Summary
Background
Sepsis is associated with T-cell exhaustion, which significantly reduces patient outcomes. Therefore, targeting of immune checkpoints (ICs) is deemed necessary for effective sepsis management. Here, we evaluated the role of SIGLEC5 as an IC ligand and explored its potential as a biomarker for sepsis.
Methods
In vitro and in vivo assays were conducted to both analyse SIGLEC5's role as an IC ligand, as well as assess its impact on survival in sepsis. A multicentre prospective cohort study was conducted to evaluate the plasmatic soluble SIGLEC5 (sSIGLEC5) as a mortality predictor in the first 60 days after admission in sepsis patients. Recruitment included sepsis patients (n = 346), controls with systemic inflammatory response syndrome (n = 80), aneurism (n = 11), stroke (n = 16), and healthy volunteers (HVs, n = 100).
Findings
SIGLEC5 expression on monocytes was increased by HIF1α and was higher in septic patients than in healthy volunteers after ex vivo LPS challenge. Furthermore, SIGLEC5-PSGL1 interaction inhibited CD8+ T-cell proliferation. Administration of sSIGLEC5r (0.8 mg/kg) had adverse effects in mouse endotoxemia models. Additionally, plasma sSIGLEC5 levels of septic patients were higher than HVs and ROC analysis revealed it as a mortality marker with an AUC of 0.713 (95% CI, 0.656–0.769; p < 0.0001). Kaplan–Meier survival curve showed a significant decrease in survival above the calculated cut-off (HR of 3.418, 95% CI, 2.380–4.907, p < 0.0001 by log-rank test) estimated by Youden Index (523.6 ng/mL).
Interpretation
SIGLEC5 displays the hallmarks of an IC ligand, and plasma levels of sSIGLEC5 have been linked with increased mortality in septic patients.
Funding
Instituto de Salud Carlos III (ISCIII) and “Fondos FEDER” to ELC (PIE15/00065, PI18/00148, PI14/01234, PI21/00869), CDF (PI21/01178), RLR (FI19/00334) and JAO (CD21/00059).
Keywords: Sepsis, Immune checkpoint, SIGLEC5, T-cell exhaustion, Biomarker, HIF1α
Research in context.
Evidence before this study
The adaptive immune system of septic patients is markedly immunosuppressed, which has been linked to the role of immune checkpoints (ICs). A majority of these ICs and their ligands show Ig-like-V-type domains, which are the structures through which they interact. SIGLEC family members, such as SIGLEC5, exhibit this Ig-like-V-type domain, with some being identified as having a role in sepsis. SIGLEC5 shows a wide recognition range of sialic acid structures and its ability to interact with highly sialylated proteins gives it a high potential to regulate the immune response in sepsis and act as a potential therapeutic target. However, there is a limited understanding of the role of SIGLEC members in sepsis and their potential for therapy. Hence, exploring the relevance of SIGLEC5 in sepsis is an important area for research, as it may lead to biomarkers for early disease prognosis and therapeutic targets.
Added value of this study
Our study revealed that SIGLEC5 is expressed at high levels in neutrophils and classical monocytes, with increased presence following stimulation with LPS. We determined that HIF1α was responsible for the overexpression of SIGLEC5. Additionally, our data demonstrated a significantly heightened expression of SIGLEC5 in both monocytes and plasma of septic patients, particularly those who perished within 60 days of infection. The deleterious effects of SIGLEC5 on survival was further validated using in vivo mouse endotoxemia models. Moreover, we discovered that SIGLEC5 obstructs CD8+ T cell proliferation in vitro without initiating apoptosis.
Implications of all the available evidence
Our study has shown that sSIGLEC5 levels can be used as a biomarker for prognosis in patients with sepsis. In addition, SIGLEC5 could be potentially involved in pharmacological strategies in sepsis.
