D, Shi D, Disulfide-bridged cleavable PEGylation in polymeric nanomedicine for controlled therapeutic delivery, Nanomedicine 10 (2015) 1941–1958, 10.2217/NNM.15.38. [DOI] [PubMed] [Google Scholar]
- [172].Xu H, Deng Y, Chen D, Hong W, Lu Y, Dong X, Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives, J. Control. Release 130 (2008) 238–245, 10.1016/J.JCONREL.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [173].Tsai RK, Discher DE, Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells, J. Cell Biol 180 (2008) 989–1003, 10.1083/JCB.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kim J, Sinha S, Solomon M, Perez-Herrero E, Hsu J, Tsinas Z, Muro S, Co-coating of receptor-targeted drug nanocarriers with anti-phagocytic moieties enhances specific tissue uptake versus non-specific phagocytic clearance, Biomaterials. 147 (2017) 14–25, 10.1016/J.BIOMATERIALS.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kishimoto TK, Ferrari JD, Lamothe RA, Kolte PN, Griset AP, O’Neil C, Chan V, Browning E, Chalishazar A, Kuhlman W, Fu FN, Viseux N, Altreuter DH, Johnston L, Maldonado R, Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles., Nat. Nanotechnol 11 (2016) 890–899, 10.1038/NNANO.2016.135. [DOI] [PubMed] [Google Scholar]
- [176].Kazi ZB, Desai AK, Troxler RB, Kronn D, Packman S, Sabbadini M, Rizzo WB, Scherer K, Abdul-Rahman O, Tanpaiboon P, Nampoothiri S, Gupta N, Feigenbaum A, Niyazov DM, Sherry L, Segel R, McVie-Wylie A, Sung C, Joseph AM, Richards S, Kishnani PS, An immune tolerance approach using transient low-dose methotrexate in the ERT-naïve setting of patients treated with a therapeutic protein: experience in infantile-onset Pompe disease, Genet. Med 21 (2019) 887–895, 10.1038/S41436-018-0270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Gaudioso Á, Silva TP, Dolores Ledesma M, Models to study basic and applied aspects of lysosomal storage disorders, Adv. Drug Deliv. Rev 190 (2022) 114532, 10.1016/J.ADDR.2022.114532. [DOI] [PubMed] [Google Scholar]
- [178].Gregoriadis G, Ryman BE, Lysosomal localization of enzyme-containing liposomes injected into rats, Biochem. J 128 (1972) 142P–143P, 10.1042/bj1280142Pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].PATEL HM, RYMAN BE, α-Mannosidase in Zinc-Deficient Rats: Possibility of Liposomal Therapy in Mannosidosis, Biochem. Soc. Trans 2 (1974) 1014–1017, 10.1042/bst0021014. [DOI] [Google Scholar]
- [180].Braidman IP, Gregoriadis G, Rapid partial purification of placental glucocerebroside β-glucosidase and its entrapment in liposomes, Biochem. J 164 (1977) 439–445, 10.1042/bj1640439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Steger LD, Desnick RJ, Enzyme therapy VI: Comparative in vivo fates and effects on lysosomal integrity of enzyme entrapped in negatively and positively charged liposomes, Biochim. Biophys. Acta - Biomembr 464 (1977) 530–546, 10.1016/0005-2736(77)90028-1. [DOI] [PubMed] [Google Scholar]
- [182].Takada G, Onodera H, Tada K, Delivery of fungal β-Galactosidase to rat brain by means of liposomes, Tohoku J. Exp. Med 136 (1982) 219–229, 10.1620/tjem.136.219. [DOI] [PubMed] [Google Scholar]
- [183].Umezawa F, Eto Y, Tokoro T, Ito F, Maekawa K, Enzyme replacement with liposomes containing beta-galactosidase from charonia lumpas in murine globoid cell leukodystrophy (twitcher), Biochem. Biophys. Res. Commun 127 (1985) 663–667, https://doi.org/ 10.1016/S0006-291X(85)80212-6. [DOI] [PubMed] [Google Scholar]
- [184].Koshkaryev A, Thekkedath R, Pagano C, Meerovich I, Torchilin VP, Targeting of lysosomes by liposomes modified with octadecyl-rhodamine B, J. Drug Target 19 (2011) 606–614, 10.3109/1061186X.2010.550921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Meerovich I, Koshkaryev A, Thekkedath R, Torchilin VP, Screening and Optimization of Ligand Conjugates for Lysosomal Targeting, Bioconjug. Chem 22 (2011) 2271–2282, 10.1021/bc200336j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Thekkedath R, Koshkaryev A, Torchilin VP, Lysosome-targeted octadecyl-rhodamine B-liposomes enhance lysosomal accumulation of glucocerebrosidase in Gaucher’s cells in vitro, Nanomedicine 8 (2012) 1055–1065, 10.2217/nnm.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sun Y, Liou B, Chu Z, Fannin V, Blackwood R, Peng Y, Grabowski GA, Davis HW, Qi X, Systemic enzyme delivery by blood-brain barrier-penetrating SapC-DOPS nanovesicles for treatment of neuronopathic Gaucher disease, EBioMedicine. 55 (2020) 102735, 10.1016/j.ebiom.2020.102735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Cabrera I, Abasolo I, Corchero JL, Elizondo E, Gil PR, Moreno E, Faraudo J, Sala S, Bueno D, González-Mira E, Rivas M, Melgarejo M, Pulido D, Albericio F, Royo M, Villaverde A, García-Parajo MF, Schwartz S, Ventosa N, Veciana J, α-Galactosidase A Loaded Nanoliposomes with Enhanced Enzymatic Activity and Intracellular Penetration, Adv. Healthc. Mater (2016) 829–840, 10.1002/adhm.201500746. [DOI] [PubMed] [Google Scholar]
- [189].Tomsen-Melero J, Passemard S, García-Aranda N, Díaz-Riascos ZV, González-Rioja R, Nedergaard Pedersen J, Lyngsø J, Merlo-Mas J, Cristóbal-Lecina E, Corchero JL, Pulido D, Cámara-Sánchez P, Portnaya I, Ionita I, Schwartz S, Veciana J, Sala S, Royo M, Córdoba A, Danino D, Pedersen JS, González-Mira E, Abasolo I, Ventosa N, Impact of Chemical Composition on the Nanostructure and Biological Activity of α-Galactosidase-Loaded Nanovesicles for Fabry Disease Treatment, ACS Appl. Mater. Interfaces 13 (2021) 7825–7838, 10.1021/acsami.0c16871. [DOI] [PubMed] [Google Scholar]
- [190].Merlo-Mas J, Tomsen-Melero J, Corchero J-L, González-Mira E, Font A, Pedersen JN, García-Aranda N, Cristóbal-Lecina E, Alcaina-Hernando M, Mendoza R, Garcia-Fruitós E, Lizarraga T, Resch S, Schimpel C, Falk A, Pulido D, Royo M, Schwartz S, Abasolo I, Pedersen JS, Danino D, Soldevila A, Veciana J, Sala S, Ventosa N, Córdoba A, Application of Quality by Design to the robust preparation of a liposomal GLA formulation by DELOS-susp method, J. Supercrit. Fluids 173 (2021) 105204, 10.1016/j.supflu.2021.105204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Aldosari MH, de Vries RP, Rodriguez LR, Hesen NA, Beztsinna N, van Kuilenburg ABP, Hollak CEM, Schellekens H, Mastrobattista E, Liposome-targeted recombinant human acid sphingomyelinase: Production, formulation, and in vitro evaluation, Eur. J. Pharm. Biopharm 137 (2019) 185–195, 10.1016/j.ejpb.2019.02.019. [DOI] [PubMed] [Google Scholar]
- [192].McGovern MM, Wasserstein MP, Kirmse B, Duvall WL, Schiano T, Thurberg BL, Richards S, Cox GF, Novel first-dose adverse drug reactions during a phase I trial of olipudase alfa (recombinant human acid sphingomyelinase) in adults with Niemann-Pick disease type B (acid sphingomyelinase deficiency), Genet. Med 18 (2016) 34–40, 10.1038/gim.2015.24. [DOI] [PubMed] [Google Scholar]
- [193].Hamill KM, Wexselblatt E, Tong W, Esko JD, Tor Y, Delivery of Cargo to Lysosomes Using GNeosomes. In: Öllinger K, Appelqvist H (Eds.) Lysosomes, Methods in Molecular Biology, vol 1594, Humana Press, New York, NY, 2017, pp. 151–163, 10.1007/978-1-4939-6934-0_9. [DOI] [PubMed] [Google Scholar]
- [194].Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y, Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles, Cells 9 (2020) 851, 10.3390/CELLS9040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Haney MJ, Klyachko NL, Harrison EB, Zhao Y, V Kabanov A, V Batrakova E, TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease, Adv. Healthc. Mater 8 (2019) 1801271, https://doi.org/ 10.1002/adhm.201801271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Do MA, Levy D, Brown A, Marriott G, Lu B, Targeted delivery of lysosomal enzymes to the endocytic compartment in human cells using engineered extracellular vesicles, Sci. Reports 9 (2019) 17274, 10.1038/s41598-019-53844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Abasolo I, Seras-Franzoso J, Díaz-Riascos ZV, Corchero JL, González P, García-Aranda N, Mandaña M, Riera R, Boullosa A, Mancilla S, Grayston A, Moltó-Abad M, Garcia-Fruitós E, Mendoza R, Pintos-Morell G, Albertazzi L, Rosell A, Casas J, Villaverde A, Schwartz S, Extracellular vesicles increase the enzymatic activity of lysosomal proteins and improve the efficacy of enzyme replacement therapy in Fabry disease, Mol. Genet. Metab 129 (2020) S16, 10.1016/J.YMGME.2019.11.010. [DOI] [Google Scholar]
- [198].Seras-Franzoso J, Díaz-Riascos ZV, Corchero JL, González P, García-Aranda N, Mandaña M, Riera R, Boullosa A, Mancilla S, Grayston A, Moltó-Abad M, Garcia-Fruitós E, Mendoza R, Pintos-Morell G, Albertazzi L, Rosell A, Casas J, Villaverde A, Schwartz S, Abasolo I, Extracellular vesicles from recombinant cell factories improve the activity and efficacy of enzymes defective in lysosomal storage disorders, J. Extracell. Vesicles 10 (2021) e12058, 10.1002/jev2.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Mayer FQ, Adorne MD, Bender EA, de Carvalho TG, Dilda AC, Beck RCR, Guterres SS, Giugliani R, Matte U, Pohlmann AR, Laronidase-Functionalized Multiple-Wall Lipid-Core Nanocapsules: Promising Formulation for a More Effective Treatment of Mucopolysaccharidosis Type I, Pharm. Res 32 (2015) 941–954, 10.1007/s11095-014-1508-y. [DOI] [PubMed] [Google Scholar]
- [200].V Álvarez J, Bravo SB, García-Vence M, De Castro MJ, Luzardo A, Colón C, Tomatsu S, Otero-Espinar FJ, Couce ML, Proteomic Analysis in Morquio A Cells Treated with Immobilized Enzymatic Replacement Therapy on Nanostructured Lipid Systems, Int. J. Mol. Sci 20 (2019) 4610, 10.3390/ijms20184610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Donida B, Tauffner B, Raabe M, Immich MF, de Farias MA, de Sá Coutinho D, Machado AZ, Kessler RG, Portugal RV, Bernardi A, Frozza R, Moura DJ, Poletto F, Vargas CR, Monoolein-based nanoparticles for drug delivery to the central nervous system: A platform for lysosomal storage disorder treatment, Eur. J. Pharm. Biopharm 133 (2018) 96–103, 10.1016/j.ejpb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- [202].Sarrazin S, Wilson B, Sly WS, Tor Y, Esko JD, Guanidinylated neomycin mediates heparan sulfate-dependent transport of active enzymes to lysosomes, Mol. Ther 18 (2010) 1268–1274, 10.1038/mt.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Tohidi Moghadam T, Ranjbar B, Khajeh K, Conformation and activity of lysozyme on binding to two types of gold nanorods: A comparative study, Int. J. Biol. Macromol 51 (2012) 91–96, 10.1016/j.ijbiomac.2012.04.020. [DOI] [PubMed] [Google Scholar]
- [204].Corchero JL, Mendoza R, Ferrer-Miralles N, Montràs A, Martínez LM, Villaverde A, Enzymatic characterization of highly stable human alpha-galactosidase A displayed on magnetic particles, Biochem. Eng. J 67 (2012) 20–27, 10.1016/j.bej.2012.05.003. [DOI] [Google Scholar]
- [205].Dekiwadia CD, Lawrie AC, Fecondo JV, Peptide-mediated cell penetration and targeted delivery of gold nanoparticles into lysosomes, J. Pept. Sci 18 (2012) 527–534, 10.1002/psc.2430. [DOI] [PubMed] [Google Scholar]
- [206].Kolodny EH, Niemann-Pick disease, Curr. Opin. Hematol 7 (2000) 48–52, 10.1097/00062752-200001000-00009. [DOI] [PubMed] [Google Scholar]
- [207].Schuchman EH, Desnick RJ, Types A and B Niemann-Pick disease, Mol. Genet. Metab 120 (2017) 27–33, 10.1016/j.ymgme.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Bajwa H, Azhar W, Niemann-Pick Disease. In: StatPearls [Internet], StatPearls Publishing, Treasure Island (FL), 2021, https://www.ncbi.nlm.nih.gov/books/NBK556129/. [Google Scholar]
- [209].Vanier MT, Niemann-Pick diseases, Handb. Clin. Neurol 113 (2013) 1717–1721, 10.1016/B978-0-444-59565-2.00041-1. [DOI] [PubMed] [Google Scholar]
- [210].Pavlů-Pereira H, Asfaw B, Poupětová H, Ledvinová J, Sikora J, Vanier MT, Sandhoff K, Zeman J, Novotná Z, Chudoba D, Elleder M, Acid sphingomyelinase deficiency. Phenotype variability with prevalence of intermediate phenotype in a series of twenty-five Czech and Slovak patients. A multi-approach study, J. Inherit. Metab. Dis 28 (2005) 203–227, 10.1007/S10545-005-5671-5. [DOI] [PubMed] [Google Scholar]
- [211].Rappaport J, Manthe RL, Solomon M, Garnacho C, Muro S, A Comparative Study on the Alterations of Endocytic Pathways in Multiple Lysosomal Storage Disorders, Mol. Pharm 13 (2016) 357–368, 10.1021/acs.molpharmaceut.5b00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Rappaport J, Garnacho C, Muro S, Clathrin-mediated endocytosis is impaired in type A-B Niemann-pick disease model cells and can be restored by ICAM-1-mediated enzyme replacement, Mol. Pharm 11 (2014) 2887–2895, 10.1021/mp500241y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Hurwitz R, Ferlinz K, Sandhofl K, The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts, Biol. Chem. Hoppe. Seyler 375 (1994) 447–450, 10.1515/BCHM3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- [214].Kölzer M, Werth N, Sandhoff K, Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine, FEBS Lett. 559 (2004) 96–98, 10.1016/S0014-5793(04)00033-X. [DOI] [PubMed] [Google Scholar]
- [215].Vapniarsky N, Wenger DA, Scheenstra D, Mete A, Sphingomyelin lipidosis (Niemann-Pick disease) in a juvenile raccoon (Procyon lotor), J. Comp. Pathol 149 (2013) 385–389, 10.1016/J.JCPA.2013.01.011. [DOI] [PubMed] [Google Scholar]
