Abstract
Background:
SARS-CoV-2 variant evolution and increasing immunity altered the impact of pediatric SARS-CoV-2 infection. Public health decision-making relies on accurate and timely reporting of clinical data.
Methods:
This international hospital-based multicenter, prospective cohort study with real-time reporting was active from March 2020 to December 2022. We evaluated longitudinal incident rates and risk factors for disease severity.
Results:
We included 564 hospitalized children with acute COVID-19 (n = 375) or multisystem inflammatory syndrome in children (n = 189) from the Netherlands, Curaçao and Surinam. In COVID-19, 134/375 patients (36%) needed supplemental oxygen therapy and 35 (9.3%) required intensive care treatment. Age above 12 years and preexisting pulmonary conditions were predictors for severe COVID-19. During omicron, hospitalized children had milder disease. During population immunity, the incidence rate of pediatric COVID-19 infection declined for older children but was stable for children below 1 year. The incidence rate of multisystem inflammatory syndrome in children was highest during the delta wave and has decreased rapidly since omicron emerged. Real-time reporting of our data impacted national pediatric SARS-CoV-2 vaccination- and booster-policies.
Conclusions:
Our data supports the notion that similar to adults, prior immunity protects against severe sequelae of SARS-CoV-2 infections in children. Real-time reporting of accurate and high-quality data is feasible and impacts clinical and public health decision-making. The reporting framework of our consortium is readily accessible for future SARS-CoV-2 waves and other emerging infections.
Keywords: COVID-19, SARS-CoV-2, MIS-C, pediatrics, real-time reporting
Most SARS-CoV-2 infections in children are mild or asymptomatic. However, severe pediatric COVID-19 can occur, predominantly in children with preexisting chronic conditions.1–3 In addition, SARS-CoV-2 infected children sometimes develop the severe hyperinflammatory postinfectious condition multisystem inflammatory syndrome in children (MIS-C).4,5 The emergence of the SARS-CoV-2 omicron variant was associated with reduced severity of COVID-19 in both adults and children, possibly related to prior immunity.6,7 Less is known about the current phase of population immunity and the impact on severe pediatric COVID-19 and MIS-C.8
Several online dashboards tracking SARS-CoV-2 infections and COVID-19 hospital admissions were available during the course of the pandemic. However, these systems lacked detail on the clinical features, risk factors and outcome of pediatric cases.9–12 At the start of the pandemic, we initiated a nationwide hospital-based multicenter, prospective cohort study with online real-time reporting and analyses to continuously track the features, risk factors and outcomes of severe pediatric acute COVID-19 and MIS-C throughout the different phases of the pandemic. Collected data was reported real-time on the study website (www.covidkids.nl).
Our aim was to provide clinicians and public health officials with real-time and freely accessible data on MIS-C and severe pediatric COVID-19, supporting clinical management and public health decisions. Here, we report the dynamics of the incidence rate and severity of pediatric acute COVID-19 and MIS-C throughout the pandemic, from wild-type to population immunity, comparing older children to infants without prior immunity. In addition, we describe the risk factors and extensive cross-sectional and longitudinal features of hospitalized pediatric patients with severe manifestations of SARS-CoV-2 infection.
MATERIALS AND METHODS
Study Design, Setting and Participants
This multicenter, prospective cohort study included patients from March 2020 to January 1 2023. All 7 academic hospitals in the Netherlands, 31/61 general hospitals, The National Center for Pediatric Oncology and 2 Caribbean hospitals (Surinam and Curaçao) included patients. Inclusion criteria were: children until the age of 18 years presenting to hospital with acute COVID-19 (polymerase chain reaction [PCR] and/or rapid antigen test), or with MIS-C. Informed consent and written approval were obtained from all parents (and children older than 12 years). The study was evaluated and approved by the local medical regional ethics committee (METC-LDD, reference number N20.043 and NL76177.058.21, protocols on the study website www.covidkids.nl). Case details were entered into an electronic data capture system. Real-time reporting and analyses were done using an automated R script (https://github.com/AJTULLING-LUMC/Real-time-reporting-framework) (Figure, Supplemental Digital Content 1, http://links.lww.com/INF/F236). Analyses, tables and figures were exported daily to the study website (www.covidkids.nl/scientific-dashboard). Children with a coincidental finding of SARS-CoV-2 infection, who were not admitted to the hospital, or who had a clinical suspicion of MIS-C without meeting the WHO criteria, were excluded from further analyses.13 The STROBE reporting guidelines were followed (Table, Supplemental Digital Content 2, http://links.lww.com/INF/F237).14
Data Collection
Data were collected using Castor Electronic Data Capture.15 Data collected included general patient characteristics (age, sex, body mass index16 and medical history), SARS-CoV-2 laboratory results (PCR/rapid antigen test/serology), laboratory markers (including estimated glomerular filtration rate17), presenting symptoms, vital signs (transformed to z scores18,19), treatment, complications and outcome. The Centers for Disease Control and Prevention criteria were used to define organ involvement in MIS-C.9 Abnormal neurocognitive developmental mainly involved patients with developmental delay related to severe underlying conditions (eg, metabolic disease, psychomotor retardation or genetic abnormality).
Outcomes
The primary outcome was the incidence rate ratio (IRR) and severity of pediatric COVID-19 and MIS-C in subsequent pandemic phases. For acute pediatric COVID-19, severe disease was defined as the need for supplemental oxygen therapy, and/or intensive care unit (ICU) admission.20 For MIS-C, severe disease was defined as needing inotropics and/or invasive ventilatory support.
Secondary outcomes were the length of hospital stay between SARS-CoV-2 variants, age groups and disease severity. We compared laboratory markers at the initial hospital visit between mild and severe cases. We evaluated if adding systemic corticosteroids to intravenous immunoglobulin (IVIG) influenced the recovery of MIS-C, as determined by reduction in C-reactive protein (CRP) level, normalization of body temperature and length of hospital stay.
SARS-CoV-2 Variants and Population Immunity
Since data on SARS-CoV-2 variants were not available for individual cases, we used data from the Dutch National Institute for Public Health and the Environment (RIVM) to determine which SARS-CoV-2 variant was dominant (>80% of all SARS-CoV-2 infections) at any given timepoint.21 For MIS-C, we used a lag period of 3 weeks, since MIS-C is a postinfectious syndrome. The wild-type variant of SARS-CoV-2 was dominant prior to March 1, 2021; the alpha variant from March 1, 2021 to July 5, 2021; the delta variant from July 6, 2021 to January 2, 2022 and the omicron variant since January 3, 2022. Based on SARS-CoV-2 seroprevalence data, and the high efficacy of vaccine- or infection-induced humoral protection,22–24 in children in the Netherlands, the United Kingdom and globally, we extrapolated that population immunity in children was reached after the BA.2 omicron wave in July 2022.25–27 At that time, almost all Dutch children >12 years had neutralizing antibodies against SARS-CoV-2, compared to ~75% of 3-year-old children and ~50% of 1-year-old children. To explore if incidence rate and severity differed in children who were less likely to have been previously infected with SARS-CoV-2, we stratified the COVID-19 cohort in children younger and older than 1 year of age.