Introduction
Sepsis is perceived as a leading cause of death in intensive care units (ICU) worldwide.1 Numerous therapeutic strategies, other than antimicrobial and fluid resuscitation treatments, have failed in clinical trials; hence, solid biomarkers of sepsis evolution remain to be identified. Though gold standards to define boundaries amongst sepsis and systemic inflammatory response syndrome (SIRS) are still needed, an increasing number of studies have indicated that patients with systemic response to infection show most of the hallmarks of immunosuppression. In addition, in the complex dynamics of sepsis, two phases have been recognized: an early inflammatory phase and a late immunosuppressive stage2,3; however, these two phases can overlap.3, 4, 5, 6, 7
Studies have demonstrated that monocytes and macrophages are key players in generating an immunosuppressive phenotype during sepsis, which can result in the development of secondary infections and, in many cases, death.3,8 T cell exhaustion, through the crosstalk between immune checkpoint (IC) ligands expressed on the monocytes and macrophages surfaces and their counterparts on T cells, is one of the main mechanisms through which this immunosuppressive phenotype occurs.9 In this regard, we and other authors have demonstrated the role of Programmed cell death-protein 1 (PD-1) and its ligand (PD-L1) in the context of sepsis.10, 11, 12, 13
Several teams have evidenced that sialic acid-binding immunoglobulin-type lectins (SIGLECs) play a crucial role in modulating the immune response, and can be involved in the pathogenesis and treatment of sepsis.14, 15, 16, 17 These lectins present sialic acid recognition sites in an Ig-like-V-type domain. Interestingly, that domain is described in other proteins known and postulated as ICs or ICs ligands, such as PD-1 and PD-L1, being the structure through which they interact.18
Some members of the SIGLEC family have already been identified as playing a role in sepsis, particularly SIGLEC1, a receptor mainly expressed on monocytes and macrophages, which has been linked to increased production of TGF-β in macrophages in vitro.19 SIGLEC2, predominantly expressed on B cells, appears to be associated with the development of sepsis, as a soluble fragment of SIGLEC2 was found to be elevated in patients with gram-negative bacterial sepsis.20 However, SIGLEC2−/− mice did not demonstrate any difference in severity, survival rate, or bacterial clearance upon infection with Staphylococcus aureus.21 SIGLEC9 (or SIGLEC-E in mice) is mainly expressed on monocytes, macrophages, neutrophils, and dendritic cells, and its expression is known to increase upon LPS stimulation. This aids in the endocytosis of TLR4 and helps to modulate the inflammatory response during sepsis.22 SIGLEC10, expressed on macrophage subsets, dendritic cells, and most B cells, is thought to inhibit DAMPs-induced inflammation and assist in regulating the immune response to infection.23,24 The paired system SIGLEC5/SIGLEC14, mainly expressed on neutrophils and monocytes, has an opposite effect on regulating the immune response despite having almost identical sialic-acid site domains. SIGLEC5 has been shown to mediate the impairment of phagocytosis and reactive oxidative species production in an infection with group B Streptococcus (GBS),25 while SIGLEC14, through interaction with adaptor proteins such as DAP12, can activate the p38-mitogen-activated protein kinase (MAPK) and Akt pathways, having a bidirectional action.26 However, since SIGLEC14 is not widely expressed,27 it is difficult to study this molecule in the general population. Despite this, our current understanding of the role of SIGLEC members in sepsis and their potential as therapeutic targets is still limited. Therefore, further research is required, including the use of clinically relevant mouse models for sepsis such as polymicrobial cecal ligation and puncture (CLP), to gain a better understanding of the role of these SIGLEC members in sepsis.
SIGLEC5 stands out among the fifteen members of the SIGLEC family reported in humans, due to its potential to regulate the immune response in cases of sepsis and its potential as a therapeutic target. This is because SIGLEC5 has a broad recognition range of sialic acid structures, including α2-3, α2-6, and α2-8 linkage conformations, as well as N-acetylneuraminic and N-glycolylneuraminic variants of sialic acid, and is able to interact with proteins highly decorated with sialic acid.28,29 Additionally, SIGLEC5 plays a multifaceted physiological role, being involved in cell-cell interactions, pathogen recognition, clearance of apoptotic cells, negative cellular signaling through soluble factors (e.g. Hsp70), and endocytosis of ligands.30,31 Furthermore, its soluble form, sSIGLEC5, has been shown to interact with the highly sialylated P selectin glycoprotein ligand-1 (PSGL1) molecule, exhibiting robust anti-inflammatory activity.32 Moreover, PSGL1 has been reported to induce CD8+ T-cell dysfunction by acting as an IC in a murine model of chronic virus infection.33
Considering these assertions, we applied a combination of in vitro, ex vivo and in vivo approaches to study the role of SIGLEC5 in the context of sepsis. We show circulating neutrophils and monocytes as the main cell types expressing surface SIGLEC5, the latter being able to modulate its expression in a greater manner upon LPS stimulation. Additionally, SIGLEC5 expression is governed by the transcription factor HIF1α. We demonstrate that SIGLEC5 acts as a CD8+ T cell-specific immune checkpoint ligand that impairs their proliferation. Besides, metalloproteinases drive the generation of a soluble form of SIGLEC5 (sSIGLEC5) that can be detected in plasma and also diminishes CD8+ T cell proliferation. Along these lines, the administration of sSIGLEC5 exacerbates the disease in two mouse models for sepsis in a CD8α T cell-dependent manner. Finally, in septic patients both monocyte-membrane-bound and sSIGLEC5 are significantly increased compared with healthy volunteers, indicating that sSIGLEC5 acts as a biomarker of severity in this pathology.