- [216].Gurda BL, Vite CH, Large animal models contribute to the development of therapies for central and peripheral nervous system dysfunction in patients with lysosomal storage diseases, Hum. Mol. Genet 28 (2019) R119–R131, 10.1093/HMG/DDZ127. [DOI] [PubMed] [Google Scholar]
- [217].Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin JP, Zhu Y, Bagel J, O’Donnell P, Sikora T, Ruane T, Wang P, Haskins ME, Wilson JM, Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I, Mol. Ther 22 (2014) 2018–2027, 10.1038/MT.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Hinderer C, Bell P, Louboutin JP, Katz N, Zhu Y, Lin G, Choa R, Bagel J, O’Donnell P, Fitzgerald CA, Langan T, Wang P, Casal ML, Haskins ME, Wilson JM, Neonatal tolerance induction enables accurate evaluation of gene therapy for MPS I in a canine model, Mol. Genet. Metab 119 (2016) 124–130, 10.1016/J.YMGME.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK, Snella EM, Mohammed EEA, Thomson CB, Raoul S, Joussemet B, Roux F, Chérel Y, Lajat Y, Piraud M, Benchaouir R, Hermening S, Petry H, Froissart R, Tardieu M, Ciron C, Moullier P, Parkes J, Kline KL, Maire I, Vanier MT, Heard JM, Colle MA, Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes, Mol. Ther 19 (2011) 251–259, 10.1038/MT.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Gray-Edwards HL, Jiang X, Randle AN, Taylor AR, Voss TL, Johnson AK, McCurdy VJ, Sena-Esteves M, Ory DS, Martin DR, Lipidomic Evaluation of Feline Neurologic Disease after AAV Gene Therapy, Mol. Ther. Methods Clin. Dev 6 (2017) 135–142, 10.1016/J.OMTM.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Gray-Edwards HL, Randle AN, Maitland SA, Benatti HR, Hubbard SM, Canning PF, Vogel MB, Brunson BL, Hwang M, Ellis LE, Bradbury AM, Gentry AS, Taylor AR, Wooldridge AA, Wilhite DR, Winter RL, Whitlock BK, Johnson JA, Holland M, Salibi N, Beyers RJ, Sartin JL, Denney TS, Cox NR, Sena-Esteves M, Martin DR, Adeno-Associated Virus Gene Therapy in a Sheep Model of Tay-Sachs Disease, Hum. Gene Ther 29 (2018) 312–326, 10.1089/HUM.2017.163. [DOI] [PubMed] [Google Scholar]
- [222].Katz ML, Tecedor L, Chen Y, Williamson BG, Lysenko E, Wininger FA, Young WM, Johnson GC, Whiting REH, Coates JR, Davidson BL, AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease, Sci. Transl. Med 7 (2015) p.313ra180, 10.1126/SCITRANSLMED.AAC6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Otterbach B, Stoffel W, Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease), Cell 81 (1995) 1053–1061, 10.1016/S0092-8674(05)80010-8. [DOI] [PubMed] [Google Scholar]
- [224].Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH, Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease, Nat. Genet 10 (1995) 288–293, 10.1038/NG0795-288. [DOI] [PubMed] [Google Scholar]
- [225].Marathe S, Miranda SRP, Devlin C, Johns A, Kuriakose G, Williams KJ, Schuchman EH, Tabas I, Creation of a mouse model for non-neurological (type B) Niemann-Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function, Hum. Mol. Genet 9 (2000) 1967–1976, 10.1093/HMG/9.13.1967. [DOI] [PubMed] [Google Scholar]
- [226].Jones I, He X, Katouzian F, Darroch PI, Schuchman EH, Characterization of common SMPD1 mutations causing types A and B Niemann-Pick disease and generation of mutation-specific mouse models, Mol. Genet. Metab 95 (2008) 152–162, 10.1016/J.YMGME.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Schuchman EH, The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann–Pick disease, J. Inherit. Metab. Dis 30 (2007) 654–663, 10.1007/S10545-007-0632-9. [DOI] [PubMed] [Google Scholar]
- [228].Miranda SRP, He X, Simonaro CM, Gatt S, Dagan A, Desnick RJ, Schuchman EH, Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology, FASEB J. 14 (2000) 1988–1995, 10.1096/FJ.00-0014COM. [DOI] [PubMed] [Google Scholar]
- [229].Keam SJ, Olipudase Alfa: First Approval, Drugs 82 (2022) 941–947, 10.1007/S40265-022-01727-X. [DOI] [PubMed] [Google Scholar]
- [230].European Medicines Agency, Xenpozyme, (2022), https://www.ema.europa.eu/en/medicines/human/EPAR/xenpozyme.
- [231].FDA Approves First Treatment for Acid Sphingomyelinase Deficiency, a Rare Genetic Disease ∣ FDA, (2022), https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-acid-sphingomyelinase-deficiency-rare-genetic-disease. [Google Scholar]
- [232].DailyMed - XENPOZYME- olipudase alfa-rpcp injection, powder, lyophilized, for solutio, (2022), https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01a910ee-a33e-4be3-ac41-322d64c34311.
- [233].Solomon M, Loeck M, Silva-Abreu M, Moscoso R, Bautista R, Vigo M, Muro S, Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases., J. Control. Release 349 (2022) 1031–1044, 10.1016/j.jconrel.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S, Delivery of Acid Sphingomyelinase in Normal and Niemann-Pick Disease Mice Using Intercellular Adhesion Molecule-1-Targeted Polymer Nanocarriers, J. Pharmacol. Exp. Ther 325 (2008) 400–408, 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- [235].Garnacho C, Dhami R, Solomon M, Schuchman EH, Muro S, Enhanced Delivery and Effects of Acid Sphingomyelinase by ICAM-1-Targeted Nanocarriers in Type B Niemann-Pick Disease Mice, Mol. Ther 25 (2017) 1686–1696, 10.1016/j.ymthe.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Rappaport J, Manthe RL, Garnacho C, Muro S, Altered clathrin-independent endocytosis in type A Niemann-Pick disease cells and rescue by ICAM-1-targeted enzyme delivery, Mol. Pharm 12 (2015) 1366–1376, 10.1021/mp5005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR, Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of ICAM-1-targeted Carriers, Mol. Ther 16 (2008) 1450–1458, 10.1038/MT.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Papademetriou J, Garnacho C, Serrano D, Bhowmick T, Schuchman EH, Muro S, Comparative binding, endocytosis, and biodistribution of antibodies and antibody-coated carriers for targeted delivery of lysosomal enzymes to ICAM-1 versus transferrin receptor, J. Inherit. Metab. Dis 36 (2013) 467–477, 10.1007/S10545-012-9534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Papademetriou IT, Garnacho C, Schuchman EH, Muro S, In Vivo Performance of Polymer Nanocarriers Dually-Targeted to Epitopes of the Same or Different Receptors, Biomaterials. 34 (2013) 3459–3466, 10.1016/j.biomaterials.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Garnacho C, Muro S, ICAM-1 targeting, intracellular trafficking, and functional activity of polymer nanocarriers coated with a fibrinogen-derived peptide for lysosomal enzyme replacement, J. Drug Target 25 (2017) 786–795, 10.1080/1061186X.2017.1349771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Wasserstein MP, Jones SA, Soran H, Diaz GA, Lippa N, Thurberg BL, Culm-Merdek K, Shamiyeh E, Inguilizian H, Cox GF, Puga AC, Successful within-patient dose escalation of olipudase alfa in acid sphingomyelinase deficiency, Mol. Genet. Metab 116 (2015) 88–97, 10.1016/J.YMGME.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Dhami R, Schuchman EH, Mannose 6-phosphate receptor-mediated uptake is defective in acid sphingomyelinase-deficient macrophages, J. Biol. Chem 279 (2004) 1526–1532, 10.1074/JBC.M309465200. [DOI] [PubMed] [Google Scholar]
- [243].Manthe RL, Rappaport JA, Long Y, Solomon M, Veluvolu V, Hildreth M, Gugutkov D, Marugan J, Zheng W, Muro S, Δ-Tocopherol effect on endocytosis and its combination with enzyme replacement therapy for lysosomal disorders: A new type of drug interaction?, J. Pharmacol. Exp. Ther 370 (2019) 823–833, 10.1124/jpet.119.257345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Hsu J, Hoenicka J, Muro S, Targeting, endocytosis, and lysosomal delivery of active enzymes to model human neurons by ICAM-1-targeted nanocarriers, Pharm. Res 32 (2015) 1264–1278, 10.1007/s11095-014-1531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Astete CE, Sabliov CM, Synthesis and characterization of PLGA nanoparticles, J. Biomater. Sci. Polym. Ed 17 (2006) 247–289, 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- [246].Li J, Rothstein SN, Little SR, Edenborn HM, Meyer TY, The effect of monomer order on the hydrolysis of biodegradable poly(lactic-co-glycolic acid) repeating sequence copolymers, J. Am. Chem. Soc 134 (2012) 16352–16359, 10.1021/ja306866w. [DOI] [PubMed] [Google Scholar]
- [247].Lü JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C, Current advances in research and clinical applications of PLGA-based nanotechnology, Expert Rev. Mol. Diagn 9 (2009) 325–341, 10.1586/ERM.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Simionescu BC, Ivanov D, Natural and synthetic polymers for designing composite materials. In: Antoniac IV (Ed.) Handbook of bioceramics and biocomposites. Springer, Cham, 2016, pp. 233–286, 10.1007/978-3-319-12460-5_11 [DOI] [Google Scholar]
- [249].Baltazar GC, Guha S, Lu W, Lim J, Boesze-Battaglia K, Laties AM, Tyagi P, Kompella UB, Mitchell CH, Acidic Nanoparticles Are Trafficked to Lysosomes and Restore an Acidic Lysosomal pH and Degradative Function to Compromised ARPE-19 Cells, PLoS One. 7 (2012) e49635, 10.1371/journal.pone.0049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Lakpa KL, Khan N, Afghah Z, Chen X, Geiger JD, Lysosomal stress response (LSR): physiological importance and pathological relevance, J. Neuroimmune Pharmacol 16 (2021) 219–237, 10.1007/s11481-021-09990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Wang Y, Qin B, Xia G, Choi SH, FDA’s poly (lactic-co-glycolic acid) research program and regulatory outcomes, AAPS J. 23 (2021) 92, 10.1208/S12248-021-00611-Y. [DOI] [PubMed] [Google Scholar]
- [252].Wiseman ME, Frank CW, Antibody adsorption and orientation on hydrophobic surfaces, Langmuir. 28 (2012) 1765–1774, 10.1021/LA203095P. [DOI] [PubMed] [Google Scholar]
- [253].Marcos-Contreras OA, Brenner JS, Kiseleva RY, Zuluaga-Ramirez V, Greineder CF, Villa CH, Hood ED, Myerson JW, Muro S, Persidsky Y, Muzykantov VR, Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain, J. Control. Release 301 (2019) 54–61, 10.1016/j.jconrel.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Glassman PM, Nong J, Myerson JW, Zuluaga-Ramirez V, Rodriguez-Garcia A, Mukalel A, Omo-Lamai S, Walsh LR, Kiseleva RY, Villa CH, Greineder CF, Kasner SE, Weissman D, Mitchell MJ, Muro S, Persidsky Y, Brenner JS, Muzykantov VR, Marcos-Contreras OA, Targeted nanocarriers coopting pulmonary leukocytes for drug delivery to the injured brain, BioRxiv (2022) 2022.02.04.479150, 10.1101/2022.02.04.479150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Gal A, Schafer E, Rohard I, The genetic basis of Fabry disease, In: Fabry Disease: Perspectives from 5 Years of FOS. Oxford PharmaGenesis, Oxford; 2006. https://www.ncbi.nlm.nih.gov/books/NBK11574/. [PubMed] [Google Scholar]
- [256].Castelli V, Stamerra CA, d’Angelo M, Cimini A, Ferri C, Current and experimental therapeutics for Fabry disease, Clin. Genet 100 (2021) 239–247, 10.1111/cge.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A Deficiency: Fabry Disease. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (Eds.), The Online Metabolic and Molecular Bases of Inherited Disease. McGraw Hill; 2019, 10.1036/ommbid.181, https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225546984.. [DOI] [Google Scholar]
- [258].Mehta A, Hughes DA, Fabry Disease. In: GeneReviews®. University of Washington, Seattle, Seattle (WA); 2022. http://europepmc.org/books/NBK1292. [Google Scholar]
- [259].Miller JJ, Kanack AJ, Dahms NM, Progress in the understanding and treatment of Fabry disease, Biochim. Biophys. Acta - Gen. Subj 1864 (2020) 129437, 10.1016/j.bbagen.2019.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Lee HJ, Park HH, Sohn Y, Ryu J, Park JH, Rhee WJ, Park TH, α-Galactosidase delivery using 30Kc19-human serum albumin nanoparticles for effective treatment of Fabry disease, Appl. Microbiol. Biotechnol 100 (2016) 10395–10402, 10.1007/s00253-016-7689-z. [DOI] [PubMed] [Google Scholar]
- [261].Sakuraba H, Murata-Ohsawa M, Kawashima I, Tajima Y, Kotani M, Ohshima T, Chiba Y, Takashiba M, Jigami Y, Fukushige T, Kanzaki T, Itoh K, Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice, J. Hum. Genet 51 (2006) 180–188, 10.1007/S10038-005-0342-9. [DOI] [PubMed] [Google Scholar]
- [262].Legler G, Pohl S, Synthesis of 5-amino-5-deoxy-D-galactopyranose and 1,5-dideoxy-1,5-imino-D-galactitol, and their inhibition of alpha- and beta-D-galactosidases, Carbohydr. Res 155 (1986) 119–129, 10.1016/S0008-6215(00)90138-1. [DOI] [PubMed] [Google Scholar]
- [263].Hsu J, Serrano D, Bhowmick T, Kumar K, Shen Y, Kuo YC, Garnacho C, Muro S, Enhanced Endothelial Delivery and Biochemical Effects of α-Galactosidase by ICAM-1-Targeted Nanocarriers for Fabry Disease, J Control Release. 149 (2011) 323–331, 10.1016/j.jconrel.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Xu M, Motabar O, Ferrer M, Marugan JJ, Zheng W, Ottinger EA, Disease models for the development of therapies for lysosomal storage diseases, Ann. N. Y. Acad. Sci 1371 (2016) 15–29, 10.1111/NYAS.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Kawagoe S, Higuchi T, Otaka M, Shimada Y, Kobayashi H, Ida H, Ohashi T, Okano HJ, Nakanishi M, Eto Y, Morphological features of iPS cells generated from Fabry disease skin fibroblasts using Sendai virus vector (SeVdp), Mol. Genet. Metab 109 (2013) 386–389, 10.1016/J.YMGME.2013.06.003. [DOI] [PubMed] [Google Scholar]