Statistical Analysis
Descriptive values are reported with numerator and denominator for categorical values, and median and interquartile ranges for continuous values. Statistical analyses were performed using R software (version 4.1.3). To determine risk factors of disease severity, a univariate logistic regression was performed. Subsequent multivariate analyses were done by including all variables that were univariately associated, with an uncorrected P < 0.10. The incidence rates were calculated per 1,000,000 pediatric person months for the different SARS-CoV-2 variant phases and adjusted for study coverage.
Incidence rates were calculated using the MIS-C and COVID-19 cases throughout the pandemic. Patients from the 2 Caribbean regions were excluded from this analysis. We corrected the incidence rates for study coverage, by determining the proportion of Dutch children living within the catchment area of hospitals participating in our study as compared to children living in the catchment area of nonparticipating hospitals (using Dutch Central Bureau for Statistics data on pediatric inhabitants per region).28 The overall study coverage of pediatric COVID-19 hospital admissions was 56.2% of the Dutch pediatric population (Figure, Supplemental Digital Content 3, http://links.lww.com/INF/F238). For MIS-C, coverage of 100% was assumed because all Dutch pediatric tertiary referral centers (including all pediatric ICUs) participated in the study. Confidence intervals (CIs) for incidence rates were calculated using the Poisson test. A univariate Poisson regression model was used to calculate the IRR per SARS-CoV-2 variant for pediatric COVID-19 hospital admissions and MIS-C. Since MIS-C is a postinfectious syndrome, which only occurs when the virus circulates, we also adjusted the IRR of MIS-C for pediatric COVID-19 hospitalizations in the same period.
To compare outcomes of different treatment regimens in MIS-C, a univariate logistic regression model, and a multivariate model with correction for disease severity, were used. To estimate the decline of CRP and body temperature over time during the MIS-C disease course, we used a generalized additive mixed model using the individual patients as a random effect. To visualize differences in laboratory markers at the initial hospital visit, we performed multiple Wilcoxon rank sum tests. Other statistical tests used in this article include the Fisher exact test and χ2 test. All P values in the main- and supplementary material, were corrected using the Benjamini–Hochberg procedure. Corrected P < 0.05 was considered statistically significant.
RESULTS
Clinical Cohort Characteristics
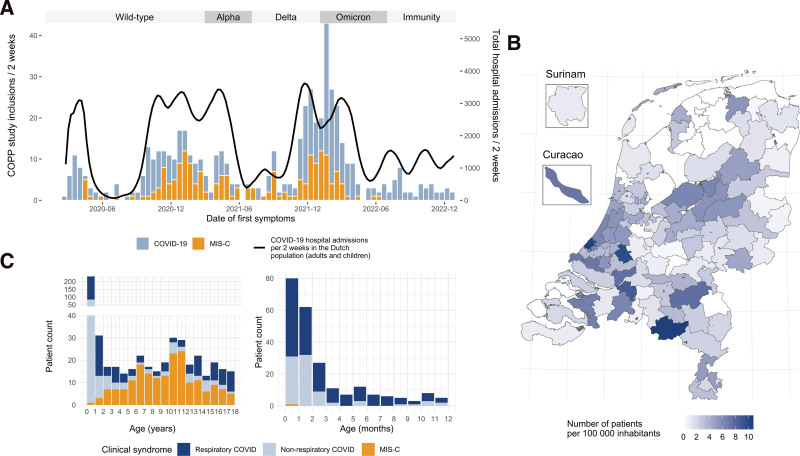
During the study period, 650 patients were included in the study. Children not admitted because of COVID-19 and children not meeting WHO criteria for MIS-C were excluded for further analysis (Figure, Supplemental Digital Content 4, http://links.lww.com/INF/F239). Data from 564 children were analyzed (189 patients with MIS-C and 375 patients with COVID-19). The dynamics of COVID-19-related pediatric hospital admissions mirrored Dutch adult hospitalizations, while MIS-C admissions lagged a few weeks behind (Fig. 1A). There were no regional differences in inclusions (Fig. 1B). Most cases were included when the wild-type variant was dominant (34%), followed by the delta variant (25%) (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). Most children admitted because of COVID-19 (239/375, 64%) had a respiratory tract infection. About a third of admitted children (136/375, 36%) had predominantly nonrespiratory symptoms related to SARS-CoV-2 infection (eg, fever and/or gastrointestinal symptoms).
FIGURE 1.
Summary panel of real-time reporting. Epidemiologic and demographic characteristics of the study cohort, as presented on the online dashboard. A: Date of first symptoms in patients with COVID-19 (blue) and MIS-C (orange). The black line shows overall (children and adults) national SARS-CoV-2 positive hospital admissions. Dominant SARS-CoV-2 variants (>80% of infections) are shown in the upper band. B: Geographical distribution of patients per 100,000 inhabitants in the Netherlands, Curaçao and Surinam. The Caribbean countries are not depicted to scale. C: Age distribution of study cohort. Patients with “Respiratory COVID-19” had predominantly respiratory symptoms at presentation. Patients with “Nonrespiratory COVID-19” had predominantly nonspecific symptoms at presentation, such as isolated fever or gastrointestinal complaints. Left pane: 0–18 years of age. Right pane: 0–12 months of age.
One patient was completely vaccinated, 2 were incompletely vaccinated and 6 had a documented prior SARS-CoV-2 infection (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). Three MIS-C cases were incompletely vaccinated, and none were completely vaccinated. There were no documented cases of MIS-C after SARS-CoV-2 reinfection.
Severe disease, defined as a necessity for oxygen suppletion and/or ICU admission, occurred in 134/375 (36%) of COVID-19 patients (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). Of these, 35/134 (26%) were admitted to ICU; 15 received mechanical ventilatory support; 7 required inotropic drug administration; 2 needed ECMO support and 1 patient with severe preexisting medical conditions died (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). In MIS-C, 81/189 cases (43%) had severe disease, defined as the need for inotropic drug treatment and/or invasive respiratory support (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). All severe MIS-C patients were admitted to the ICU, 5 patients received mechanical ventilatory support; 77/81 (95%) received inotropic drug treatment; and none died.