Methods
Study design
This study aimed to reveal the role of SIGLEC5 in the context of sepsis. This protein belongs to the SIGLEC family, which plays a relevant role in the modulation of the immune response and could have an important effect on the sepsis disease. Monocytes and macrophages are determinants in orchestrating the immunosuppressive phenotype in sepsis, we hypothesized that SIGLEC5, mainly expressed on monocytes and neutrophils, could be acting as an immune checkpoint in this disease. The combination of in vitro, ex vivo, and in vivo approaches to study the impact of SIGLEC5 in the context of sepsis compared to healthy volunteers suggest that SIGLEC5 plays a role in the immunosuppressive effect, dampening the proliferation capacity of CD8+ T cells, accelerating the mortality in mouse models of endotoxemia, and showing a prognostic effect of mortality through its soluble form in plasma.
Healthy volunteers and patients’ samples
Patients older than 18 years meeting the diagnostic criteria for sepsis according to the Third International Consensus Definitions for Sepsis and Septic-Shock34 (Sepsis-3) were enrolled in this study upon arrival to Emergencies (Table 1 and Supplementary Table S1, n = 426). Sepsis picture was primarily identified by SOFA (tachypnea, altered level of consciousness and hypotension) and then thoroughly corroborated following International Sepsis Definitions Conference criteria35 and classified according to their severity in sepsis and septic shock (operationally defined as requiring vasopressor therapy to maintain a mean arterial blood pressure of >65 mmHg and an increased plasma lactate level of >2 mmol/L). Patients were followed up for 60 days and classified according to their outcome into survivors or exitus. Blood samples were promptly obtained from patients upon their admission to the Emergency Department of the University Hospital La Paz (Madrid, Spain) within the first hour of their arrival and peripheral blood mononuclear cells (PBMCs) and plasma were isolated by standardized procedures.36 Plasma samples from septic patients collected during the first hour of patient admission to the Emergency Department as well as clinical data and evolution information were provided by the University Hospital La Paz (n = 104), the Sepsis Bank of Vall d'Hebron University Hospital Biobank (n = 303) and Virgen de la Arrixaca University Clinical Hospital Biobank (n = 19) (Spanish National Biobanks Network). As controls, healthy volunteers (HV, n = 100) were recruited in person from the Blood Donor Services of La Paz University Hospital and patients with aneurysm (n = 11) and stroke (n = 16) were also enrolled from The Emergency Department and Department of Neurology of La Paz University Hospital, respectively (see Supplementary Table S2). All of them were free of pathogen colonization and recruited randomly but using an age and gender matching. Considering that our study is not a clinical trial, we employed Andrew Fisher’s Formula37 to calculate our sample size. We used a confidence level of 90% (corresponding to a Z-score of 1.65), a standard deviation of 0.5, and a confidence interval (marginal error) of ±5%.
Table 1.
Baseline characteristics of SIRS and septic patients included in the study.
| Characteristica | All patients (n = 426) | Sepsis (n = 188) | Septic shock (n = 158) | SIRSb (n = 80) | p-valuec |
|---|---|---|---|---|---|
| Age–yr | 67.4 ± 16.2 | 67.2 ± 17.2 | 69 ± 15.1 | 65 ± 15.7 | |
| Male sex—no. (%) | 240 (56.47) | 106 (56.68) | 89 (56.33) | 45 (56.2) | |
| APACHE II Score | 17.9 ± 7.6 | 17.3 ± 6.2 | 22.2 ± 8.3 | 13.3 ± 4.4 | <0.0001 |
| SOFA Score | 5.3 ± 3.2 | 4 ± 2.4 | 7.5 ± 3.3 | 4 ± 2.1 | <0.0001 |
| Lactate, nmol/L | 3.28 ± 3.21 | 2.95 ± 2.39 | 4.49 ± 4.28 | 1.66 ± 0.6 | <0.0001 |
| CRP, mg/L | 73.37 ± 105.39 | 75.65 ± 103.17 | 70.62 ± 107.95 | – | |
| PCT, ng/mL | 25.73 ± 51.73 | 20.52 ± 51.54 | 31.94 ± 51.35 | – | <0.0001 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; CRP: C-reactive protein; INR: International Normalized Ratio; PCT: Procalcitonin; SIRS: Systemic Inflammatory Response Syndrome; SOFA: Sequential Organ Failure Assessment.