- [266].Kido J, Nakamura K, Era T, Role of induced pluripotent stem cells in lysosomal storage diseases, Mol. Cell. Neurosci 108 (2020) 103540, 10.1016/j.mcn.2020.103540. [DOI] [PubMed] [Google Scholar]
- [267].Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB, α-Galactosidase A deficient mice: a model of Fabry disease, Proc. Natl. Acad. Sci. U.S.A 94 (1997) 2540–2544, 10.1073/PNAS.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Ohshima T, Schiffmann R, Murray GJ, Kopp J, Quirk JM, Stahl S, Chan CC, Zerfas P, Tao-Cheng JH, Ward JM, Brady RO, Kulkarni AB, Aging accentuates and bone marrow transplantation ameliorates metabolic defects in Fabry disease mice, Proc. Natl. Acad. Sci. U.S.A 96 (1999) 6423–6427, 10.1073/PNAS.96.11.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Miller JJ, Aoki K, Moehring F, Murphy CA, O’Hara CL, Tiemeyer M, Stucky CL, Dahms NM, Neuropathic pain in a Fabry disease rat model, JCI Insight. 3 (2018) e99171, 10.1172/JCI.INSIGHT.99171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Miller JJ, Aoki K, Mascari CA, Beltrame AK, Sokumbi O, North PE, Tiemeyer M, Kriegel AJ, Dahms NM, α-Galactosidase A-deficient rats accumulate glycosphingolipids and develop cardiorenal phenotypes of Fabry disease, FASEB J. 33 (2019) 418–429, 10.1096/FJ.201800771R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Miller JJ, Aoki K, Reid CA, Tiemeyer M, Dahms NM, Kassem IS, Rats deficient in α-galactosidase A develop ocular manifestations of Fabry disease, Sci. Rep 9 (2019) 9392, 10.1038/S41598-019-45837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Giannotti MI, Abasolo I, Oliva M, Andrade F, García-Aranda N, Melgarejo M, Pulido D, Corchero JL, Fernández Y, Villaverde A, Royo M, García-Parajo MF, Sanz F, Schwartz S, Highly Versatile Polyelectrolyte Complexes for Improving the Enzyme Replacement Therapy of Lysosomal Storage Disorders, ACS Appl. Mater. Interfaces 8 (2016) 25741–25752, 10.1021/acsami.6b08356. [DOI] [PubMed] [Google Scholar]
- [273].Arends M, Biegstraaten M, Wanner C, Sirrs S, Mehta A, Elliott PM, Oder D, Watkinson OT, Bichet DG, Khan A, Iwanochko M, Vaz FM, van Kuilenburg ABP, West ML, Hughes DA, Hollak CEM, Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: an international cohort study, J. Med. Genet 55 (2018) 351–358, 10.1136/JMEDGENET-2017-104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Parini R, Pintos-Morell G, Hennermann JB, Hsu TR, Karabul N, Kalampoki V, Gurevich A, Ramaswami U, Analysis of renal and cardiac outcomes in male participants in the Fabry outcome survey starting Agalsidase Alfa enzyme replacement therapy before and after 18 years of age, Drug Des. Devel. Ther 14 (2020) 2149–2158, 10.2147/DDDT.S249433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Hristine C, Ng ME, Uffon AG, Ilcox IRW, Ominique D, Ermain PG, Hilip P, Ee L, Teve S, Aldek W, Ouis L, Aplan C, Abor G, Inthorst EL, Obert R, Esnick JD, Safety and efficacy of recombinant human α-Galactosidase A replacement therapy in Fabry’s disease, N. Engl. J. Med 345 (2001) 9–16, 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- [276].Beck M, Ricci R, Widmer U, Dehout F, García De Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Houge G, Ramaswami U, Gal A, Mehta A, Fabry disease: overall effects of agalsidase alfa treatment, Eur. J. Clin. Invest 34 (2004) 838–844, 10.1111/J.1365-2362.2004.01424.X. [DOI] [PubMed] [Google Scholar]
- [277].Lenders M, Brand E, Effects of Enzyme Replacement Therapy and Antidrug Antibodies in Patients with Fabry Disease, J. Am. Soc. Nephrol 29 (2018) 2265–2278, 10.1681/ASN.2018030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Germain DP, Nicholls K, Giugliani R, Bichet DG, Hughes DA, Barisoni LM, Colvin RB, Jennette JC, Skuban N, Castelli JP, Benjamin E, Barth JA, Viereck C, Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study, Genet. Med 21 (2019) 1987–1997, 10.1038/S41436-019-0451-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Giannotti MI, Esteban O, Oliva M, García-Parajo MF, Sanz F, PH-responsive polysaccharide-based polyelectrolyte complexes as nanocarriers for lysosomal delivery of therapeutic proteins, Biomacromolecules 12 (2011) 2524–2533, 10.1021/bm2003384. [DOI] [PubMed] [Google Scholar]
- [280].Kizhner T, Azulay Y, Hainrichson M, Tekoah Y, Arvatz G, Shulman A, Ruderfer I, Aviezer D, Shaaltiel Y, Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease, Mol. Genet. Metab 114 (2015) 259–267, 10.1016/J.YMGME.2014.08.002. [DOI] [PubMed] [Google Scholar]
- [281].Hsu J, Bhowmick T, Burks SR, Kao JPY, Muro S, Enhancing Biodistribution of Therapeutic Enzymes In Vivo by Modulating Surface Coating and Concentration of ICAM-1-Targeted Nanocarriers, J Biomed Nanotechnol. 10 (2014) 345–354, 10.1166/jbn.2014.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].George M, Abraham TE, Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan–a review, J. Control. Release 114 (2006) 1–14, 10.1016/J.JCONREL.2006.04.017. [DOI] [PubMed] [Google Scholar]
- [283].Basu SK, Rajendran A, Studies in the Development of Nateglinide Loaded Calcium Alginate and Chitosan Coated Calcium Alginate Beads, Chem. Pharm. Bull 56 (2008) 1077–1084, 10.1248/CPB.56.1077. [DOI] [PubMed] [Google Scholar]
- [284].Hou JY, Gao LN, Meng FY, Cui YL, Mucoadhesive microparticles for gastroretentive delivery: preparation, biodistribution and targeting evaluation, Mar. Drugs 12 (2014) 5764–5787, 10.3390/MD12125764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Zhang Y, Wei W, Lv P, Wang L, Ma G, Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin, Eur. J. Pharm. Biopharm 77 (2011) 11–19, 10.1016/J.EJPB.2010.09.016. [DOI] [PubMed] [Google Scholar]
- [286].Mane V, Muro S, Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice, Int. J. Nanomedicine 7 (2012) 4223–4237, 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Budd PM, Polyelectrolytes, Compr. Polym. Sci. Suppl 1 (1989) 215–230, 10.1016/B978-0-08-096701-1.00011-2. [DOI] [Google Scholar]
- [288].Cohen Stuart MA, Supramolecular perspectives in colloid science, Colloid Polym. Sci 286 (2008) 855–864, 10.1007/S00396-008-1861-7. [DOI] [Google Scholar]
- [289].Amidi M, Mastrobattista E, Jiskoot W, Hennink WE, Chitosan-based delivery systems for protein therapeutics and antigens, Adv. Drug Deliv. Rev 62 (2010) 59–82, 10.1016/J.ADDR.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [290].Raub TJ, Audus KL, Adsorptive endocytosis and membrane recycling by cultured primary bovine brain microvessel endothelial cell monolayers, J. Cell Sci 97 (1990) 127–138, 10.1242/JCS.97.1.127. [DOI] [PubMed] [Google Scholar]
- [291].Okajima F, Regulation of inflammation by extracellular acidification and proton-sensing GPCRs, Cell. Signal 25 (2013) 2263–2271, 10.1016/J.CELLSIG.2013.07.022. [DOI] [PubMed] [Google Scholar]
- [292].Jiang Y, Stenzel M, Drug Delivery Vehicles Based on Albumin-Polymer Conjugates, Macromol. Biosci 16 (2016) 791–802, 10.1002/MABI.201500453. [DOI] [PubMed] [Google Scholar]
- [293].Lee HJ, Park HH, Kim JA, Park JH, Ryu J, Choi J, Lee J, Rhee WJ, Park TH, Enzyme delivery using the 30Kc19 protein and human serum albumin nanoparticles, Biomaterials 35 (2014) 1696–1704, 10.1016/j.biomaterials.2013.11.001. [DOI] [PubMed] [Google Scholar]
- [294].Park JH, Lee JH, Park HH, Rhee WJ, Choi SS, Park TH, A protein delivery system using 30Kc19 cell-penetrating protein originating from silkworm, Biomaterials 33 (2012) 9127–9134, 10.1016/J.BIOMATERIALS.2012.08.063. [DOI] [PubMed] [Google Scholar]
- [295].Schnitzer JE, gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis, Am. J. Physiol 262 (1992) H246–H254, 10.1152/AJPHEART.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- [296].Genzyme Corporation. Google Scholar, Fabrazyme® (Agalsidase Beta for Intravenous Infusion) Prescribing Information. , Cambridge, MA, 2010, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103979s5303lbl.pdf. [Google Scholar]
- [297].Shire Human Genetic Therapies AB, Replagal, INN-agalsidase alfa (EPAR—Product Information), 2022, https://www.ema.europa.eu/en/medicines/human/EPAR/replagal#product-information-section.
- [298].Schiffmann R, Goker-Alpan O, Holida M, Giraldo P, Barisoni L, Colvin RB, Jennette CJ, Maegawa G, Boyadjiev SA, Gonzalez D, Nicholls K, Tuffaha A, Atta MG, Rup B, Charney MR, Paz A, Szlaifer M, Alon S, Brill-Almon E, Chertkoff R, Hughes D, Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial, J. Inherit. Metab. Dis 42 (2019) 534–544, 10.1002/JIMD.12080. [DOI] [PubMed] [Google Scholar]
- [299].Kozma GT, Shimizu T, Ishida T, Szebeni J, Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals, Adv. Drug Deliv. Rev 154–155 (2020) 163–175, 10.1016/J.ADDR.2020.07.024. [DOI] [PubMed] [Google Scholar]
- [300].Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE, Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol, Cell 137 (2009) 1213–1224, 10.1016/J.CELL.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Xu S, Benoff B, Liou HL, Lobel P, Stock AM, Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease, J. Biol. Chem 282 (2007) 23525–23531, 10.1074/JBC.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Xu Y, Zhang Q, Tan L, Xie X, Zhao Y, The characteristics and biological significance of NPC2: Mutation and disease, Mutat. Res. Rev. Mutat. Res 782 (2019) 108284, 10.1016/J.MRREV.2019.108284. [DOI] [PubMed] [Google Scholar]
- [303].Patterson M. Niemann-Pick Disease Type C. 2000. In: Adam MP, Everman DB, Mirzaa GM, et al. (Eds.) GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993–2023. https://www.ncbi.nlm.nih.gov/books/NBK1296/. [Google Scholar]
- [304].Matencio A, Navarro-Orcajada S, González-Ramón A, García-Carmona F, López-Nicolás JM, Recent advances in the treatment of Niemann pick disease type C: A mini-review, Int. J. Pharm 584 (2020) 119440, 10.1016/j.ijpharm.2020.119440. [DOI] [PubMed] [Google Scholar]
- [305].Pallottini V, Pfrieger FW, Understanding and Treating Niemann–Pick Type C Disease: Models Matter, Int. J. Mol. Sci 21 (2020) 8979, 10.3390/IJMS21238979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Brown A, Patel S, Ward C, Lorenz A, Ortiz M, DuRoss A, Wieghardt F, Esch A, Otten EG, Heiser LM, Korolchuk VI, Sun C, Sarkar S, Sahay G, PEG-lipid micelles enable cholesterol efflux in Niemann-Pick Type C1 disease-based lysosomal storage disorder, Sci. Reports 6 (2016) 31750, 10.1038/srep31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Tamura A, Yui N, Polyrotaxane-based systemic delivery of β-cyclodextrins for potentiating therapeutic efficacy in a mouse model of Niemann-Pick type C disease, J. Control. Release 269 (2018) 148–158, 10.1016/j.jconrel.2017.11.016. [DOI] [PubMed] [Google Scholar]
- [308].Pipalia NH, Huang A, Ralph H, Rujoi M, Maxfield FR, Automated microscopy screening for compounds that partially revert cholesterol accumulation in Niemann-Pick C cells, J. Lipid Res 47 (2006) 284–301, 10.1194/JLR.M500388-JLR200. [DOI] [PubMed] [Google Scholar]
- [309].Soga M, Ishitsuka Y, Hamasaki M, Yoneda K, Furuya H, Matsuo M, Ihn H, Fusaki N, Nakamura K, Nakagata N, Endo F, Irie T, Era T, HPGCD Outperforms HPBCD as a Potential Treatment for Niemann-Pick Disease Type C During Disease Modeling with iPS Cells, Stem Cells 33 (2015) 1075–1088, 10.1002/STEM.1917. [DOI] [PubMed] [Google Scholar]
- [310].Trilck M, Peter F, Zheng C, Frank M, Dobrenis K, Mascher H, Rolfs A, Frech MJ, Diversity of glycosphingolipid GM2 and cholesterol accumulation in NPC1 patient-specific iPSC-derived neurons, Brain Res. 1657 (2017) 52–61, 10.1016/J.BRAINRES.2016.11.031. [DOI] [PubMed] [Google Scholar]
- [311].Sung E-A, Yu K-R, Shin J-H, Seo Y, Kim H-S, Koog MG, Kang I, Kim J-J, Lee B-C, Shin T-H, Lee JY, Lee S, Kang T-W, Choi SW, Kang K-S, Sung E-A, Yu K-R, Shin J-H, Seo Y, Kim H-S, Guen Koog M, Kang I, Kim J-J, Lee B-C, Shin T-H, Young Lee J, Lee S, Kang T-W, Won Choi S, Kang K-S, Generation of patient specific human neural stem cells from Niemann-Pick disease type C patient-derived fibroblasts, Oncotarget 8 (2017) 85428–85441, 10.18632/ONCOTARGET.19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ, Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene, Science 277 (1997) 232–235, 10.1126/SCIENCE.277.5323.232. [DOI] [PubMed] [Google Scholar]
- [313].Pentchev PG, Gal AE, Booth AD, Omodeo-Sale F, Fours J, Neumeyer BA, Quirk JM, Dawson G, Brady RO, A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase, Biochim. Biophys. Acta - Lipids Lipid Metab 619 (1980) 669–679, 10.1016/0005-2760(80)90116-2. [DOI] [PubMed] [Google Scholar]
- [314].Miyawaki S, Mitsuoka S, Sakiyama T, Kitagawa T, Sphingomyelinosis, a new mutation in the mouse: A model of Niemann-Pick disease in humans, J. Hered 73 (1982) 257–263, 10.1093/OXFORDJOURNALS.JHERED.A109635. [DOI] [PubMed] [Google Scholar]