All MIS-C patients had multisystem involvement, most often gastrointestinal (90%) and cardiac (75%) (Table, Supplemental Digital Content 5, http://links.lww.com/INF/F240). Recovery of MIS-C, as determined by reduction in CRP level, normalization of body temperature and length of hospital stay, was not different between the different treatment regimens (IVIG only vs IVIG and steroids, Figure, Supplemental Digital Content 6, http://links.lww.com/INF/F241 and Figure, Supplemental Digital Content 7, http://links.lww.com/INF/F242), also when adjusted for severity of MIS-C.
Incidence Rate During Different Phases of the Pandemic
The incidence rate of hospitalized pediatric COVID-19 patients was highest when the omicron variant was dominant, both for children above 1 year of age and infants under 1 year of age, 7.2 (95% CI: 6.0–8.7) and 4.9 (95% CI: 3.9–6.2) per 1,000,000 pediatric person months, respectively. After the omicron wave, during population immunity, the incidence rate of pediatric COVID-19 admissions (IR: 0.7; 95% CI: 0.3–1.2) and IRR (as compared to wild-type) were strongly reduced in older children (IRR: 0.3; 95% CI: 0.1–0.5). In contrast, in infants younger than 1 year, who are more likely to be COVID-19 naive, the incidence rate and IRR of COVID-19-related hospital admissions was stable throughout the pandemic and population immunity, with the exception of an increase during omicron (Table 1).
TABLE 1.
Incidence and Incidence Rate Ratios of Pediatric COVID-19 and MIS-C Hospitalizations Throughout the Pandemic
| I: Cases | II: Incidence Rate Per 1,000,000 Pediatric Person Months | III: Incidence Rate Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Hospitalizations Infants (<1 Year) | COVID-19 Hospitalizations Children (≥1 Year) | MIS-C | COVID-19 Hospitalizations Infants (<1 Year) | COVID-19 Hospitalizations Children (≥1 Year) | MIS-C | COVID-19 Hospitalizations Infants (<1 year) | COVID-19 Hospitalizations Children (≥1 year) | MIS-C | MIS-C Adjusted for Pediatric COVID-19 Hospitalizations | |
| wild-type (12.4 months) | 66 | 48 | 75 | 3.4 (CI: 2.8–4.1) | 2.5 (CI: 2.0–3.1) | 2.2 (CI: 1.7–2.8) | Ref | Ref | Ref | Ref |
| Alpha (4.1 months) | 17 | 9 | 26 | 2.6 (CI: 1.8–3.8) | 1.4 (CI: 0.8–2.3) | 2.3 (CI: 1.5–3.3) | 0.8 (0.5–1.1; ns) | 0.6 (0.3–0.9; ns) | 1.0 (0.7–1.6; ns) | 1.5 (1.0–2.4; ns) |
| Delta (6.0 months) | 44 | 28 | 65 | 4.7 (CI: 3.7–5.9) | 3.0 (CI: 2.3–4.0) | 4.0 (CI: 3.1–5.0) | 1.4 (1.0–1.8; ns) | 1.2 (0.9–1.7; ns) | 1.8 (1.3–2.5; **) | 1.4 (1.0–1.9; ns) |
| Omicron (5.9 months) | 66 | 45 | 19 | 7.2 (CI: 6.0–8.7) | 4.9 (CI: 3.9–6.2) | 1.2 (CI: 0.7–1.8) | 2.1 (1.6–2.7; ***) | 2.0 (1.5–2.7; ***) | 0.5 (0.3–0.9; *) | 0.3 (0.2–0.4; ***) |
| Population immunity (6.0 months) | 33 | 6 | 0 | 3.5 (CI: 2.7–4.6) | 0.7 (CI: 0.3–1.2) | 0.0 (CI: 0.0–0.2) | 1.0 (0.8–1.4; ns) | 0.3 (0.1–0.5; ***) | No cases | No cases |
I: Number of children with COVID-19 and MIS-C included in our study during the different phases of the pandemic, excluding patients from Curaçao and Surinam. II: Incidence rate per 1,000,000 pediatric person months. COVID-19 incidence rate was corrected for study coverage. III: Incidence rate ratio (IRR) during the different phases of the pandemic, as compared to wild-type. For MIS-C incidence rate ratio is also shown adjusted for pediatric COVID-19 hospital admissions in the different pandemic phases. IRR (95% confidence interval, P value). The P value was calculated using the z score obtained by the Poisson distribution model (P values: ≥0.05 ns, <0.05*, <0.01**, <0.001***). No MIS-C cases were observed during the population immunity phase.
MIS-C indicates multisystem inflammatory syndrome in children.
The incidence rate of MIS-C was highest when the delta variant was dominant (4.0 per 1,000,000 person months, 95% CI: 3.1–5.0), with a strong reduction when omicron emerged (1.2 per 1,000,000 person months, 95% CI: 0.7–1.8). No cases of MIS-C were registered after July 2022. When adjusting for COVID-19-related pediatric hospital admissions, the IRR of MIS-C was lowest during omicron (0.3, 95% CI: 0.2–0.4, Table 1).
Risk Factors for Severe Disease
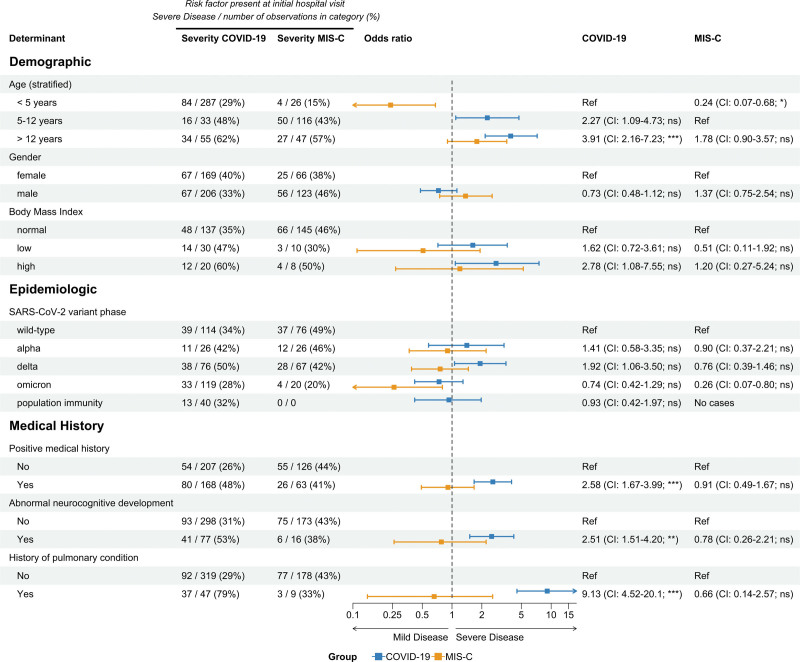
Significant risk factors for severe acute pediatric COVID-19 were higher age (>12 years), a history of neurocognitive developmental abnormalities, and preexisting pulmonary conditions (OR: 3.91, 2.51 and 9.13, respectively, see Fig. 2). In a multivariate model, with the inclusion of age, general medical history, neurocognitive developmental disease and history of pulmonary conditions, older children and children with preexisting pulmonary conditions had significantly more severe COVID-19 (Table, Supplemental Digital Content 8, http://links.lww.com/INF/F243).