Data are presented as mean ± SD, or number (%).
Non-infectious SIRS.
p values were calculated by ANOVA test.
Mouse strains
Wild-type female C57BL/6 mice (6–9 weeks old) were purchased from Charles River Laboratories (France). Rag1−/− female C57BL/6 mice (8–9 weeks old) were a gift from Carlos del Fresno (CNIC, Madrid, Spain). Mice were properly housed in temperature and light-regulated rooms (12:12 h light/dark cycle, 21–24 °C) with food and water ad libitum. All mice were randomly assigned to experimental groups. Mice were followed-up for survival considering mice to have died when they reached the humane endpoint, in our case, when they lost more than 10% of their initial weight. In addition, post-mortem samples were collected.
Cell culture conditions
PBMCs and plasma from healthy volunteers and patients were isolated by Ficoll-Plus (GE Healthcare Bio-Sciences) gradient. Monocytes were enriched by: (1) adherence by plating PBMCs in FBS-free RPMI 1640 media with antibiotics for 1 h, and then non-adherent cells were removed by washing with Phosphate Buffered Saline (PBS) three times; or (2) negative isolation using magnetic beads of the Monocyte Isolation Kit (Mintenyi Biotec) according to the manufacturer’s instructions. The purity of the monocyte cultures was tested by CD14 labelling and FACS analysis (average 89% and >95% of CD14+ cells, respectively). Neutrophils isolation was performed using 3% Dextran 500 in NaCl 0.9% of the polymorph nuclear and red cells phase after the isolation of the PBMCs by Ficoll-Plus gradient. All primary human and mice cultures were incubated at 37 °C 5% CO2 in RPMI 1640 media containing 10% foetal bovine serum (FBS), 25 Mm HEPES, l-glutamine and 1% Penicillin and Streptomycin Mix (Gibco).
ELISA protocol
Concentrations of sSIGLEC5 in supernatants of human monocyte cultures and plasma samples were determined using a commercially available ELISA kit (Sigma-Aldrich), following the manufacturer’s instructions.
Antibodies and flow cytometry analysis
FACS analysis for all in vitro assays were developed using specific anti-human antibodies (Abs) to the following surface molecules: CD8-Allophycocianin (APC, RRID:AB_11140266), CD14-APC (RRID:AB_11140663), HLA-DR-Fluorescein-isothiocyanate (FITC, RRID:AB_11140598) (all three from ImmunoStep), CD3-Brilliant Violet (BV)-786 (RRID:AB_2739260), CD4-Peridinin-chlorophyll-A protein (PerCP, RRID:AB_393791), CD8-BV510 (RRID:AB_2722546), CD14-BUV395 (RRID:AB_2744288), PSGL1-Phycoerythrin (PE, RRID:AB_396325) (all five from BD Biosciences) and SIGLEC5-PE (RRID:AB_2905383, Miltenyi Biotec), as well as specific mouse CD8α-APC (RRID:AB_2728039, Miltenyi Biotec). Cells were stained with proper Abs for 30 min at 4 °C in the dark, and washed with Phosphate Buffered Saline (PBS) twice. All antibodies used were titrated and matched isotype antibodies were used as negative controls. For all in vitro assays, samples were run in FACS Calibur or FACS Celesta (BD Biosciences) flow cytometers, and data were analysed with FlowJo (TreeStar) v. X.07 software or BD FACSDiva v8, respectively.
For the SIGLEC5 expression characterization in the main immune populations, whole blood was lysed by Pharm Lyse 1X (BD) for 20 min, washed with PBS twice and then were stained with fluorochrome-conjugated antibodies to a multi-colour panel of surface markers listed in Supplementary Table S3. Dead cells were excluded using LIVE/DEAD Blue fluorescent reactive dye purchased from Invitrogen and True-Stain Monocyte Blocker (BioLegend) reagent was added prior to the label protocol to block the nonspecific binding of some fluorochrome on monocytes. Labelled cells were acquired on a Cytek Aurora Spectral Cytometer (Cytek Biosciences). Data were analysed using FlowJo (TreeStar) v10.6.2 software.