- [315].Maue RA, Burgess RW, Wang B, Wooley CM, Seburn KL, Vanier MT, Rogers MA, Chang CC, Chang TY, Harris BT, Graber DJ, Penatti CAA, Porter DM, Szwergold BS, Henderson LP, Totenhagen JW, Trouard TP, Borbon IA, Erickson RP, A novel mouse model of Niemann–Pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations, Hum. Mol. Genet 21 (2012) 730–750, 10.1093/HMG/DDR505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Sleat DE, Wiseman JA, El-Banna M, Price M, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P, Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport, Proc. Natl. Acad. Sci. U.S.A 101 (2004) 5886–5891, 10.1073/PNAS.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Schrantz N, Sagiv Y, Liu Y, Savage PB, Bendelac A, Teyton L, The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells, J. Exp. Med 204 (2007) 841–852, 10.1084/JEM.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Nielsen GK, Dagnaes-Hansen F, Holm IE, Meaney S, Symula D, Andersen NT, Heegaard CW, Protein replacement therapy partially corrects the cholesterol-storage phenotype in a mouse model of Niemann-Pick type C2 disease, PLoS One 6 (2011) e27287, 10.1371/JOURNAL.PONE.0027287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Acuña M, González-Hódar L, Amigo L, Castro J, Morales MG, Cancino GI, Groen AK, Young J, Miquel JF, Zanlungo S, Transgenic overexpression of Niemann-Pick C2 protein promotes cholesterol gallstone formation in mice, J. Hepatol 64 (2016) 361–369, 10.1016/J.JHEP.2015.10.002. [DOI] [PubMed] [Google Scholar]
- [320].Lowenthal AC, Cummings JF, Wenger DA, Thrall MA, Wood PA, de Lahunta A, Feline sphingolipidosis resembling Niemann-Pick disease type C, Acta Neuropathol. 81 (1990) 189–197, 10.1007/BF00334507. [DOI] [PubMed] [Google Scholar]
- [321].March PA, Thrall MA, Brown DE, Mitchell TW, Lowenthal AC, Walkley SU, GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C, Acta Neuropathol. 94 (1997) 164–172, 10.1007/S004010050689. [DOI] [PubMed] [Google Scholar]
- [322].Vite CH, Ding W, Bryan C, O’Donnell P, Cullen K, Aleman D, Haskins ME, Van Winkle T, Clinical, electrophysiological, and serum biochemical measures of progressive neurological and hepatic dysfunction in feline Niemann-Pick type C disease, Pediatr. Res 64 (2008) 544–549, 10.1203/pdr.0b013e318184d2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Bagel JH, Sikora TU, Prociuk M, Pesayco JP, Mizisin AP, Shelton GD, Vite CH, Electrodiagnostic testing and histopathologic changes confirm peripheral nervous system myelin abnormalities in the feline model of Niemann-Pick disease type C, J. Neuropathol. Exp. Neurol 72 (2013) 256–262, 10.1097/NEN.0B013E318286587F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Roszell BR, Tao JQ, Yu KJ, Gao L, Huang S, Ning Y, Feinstein SI, Vite CH, Bates SR, Pulmonary abnormalities in animal models due to Niemann-Pick type C1 (NPC1) or C2 (NPC2) disease, PLoS One 8 (2013) e67084, 10.1371/JOURNAL.PONE.0067084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Geberhiwot T, Moro A, Dardis A, Ramaswami U, Sirrs S, Marfa MP, Vanier MT, Walterfang M, Bolton S, Dawson C, Héron B, Stampfer M, Imrie J, Hendriksz C, Gissen P, Crushell E, Coll MJ, Nadjar Y, Klünemann H, Mengel E, Hrebicek M, Jones SA, Ory D, Bembi B, Patterson M , Consensus clinical management guidelines for Niemann-Pick disease type C, Orphanet J. Rare Dis 13 (2018) 50, 10.1186/S13023-018-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE, Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study, Lancet Neurol. 6 (2007) 765–772, 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- [327].Pineda M, Walterfang M, Patterson MC, Miglustat in Niemann-Pick disease type C patients: a review, Orphanet J. Rare Dis 13 (2018) 140, 10.1186/S13023-018-0844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Patterson MC, Garver WS, Giugliani R, Imrie J, Jahnova H, Meaney FJ, Nadjar Y, Vanier MT, Moneuse P, Morand O, Rosenberg D, Schwierin B, Héron B, Long-term survival outcomes of patients with Niemann-Pick disease type C receiving miglustat treatment: A large retrospective observational study, J. Inherit. Metab. Dis 43 (2020) 1060–1069, 10.1002/JIMD.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].López CA, de Vries AH, Marrink SJ, Molecular Mechanism of Cyclodextrin Mediated Cholesterol Extraction, PLOS Comput. Biol 7 (2011) e1002020, 10.1371/JOURNAL.PCBI.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, Rao R, Soldatos A, Sidhu R, Walters KA, Xu X, Thurm A, Solomon B, Pavan WJ, Machielse BN, Kao M, Silber SA, McKew JC, Brewer CC, Vite CH, Walkley SU, Austin CP, Porter FD, Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial, Lancet 390 (2017) 1758–1768, 10.1016/S0140-6736(17)31465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Vite CH, Bagel JH, Swain GP, Prociuk M, Sikora TU, Stein VM, O’Donnell P, Ruane T, Ward S, Crooks A, Li S, Mauldin E, Stellar S, De Meulder M, Kao ML, Ory DS, Davidson C, Vanier MT, Walkley SU, Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease, Sci. Transl. Med 7 (2015) p.276ra26, 10.1126/SCITRANSLMED.3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Maarup TJ, Chen AH, Porter FD, Farhat NY, Ory DS, Sidhu R, Jiang X, Dickson PI, Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1, Mol. Genet. Metab 116 (2015) 75–79, 10.1016/J.YMGME.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Bremova-Ertl T, Claassen J, Foltan T, Gascon-Bayarri J, Gissen P, Hahn A, Hassan A, Hennig A, Jones SA, Kolnikova M, Martakis K, Raethjen J, Ramaswami U, Sharma R, Schneider SA, Efficacy and safety of N-acetyl-L-leucine in Niemann-Pick disease type C, J. Neurol 269 (2022) 1651–1662, 10.1007/S00415-021-10717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Mengel E, Patterson MC, Da Riol RM, Del Toro M, Deodato F, Gautschi M, Grunewald S, Grønborg S, Harmatz P, Héron B, Maier EM, Roubertie A, Santra S, Tylki-Szymanska A, Day S, Andreasen AK, Geist MA, Havnsøe Torp Petersen N, Ingemann L, Hansen T, Blaettler T, Kirkegaard T, í Dali C, Efficacy and safety of arimoclomol in Niemann-Pick disease type C: Results from a double-blind, randomised, placebo-controlled, multinational phase 2/3 trial of a novel treatment, J. Inherit. Metab. Dis 44 (2021) 1463–1480, 10.1002/JIMD.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Collins CJ, McCauliff LA, Hyun SH, Zhang Z, Paul LN, Kulkarni A, Zick K, Wirth M, Storch J, Thompson DH, Synthesis, characterization, and evaluation of pluronic-based β-cyclodextrin polyrotaxanes for mobilization of accumulated cholesterol from Niemann-Pick Type C fibroblasts, Biochemistry 52 (2013) 3242–3253, 10.1021/BI3010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Egele K, Samaddar S, Schneider N, Thompson D, Wenz G, Synthesis of the anionic hydroxypropyl-β-cyclodextrin:poly(decamethylenephosphate) polyrotaxane and evaluation of its cholesterol efflux potential in Niemann-Pick C1 cells, J. Mater. Chem. B 7 (2019) 528–537, 10.1039/C8TB02950D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Collins CJ, Loren BP, Alam MS, Mondjinou Y, Skulsky JL, Chaplain CR, Haldar K, Thompson DH, Pluronic based β-cyclodextrin polyrotaxanes for treatment of Niemann-Pick Type C disease, Sci. Reports 7 (2017) 46737, 10.1038/srep46737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Mondjinou YA, McCauliff LA, Kulkarni A, Paul L, Hyun SH, Zhang Z, Wu Z, Wirth M, Storch J, Thompson DH, Synthesis of 2-hydroxypropyl-β-cyclodextrin/pluronic-based polyrotaxanes via heterogeneous reaction as potential niemann-pick type C therapeutics, Biomacromolecules 14 (2013) 4189–4197. 10.1021/BM400922A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Tamura A, Yui N, Lysosomal-specific Cholesterol Reduction by Biocleavable Polyrotaxanes for Ameliorating Niemann-Pick Type C Disease, Sci. Rep 4 (2014) 4356, 10.1038/SREP04356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Tamura A, Nishida K, Yui N, Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease, Sci. Technol. Adv. Mater 17 (2016) 361–374, 10.1080/14686996.2016.1200948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Bonam SR, Wang F, Muller S, Lysosomes as a therapeutic target, Nat. Rev. Drug Discov 18 (2019) 923–948, 10.1038/s41573-019-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Sheth J, Bhavsar R, Mistri M, Pancholi D, Bavdekar A, Dalal A, Ranganath P, Girisha KM, Shukla A, Phadke S, Puri R, Panigrahi I, Kaur A, Muranjan M, Goyal M, Ramadevi R, Shah R, Nampoothiri S, Danda S, Datar C, Kapoor S, Bhatwadekar S, Sheth F, Gaucher disease: Single gene molecular characterization of one-hundred Indian patients reveals novel variants and the most prevalent mutation, BMC Med. Genet 20 (2019) 31, 10.1186/S12881-019-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Platt FM, d’Azzo A, Davidson BL, Neufeld EF, Tifft CJ, Lysosomal storage diseases, Nat. Rev. Dis. Prim 4 (2018) 27, 10.1038/s41572-018-0025-4. [DOI] [PubMed] [Google Scholar]
- [344].Horowitz M, Braunstein H, Zimran A, Revel-Vilk S, Goker-Alpan O, Lysosomal functions and dysfunctions: Molecular and cellular mechanisms underlying Gaucher disease and its association with Parkinson disease, Adv. Drug Deliv. Rev 187 (2022) 114402, 10.1016/J.ADDR.2022.114402. [DOI] [PubMed] [Google Scholar]
- [345].Stern G, Niemann-Pick’s and Gaucher’s diseases, Park. Relat. Disord 20 (2014) S143–S146, 10.1016/S1353-8020(13)70034-8. [DOI] [PubMed] [Google Scholar]
- [346].Martín-Banderas L, Holgado MA, Durán-Lobato M, Infante JJ, Álvarez-Fuentes J, Fernández-Arévalo M, Role of nanotechnology for enzyme replacement therapy in lysosomal diseases. A focus on Gaucher’s disease, Curr. Med. Chem 23 (2016) 929–952, 10.2174/0929867323666160210130608. [DOI] [PubMed] [Google Scholar]
- [347].Mignot C, Gelot A, Bessières B, Daffos F, Voyer M, Menez F, Fallet Bianco C, Odent S, Le Duff D, Loget P, Fargier P, Costil J, Josset P, Roume J, Vanier MT, Maire I, Billette de Villemeur T, Perinatal-lethal Gaucher disease, Am. J. Med. Genet. Part A 120A (2003) 338–344, 10.1002/AJMG.A.20117. [DOI] [PubMed] [Google Scholar]
- [348].Mohamed FE, Ali A, Al-Tenaiji A, Al-Jasmi A, Al-Jasmi F, A Type 3 Gaucher-Like Disease Due To Saposin C Deficiency in Two Emirati Families Caused by a Novel Splice Site Variant in the PSAP Gene, J. Mol. Neurosci 72 (2022) 1322–1333, 10.1007/S12031-022-01987-Y. [DOI] [PubMed] [Google Scholar]
- [349].Sasagasako N, Kobayashi T, Yamaguchi Y, Shinnoh N, Goto I, Glucosylceramide and Glucosylsphingosine Metabolism in Cultured Fibroblasts Deficient in Acid β-Glucosidase Activity, J. Biochem 115 (1994) 113–119, 10.1093/OXFORDJOURNALS.JBCHEM.A124284. [DOI] [PubMed] [Google Scholar]
- [350].Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, Marugan J, Patnaik S, Dutra A, Southall N, Zheng W, Tayebi N, Sidransky E, Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs, Sci. Transl. Med 6 (2014) p.240ra73, 10.1126/SCITRANSLMED.3008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J, Sidransky E, A New Glucocerebrosidase Chaperone Reduces α-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism, J. Neurosci 36 (2016) 7441–7452, 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Awad O, Sarkar C, Panicker LM, Miller D, Zeng X, Sgambato JA, Lipinski MM, Feldman RA, Altered TFEB-mediated lysosomal biogenesis in Gaucher disease iPSC-derived neuronal cells, Hum. Mol. Genet 24 (2015) 5775–5788, 10.1093/HMG/DDV297. [DOI] [PubMed] [Google Scholar]
- [353].Mistry PK, Lopez G, Schiffmann R, Barton NW, Weinreb NJ, Sidransky E, Gaucher disease: Progress and ongoing challenges, Mol. Genet. Metab 120 (2017) 8–21, 10.1016/j.ymgme.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, Gasser T, Berg D, GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study, Mov. Disord 30 (2015) 407–411, 10.1002/MDS.26071. [DOI] [PubMed] [Google Scholar]
- [355].Das PK, Murray GJ, Gal AE, Barranger JA, Glucocerebrosidase deficiency and lysosomal storage of glucocerebroside induced in cultured macrophages, Exp. Cell Res 168 (1987) 463–474, 10.1016/0014-4827(87)90019-X. [DOI] [PubMed] [Google Scholar]
- [356].Lamghari M, Barrias CC, Sá Miranda C, Barbosa MA, Recombinant glucocerebrosidase uptake by Gaucher disease human osteoblast culture model, Blood Cells, Mol. Dis 35 (2005) 348–354, 10.1016/j.bcmd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- [357].Farfel-Becker T, Vitner EB, Futerman AH, Animal models for Gaucher disease research, Dis. Model. Mech 4 (2011) 746–752, 10.1242/DMM.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Kanfer JN, Legler G, Sullivan J, Raghavan SS, Mumford RA, The Gaucher mouse, Biochem. Biophys. Res. Commun 67 (1975) 85–90, 10.1016/0006-291X(75)90286-7. [DOI] [PubMed] [Google Scholar]
- [359].Tybulewicz VLJ, Tremblay ML, Lamarca ME, Willemsen R, Stubblefield BK, Winfield S, Zablocka B, Sidransky E, Martin BM, Huang SP, Mintzer KA, Westphal H, Mulligan RC, Ginns EI, Animal model of Gaucher’s disease from targeted disruption of the mouse glucocerebrosidase gene, Nature 357 (1992) 407–410, 10.1038/357407A0. [DOI] [PubMed] [Google Scholar]
- [360].Sidransky E, Sherer DM, Ginns EI, Gaucher Disease in the Neonate: A distinct Gaucher phenotype is analogous to a mouse model created by targeted disruption of the glucocerebrosidase gene, Pediatr. Res 32 (1992) 494–498, 10.1203/00006450-199210000-00023. [DOI] [PubMed] [Google Scholar]