FIGURE 2.
Risk factors of severe disease in COVID-19 and MIS-C. Demographic, epidemiologic and clinical characteristics of children admitted with COVID-19 or MIS-C. Results from a univariate logistic regression model, with disease severity as a dependent variable, are presented in the forest plot, with corresponding odds ratio, confidence intervals and P value. Proportions of severe cases over all observations in that group are shown with counts and percentages. P values were corrected using the Benjamini–Hochberg procedure (P values: ≥0.05 ns, <0.05*, <0.01**, <0.001***). CI indicates confidence interval; MIS-C, multisystem inflammatory syndrome in children.
In MIS-C, children younger than 5 years of age had a milder disease course than older children (OR: 0.24; 95% CI: 0.07–0.68). During omicron, there was a trend for milder MIS-C (OR: 0.26; 95% CI: 0.07–0.80), although this was not statistically significant after multivariate correction, with the inclusion of age and SARS-CoV-2 variant type into the model (Fig. 2 and Table, Supplemental Digital Content 8, http://links.lww.com/INF/F243).
Next, we evaluated which biomarkers and physiological parameters were associated with severe disease. In COVID-19, high respiratory rate, high CRP and high neutrophile count at admission were associated with severe disease (Figure, Supplemental Digital Content 9, http://links.lww.com/INF/F244). In MIS-C, high respiratory and cardiac rate, low blood pressure and high inflammatory biomarkers (CRP and ferritin), lymphocytopenia, elevated cardiac biomarkers (NT Pro-BNP and troponin T), and reduced estimated glomerular filtration rate at admission were associated with severe disease (Figure, Supplemental Digital Content 9, http://links.lww.com/INF/F244). Cardiac and renal organ system involvement were predictors for severe MIS-C (Table, Supplemental Digital Content 10, http://links.lww.com/INF/F245). Positive SARS-CoV-2 PCR in nasopharyngeal swab at diagnosis of MIS-C was not a predictor for severe MIS-C (Table, Supplemental Digital Content 10, http://links.lww.com/INF/F245).
Dynamics of Disease Severity Over Time
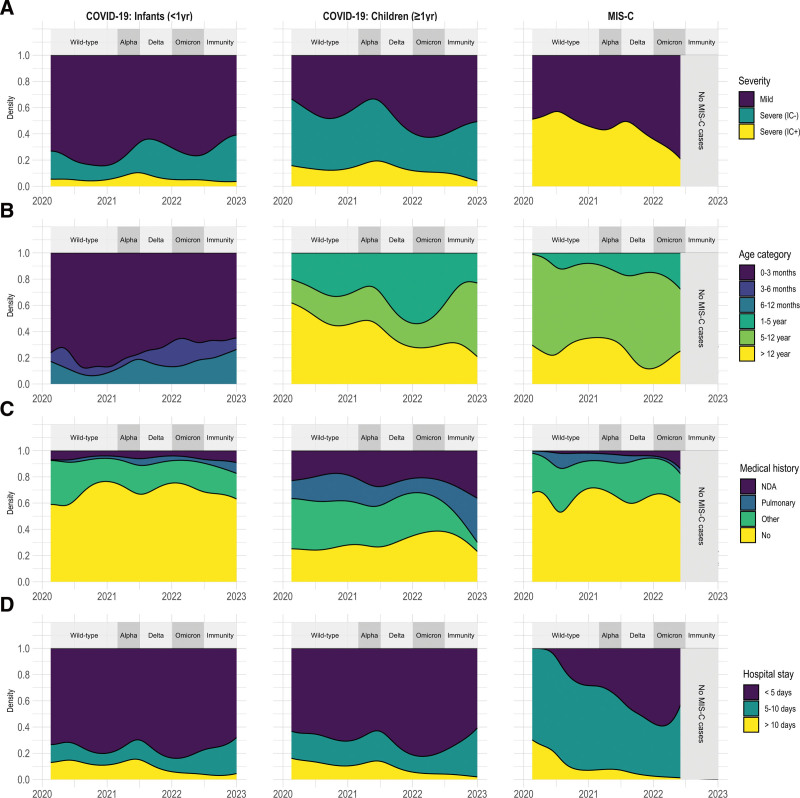
To evaluate if there was a difference in severity and risk factors of COVID-19 in children younger than 1 year of age (who are presumed to be more likely SARS-CoV-2 naive) as compared to older children (who, as the pandemic progressed, may have had prior SARS-CoV-2 exposure), we stratified our cohort by age below or above 1 year. For children younger than 1 year, length of hospital stay, proportion with severe disease and proportion needing ICU care was stable throughout the pandemic (Fig. 3 and Table, Supplemental Digital Content 11, http://links.lww.com/INF/F246). For children older than 1 year of age, there was less frequent severe disease (38% vs. 60%, P = 0.03) after omicron emerged and a significant reduction in length of hospital stay (median of 2 days vs. 3 days, P = 0.005) (Fig. 3 and Table, Supplemental Digital Content 12, http://links.lww.com/INF/F247).
FIGURE 3.
Patient dynamics throughout COVID-19 pandemic phases. Demographic and clinical characteristics of hospitalized COVID-19 and MIS-C patients during pandemic course by date of disease onset. Dominant SARS-CoV-2 variants (>80% of infections) are shown in the upper band. A: Disease severity. B: Age (stratified). C: Preexisting medical history. D: Hospital stay in days. IC indicates intensive care; MIS-C, multisystem inflammatory syndrome in children; NDA, neurocognitive developmental abnormalities.
For MIS-C, there was a trend for less severe disease during omicron as compared to earlier variants and a significantly reduced length of hospital stay (median of 4 days vs. 5 days, P = 0.04) (Fig. 3 and Table, Supplemental Digital Content 13, http://links.lww.com/INF/F248). Only 40 children were included with COVID-19 after July 2022, of whom only 6 were older than 1 year of age. Three of these were admitted because of an asthma exacerbation and 2 had prior severe neurocognitive developmental disorders.
Real-time Reporting and Public Health Impact
Daily updates on the dashboard on www.covidkids.nl provided the public, policymakers and physicians with real-time insight into the course of the pandemic in Dutch and Caribbean children. The National Health Council of the Netherlands referred to our dashboard on multiple occasions with regard to pediatric vaccination policy (2021, 4 times), and booster vaccination for specific subgroups of children (2022–2023, 3 times, Table, Supplemental Digital Content 14, http://links.lww.com/INF/F249).