In vitro LPS stimulation
Monocytes or neutrophils (3 × 105 cells per well) were stimulated or not with 10 ng/mL lipopolysaccharide (LPS, from Escherichia coli, Sigma-Aldrich) for 16 h in flat bottom 24-wells plates, in a final volume of 0.5 mL. For the time-course of extracellular SIGLEC5 expression, monocytes were treated or not with 10 ng/mL LPS, for periods ranging from 3 to 48 h. For the matrix metalloproteinase (MMP) and ADAM-family inhibition, the specific inhibitor GM6001 (Merck Millipore) was added 6 h after the LPS challenge, and cultures were maintained for a maximum of 48 h. For HIF1α inhibition, the potent inhibitor PX-478 (Cayman Chemical) was added for 3 h, and then challenged or not with LPS (10 ng/mL) for another 16 h.
In silico analysis of genomic and protein sequences
Bioinformatics analysis, such as protein and genomic structure of SIGLEC5, were performed using the ENSEMBL, Uniprot and NextProt online tools. The proximal promoter region and flanking promoters of the human SIGLEC5 were localized using the ENSEMBL online tool, and the Hypoxia Response Elements (HREs) using a self-generated Python script with the consensus site to the binding of the HIF1α factor (5′-ACGTG-3′). The multiple sequence alignments were performed with ClustalW (NCBI). To check the sequence identity and similarity of SIGLEC5 with the other proteins, the EMBOSS-Needle online tool was used.
Monocyte nucleofection procedure
Magnetic negative sorted monocytes by Pan Monocyte Isolation Kit (Miltenyi Biotec) were used for HIF1α and SIGLEC5 overexpression assays (see Cell Culture above) by nucleofection as previously described,38 using an Alexa Nucleofector (Lonza Group). HIF1α overexpression was done with an expression vector, which was a kind gift from Dr. del Peso (Institute for Biomedical Research Alberto Sols). SIGLEC5 overexpression was done with an expression vector (pUNO1-hSIGLEC5, InvivoGen).
Chromatin immunoprecipitation (ChIP) assay
Human/Mouse HIF1α ExactaChIP Chromatin IP Kit (R&D Systems) was used to perform ChIP on lysates from isolated human blood monocytes, previously nucleofected or not with a HIF1α overexpression vector.38 Resultant DNA was isolated using either an unspecific IgG antibody or a specific antibody against HIF1α, in order to perform the immunoprecipitation according to the manufacturer’s protocol.
The products (Hypoxia Response Elements [HRE] sites) were amplified with primers shown in Supplementary Table S4, using the NZY Jaq 2X Colourless Master Mix, in an Eppendorf AG Mastercycler Thermocycler. The following program was used: a first denaturation step at 94 °C for 5 min, 40 cycles of denaturation at 94 °C for 1 min (primer annealing at 56 °C for 50 s) with primer extension at 72 °C for 45 s, and a final extension at 72 °C for 5 min after completion of the cycling steps.
RNA isolation and RT-qPCR
Adherent monocytes were washed twice with PBS and the RNA was extracted using the High Pure RNA Isolation Kit (Roche Diagnostics). The RNA concentration of each sample was measured using a NanoDrop 2000 (Thermo Fisher Scientific). cDNA was synthesized from 0.25 μg total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-qPCRs were performed using the QuantiMix Easy SYG Kit (Biotools) according to the manufacturer’s instructions. Gene expression levels were analysed using the LightCycler system (Roche Diagnostics). Reactions were run in triplicate and expression level of β-ACTIN housekeeping was used as internal standard to normalize data. The cDNA copy number of each gene of interest was determined using a 7-point standard curve. All the primers were synthesized by Eurofins Genomics. Specific primers for each gene are shown in Supplementary Table S5.
Lymphocyte proliferation assays
Carboxyfluorescein succinimidyl ester (CFSE) was purchased from ThermoFisher Scientific, and used following the manufacturer’s protocol to assess T cells lymphocytes proliferation. Isolated or nucleofected monocytes (104 cells per well) were seeded into round bottom 96-wells plates, and then stimulated or not with LPS (10 ng/mL) for 16 h. Afterwards, monocytes were washed once with PBS and autologous CFSE-labelled T cells were added to a ratio 1:5 (monocyte:lymphocyte) in fresh complete RPMI 1640 medium. Cells were then stimulated or not with Pokeweed (PWD, 2.5 μg/mL), and treated or not with fully mouse anti-PSGL1 antibody (0.25 μg/mL) or anti-SIGLEC5 antibody (1 μg/mL).