- [361].Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, Döring T, Sandhoff K, Proia RL, Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure (SIMP), Proc. Natl. Acad. Sci. U.S.A 95 (1998) 2503–2508, 10.1073/PNAS.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Enquist IB, Nilsson E, Ooka A, Månsson JE, Olsson K, Ehinger M, Brady RO, Richter J, Karlsson S, Effective cell and gene therapy in a murine model of Gaucher disease, Proc. Natl. Acad. Sci. U.S.A 103 (2006) 13819–13824, 10.1073/PNAS.0606016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Hartley WJ, Blakemore WF, Neurovisceral glucocerebroside storage (Gaucher’s disease) in a dog, Vet. Pathol 10 (1973) 191–201, 10.1177/030098587301000302. [DOI] [PubMed] [Google Scholar]
- [364].Karageorgos L, Lancaster MJ, Nimmo JS, Hopwood JJ, Gaucher disease in sheep, J. Inherit. Metab. Dis 34 (2011) 209–215, 10.1007/S10545-010-9230-3. [DOI] [PubMed] [Google Scholar]
- [365].Gary SE, Ryan E, Steward AM, Sidransky E, Recent advances in the diagnosis and management of Gaucher disease, Expert Rev. Endocrinol. Metab 13 (2018) 107–118, 10.1080/17446651.2018.1445524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Schuchman E, Muro S, The development of enzyme replacement therapy for lysosomal diseases: Gaucher disease and beyond. In: Futerman T, Zimran A (Eds.), Gaucher Disease: Lessons Learned about Therapy of Lysosomal Diseases. CRC Press, T.& F. Group, Boca Raton, (FL), 2006, pp. 125–140. [Google Scholar]
- [367].Pastores GM, Rosenbloom B, Weinreb N, Goker-Alpan O, Grabowski G, Cohn GM, Zahrieh D, A multicenter open-label treatment protocol (HGT-GCB-058) of velaglucerase alfa enzyme replacement therapy in patients with Gaucher disease type 1: safety and tolerability, Genet. Med 16 (2014) 359–366, 10.1038/GIM.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Zimran A, Pastores GM, Tylki-Szymanska A, Hughes DA, Elstein D, Mardach R, Eng C, Smith L, Heisel-Kurth M, Charrow J, Harmatz P, Fernhoff P, Rhead W, Longo N, Giraldo P, Ruiz JA, Zahrieh D, Crombez E, Grabowski GA, Safety and efficacy of velaglucerase alfa in Gaucher disease type 1 patients previously treated with imiglucerase, Am. J. Hematol 88 (2013) 172–178, 10.1002/AJH.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Pastores GM, Shankar SP, Petakov M, Giraldo P, Rosenbaum H, Amato DJ, Szer J, Chertkoff R, Brill-Almon E, Zimran A, Enzyme replacement therapy with taliglucerase alfa: 36-month safety and efficacy results in adult patients with Gaucher disease previously treated with imiglucerase, Am. J. Hematol 91 (2016) 661–665, 10.1002/AJH.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Jain D, Bar-Shalom D, Alginate drug delivery systems: application in context of pharmaceutical and biomedical research, Drug Dev. Ind. Pharm 40 (2014) 1576–1584, 10.3109/03639045.2014.917657. [DOI] [PubMed] [Google Scholar]
- [371].Ribeiro C, Barrias C, Barbosa M, Calcium phosphate-alginate microspheres as enzyme delivery matrices, Biomaterials 25 (2004) 4363–4373, 10.1016/j.biomaterials.2003.11.028. [DOI] [PubMed] [Google Scholar]
- [372].Barrias CC, Ribeiro CC, Martins MCL, Barbosa MA, Rodrigues D, Sá Miranda MC, Calcium phosphate microspheres for localised delivery of a therapeutic enzyme, Key Eng. Mater 309–311 (2006) 903–906, 10.4028/WWW.SCIENTIFIC.NET/KEM.309-311.903. [DOI] [Google Scholar]
- [373].Barrias CC, Ribeiro CC, Rodrigues D, Sá Miranda MC, Barbosa MA, Effect of calcium phosphate addition to alginate microspheres: modulation of enzyme release kinetics and improvement of cell adhesion, Key Eng. Mater 284–286 (2005) 689–692, 10.4028/www.scientific.net/kem.284-286.689. [DOI] [Google Scholar]
- [374].Rowley JA, Madlambayan G, Mooney DJ, Alginate hydrogels as synthetic extracellular matrix materials, Biomaterials 20 (1999) 45–53, 10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- [375].Lee D, Hong JH, Nanoparticle-mediated therapeutic application for modulation of lysosomal ion channels and functions, Pharmaceutics 12 (2020) 217, 10.3390/pharmaceutics12030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Schultz ML, Tecedor L, Chang M, Davidson BL, Clarifying lysosomal storage diseases, Trends Neurosci. 34 (2011) 401–410, 10.1016/J.TINS.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Zeng J, Shirihai OS, Grinstaff MW, Degradable nanoparticles restore lysosomal pH and autophagic flux in lipotoxic pancreatic beta cells, Adv. Healthc. Mater 8 (2019) 1801511, 10.1002/adhm.201801511. [DOI] [PubMed] [Google Scholar]
- [378].Frederick Z, James A, E Dale A, Lysosomal reacidification via degradation of PLGA nanoparticles in a lipotoxic cardiomyopathy model, Front. Bioeng. Biotechnol 4 (2016) Conference Abstract: 10th World Biomaterials Congress, 10.3389/CONF.FBIOE.2016.01.01307. [DOI] [Google Scholar]
- [379].Zeng J, Martin A, Han X, Shirihai OS, Grinstaff MW, Biodegradable PLGA nanoparticles restore lysosomal acidity and protect neural pc-12 cells against mitochondrial toxicity, Ind. Eng. Chem. Res 58 (2019) 13910–13917, 10.1021/acs.iecr.9b02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Bourdenx M, Daniel J, Genin E, Soria FN, Blanchard-Desce M, Bezard E, Dehay B, Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases, Autophagy 12 (2016) 472–483, 10.1080/15548627.2015.1136769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Prévot G, Soria FN, Thiolat ML, Daniel J, Verlhac JB, Blanchard-Desce M, Bezard E, Barthélémy P, Crauste-Manciet S, Dehay B, Harnessing lysosomal pH through PLGA nanoemulsion as a treatment of lysosomal-related neurodegenerative diseases, Bioconjug. Chem 29 (2018) 4083–4089, 10.1021/acs.bioconjchem.8b00697. [DOI] [PubMed] [Google Scholar]
- [382].Arotcarena M-L, Soria FN, Cunha A, Doudnikoff E, Prévot G, Daniel J, Blanchard-Desce M, Barthélémy P, Bezard E, Crauste-Manciet S, Dehay B, Benjamin Dehay C, Basque A, Acidic nanoparticles protect against α-synuclein-induced neurodegeneration through the restoration of lysosomal function, Aging Cell 21 (2022) e13584, 10.1111/acel.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Dean RT, Lysosomes and protein degradation, Acta Biol. Med. Ger 36 (1977) 1815–1820, 10.1515/9783112650080-037. [DOI] [PubMed] [Google Scholar]
- [384].Usenovic M, Krainc D, Lysosomal dysfunction in neurodegeneration: the role of ATP13A2/PARK9, Autophagy 8 (2012) 987–988, 10.4161/AUTO.20256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen C-M, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung H-C, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen G-J, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan E-K, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu Y-R, Zabetian CP, Zhao Y, Ziegler SG, Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease, N. Engl. J. Med 361 (2009) 1651–1661. 10.1056/NEJMOA0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Klein AD, Mazzulli JR, Is Parkinson’s disease a lysosomal disorder?, Brain 141 (2018) 2255–2262, 10.1093/BRAIN/AWY147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Nicoli ER, Annunziata I, d’Azzo A, Platt FM, Tifft CJ, Stepien KM, GM1 Gangliosidosis—A Mini-Review, Front. Genet 12 (2021) 1652, 10.3389/FGENE.2021.734878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Brunetti-Pierri N, Scaglia F, GM1 gangliosidosis: Review of clinical, molecular, and therapeutic aspects, Mol. Genet. Metab 94 (2008) 391–396, 10.1016/j.ymgme.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [389].Gupta M, Pandey H, Sivakumar S, Intracellular Delivery of β-Galactosidase Enzyme Using Arginase-Responsive Dextran Sulfate/Poly- l -arginine Capsule for Lysosomal Storage Disorder, ACS Omega. 2 (2017) 9002–9012, 10.1021/acsomega.7b01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Kajihara R, Numakawa T, Odaka H, Yaginuma Y, Fusaki N, Okumiya T, Furuya H, Inui S, Era T, Novel drug candidates improve ganglioside accumulation and neural dysfunction in GM1 gangliosidosis models with autophagy activation, Stem Cell Reports 14 (2020) 909–923, 10.1016/j.stemcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Son MY, Kwak JE, Seol B, Lee DY, Jeon H, Cho YS, A novel human model of the neurodegenerative disease GM1 gangliosidosis using induced pluripotent stem cells demonstrates inflammasome activation, J. Pathol 237 (2015) 98–110, 10.1002/PATH.4551. [DOI] [PubMed] [Google Scholar]
- [392].Hahn CN, Del Pilar Martin M, Schröder M, Vanier MT, Hara Y, Suzuki K, Suzuki K, D’Azzo A, Generalized CNS disease and massive GM1-ganglioside accumulation in mice defective in lysosomal acid β-galactosidase, Hum. Mol. Genet 6 (1997) 205–211, 10.1093/HMG/6.2.205. [DOI] [PubMed] [Google Scholar]
- [393].Matsuda J, Suzuki O, Oshima A, Ogura A, Naiki M, Suzuki Y, Neurological manifestations of knockout mice with β-galactosidase deficiency, Brain Dev. 19 (1997) 19–20, 10.1016/S0387-7604(96)00077-0. [DOI] [PubMed] [Google Scholar]
- [394].Matsuda J, Suzuki O, Oshima A, Ogura A, Noguchi Y, Yamamoto Y, Asano T, Takimoto K, Sukegawa K, Suzuki Y, Naiki M, β-Galactosidase-deficient mouse as an animal model for GM1-gangliosidosis, Glycoconjugate J. 14 (1997) 729–736, 10.1023/A:1018573518127. [DOI] [PubMed] [Google Scholar]
- [395].Przybilla MJ, Ou L, Tăbăran AF, Jiang X, Sidhu R, Kell PJ, Ory DS, O’Sullivan MG, Whitley CB, Comprehensive behavioral and biochemical outcomes of novel murine models of GM1-gangliosidosis and Morquio syndrome type B, Mol. Genet. Metab 126 (2019) 139–150, 10.1016/J.YMGME.2018.11.002. [DOI] [PubMed] [Google Scholar]
- [396].Eikelberg D, Lehmbecker A, Brogden G, Tongtako W, Hahn K, Habierski A, Hennermann JB, Naim HY, Felmy F, Baumgärtner W, Gerhauser I, Axonopathy and reduction of membrane resistance: key features in a new murine model of human GM1-gangliosidosis, J. Clin. Med 9 (2020) 1004, 10.3390/JCM9041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Liu S, Feng Y, Huang Y, Jiang X, Tang C, Tang F, Zeng C, Liu L, A GM1 gangliosidosis mutant mouse model exhibits activated microglia and disturbed autophagy, Exp. Biol. Med 246 (2021) 1330–1341, 10.1177/1535370221993052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Bradbury AM, Gurda BL, Casal ML, Ponder KP, Vite CH, Haskins ME, A review of gene therapy in canine and feline models of lysosomal storage disorders, Hum. Gene Ther. Clin. Dev 26 (2015) 27–37, 10.1089/humc.2015.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Baker HJ, Russell Lindsey J, McKhann GM, Farrell DF, Neuronal GM1 gangliosidosis in a Siamese cat with beta-galactosidase deficiency, Science 174 (1971) 838–839, 10.1126/SCIENCE.174.4011.838. [DOI] [PubMed] [Google Scholar]
- [400].Farrell DF, Raker HJ, Herndon RM, Lindsey JR, McKhann GM, Feline GM1 gangliosidosis: Biochemical and ultrastructukal comparisons with the disease in man, J. Neuropathol. Exp. Neurol 32 (1973) 1–18, 10.1097/00005072-197301000-00001. [DOI] [PubMed] [Google Scholar]
- [401].McCurdy VJ, Johnson AK, Gray-Edwards HL, Randle AN, Brunson BL, Morrison NE, Salibi N, Johnson JA, Hwang M, Beyers RJ, Leroy SG, Maitland S, Denney TS, Cox NR, Baker HJ, Sena-Esteves M, Martin DR, Sustained normalization of neurological disease after intracranial gene therapy in a feline model, Sci. Transl. Med 6 (2014) 231ra48, 10.1126/SCITRANSLMED.3007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Yamato O, Ochiai K, Masuoka Y, Hayashida E, Tajima M, Omae S, Iijima M, Umemura T, Maede Y, GM1 gangliosidosis in shiba dogs, Vet. Rec 146 (2000) 493–496, 10.1136/VR.146.17.493. [DOI] [PubMed] [Google Scholar]
- [403].Yamato O, Endoh D, Kobayashi A, Masuoka Y, Yonemura M, Hatakeyama A, Satoh H, Tajima M, Yamasaki M, Maede Y, A novel mutation in the gene for canine acid β-galactosidase that causes GM1-gangliosidosis in Shiba dogs, J. Inherit. Metab. Dis 25 (2002) 525–526, 10.1023/A:1021280007739. [DOI] [PubMed] [Google Scholar]
- [404].Alroy J, Orgad U, DeGasperi R, Richard R, Warren CD, Knowles K, Thalhammer JG, Raghavan SS, Canine GM1-gangliosidosis. A clinical, morphologic, histochemical, and biochemical comparison of two different models, Am. J. Pathol 140 (1992) 675–689. [PMC free article] [PubMed] [Google Scholar]
- [405].Rha AK, Maguire AS, Martin DR, GM1 Gangliosidosis: Mechanisms and Management, Appl. Clin. Genet 14 (2021) 209–233. 10.2147/TACG.S206076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Kasperzyk JL, El-Abbadi MM, Hauser EC, D’Azzo A, Platt FM, Seyfried TN, N-butyldeoxygalactonojirimycin reduces neonatal brain ganglioside content in a mouse model of GM1 gangliosidosis, J. Neurochem 89 (2004) 645–653, 10.1046/J.1471-4159.2004.02381.X. [DOI] [PubMed] [Google Scholar]
- [407].Kasperzyk JL, D’Azzo A, Platt FM, Alroy J, Seyfried TN, Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice, J. Lipid Res 46 (2005) 744–751, 10.1194/JLR.M400411-JLR200. [DOI] [PubMed] [Google Scholar]
- [408].Elliot-Smith E, Speak AO, Lloyd-Evans E, Smith DA, va. der Spoel AC, Jeyakumar M, Butters TD, Dwek RA, d’Azzo A, Platt FM, Beneficial effects of substrate reduction therapy in a mouse model of GM1 gangliosidosis, Mol. Genet. Metab 94 (2008) 204–211, 10.1016/J.YMGME.2008.02.005. [DOI] [PubMed] [Google Scholar]
- [409].Jarnes Utz JR, Kim S, King K, Ziegler R, Schema L, Redtree ES, Whitley CB, Infantile gangliosidoses: Mapping a timeline of clinical changes, Mol. Genet. Metab 121 (2017) 170–179, 10.1016/J.YMGME.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Suzuki Y, Ichinomiya S, Kurosawa M, Ohkubo M, Watanabe H, Iwasaki H, Matsuda J, Noguchi Y, Takimoto K, Itoh M, Tabe M, Iida M, Kubo T, Ogawa S, Nanba E, Higaki K, Ohno K, Brady RO, Chemical chaperone therapy: clinical effect in murine GM1-gangliosidosis, Ann. Neurol 62 (2007) 671–675, 10.1002/ANA.21284. [DOI] [PubMed] [Google Scholar]