SARS-CoV-2 vaccination for children in the Netherlands was introduced in a stepwise approach, with the monovalent BioNTech/Pfizer vaccine. Children older than 12 years of age were eligible for vaccination from July 2021 onwards. The vaccination rate in this age group was 56%, which is reflected by a relative reduction in hospitalized teenagers since the autumn of 2021 (Fig. 3B).29 Universal vaccination for children between 5 and 12 years of age was offered in March 2022, but only 3% of children in this age group were vaccinated. Nevertheless, because of high circulation of the omicron variant in the spring of 2022, most children have developed immunity to SARS-CoV-2, regardless of vaccination.25 In the last few months of our study, most hospitalized children were infants encountering the virus for the first time with a relatively mild disease course, and children with a history of pulmonary disease (and/or neurocognitive developmental disorder).
DISCUSSION
The COVID-19 pandemic has impacted social and healthcare-related aspects of life on a global scale. Healthcare policymakers, medical staff and hospital management depend on reliable and up-to-date information to guide decision-making. Since the start of the pandemic, Dutch pediatricians collected data on pediatric COVID-19 and MIS-C, in a concerted effort to determine the incidence and risk factors for a severe course. All clinical, demographic and epidemiological characteristics were continuously and automatically updated on an online dashboard. Here, we present the findings from this prospective cohort study.
Multiple COVID-19 dashboards have been set up globally, some of which also include pediatric patients, but none provided detailed clinical information on children of the present study.9–12 Our real-time reporting facilitated monitoring of the magnitude, risk factors and outcome of pediatric COVID-19 and MIS-C. The analyses on our dashboard were of great interest to medical professionals and the general public. In addition, our data were instrumental to national SARS-CoV-2 vaccination policies in children.30
In this study, we confirm that adolescents and children with abnormal neurocognitive development or preexisting pulmonary conditions are more likely to develop severe COVID-19.31 In line with previous studies, most children with MIS-C were previously healthy.32 Although this is not the first study to evaluate risk factors for severe disease in pediatric COVID-19 and MIS-C, it is unique in covering all sequential phases of the SARS-CoV-2 pandemic, from wild-type to population immunity.
In line with other studies,6,7 the SARS-CoV-2 omicron variant phase was associated with reduced severity. For adults, the reduced severity of omicron has been linked to increasing immunity as a result of natural infection and vaccination, but in children, this is less clear.33
Incidence rates dropped dramatically during the course of the pandemic, both for children above 1 year of age admitted for COVID-19 and children with MIS-C. In contrast, incidence rate and severity remained stable through time for children younger than 1 year of age. These youngest children are not eligible for vaccination and are less likely to have been previously infected with SARS-CoV-2 than older children, and can therefore be presumed to lack protective immunity. In addition, infants (<1 year of age) very rarely develop reinfection with SARS-CoV-2 within the first year of life.22 Although a lower pathogenicity of SARS-CoV-2 omicron variant is not completely ruled out, this supports the notion that similar to adults, prior immunity protects against severe sequelae of SARS-CoV-2 infections in children.
Due to the observational nature of the study design, not all parameters were available for all patients, which may limit the generalizability of risk factor identification. Another limitation is, that because of ethical considerations, we did not collect data on ethnicity, which has been reported as a risk factor for MIS-C in multiple reports.5,34 Additionally, our study may be limited by inclusion biases. Informed consent may have been more difficult to obtain for deceased patients as well as for patients who were discharged shortly after presentation. Finally, there could be a reporting bias towards more severe cases both at the individual study site level and at the study level, since all academic hospitals and pediatric ICUs participated in the study, accounting for the most severe COVID-19 cases and many MIS-C cases, and the majority -but not all- general hospitals included cases.
Currently, pediatric hospital admissions for severe SARS-CoV-2-related conditions are very rare, potentially due to a high degree of protective immunity in children. Should future SARS-CoV-2 variants or declining immunity result in an increased burden of disease, the reporting framework of our consortium is readily accessible. Other (re-)emerging pediatric infectious diseases also warrant a collaborative effort with real-time reporting of data. Therefore, our consortium is currently using this infrastructure to study the recent increase in invasive group A streptococcal infections in children.35 Our study shows that real-time reporting of accurate and high-quality data is feasible and can be implemented rapidly in rare, life-threatening diseases, and impacts clinical and public health decision-making.
ACKNOWLEDGMENTS
The authors thank Estefanía Laney (Trialbureau Willem-Alexander Children’s Hospital) and Tessa van der Geest (Pedmed-NL), Károly Illy and Willem de Vries (Dutch Pediatric Society), and Hester Rippen (Stichting Kind & Ziekenhuis) for practical support, Ron Verzijden for designing the website and Lieke Sanders from the National Institute for Public Health and the Environment for strategic advice. The following persons helped in recruiting patients and in data-management: Alma Qureshi, Amy Sieben, Anita Boorsma, Anne Vos, Anneloes Boers, Astrid Ritman, Bas Harzing, Bregje Raap, Carole Brouwer, Ellen Devilee-Massaar, Fleur Meijs, Ilka van Dalen-Vink, Ivo Hoefnagels, Jacqueline Zonneveld, Janneke van Oosterhout, Joke Dunk, Karin Miedema, Kimberly Bodaar, Laura de Jong, Laura Overdam, Lieke Noij, Lies Kosters, Marlou van der Heijden, Maureen Heijne den Bak, Merel Huiskamp, Merel van Pieterson, Merian van Overveld, Nellie van Eijk, Nienke Wassink, Pauline Lindhout, Ritoja Bhattacharya, Sanne Graaf, Tiny Dauven, Wendy Wittenberg. The authors thank Simon Tulling for data analysis assistance.