Apoptosis assay
PBMCs (2 × 105 cells per well) from HVs were cultured with or without sSIGLEC5r (500 or 1000 ng/mL) for 24 h, and then stained with AnnexinV-FITC and Propidium Iodide (PI), for measuring apoptosis by flow cytometry, following manufacturers’ recommendations (ImmunoStep).
Binding assay protocol
A polyclonal, FITC-labelled antibody against human IgG-Fc (Abcam), hereinafter α-Fc-FITC, was purchased. A recombinant human SIGLEC5 protein, with a crystallisable fragment (Fc) region of antibody domain (R&D Systems), hereinafter sSIGLEC5r, was purchased.
Binding of sSIGLEC5r was performed as previously reported.39 Briefly, PBMCs from HVs or septic patients, treated or not with neuraminidase (NM, 0.01 U/mL for 24 h), were washed twice with 10 mL of ice-cold Ligand Binding Buffer (LBB, PBS with 1% BSA, 0.05% Sodium Azide and 0.1 mM CaCl2 2 H2O), fixed using 4% paraformaldehyde (PFA), and 106 cells resuspended in 1 mL LBB. Afterwards, fixed PBMCs were washed twice with ice-cold LBB and incubated with the recombinant protein sSIGLEC5r (500 ng in 100 μL of LBB) or LBB alone for 1 h and hand-mixed each 15 min on ice. Next, sSIGLEC5r binding was detected using α-Fc-FITC (1 μL in 100 μL of LBB). Possible Fc-Receptors were blocked using a Fc-blocking (ImmunoStep) for 30 min before incubation with the recombinant protein. PBMCs incubated only with α-Fc-FITC were used as the negative control.
Mouse endotoxin in vivo model
C57BL/6, 8–9 weeks old (purchased to Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain) female mice were administered a peritoneal injection with either LPS of E. coli (055:B5, Sigma Aldrich) (20 mg/kg), sSIGLEC5r (0.8 mg/kg) or both LPS (20 mg/kg) plus sSIGLEC5r (0.8 mg/kg) using sterile saline solution (0.90% w/v of NaCl) as vehicle. To deplete CD8α T cells, an anti-mouse CD8α antibody (2.43 clone, Bioxcell) was administered by intraperitoneal injection (20 μg per mice) 24 h before the peritoneal injection of LPS and/or sSIGLEC5r. Mice were followed up for survival and post-mortem lung samples were collected.
Mouse cecal ligation and puncture (CLP) in vivo model
C57BL/6 wild type (8–9 weeks old) female mice and Rag1−/− C57BL/6 (8–9 weeks old; purchased to Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain) were modelled. Cecal ligation and puncture protocol was used to induce polymicrobial sepsis as described.40 Briefly, a 2-cm midline incision was performed to exteriorize the caecum, before being ligated with a 3.0-silk suture at 1 cm of its base, then punctured once with a 25-gauge needle and a small drop of cecal content from the perforation site extruded. Caecum was then returned to the peritoneal cavity and the abdominal incision closed with 4.0-silk sutures. Mice were administered or not a retro-orbital injection of sSIGLEC5r (0.8 mg/kg) using sterile saline solution (0.90% w/v of NaCl) as vehicle.
Histological analysis
Following sacrifice, lungs from mice were fixed in 4% paraformaldehyde embedded in paraffin and cut into 5 μm sections in a microtome (RM2255, Lefor histopathological analysis, Leica Biosystems, Wetzlar, Germany). Sections were stained with haematoxylin and eosin (H&E) and images were captured using light microscopy (BX41, Olympus Optical Co., Ltd., Tokyo, Japan). Briefly, the acute lung injury score of mice, on a scale of 0 (optimal) to 12 (severe), were estimated in accordance with combined assessments of alveolar congestion, haemorrhage, fibrin and infiltrates as previously described.41 Five randomly selected fields from each slide were analysed by three independent observers.
Statistical analysis
Data are presented as numbers and percentages, means and standard deviations. D’Agostino & Pearson Normality test was performed to all the studied variables. Accordingly, differences among the groups were evaluated with the use of Chi-square test, Student’s t-test or an analysis of variance (ANOVA) model, followed by the Kruskal–Wallis or Friedman test was used to evaluate multiple comparisons in the ANOVA models, without assuming a Gaussian distribution or normality after a pairing or non-pairing experimental design, respectively. To ascertain the accuracy of the results, Dunn's multiple comparison test was implemented.