- [411].Reynolds GD, Baker HJ, Reynolds RH, Enzyme replacement using liposome carriers in feline GM1 gangliosidosis fibroblasts, Nature 275 (1978) 754–755, 10.1038/275754a0. [DOI] [PubMed] [Google Scholar]
- [412].Samoylova TI, Martin DR, Morrison NE, Hwang M, Cochran AM, Samoylov AM, Baker HJ, Cox NR, Generation and characterization of recombinant feline β-galactosidase for preclinical enzyme replacement therapy studies in GM1 gangliosidosis, Metab. Brain Dis 23 (2008) 161–173, 10.1007/S11011-008-9086-5. [DOI] [PubMed] [Google Scholar]
- [413].Gupta M, Sivakumar S, Light responsive Gold NPs-polymer hybrid LBL capsules for the Lysosomal Storage Disorder, International Journal of Community Science and Technology. 4 N°1 (2021) 1–15. ISSN: 2455-7536 (Online). Available from: https://www.jjss.co.in/journal/index.php/PH/article/view/85. [Google Scholar]
- [414].Mole SE, Anderson G, Band HA, Berkovic SF, Cooper JD, Kleine Holthaus S-M, McKay TR, Medina DL, Rahim AA, Schulz A, Smith AJ, Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis, Lancet Neurol. 18 (2019) 107–116, 10.1016/S1474-4422(18)30368-5. [DOI] [PubMed] [Google Scholar]
- [415].Mole SE, Williams RE, Goebel HH, Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses, Neurogenetics 6 (2005) 107–126, 10.1007/S10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- [416].Lojewski X, Staropoli JF, Biswas-legrand S, Simas AM, Haliw L, Selig MK, Coppel SH, Goss KA, Petcherski A, Chandrachud U, Sheridan SD, Lucente D, Sims KB, Gusella JF, Sondhi D, Crystal RG, Reinhardt P, Sterneckert J, Schöler H, Haggarty SJ, Storch A, Hermann A, Cotman SL, Human iPSC models of neuronal ceroid lipofuscinosis capture distinct effects of TPP1 and CLN3 mutations on the endocytic pathway, Hum. Mol. Genet 23 (2014) 2005–2022, 10.1093/HMG/DDT596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Uusi-Rauva K, Blom T, Von Schantz-Fant C, Blom T, Jalanko A, Kyttälä A, Induced pluripotent stem cells derived from a CLN5 patient manifest phenotypic characteristics of neuronal ceroid lipofuscinoses, Int. J. Mol. Sci 18 (2017) 955, 10.3390/IJMS18050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Chaterji S, Ahn EH, Kim DH, CRISPR genome engineering for human pluripotent stem cell research, Theranostics 7 (2017) 4445–4469, 10.7150/THNO.18456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [419].Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL, Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice, Proc. Natl. Acad. Sci. U.S.A 98 (2001) 13566–13571, 10.1073/PNAS.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [420].Miller JN, Kovács AD, Pearce DA, The novel Cln1(R151X) mouse model of infantile neuronal ceroid lipofuscinosis (INCL) for testing nonsense suppression therapy, Hum. Mol. Genet 24 (2015) 185–196, 10.1093/HMG/DDU428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Sanders DN, Farias FH, Johnson GS, Chiang V, Cook JR, O’Brien DP, Hofmann SL, Lu JY, Katz ML, A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund, Mol. Genet. Metab 100 (2010) 349–356, 10.1016/J.YMGME.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Kolicheski A, Barnes Heller HL, Arnold S, Schnabel RD, Taylor JF, Knox CA, Mhlanga-Mutangadura T, O’Brien DP, Johnson GS, Dreyfus J, Katz ML, Homozygous PPT1 splice donor mutation in a cane corso dog with neuronal ceroid lipofuscinosis, J. Vet. Intern. Med 31 (2017) 149–157, 10.1111/JVIM.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [423].Galliani M, Santi M, Del Grosso A, Cecchettini A, Santorelli FM, Hofmann SL, Lu JY, Angella L, Cecchini M, Signore G, Cross-linked enzyme aggregates as versatile tool for enzyme delivery: application to polymeric nanoparticles, Bioconjug. Chem 29 (2018) 2225–2231, 10.1021/acs.bioconjchem.8b00206. [DOI] [PubMed] [Google Scholar]
- [424].Wenger DA, Luzi P, Krabbe disease: globoid cell leukodystrophy, in: Rosenberg RN, Pascual JM (Eds.), Rosenberg’s Mol. Genet. Basis Neurol. Psychiatr. Dis (Sixth Edition), Elsevier, 2020, Vol.1, pp. 481–491, 10.1016/B978-0-12-813955-4.00034-9. [DOI] [Google Scholar]
- [425].Orsini JJ, Escolar ML, Wasserstein MP, Caggana M, Krabbe Disease, In: GeneReviews® 2018, University of Washington, Seattle, Seattle (WA), http://europepmc.org/books/NBK1238. [Google Scholar]
- [426].Del Grosso A, Galliani M, Angella L, Santi M, Tonazzini I, Parlanti G, Signore G, Cecchini M, Brain-targeted enzyme-loaded nanoparticles: A breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease, Sci. Adv 5 (2019) eaax7462, 10.1126/sciadv.aax7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Suzuki K, Suzuki K, The twitcher mouse. A model of human globoid cell leukodystrophy (krabbe’s disease), Am. J. Pathol 111 (1983) 394–397, Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1916270/. [PMC free article] [PubMed] [Google Scholar]
- [428].Avola R, Graziano ACE, Pannuzzo G, Alvares E, Cardile V, Krabbe’s leukodystrophy: Approaches and models in vitro, J. Neurosci. Res 94 (2016) 1284–1292, 10.1002/jnr.23846. [DOI] [PubMed] [Google Scholar]
- [429].Wenger DA, Rafi MA, Luzi P, Molecular genetics of krabbe disease (Globoid cell Leukodystrophy): Diagnostic and clinical implications, Hum. Mutat 10 (1997) 268–279, . [DOI] [PubMed] [Google Scholar]
- [430].Potter GB, Santos M, Davisson MT, Rowitch DH, Marks DL, Bongarzone ER, Petryniak MA, Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease, Hum. Mol. Genet 22 (2013) 3397–3414, 10.1093/HMG/DDT190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].Weinstock NI, Kreher C, Favret J, Nguyen D, Bongarzone ER, Wrabetz L, Laura Feltri M, Shin D, Brainstem development requires galactosylceramidase and is critical for pathogenesis in a model of Krabbe disease, Nat. Commun 11 (2020) 5356, 10.1038/s41467-020-19179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].Fankhauser R, Luginbuhl H, Hartley WJ, Leukodystrophie vom typus Krabbe beim hund, Schweiz Arch Tierheilkd. 105 (1963) 198–207, 10.5169/seals-591039. [DOI] [Google Scholar]
- [433].Baskin GB, Ratterree M, Davison BB, Falkenstein KP, Clarke MR, England JD, Vanier MT, Luzi P, Rafi MA, Wenger DA, Genetic Galactocerebrosidase Deficiency (Globoid Cell Leukodystrophy, Krabbe Disease) in Rhesus Monkeys (Macaca mulatta), Lab. Anim. Sci 48 (1998) 476–482. ISSN: 00236764, Available from: https://www.scopus.com/record/display.uri?eid=2-s2.0-0031770855&origin=inward#abstract [PubMed] [Google Scholar]
- [434].Wenger DA, Murine, canine and non-human primate models of Krabbe disease, Mol. Med. Today 6 (2000) 449–451, 10.1016/S1357-4310(00)01800-1. [DOI] [PubMed] [Google Scholar]
- [435].Demeule M, Currie JC, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne JP, Béliveau R, Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2, J. Neurochem 106 (2008) 1534–1544, 10.1111/J.1471-4159.2008.05492.X. [DOI] [PubMed] [Google Scholar]
- [436].Santi M, Maccari G, Mereghetti P, Voliani V, Rocchiccioli S, Ucciferri N, Luin S, Signore G, Rational design of a transferrin-binding peptide sequence tailored to targeted nanoparticle internalization, Bioconjug. Chem 28 (2017) 471–480, 10.1021/ACS.BIOCONJCHEM.6B00611. [DOI] [PubMed] [Google Scholar]
- [437].Costantino L, Gandolfi F, Tosi G, Rivasi F, Vandelli MA, Forni F, Peptide-derivatized biodegradable nanoparticles able to cross the blood-brain barrier, J. Control. Release 108 (2005) 84–96, 10.1016/J.JCONREL.2005.07.013. [DOI] [PubMed] [Google Scholar]
- [438].Salvalaio M, Rigon L, Belletti D, D’Avanzo F, Pederzoli F, Ruozi B, Marin O, Vandelli MA, Forni F, Scarpa M, Tomanin R, Tosi G, Targeted polymeric nanoparticles for brain delivery of high molecular weight molecules in lysosomal storage disorders, PLoS ONE 11 (2016) e0156452, 10.1371/journal.pone.0156452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Rigon L, Salvalaio M, Pederzoli F, Legnini E, Duskey JT, D’Avanzo F, De Filippis C, Ruozi B, Marin O, Vandelli MA, Ottonelli I, Scarpa M, Tosi G, Tomanin R, Targeting brain disease in MPSII: Preclinical evaluation of IDS-loaded PLGA nanoparticles, Int. J. Mol. Sci 20 (2019) 2014, 10.3390/ijms20082014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Xiao G, Gan LS, Receptor-mediated endocytosis and brain delivery of therapeutic biologics, Int. J. Cell Biol (2013) 703545, 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [441].Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T, Targeting the transferrin receptor for brain drug delivery, Prog. Neurobiol 181 (2019) 101665, 10.1016/j.pneurobio.2019.101665. [DOI] [PubMed] [Google Scholar]
- [442].Kawabata H, Transferrin and transferrin receptors update, Free Radic. Biol. Med 133 (2019) 46–54, 10.1016/J.FREERADBIOMED.2018.06.037. [DOI] [PubMed] [Google Scholar]
- [443].Vilella A, Ruozi B, Belletti D, Pederzoli F, Galliani M, Semeghini V, Forni F, Zoli M, Vandelli MA, Tosi G, Endocytosis of nanomedicines: the case of glycopeptide engineered PLGA nanoparticles, Pharm. 7 (2015) 74–89, 10.3390/PHARMACEUTICS7020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [444].Safary A, Akbarzadeh Khiavi M, Omidi Y, Rafi MA, Targeted enzyme delivery systems in lysosomal disorders: an innovative form of therapy for mucopolysaccharidosis, Cell. Mol. Life Sci 76 (2019) 3363–3381, 10.1007/s00018-019-03135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Casale J, Crane JS, Biochemistry, Glycosaminoglycans. In: StatPearls [Internet], StatPearls Publishing, Treasure Island (FL), 2022, Available from: https://www.ncbi.nlm.nih.gov/books/NBK544295/. [PubMed] [Google Scholar]
- [446].Bie H, Yin J, He X, Kermode AR, Goddard-Borger ED, Withers SG, James MNG, Insights into mucopolysaccharidosis I from the structure and action of α-L-iduronidase, Nat. Chem. Biol 9 (2013) 739–745, 10.1038/nchembio.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [447].Clarke LA, Mucopolysaccharidosis Type I, In: GeneReviews®. University of Washington, Seattle (WA), 2021, Available from: http://europepmc.org/books/NBK1162. [Google Scholar]
- [448].Dʹavanzo F, Rigon L, Zanetti A, Tomanin R, Mucopolysaccharidosis type II: One hundred years of research, diagnosis, and treatment, Int. J. Mol. Sci 21 (2020) 1258, 10.3390/ijms21041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [449].Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van Der Ploeg AT, Zeman J, Mucopolysaccharidosis type II (Hunter syndrome): A clinical review and recommendations for treatment in the era of enzyme replacement therapy, Eur. J. Pediatr 167 (2008) 267–277, 10.1007/S00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].D’avanzo F, Zanetti A, De Filippis C, Tomanin R, Mucopolysaccharidosis Type VI, an updated overview of the disease, Int. J. Mol. Sci 22 (2021) 13456, 10.3390/IJMS222413456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Tomatsu S, Montano AM, Dung VC, Grubb JH, Sly WS, Mutations and polymorphisms in GUSB gene in mucopolysaccharidosis VII (Sly Syndrome), Hum. Mutat 30 (2009) 511–519, 10.1002/HUMU.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Imundo L, LeDuc CA, Guha S, Brown M, Perino G, Gushulak L, Triggs-Raine B, Chung WK, A complete deficiency of Hyaluronoglucosaminidase 1 (HYAL1) presenting as familial juvenile idiopathic arthritis, J. Inherit. Metab. Dis 34 (2011) 1013–1022, 10.1007/S10545-011-9343-3. [DOI] [PubMed] [Google Scholar]
- [453].Triggs-Raine B, Salo TJ, Zhang H, Wicklow BA, Natowicz MR, Mutations in HYAL1, a member of a tandemly distributed multigene family encoding disparate hyaluronidase activities, cause a newly described lysosomal disorder, mucopolysaccharidosis IX, Proc. Natl. Acad. Sci. U.S.A 96 (1999) 6296–6300, 10.1073/PNAS.96.11.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [454].Natowicz MR, Short MP, Wang Y, Dickersin GR, Gebhardt MC, Rosenthal DI, Sims KB, Rosenberg AE, Clinical and biochemical manifestations of hyaluronidase deficiency, N. Engl. J. Med 335 (1996) 1029–1033, 10.1056/NEJM199610033351405. [DOI] [PubMed] [Google Scholar]
- [455].Shannon Danes B, Bearn AG, Hurler’s Syndrome: Demonstration of an Inherited Disorder of Connective Tissue in Cell Culture, Science (80-. ). 149 (1965) 987–989. 10.1126/SCIENCE.149.3687.987. [DOI] [PubMed] [Google Scholar]
- [456].Danes BS, Bearn AG, Hurler's syndrome: a genetic study in cell culture, J. Exp. Med 123 (1966) 1–16, 10.1084/JEM.123.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Kobolák J, Molnár K, Varga E, Bock I, Jezsó B, Téglási A, Zhou S, Lo Giudice M, Hoogeveen-Westerveld M, Pijnappel WP, Phanthong P, Varga N, Kitiyanant N, Freude K, Nakanishi H, László L, Hyttel P, Dinnyés A, Modelling the neuropathology of lysosomal storage disorders through disease-specific human induced pluripotent stem cells, Exp. Cell Res 380 (2019) 216–233, 10.1016/J.YEXCR.2019.04.021. [DOI] [PubMed] [Google Scholar]
- [458].Lito S, Sidibe A, Ilmjarv S, Burda P, Baumgartner M, Wehrle-Haller B, Krause KH, Marteyn A, Induced pluripotent stem cells to understand mucopolysaccharidosis. I: demonstration of a migration defect in neural precursors, Cells 9 (2020) 2593, 10.3390/CELLS9122593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [459].Suga M, Kondo T, Imamura K, Shibukawa R, Okanishi Y, Sagara Y, Tsukita K, Enami T, Furujo M, Saijo K, Nakamura Y, Osawa M, Saito MK, Yamanaka S, Inoue H, Generation of a human induced pluripotent stem cell line, BRCi001-A, derived from a patient with mucopolysaccharidosis type I, Stem Cell Res. 36 (2019) 101406, 10.1016/J.SCR.2019.101406. [DOI] [PubMed] [Google Scholar]