COPP-study group:
Leontien B. van der Aa, MD, PhD, Department of Pediatrics, Zaans Medical Center, Zaandam, the Netherlands; Koen J. van Aerde, MD, Department of Pediatric Infectious Disease and Immunology, Radboud University Medical Center, Nijmegen, the Netherlands; Bettina Auffarth-Smedema, MD, PhD, Department of Pediatrics, Ommelander Hospital, Groningen, the Netherlands; Ingeborg Y. Bart, MD, Department of Pediatrics, Canisius-Wilhelmina Hospital, Nijmegen, the Netherlands; Cherise Beek, MD, Department of Pediatrics, Academic Hospital Paramaribo, Paramaribo, Surinam; Gitanjali I. Bechan, MD, PhD, Department of Pediatrics, Alrijne Hospital, Leiderdorp, the Netherlands; J. Merlijn van den Berg, MD, PhD, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands; Venje H. Boonstra, MD, PhD, Department of Pediatrics, BovenIJ Hospital, Amsterdam, the Netherlands; Mijke Breukels, MD, PhD, Department of Pediatrics, Elkerliek Hospital, Helmond, the Netherlands; Danielle M.C. Brinkman, MD, PhD, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands; Patricia C.J.L. Bruijning-Verhagen, MD, PhD, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands; Stephanie C. de Crom, MD, PhD, Department of Pediatrics, Bravis Hospital, Roosendaal, the Netherlands; Margot R. Ernst-Kruis, MD, Department of Pediatrics, Meander Medical Center, Amersfoort, the Netherlands; Pieter L.A. Fraaij, MD, PhD, Department of Pediatric Immunology and Infectious Diseases, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherland; Joyce Goris, MD, Department of Pediatrics, ZorgSaam Hospital, Terneuzen, the Netherlands; Michael Groeneweg, MD, PhD, Department of Pediatrics, Maasstad Hospital, Rotterdam, the Netherlands; Mariken Gruppen, MD, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands; Sanne C. Hammer, MD, Department of Pediatrics, Amphia Hospital, Breda, the Netherlands; Petra C.E. Hissink Muller, MD, PhD, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands; Jenneke Homan-van der Veen, MD, Department of Pediatrics, Deventer Hospital, Deventer, the Netherlands; Monique A.M. Jacobs, MD, Department of Pediatrics, Slingeland Hospital, Doetinchem, the Netherlands; Lindy Janssen, MD, Department of Pediatrics, Curaçao Medical Center, Willemstad, Curaçao; Arvid W.A. Kamps, MD, PhD, Department of Pediatrics, Martini Hospital, Groningen, the Netherlands; Naomi Ketharanathan, MD, Department of Pediatric Intensive Care, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherlands; Kevin H. van ‘t Kruys, MD, Department of Pediatrics, Academic Hospital Paramaribo, Paramaribo, Surinam; Martijn van der Kuip, MD, PhD, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands; Taco W. Kuijpers, MD, PhD, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands; Elizabeth G. Legger, MD, Department of Pediatric Rheumatology, University Medical Center Groningen, Groningen, the Netherlands; Shirley Lo-A-Njoe, MD, Department of Pediatrics, Curaçao Medical Center, Willemstad, Curaçao; Meindert E. Manshande, MD, Department of Pediatrics, Curaçao Medical Center, Willemstad, Curaçao; Carien J. Miedema, MD, Department of Pediatrics, Catharina Hospital, Eindhoven, the Netherlands; Charlie C. Obihara, MD, PhD, Department of Pediatrics, Elisabeth-TweeStedenziekenhuis, Tilburg, the Netherlands; Gideon O. Olivieira, MD, Department of Pediatrics, Academic Hospital Paramaribo, Paramaribo, Surinam; Annemarie Oudshoorn, MD, Department of Pediatrics, Gelre Hospital, Apeldoorn, the Netherlands; Esther J.E. Peeters, MD, Department of Pediatrics, Juliana Children’s Hospital, Hagaziekenhuis, the Hague, the Netherlands; Ronald Petru, MD, Department of Pediatric Intensive Care, Radboud University Medical Center, Nijmegen, the Netherlands; Marielle W.H. Pijnenburg, MD, PhD, Department of Pediatric Pulmonology, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherlands; Denise Rook, MD, PhD, Department of Pediatrics, Juliana Children’s Hospital, Hagaziekenhuis, the Hague, the Netherlands; Kim Schilleman, MD, PhD, Department of Pediatrics, Admiraal de Ruyter Hospital, Goes, the Netherlands; Rian Schopmeijer, BSc, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands; David Slotboom, MD, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands; Manouk van der Steen, MD, Department of Pediatrics, University Medical Center Maastricht, Maastricht, the Netherlands; Kim Stol, MD, PhD, Department of Pediatric Infectious Disease and Immunology, Radboud University Medical Center, Nijmegen, Netherlands; Yolande E.M. Thomasse, MD, Department of Pediatrics, Dijklander Hospital, Hoorn, the Netherlands; Wim J.E. Tissing, MD, PhD, Prinses Máxima Center for Pediatric Oncology, Utrecht, the Netherlands; Xandra W. van den Tweel, MD, PhD, Department of Pediatrics, Maasstad Hospital, Rotterdam, the Netherlands; Sebastiaan J. Vastert, MD, PhD, Department of Pediatric Rheumatology, University Medical Center Utrecht, Utrecht, the Netherlands; Anne B. Verbeek, BSc, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands; Annette M.M. Vernooij-van Langen, MD, PhD, Department of Pediatrics, St Jansdal Hospital, Harderwijk, the Netherlands; Jantien W. Wieringa, MD, Department of Pediatrics, Haaglanden Medical Center, the Hague, the Netherlands; Joanne G. Wildenbeest, MD, PhD, Department of Pediatric Infectious Diseases, University Medical Center Utrecht, Utrecht, the Netherlands; Saskia N. de Wildt, MD, PhD, Department of Pharmacology and Toxicology, Radboud University Medical Center, Nijmegen, the Netherlands; Christiaan van Woerden, MD, PhD, Department of Pediatrics, Bravis Hospital, Roosendaal, the Netherlands.
Supplementary Material
Footnotes
This study was funded by the #wakeuptocorona crowdfund initiative of the Bontius Stichting and the Leiden University Fund, and by ZonMw (10430072110007 and 10430102110009). The study sponsors had no role in the design, collection, analysis and interpretation of data, nor in the writing of the report and in the submission.
The authors have no conflicts of interest to disclose.
Some of the data presented here is also shown on our study website www.covidkids.nl. A modified version of the abstract was submitted to the European Society of Pediatric Infectious Diseases (ESPID) and was accepted for an oral presentation at the ESPID 2023 congress in Lisbon. An online preprint version of the manuscript was posted on the SSRN website (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4401713).
The study is listed in the Health-RI COVID-19 data portal, in which federated metadata is deposited (https://covid19initiatives.health-ri.nl/p/Project/27866022694505020). Data will be shared with researchers who provide a methodologically sound proposal. Shared data can be used to achieve aims in the approved proposal. Only anonymous data will be shared. Proposals should be directed to e.p.buddingh@lumc.nl; to gain access, data requestors will need to sign a data access agreement. Supplementary code used for the real-time reporting framework will be deposited upon acceptance on: https://github.com/AJTULLING-LUMC/Real-time-reporting-framework. The code can also be provided upon request of the editor or reviewer.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Adam J. Tulling, Email: a.j.tulling@lumc.nl.
Gertjan Lugthart, Email: G.Lugthart@lumc.nl.
Miriam G. Mooij, Email: m.mooij@erasmusmc.nl.
Caroline L. H. Brackel, Email: ckosterinkbrackel@tergooi.nl.
Suzanne W. J. Terheggen-Lagro, Email: s.w.terheggenlagro@amsterdamumc.nl.