For patients with sepsis, Receiver-Operating Characteristic (ROC) curve analysis and its area under the curve (AUC) were used to determine whether the sSIGLEC5 levels could be used as a predictor of mortality in patients with sepsis. The area under the ROC curve measures how well the model discriminates between Survival and Exitus groups of patients using only the sSIGLEC5 levels in plasma. We also indicated sensitivity and specificity and the optimal threshold (in terms of maximum sensitivity and specificity) in the primary study cohort were determined on by the Youden Index. Considering sSIGLEC5 levels, we used the Kaplan–Meier method to estimate survival rate over time (60 days) and hazard ratio.
Pearson's analysis was then applied to analyse correlations between plasmatic sSIGLEC5, lactate levels and two severity scores (APACHE-II and SOFA) to determine how variables behave in relation to one another; through the use of the normality test, each variable was ascertained to have a normal distribution. The correlation coefficient (r) was interpreted based on prior studies without having to utilise any distinct cut-off.42
The Kaplan–Meier method was employed to assess the survival rate of mice after injection of bacterial lipopolysaccharide (endotoxin) and when surgery (CLP) had been initiated, up to a humane endpoint of 10% weight loss. Data for hazard ratio was collected over a period of 168 h.
p values of less than 0.05 were considered to indicate statistical significance. All p-values are two-sided, and the 95% Confidence Intervals (95% CI) are also presented. Statistical analyses were conducted using Prism 8.0 (GraphPad), SPSS version 23 (IBM) software. Youden Index was calculated with MedCalc statistical software. Sample sizes are detailed in the figure legends.
Ethics
This study was conducted following the guidelines of the 1975 Declaration of Helsinki and was approved by the Local Committee for Ethics (La Paz University Hospital, Madrid, PI-3761). Informed consent was acquired from all participants, and all data collected were treated with the utmost confidentiality. The animal experiments within this study were designed and performed following approval from the Institutional Animal Care and Use Committee at Biomedical Research Institute (IIB, PI-2599).
Role of the funding source
This research project was conducted with its funders having no oversight of the study design, data collection, data analysis, data interpretation, report writing, or the decision to submit the article for publication. All stages of the project were carried out independently of the research funding, guaranteeing the integrity of the results, conclusions, and interpretations.
Results
LPS induces SIGLEC5 expression in human monocytes in a HIF1α-dependent manner
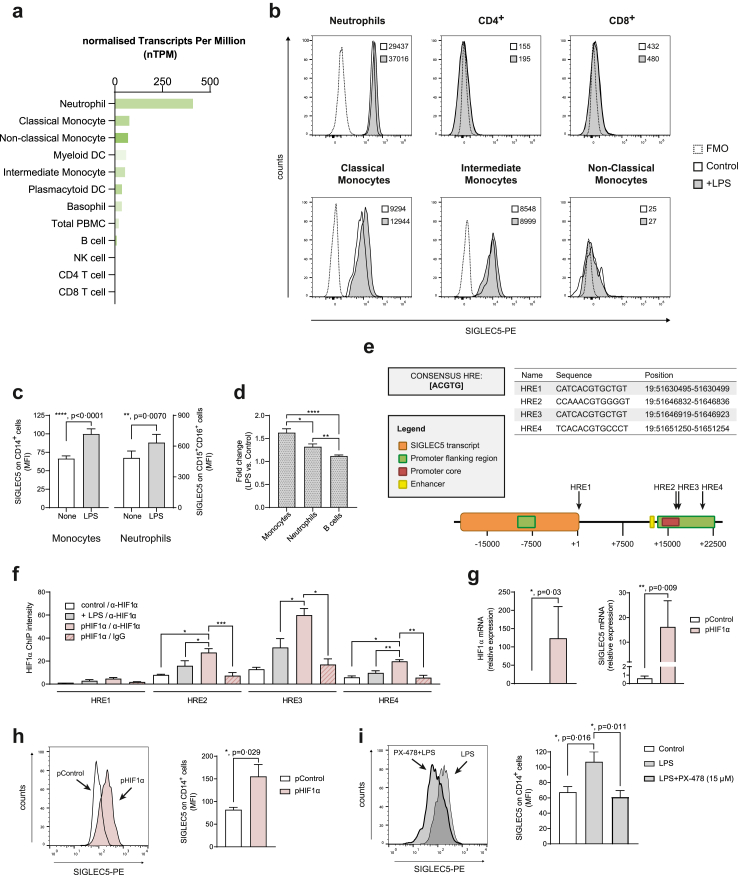
Based on the values reported in the Monaco database,43 Fig. 1a illustrates a high SIGLEC5 RNA expression in human neutrophils, monocytes, dendritic cells, and a milder expression in B cells. We confirmed the expression of membrane-bound SIGLEC5 by full-spectrum flow cytometry on a wide panel of blood circulating immune populations stimulated or not with lipopolysaccharide (LPS), simulating an encounter with Gram-negative bacteria. Fig. 1b shows a strong SIGLEC5 expression on neutrophils and classical and intermediate monocytes. Note that a low expression on B cells (CD19+CD20+) and both myeloid and plasmacytoid dendritic cells (mDC and pDC) was also detected (Supplementary Figure S1a). In addition, an increase in SIGLEC5 expression was observed when both monocytes and neutrophils were stimulated with LPS (Fig. 1b and c), with a greater increase in monocytes than neutrophils (Fig. 1d).