- [460].Varga E, Nemes C, Bock I, Varga N, Fehér A, Dinnyés A, Kobolák J, Generation of Mucopolysaccharidosis type II (MPS II) human induced pluripotent stem cell (iPSC) line from a 1-year-old male with pathogenic IDS mutation, Stem Cell Res. 17 (2016) 482–484, 10.1016/J.SCR.2016.09.033. [DOI] [PubMed] [Google Scholar]
- [461].Řeboun M, Rybová J, Dobrovolný R, Včelák J, Veselková T, Štorkánová G, Mušálková D, Hřebíček M, Ledvinová J, Magner M, Zeman J, Pešková K, Dvořáková L, X-Chromosome Inactivation Analysis in Different Cell Types and Induced Pluripotent Stem Cells Elucidates the Disease Mechanism in a Rare Case of Mucopolysaccharidosis Type II in a Female., Folia Biol. 62 (2016) 82–89, Available from: https://europepmc.org/article/med/27187040. [DOI] [PubMed] [Google Scholar]
- [462].Rybová J, Ledvinová J, Sikora J, Kuchař L, Dobrovolný R, Neural cells generated from human induced pluripotent stem cells as a model of CNS involvement in mucopolysaccharidosis type II, J. Inherit. Metab. Dis 41 (2018) 221–229, 10.1007/S10545-017-0108-5. [DOI] [PubMed] [Google Scholar]
- [463].Griffin TA, Anderson HC, Wolfe JH, Ex Vvvo gene therapy using patient iPSC-derived NSCs reverses pathology in the brain of a homologous mouse model, Stem Cell Reports 4 (2015) 835–846, 10.1016/J.STEMCR.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [464].Fusar Poli E, Zalfa C, D’Avanzo F, Tomanin R, Carlessi L, Bossi M, Rota Nodari L, Binda E, Marmiroli P, Scarpa M, Delia D, Vescovi AL, De Filippis L, Murine neural stem cells model Hunter disease in vitro: glial cell-mediated neurodegeneration as a possible mechanism involved, Cell Death Dis. 4 (2013) e906, 10.1038/cddis.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [465].Muenzer J, Fu H, Targeting disruption of the mouse iduronate sulfatase gene, Am. J. Hum. Genet 65 (1999) A427, ISSN/ISBN: 0002-9297. [Google Scholar]
- [466].Muenzer J, Lamsa J, Garcia A, Dacosta J, Garcia J, Treco D, Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report, Acta Paediatr. Suppl 91 (2002) 98–99, 10.1111/j.1651-2227.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- [467].Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF, Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB, Proc. Natl. Acad. Sci. U.S.A 100 (2003) 1902–1907, 10.1073/PNAS.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Jung SC, Park ES, Choi EN, Kim CH, Kim SJ, Jin DK, Characterization of a novel mucopolysaccharidosis type II mouse model and recombinant AAV2/8 vector-mediated gene therapy, Mol. Cells 30 (2010) 13–18, 10.1007/S10059-010-0083-2. [DOI] [PubMed] [Google Scholar]
- [469].Higuchi T, Shimizu H, Fukuda T, Kawagoe S, Matsumoto J, Shimada Y, Kobayashi H, Ida H, Ohashi T, Morimoto H, Hirato T, Nishino K, Eto Y, Enzyme replacement therapy (ERT) procedure for mucopolysaccharidosis type II (MPS II) by intraventricular administration (IVA) in murine MPS II, Mol. Genet. Metab 107 (2012) 122–128, 10.1016/J.YMGME.2012.05.005. [DOI] [PubMed] [Google Scholar]
- [470].Motas S, Haurigot V, Garcia M, Marcó S, Ribera A, Roca C, Sánchez X, Sánchez V, Molas M, Bertolin J, Maggioni L, León X, Ruberte J, Bosch F, CNS-directed gene therapy for the treatment of neurologic and somatic mucopolysaccharidosis type II (Hunter syndrome), JCI Insight 1 (2016) e86696, 10.1172/JCI.INSIGHT.86696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Curtain MM, Donahue LR, A mutation in the Arsb gene; a mouse model that resembles Maroteaux-Lamy syndrome, MGI Direct Data Submission (2009) J:149960. Available from: http://www.informatics.jax.org/reference/J:149960. [Google Scholar]
- [472].Entchev E, Jantzen I, Masson P, Bocart S, Bournique B, Luccarini JM, Bouchot A, Lacombe O, Junien JL, Broqua P, Tallandier M, Odiparcil, a potential glycosaminoglycans clearance therapy in mucopolysaccharidosis VI—Evidence from in vitro and in vivo models, PLoS ONE 15 (2020) e0233032, 10.1371/JOURNAL.PONE.0233032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [473].Birkenmeier EH, Davisson MT, Beamer WG, Ganschow RE, Vogler CA, Gwynn B, Lyford KA, Maltais LM, Wawrzyniak CJ, Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency, J. Clin. Invest 83 (1989) 1258–1266, 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [474].Sands MS, Birkenmeier EH, A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII, Proc. Natl. Acad. Sci. U.S.A 90 (1993) 6567–6571, 10.1073/PNAS.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [475].Gwynn B, Lueders K, Sands MS, Birkenmeier EH, Intracisternal A-particle element transposition into the murine beta-glucuronidase gene correlates with loss of enzyme activity: a new model for beta-glucuronidase deficiency in the C3H mouse, Mol. Cell. Biol 18 (1998) 6474–6481, 10.1128/MCB.18.11.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [476].Vogler C, Barker J, Sands MS, Levy B, Galvin N, Sly WS, Murine mucopolysaccharidosis VIL: impact of therapies on the phenotype, clinical course, and pathology in a model of a lysosomal storage disease, Pediatr. Dev. Pathol 4 (2001) 421–433, 10.1007/S10024001-0079-1. [DOI] [PubMed] [Google Scholar]
- [477].Sly WS, Vogler C, Grubb JH, Zhou M, Jiang J, Zhou XY, Tomatsu S, Bi Y, Snella EM, Active site mutant transgene confers tolerance to human beta-glucuronidase without affecting the phenotype of MPS VII mice, Proc. Natl. Acad. Sci. U.S.A 98 (2001) 2205–2210, 10.1073/PNAS.051623698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [478].Tomatsu S, Orii KO, Vogler C, Grubb JH, Snella EM, Gutierrez M, Dieter T, Holden CC, Sukegawa K, Orii T, Kondo N, Sly WS, Production of MPS VII mouse (Gus(tm(hE540A x mE536A)Sly)) doubly tolerant to human and mouse beta-glucuronidase, Hum. Mol. Genet 12 (2003) 961–973, 10.1093/HMG/DDG119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [479].Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S, Hombach-Klonisch S, Csoka AB, Stern R, Triggs-Raine BL. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet. 17 (2008) 1904–1915, 10.1093/hmg/ddn207. [DOI] [PubMed] [Google Scholar]
- [480].Spellacy E, Shull RM, Constantopoulos G, Neufeld EF, A canine model of human alpha-L-iduronidase deficiency, Proc. Natl. Acad. Sci. U.S.A 80 (1983) 6091–6095, 10.1073/PNAS.80.19.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Haskins ME, Desnick RJ, Diferrante N, Jezyk PF, Patterson DF, Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII, Pediatr. Res 18 (1984) 980–984, 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- [482].Silverstein Dombrowski DC, Carmichael KP, Wang P, O’Malley TM, Haskins ME, Giger U, Mucopolysaccharidosis type VII in a German Shepherd dog, J. Am. Vet. Med. Assoc 224 (2004) 553–557, 10.2460/JAVMA.2004.224.553. [DOI] [PubMed] [Google Scholar]
- [483].Neer TM, Dial SM, Pechman R, Wang P, Oliver JL, Giger U, Mucopolysaccharidosis VI in a miniature pischer, J. Vet. Intern. Med 9 (1995) 429–433, 10.1111/J.1939-1676.1995.TB03306.X. [DOI] [PubMed] [Google Scholar]
- [484].Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF, Alpha-L-iduronidase deficiency in a cat: a model of mucopolysaccharidosis I, Pediatr. Res 13 (1979) 1294–1297, 10.1203/00006450-197911000-00018. [DOI] [PubMed] [Google Scholar]
- [485].Gitzelmann R, Bosshard NU, Superti-Furga A, Spycher MA, Briner J, Lutz H, Wiesmann U, Litschi B, Feline mucopolysaccharidosis VII due to beta-glucuronidase deficiency, Vet. Pathol 31 (1994) 435–443, 10.1177/030098589403100405. [DOI] [PubMed] [Google Scholar]
- [486].Fyfe JC, Kurzhals RL, Lassaline ME, Henthorn PS, Alur PRK, Wang P, Wolfe JH, Giger U, Haskins ME, Patterson DF, Sun H, Jain S, Yuhki N, Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII, Genomics 58 (1999) 121–128, 10.1006/GENO.1999.5825. [DOI] [PubMed] [Google Scholar]
- [487].Jezyk PF, Haskins ME, Patterson DF, Mellman WJ, Greenstein M, Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: a model of Maroteaux-Lamy syndrome, Science 198 (1977) 834–836, 10.1126/SCIENCE.144321. [DOI] [PubMed] [Google Scholar]
- [488].Moro E, Tomanin R, Friso A, Modena N, Tiso N, Scarpa M, Argenton F, A novel functional role of iduronate-2-sulfatase in zebrafish early development, Matrix Biol. 29 (2010) 43–50, 10.1016/J.MATBIO.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [489].Bellesso S, Salvalaio M, Lualdi S, Tognon E, Costa R, Braghetta P, Giraudo C, Stramare R, Rigon L, Filocamo M, Tomanin R, Moro E, FGF signaling deregulation is associated with early developmental skeletal defects in animal models for mucopolysaccharidosis type II (MPSII), Hum. Mol. Genet 27 (2018) 2262–2275, 10.1093/HMG/DDY131. [DOI] [PubMed] [Google Scholar]
- [490].Fachel FNS, Frâncio L, Poletto É, Schuh RS, Teixeira HF, Giugliani R, Baldo G, Matte U, Gene editing strategies to treat lysosomal disorders: The example of mucopolysaccharidoses, Adv. Drug Deliv. Rev 191 (2022) 114616, 10.1016/J.ADDR.2022.114616. [DOI] [PubMed] [Google Scholar]
- [491].Sawamoto K, Stapleton M, Alméciga-Díaz CJ, Espejo-Mojica AJ, Losada JC, Suarez DA, Tomatsu S, Therapeutic options for mucopolysaccharidoses: current and emerging treatments, Drugs 79 (2019) 1103–1134. 10.1007/S40265-019-01147-4. [DOI] [PubMed] [Google Scholar]
- [492].Keller BL, Lohmann CA, Kyeremateng SO, Fricker G, Synthesis and characterization of biodegradable poly(butyl cyanoacrylate) for drug delivery applications, Polymers 14 (2022) 998, 10.3390/POLYM14050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [493].Alyautdin RN, Petrov VE, Langer K, Berthold A, Kharkevich DA, Kreuter J, Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles, Pharm. Res 14 (1997) 325–328, 10.1023/A:1012098005098. [DOI] [PubMed] [Google Scholar]
- [494].Alyautdin RN, Tezikov EB, Ramge P, Kharkevich DA, Begley DJ, Kreuter J, Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: an in situ brain perfusion study, J. Microencapsul 15 (1998) 67–74, 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- [495].Kurakhmaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS, Kreuter J, Gelperina S, Begley D, Alyautdin RN, Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles, J. Drug Target 17 (2009) 564–574, 10.1080/10611860903112842. [DOI] [PubMed] [Google Scholar]
- [496].Steiniger SCJ, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD, Gelperina SE, Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles, Int. J. Cancer 109 (2004) 759–767, 10.1002/IJC.20048. [DOI] [PubMed] [Google Scholar]
- [497].Mühlstein A, Gelperina S, Kreuter J, Development of nanoparticle-bound arylsulfatase B for enzyme replacement therapy of mucopolysaccharidosis VI, Pharmazie 68 (2013) 549–554, 10.1691/PH.2013.6502. [DOI] [PubMed] [Google Scholar]
- [498].Tosi G, Ruozi B, Belletti D, Vilella A, Zoli M, Vandelli MA, Forni F, Brain-targeted polymeric nanoparticles: In vivo evidence of different routes of administration in rodents, Nanomedicine 8 (2013) 1373–1383, 10.2217/nnm.12.172. [DOI] [PubMed] [Google Scholar]
- [499].Peruzzo P, Pavan E, Dardis A, Molecular genetics of Pompe disease: a comprehensive overview, Ann. Transl. Med 7 (2019) 278, 10.21037/ATM.2019.04.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [500].Kishnani PS, Howell RR, Pompe disease in infants and children, J. Pediatr 144 (2004) S35–S43, 10.1016/J.JPEDS.2004.01.053. [DOI] [PubMed] [Google Scholar]
- [501].Reuser AJ, Hirschhorn R, Kroos MA. Pompe Disease: Glycogen Storage Disease Type II, Acid α-Glucosidase (Acid Maltase) Deficiency. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (Eds.), The Online Metabolic and Molecular Bases of Inherited Disease, McGraw Hill, 2019. Available from: https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225890450. [Google Scholar]
- [502].Leslie N, Bailey L, Pompe Disease, Pompe Disease. In: GeneReviews® [Internet]. University of Washington, Seattle, Seattle (WA), 2017. Available from: https://europepmc.org/article/nbk/nbk1261. [Google Scholar]
- [503].Cardone M, Porto C, Tarallo A, Vicinanza M, Rossi B, Polishchuk E, Donaudy F, Andria G, De Matteis MA, Parenti G, Abnormal mannose-6-phosphate receptor trafficking impairs recombinant alpha-glucosidase uptake in Pompe disease fibroblasts, Pathogenetics 1 (2008) 6, 10.1186/1755-8417-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [504].Kang JY, Shin KK, Kim HH, Min JK, Ji ES, Kim JY, Kwon O, Oh DB, Lysosomal targeting enhancement by conjugation of glycopeptides containing mannose-6-phosphate glycans derived from glyco-engineered yeast, Sci. Rep 8 (2018) 8730, 10.1038/s41598-018-26913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [505].Tancini B, Tosi G, Bortot B, Dolcetta D, Magini A, De Martino E, Urbanelli L, Ruozi B, Forni F, Emiliani C, Vandelli MA, Severini GM, Use of Polylactide-Co-Glycolide-Nanoparticles for Lysosomal Delivery of a Therapeutic Enzyme in Glycogenosis Type II Fibroblasts, J. Nanosci. Nanotechnol 15 (2015) 2657–2666, 10.1166/jnn.2015.9251. [DOI] [PubMed] [Google Scholar]