Rianne Oostenbrink, Email: r.oostenbrink@erasmusmc.nl.
Corinne M. P. Buysse, Email: c.buysse@erasmusmc.nl.
Simone Hashimoto, Email: s.hashimoto@amsterdamumc.nl.
Wineke Armbrust, Email: w.armbrust@umcg.nl.
Michiel A. G. E. Bannier, Email: michiel.bannier@mumc.nl.
Jolita Bekhof, Email: j.bekhof@isala.nl.
Helma B. van Gameren-Oosterom, Email: Helma.Gameren@ghz.nl.
Han Hendriks, Email: hanhendriks58@gmail.com.
Marlies A. van Houten, Email: MvanHouten2@spaarnegasthuis.nl.
Jan W. van der Linden, Email: j.vanderlinden@bernhoven.nl.
Ankie Lebon, Email: A.Lebon@asz.nl.
Lonneke van Onzenoort-Bokken, Email: Lonneke.Bokken@mmc.nl.
Gerdien A. Tramper-Stranders, Email: G.Tramper@franciscus.nl.
Mirjam van Veen, Email: mi.vanveen@hagaziekenhuis.nl.
Erik G. J. von Asmuth, Email: E.G.J.von_Asmuth@lumc.nl.
Leontien B. van der Aa, Department of Pediatrics, Zaans Medical Center, Zaandam, the Netherlands.
Koen J. van Aerde, Department of Pediatric Infectious Disease and Immunology, Radboud University Medical Center, Nijmegen, the Netherlands.
Bettina Auffarth-Smedema, Department of Pediatrics, Ommelander Hospital, Groningen, the Netherlands.
Ingeborg Y. Bart, Department of Pediatrics, Canisius-Wilhelmina Hospital, Nijmegen, the Netherlands.
Cherise Beek, Department of Pediatrics, Academic Hospital Paramaribo, Paramaribo, Surinam.
Gitanjali I. Bechan, Department of Pediatrics, Alrijne Hospital, Leiderdorp, the Netherlands.
J. Merlijn van den Berg, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Venje H. Boonstra, Department of Pediatrics, BovenIJ Hospital, Amsterdam, the Netherlands.
Mijke Breukels, Department of Pediatrics, Elkerliek Hospital, Helmond, the Netherlands.
Danielle M.C. Brinkman, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands.
Patricia C.J.L. Bruijning-Verhagen, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands.
Stephanie C. de Crom, Department of Pediatrics, Bravis Hospital, Roosendaal, the Netherlands.
Margot R. Ernst-Kruis, Department of Pediatrics, Meander Medical Center, Amersfoort, the Netherlands.
Pieter L.A. Fraaij, Department of Pediatric Immunology and Infectious Diseases, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherland.
Joyce Goris, Department of Pediatrics, ZorgSaam Hospital, Terneuzen, the Netherlands.
Michael Groeneweg, Department of Pediatrics, Maasstad Hospital, Rotterdam, the Netherlands.
Mariken Gruppen, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Sanne C. Hammer, Department of Pediatrics, Amphia Hospital, Breda, the Netherlands.
Petra C.E. Hissink Muller, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands.
Jenneke Homan-van der Veen, Department of Pediatrics, Deventer Hospital, Deventer, the Netherlands.
Monique A.M. Jacobs, Department of Pediatrics, Slingeland Hospital, Doetinchem, the Netherlands.
Arvid W.A. Kamps, Department of Pediatrics, Martini Hospital, Groningen, the Netherlands.
Naomi Ketharanathan, Department of Pediatric Intensive Care, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherlands.
Martijn van der Kuip, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Taco W. Kuijpers, Department of Pediatric Immunology, Rheumatology and Infectious Disease, Emma Children’s Hospital, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Elizabeth G. Legger, Department of Pediatric Rheumatology, University Medical Center Groningen, Groningen, the Netherlands.
Shirley Lo-A-Njoe, Department of Pediatrics, Curaçao Medical Center, Willemstad, Curaçao.
Meindert E. Manshande, Department of Pediatrics, Curaçao Medical Center, Willemstad, Curaçao.
Carien J. Miedema, Department of Pediatrics, Catharina Hospital, Eindhoven, the Netherlands.
Charlie C. Obihara, Department of Pediatrics, Elisabeth-TweeStedenziekenhuis, Tilburg, the Netherlands.
Gideon O. Olivieira, Department of Pediatrics, Academic Hospital Paramaribo, Paramaribo, Surinam.
Annemarie Oudshoorn, Department of Pediatrics, Gelre Hospital, Apeldoorn, the Netherlands.
Esther J.E. Peeters, Department of Pediatrics, Juliana Children’s Hospital, Hagaziekenhuis, the Hague, the Netherlands.
Ronald Petru, Department of Pediatric Intensive Care, Radboud University Medical Center, Nijmegen, the Netherlands.
Marielle W.H. Pijnenburg, Department of Pediatric Pulmonology, Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherlands.
Denise Rook, Department of Pediatrics, Juliana Children’s Hospital, Hagaziekenhuis, the Hague, the Netherlands.
Kim Schilleman, Department of Pediatrics, Admiraal de Ruyter Hospital, Goes, the Netherlands.
Rian Schopmeijer, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands.
David Slotboom, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands.
Manouk van der Steen, Department of Pediatrics, University Medical Center Maastricht, Maastricht, the Netherlands.
Kim Stol, Department of Pediatric Infectious Disease and Immunology, Radboud University Medical Center, Nijmegen, Netherlands.
Yolande E.M. Thomasse, Department of Pediatrics, Dijklander Hospital, Hoorn, the Netherlands.
Wim J.E. Tissing, Prinses Máxima Center for Pediatric Oncology, Utrecht, the Netherlands.
Xandra W. van den Tweel, Department of Pediatrics, Maasstad Hospital, Rotterdam, the Netherlands.
Sebastiaan J. Vastert, Department of Pediatric Rheumatology, University Medical Center Utrecht, Utrecht, the Netherlands.
Anne B. Verbeek, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Leiden, the Netherlands.
Annette M.M. Vernooij-van Langen, Department of Pediatrics, St Jansdal Hospital, Harderwijk, the Netherlands.
Jantien W. Wieringa, Department of Pediatrics, Haaglanden Medical Center, the Hague, the Netherlands.
Joanne G. Wildenbeest, Department of Pediatric Infectious Diseases, University Medical Center Utrecht, Utrecht, the Netherlands.
Saskia N. de Wildt, Department of Pharmacology and Toxicology, Radboud University Medical Center, Nijmegen, the Netherlands.
Christiaan van Woerden, Department of Pediatrics, Bravis Hospital, Roosendaal, the Netherlands.