- [506].Broadhead DM, Butterworth J, α-Glucosidase in Pompe's disease, J. Inherit. Metab. Dis 1 (1978) 153–154, 10.1007/BF01805584. [DOI] [PubMed] [Google Scholar]
- [507].Hsu J, Northrup L, Bhowmick T, Muro S, Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: comparative performance of a strategy for three distinct lysosomal storage disorders, Nanomed. Nanotech. Biol. Med 8 (2012) 731–739, 10.1016/j.nano.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [508].Huang HP, Chen PH, Hwu WL, Chuang CY, Chien YH, Stone L, Chien CL, Li LT, Chiang SC, Chen HF, Ho HN, Chen CH, Kuo HC, Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification, Hum. Mol. Genet 20 (2011) 4851–4864,. 10.1093/HMG/DDR424. [DOI] [PubMed] [Google Scholar]
- [509].Raval KK, Tao R, White BE, De Lange WJ, Koonce CH, Yu J, Kishnani PS, Thomson JA, Mosher DF, Ralphe JC, Kamp TJ, Pompe Disease Results in a Golgi-based Glycosylation Deficit in Human Induced Pluripotent Stem Cell-derived Cardiomyocytes, J. Biol. Chem 290 (2015) 3121–3136, 10.1074/JBC.M114.628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [510].Sato Y, Kobayashi H, Higuchi T, Shimada Y, Ida H, Ohashi T, TFEB overexpression promotes glycogen clearance of Pompe disease iPSC-derived skeletal muscle, Mol. Ther. - Methods Clin. Dev 3 (2016) 16054, 10.1038/MTM.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [511].Sato Y, Kobayashi H, Higuchi T, Shimada Y, Ida H, Ohashi T, Metabolomic profiling of Pompe disease-induced pluripotent stem cell-derived cardiomyocytes reveals that oxidative stress is associated with cardiac and skeletal muscle pathology, Stem Cells Transl. Med 6 (2017) 31–39, 10.5966/SCTM.2015-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [512].Yoshida T, Awaya T, Jonouchi T, Kimura R, Kimura S, Era T, Heike T, Sakurai H, A skeletal muscle model of infantile-onset Pompe disease with patient-specific iPS cells, Sci. Rep 7 (2017) 13473, 10.1038/S41598-017-14063-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [513].Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, Plotz P, Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II, J. Biol. Chem 273 (1998) 19086–19092, 10.1074/JBC.273.30.19086. [DOI] [PubMed] [Google Scholar]
- [514].Baik AD, Calafati P, Zhang X, Aaron NA, Mehra A, Moller-Tank S, Miloscio L, Praggastis M, Giovannone N, Pan C, Tang Y, Bridges S, Mujica A, Barbounis P, Yanolatos J, Gale N, Li N, Kyratsous CA, Schoenherr CJ, Murphy AJ, Economides AN, Cygnar KD, Cell type-selective targeted delivery of a recombinant lysosomal enzyme for enzyme therapies, Mol. Ther 29 (2021) 3512–3524, 10.1016/j.ymthe.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [515].Seppälä EH, Reuser AJJ, Lohi H, A nonsense mutation in the acid α-glucosidase gene causes Pompe disease in Finnish and Swedish Lapphunds, PLoS ONE 8 (2013) e56825, 10.1371/JOURNAL.PONE.0056825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [516].Lyons RE, Johnston DJ, McGowan MR, Laing A, Robinson B, Owen H, Hill BD, Burns BM, E7 (1057ΔTA) mutation of the acidic α-glucosidase gene causes Pompe’s disease in Droughtmaster cattle, Aust. Vet. J 95 (2017) 138–142, 10.1111/AVJ.12575. [DOI] [PubMed] [Google Scholar]
- [517].Tanaka S, Suzuki R, Koyama H, Machida N, Yabuki A, Yamato O, Glycogen storage disease in a young cat with heart failure, J. Vet. Intern. Med 36 (2022) 259–263, 10.1111/JVIM.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [518].Kishnani PS, Steiner RD, Bali D, Berger K, Byrne BJ, Case L, Crowley JF, Downs S, Howell RR, Kravitz RM, Mackey J, Marsden D, Martins AM, Millington DS, Nicolino M, O’Grady G, Patterson MC, Rapoport DM, Slonim A, Spencer CT, Tifft CJ, Watson MS, Pompe disease diagnosis and management guideline, Genet. Med 8 (2006) 267–288, 10.1097/01.GIM.0000218152.87434.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [519].Lachmann RH, Treating lysosomal storage disorders: What have we learnt?, J. Inherit. Metab. Dis 43 (2020) 125–132, 10.1002/jimd.12131. [DOI] [PubMed] [Google Scholar]
- [520].Chen M, Zhang L, Liang Y, Enzyme replacement therapy for infantile-onset Pompe disease, Cochrane Database Syst. Rev 11 (2017) CD011539, 10.1002/14651858.CD011539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [521].Schoser B, Stewart A, Kanters S, Hamed A, Jansen J, Chan K, Karamouzian M, Toscano A, Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis, J. Neurol 264 (2017) 621–630, 10.1007/S00415-016-8219-8. [DOI] [PubMed] [Google Scholar]
- [522].Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT, Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants, Mol. Genet. Metab 99 (2010) 26–33, 10.1016/J.YMGME.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [523].de Vries JM, van der Beek NAME, Kroos MA, Özkan L, van Doorn PA, Richards SM, Sung CCC, Brugma JDC, Zandbergen AAM, van der Ploeg AT, Reuser AJJ, High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa, Mol. Genet. Metab 101 (2010) 338–345, 10.1016/J.YMGME.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [524].Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, Chen YT, Rosenberg AS, Kishnani PS, The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease, Genet. Med 13 (2011) 729–736, 10.1097/GIM.0B013E3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [525].Sun B, Li S, Bird A, Yi H, Kemper A, Thurberg BL, Koeberl DD, Antibody formation and mannose-6-phosphate receptor expression impact the efficacy of muscle-specific transgene expression in murine Pompe disease, J. Gene Med 12 (2010) 881–891, 10.1002/JGM.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [526].Schoser B, Roberts M, Byrne BJ, Sitaraman S, Jiang H, Laforêt P, Toscano A, Castelli J, Díaz-Manera J, Goldman M, van der Ploeg AT, Bratkovic D, Kuchipudi S, Mozaffar T, Kishnani PS, Sebok A, Pestronk A, Dominovic-Kovacevic A, Khan A, Koritnik B, Tard C, Lindberg C, Quinn C, Eldridge C, Bodkin C, Reyes-Leiva D, Hughes D, Stefanescu E, SALORT-CAMPANA E, Butler E, Bouhour F, Kim G, Konstantinos Papadimas G, Parenti G, Bartosik-Psujek H, Kushlaf H, Akihiro H, Lau H, Pedro H, Andersen H, Amartino H, Shiraishi H, Kobayashi H, Tarnev I, Vengoechea J, Avelar J, Shin JH, Cauci J, Alonso-Pérez J, Janszky J, Berthy J, Cornelia K, Gutschmidt K, Claeys K, Judit Molnar M, Wencel M, Tarnopolsky M, Dimachkie M, Tchan M, Freimer M, Longo N, Vidal-Fernandez N, Musumeci O, Goker-Alpan O, Deegan P, Clemens PR, Roxburgh R, Henderson R, Hopkin R, Sacconi S, Fecarotta S, Attarian S, Wenninger S, Dearmey S, Hiwot T, Burrow T, Ruck T, Sawada T, Laszlo V, Löscher W, Chien YH, Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, phase 3 trial, Lancet Neurol. 20 (2021) 1027–1037, 10.1016/S1474-4422(21)00331-8. [DOI] [PubMed] [Google Scholar]
- [527].Talelli M, Vicent MJ, Reduction sensitive poly(l-glutamic acid) (PGA)-protein conjugates designed for polymer masked-unmasked protein therapy, Biomacromolecules 15 (2014) 4168–4177. 10.1021/BM5011883. [DOI] [PubMed] [Google Scholar]
- [528].Escalona GR, Sanchis J, Vicent MJ, pH-Responsive polyacetal–protein conjugates designed for polymer masked–unmasked protein therapy (PUMPT), Macromol. Biosci 18 (2018) 1700302, 10.1002/MABI.201700302. [DOI] [PubMed] [Google Scholar]
- [529].Duncan R, Gilbert HRP, Carbajo RJ, Vicent MJ, Polymer masked-unmasked protein therapy. 1. Bioresponsive dextrin-trypsin and -melanocyte stimulating hormone conjugates designed for α-amylase activation, Biomacromolecules. 9 (2008) 1146–1154, 10.1021/BM701073N. [DOI] [PubMed] [Google Scholar]
- [530].Buescher JM, Margaritis A, Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries, Crit. Rev. Biotechnol 27 (2007) 1–19, 10.1080/07388550601166458. [DOI] [PubMed] [Google Scholar]
- [531].Duncan R, Vicent MJ, Polymer therapeutics-prospects for 21st century: the end of the beginning, Adv. Drug Deliv. Rev 65 (2013) 60–70, 10.1016/J.ADDR.2012.08.012. [DOI] [PubMed] [Google Scholar]
- [532].Duncan R, Polymer therapeutics: Top 10 selling pharmaceuticals - what next?, J. Control. Release 190 (2014) 371–380, 10.1016/J.JCONREL.2014.05.001. [DOI] [PubMed] [Google Scholar]
- [533].Sun Y, Sha Y, Cui G, Meng F, Zhong Z, Lysosomal-mediated drug release and activation for cancer therapy and immunotherapy. Adv Drug Deliv Rev. 192 (2023) 114624, 10.1016/j.addr.2022.114624. [DOI] [PubMed] [Google Scholar]
- [534].Dowdy SF, Setten RL, Cui X-S, Jadhav SG, Delivery of RNA Therapeutics: The Great Endosomal Escape!, Nucleic Acid Ther. 32 (2022) 361–368, 10.1089/NAT.2022.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [535].Tosi G, Fano RA, Bondioli L, Badiali L, Benassi R, Rivasi F, Ruozi B, Forni F, Vandelli MA, Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood-brain barrier, Nanomedicine 6 (2011) 423–436, 10.2217/NNM.11.11. [DOI] [PubMed] [Google Scholar]
- [536].Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD, Tor Y, Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway, J. Biol. Chem 282 (2007) 13585–13591. 10.1074/JBC.M700463200. [DOI] [PubMed] [Google Scholar]
- [537].Gidwani B, Sahu V, Shukla SS, Pandey R, Joshi V, Jain VK, Vyas A, Quantum dots: Prospectives, toxicity, advances and applications, J. Drug Deliv. Sci. Technol 61 (2021) 102308, 10.1016/J.JDDST.2020.102308. [DOI] [Google Scholar]
- [538].Makadia HK, Siegel SJ, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier, Polymers 3 (2011) 1377–1397, 10.3390/POLYM3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [539].Wongpinyochit T, Johnston BF, Seib FP, Degradation behavior of silk nanoparticles-enzyme responsiveness, ACS Biomater. Sci. Eng 4 (2018) 942–951, 10.1021/ACSBIOMATERIALS.7B01021. [DOI] [PubMed] [Google Scholar]
- [540].Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, Forni F, Targeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123, J. Control. Release 122 (2007) 1–9, 10.1016/J.JCONREL.2007.05.022. [DOI] [PubMed] [Google Scholar]
- [541].Muro S, Alterations in Cellular Processes Involving Vesicular Trafficking and Implications in Drug Delivery, Biomimetics 3 (2018) 19, 10.3390/BIOMIMETICS3030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [542].Gaudioso Á, P Silva T, D Ledesma M. Models to study basic and applied aspects of lysosomal storage disorders. Adv. Drug Deliv. Rev 190 (2022) 114532, 10.1016/j.addr.2022.114532. [DOI] [PubMed] [Google Scholar]
- [543].Long Y, Xu M, Li R, Dai S, Beers J, Chen G, Soheilian F, Baxa U, Wang M, Marugan JJ, Muro S, Li Z, Brady R, Zheng W, Induced pluripotent stem cells for disease modeling and evaluation of therapeutics for Niemann-Pick disease type A, Stem Cells Transl. Med 5 (2016) 1644–1655, 10.5966/SCTM.2015-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [544].Luciani M, Gritti A, Meneghini V, Human iPSC-Based Models for the Development of Therapeutics Targeting Neurodegenerative Lysosomal Storage Diseases, Front. Mol. Biosci 7 (2020) 224, 10.3389/fmolb.2020.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [545].Lee SE, Shin N, Kook MG, Kong D, Kim NG, Choi SW, Kang KS, Human iNSC-derived brain organoid model of lysosomal storage disorder in Niemann-Pick disease type C, Cell Death Dis. 11 (2020) 1059, 10.1038/S41419-020-03262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [546].Leung CM, de Haan P, Ronaldson-Bouchard K, Kim GA, Ko J, Rho HS, Chen Z, Habibovic P, Jeon NL, Takayama S, Shuler ML, Vunjak-Novakovic G, Frey O, Verpoorte E, Toh YC, A guide to the organ-on-a-chip, Nat. Rev. Methods Primers 2 (2022) 3, 10.1038/s43586-022-00118-6. [DOI] [Google Scholar]
- [547].Schuster T, Mühlstein A, Yaghootfam C, Maksimenko O, Shipulo E, Gelperina S, Kreuter J, Gieselmann V, Matzner U, Potential of surfactant-coated nanoparticles to improve brain delivery of arylsulfatase A, J. Control. Release 253 (2017) 1–10, 10.1016/j.jconrel.2017.02.016. [DOI] [PubMed] [Google Scholar]
- [548].van Haasteren J, Li J, Scheideler OJ, Murthy N, Schaffer DV, The delivery challenge: fulfilling the promise of therapeutic genome editing, Nat. Biotechnol 38 (2020) 845–855, 10.1038/S41587-020-0565-5. [DOI] [PubMed] [Google Scholar]
- [549].Hong L, Wang Z, Wei X, Shi J, Li C, Antibodies against polyethylene glycol in human blood: A literature review, J. Pharmacol. Toxicol. Methods 102 (2020) 106678, 10.1016/J.VASCN.2020.106678. [DOI] [PubMed] [Google Scholar]
- [550].Wang F, Ullah A, Fan X, Xu Z, Zong R, Wang X, Chen G, Delivery of nanoparticle antigens to antigen-presenting cells: from extracellular specific targeting to intracellular responsive presentation, J. Control. Release 333 (2021) 107–128, 10.1016/J.JCONREL.2021.03.027. [DOI] [PubMed] [Google Scholar]
- [551].Kishimoto TK, Maldonado RA, Nanoparticles for the induction of antigen-specific immunological tolerance, Front. Immunol 9 (2018) 230, 10.3389/FIMMU.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [552].Nieto González N, Obinu A, Rassu G, Giunchedi P, Gavini E, Polymeric and lipid nanoparticles: which applications in pediatrics?, Pharmaceutics 13 (2021) 670, 10.3390/PHARMACEUTICS13050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [553].Garbade SF, Zielonka M, Mechler K, Kölker S, Hoffmann GF, Staufner C, Mengel E, Ries M, FDA orphan drug designations for lysosomal storage disorders – a cross-sectional analysis, PLoS ONE 15 (2020) e0230898, 10.1371/JOURNAL.PONE.0230898. [DOI] [PMC free article] [PubMed] [Google Scholar]