Collaborators: Leontien B. van der Aa, Koen J. van Aerde, Bettina Auffarth-Smedema, Ingeborg Y. Bart, Cherise Beek, Gitanjali I. Bechan, J. Merlijn van den Berg, Venje H. Boonstra, Mijke Breukels, Danielle M.C. Brinkman, Patricia C.J.L. Bruijning-Verhagen, Stephanie C. de Crom, Margot R. Ernst-Kruis, Pieter L.A. Fraaij, Joyce Goris, Michael Groeneweg, Mariken Gruppen, Sanne C. Hammer, Petra C.E. Hissink Muller, Jenneke Homan-van der Veen, Monique A.M. Jacobs, Arvid W.A. Kamps, Naomi Ketharanathan, Martijn van der Kuip, Taco W. Kuijpers, Elizabeth G. Legger, Shirley Lo-A-Njoe, Meindert E. Manshande, Carien J. Miedema, Charlie C. Obihara, Gideon O. Olivieira, Annemarie Oudshoorn, Esther J.E. Peeters, Ronald Petru, Marielle W.H. Pijnenburg, Denise Rook, Kim Schilleman, Rian Schopmeijer, David Slotboom, Manouk van der Steen, Kim Stol, Yolande E.M. Thomasse, Wim J.E. Tissing, Xandra W. van den Tweel, Sebastiaan J. Vastert, Anne B. Verbeek, Annette M.M. Vernooij-van Langen, Jantien W. Wieringa, Joanne G. Wildenbeest, Saskia N. de Wildt, and Christiaan van Woerden
REFERENCES
- 1.Woodruff RC, Campbell AP, Taylor CA, et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022;149:e2021053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40:e137–e145. [DOI] [PubMed] [Google Scholar]
- 3.Wanga V, Gerdes ME, Shi DS, et al. ; BMBS1. Characteristics and clinical outcomes of children and adolescents aged <18 years hospitalized with COVID-19 - six hospitals, United States, July–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhedin S, Lundholm C, Horne A, et al. ; Swedish Pediatric MIS-C Consortium. Risk factors for multisystem inflammatory syndrome in children - a population-based cohort study of over 2 million children. Lancet Reg Health Eur. 2022;19:100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hani E, Bertran M, Powell A, et al. Significantly lower infection fatality rates associated with SARS-CoV-2 Omicron (B.1.1.529) infection in children and young people: active, prospective national surveillance, January–March 2022, England. J Infect. 2023;86:397–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahl A, Mielke N, Johnson S, et al. Severe COVID-19 outcomes in pediatrics: an observational cohort analysis comparing Alpha, Delta, and Omicron variants. Lancet Reg Health Am. 2023;18:100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386:1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. 2020. Available at: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed March 23, 2023.
- 10.Women’s Institute for Independent Social Enquiry (WiiSE). Coronavirus in Kids Tracking and Education Project. The COVKID Project. 2021. Available at: https://www.covkidproject.org/. Accessed March 23, 2023. [Google Scholar]
- 11.Martin B, DeWitt PE, Russell S, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID Cohort Collaborative. JAMA Netw Open . 2022;5:e2143151.. [Google Scholar]
- 12.World Health Organization (WHO). WHO Global Clinical Platform: COVID-19. 2021. Available at: https://www.who.int/teams/health-care-readiness/covid-19/data-platform. Accessed March 23, 2023.
- 13.World Health Organization (WHO). Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19: Scientific Brief. 2020. Available at: https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed March 23, 2023.
- 14.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 15.Castor EDC. Castor Electronic Data Capture. 2019. Available at: https://castoredc.com. Accessed March 23, 2023.
- 16.Roede MJ, Van Wieringen JC. Growth Diagrams 1980: Netherlands Third Nation-wide Survey. Tijl Tijdschriften; 1985. [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin B, DeWitt PE, Albers D, et al. Development of a pediatric blood pressure percentile tool for clinical decision support. JAMA Netw Open. 2022;5:e2236918–e22318-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepanski RJ, Godambe SA, Zaritsky AL. Pediatric vital sign distribution derived from a multi-centered emergency department database. Front Pediatr. 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter interim guidance on use of antivirals for children with coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc. 2021;10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Public Health and the Environment. Rijksinstituut voor Volksgezondheid en Milieu (RIVM). COVID-19. Available at: https://www.rivm.nl/coronavirus-covid-19. Accessed March 23, 2023. [PubMed]
- 22.Medic S, Anastassopoulou C, Lozanov-Crvenkovic Z, et al. Incidence, risk, and severity of SARS-CoV-2 reinfections in children and adolescents between March 2020 and July 2022 in Serbia. JAMA Netw Open. 2023;6:e2255779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenck RW, Jr, Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeser C, Whitaker H, Linley E, et al. Large increases in SARS-CoV-2 seropositivity in children in England: effects of the delta wave and vaccination. J Infect. 2022;84:418–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute for Public Health and the Environment. Rijksinstituut voor Volksgezondheid en Milieu (RIVM). PIENTER Corona study. 2022. Available at: https://www.rivm.nl/en/pienter-corona-study. Accessed March 20, 2023. [PubMed] [Google Scholar]
- 27.Naeimi R, Sepidarkish M, Mollalo A, et al. SARS-CoV-2 seroprevalence in children worldwide: a systematic review and meta-analysis. EClinicalMedicine. 2023;56:101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutch Central Bureau for Statistics (CBS). Population Distribution in the Netherlands. 2022. Available at: https://www.cbs.nl/nl-nl/dossier/nederland-regionaal/geografische-data/gegevens-per-postcode. Accessed March 23, 2023.
- 29.Dutch Ministry of Health, Welfare and Sport. Coronavirus Dashboard. 2020. Available at: https://coronadashboard.government.nl/landelijk/vaccinaties. Accessed March 23, 2023.
- 30.Gezondheidsraad; Independent Scientific Advisory Body for Government and Parliament. COVID-19 Advices. 2023. Available at: https://www.gezondheidsraad.nl/over-ons/de-gezondheidsraad-en-covid-19. Accessed March 23, 2023. [Google Scholar]
- 31.Schober T, Caya C, Barton M, et al. Risk factors for severe PCR-positive SARS-CoV-2 infection in hospitalised children. BMJ Paediatr Open. 2022;6:e001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stierman B, Abrams JY, Godfred-Cato SE, et al. Racial and ethnic disparities in multisystem inflammatory syndrome in children in the United States, March 2020 to February 2021. Pediatr Infect Dis J. 2021;40:e400–e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kempen EB, Bruijning-Verhagen PCJ, Borensztajn D, et al. Increase in invasive group a streptococcal infections in children in the Netherlands, a survey among 7 hospitals in 2022. Pediatr Infect Dis J. 2023;42:e122–e124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.