Fig. 1.
LPS induces SIGLEC5 expression on human monocytes under Hypoxia Inducible Factor 1 alpha (HIF1α) control. (a) Normalized SIGLEC5 RNA expression on the main immune cells in the blood from the Monaco database.43 (b) SIGLEC5 expression on the cell surface of a panel of blood circulating immune populations stimulated (grey filled line) or not (black clear line) with lipopolysaccharide (LPS), including neutrophils, T lymphocytes, classical monocytes (CD14+CD16–), intermediate monocytes (CD14+CD16+) and non-classical monocytes (CD14–CD16+); MFI of LPS-stimulated or not conditions were indicated into the plots. A fluorescence minus one (FMO) was used as negative control of SIGLEC5 expression represented in dotted line. (c) SIGLEC5 expression on monocytes (left panel, n = 35) and neutrophils (right panel, n = 14) from HVs challenged or not with 10 ng/mL of LPS for 16 h. (d) Fold change of SIGLEC5 expression on monocytes, neutrophils and B cells from HVs challenged stimulated with LPS vs. control without stimulation. (e) Hypoxia Response Elements (HRE) in the human SIGLEC5 sequence (Ensembl, ENSG00000268500) based on the consensus sequence [5′-ACGTG-3′]. Sequences and positions are shown. (f) A Chromatin immunoprecipitation (ChIP) assay was conducted on: control monocytes (white column), LPS-stimulated monocytes for 16 h (grey filled column), HIF1α-transfected monocytes (burgundy filled column), all of them immunoprecipitated with an antibody against HIF1α, and HIF1α-transfected monocytes immunoprecipitated with an unspecific IgG antibody (burgundy filled and lined column). HREs band intensities of PCR products normalized to one of the negative controls (n = 4). (g) Relative expression (mRNA) by RT-qPCR of HIF1α (left panel) and SIGLEC5 (right panel) on CD14+ cells from HVs nucleofected with pHIF1α (burgundy filled column) or a control plasmid (white column) (n = 6). (h) Representative histogram overlay of SIGLEC5 expression (left panel) on CD14+ cells nucleofected with pControl (white histogram) or pHIF1α (burgundy histogram) and MFI of SIGLEC5 (right panel) on CD14+ cells after 16 h of nucleofection with pControl (white column) or pHIF1α (burgundy filled column) (n = 4). (i) Representative histogram overlay of SIGLEC5 on CD14+ cells pre-treated (grey thickest line) or not (grey thinnest line) with a specific inhibitor (PX-478) of HIF1α for 3 h, and then challenged with LPS for 16 h (left panel), and MFI of SIGLEC5 on CD14+ cells pre-treated (grey filled columns) or not (black clear column) with a specific HIF1α inhibitor (PX-478) for 3 h, and then challenged (grey filled and thickest line) or not (grey filled and thinnest line) with LPS for 16 h (right panel) (n = 7). Data shown as mean ± SEM. (c, d, and g–i) Paired t-test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001). (f) One-way ANOVA test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
Due to the important role of classical monocytes and macrophages in orchestrating the immunosuppressive phenotype during sepsis3,8 and the significant increment of SIGLEC5 on these cells after LPS stimulation, we focused on characterizing the regulation of SIGLEC5 transcription in these cells. Previously, we reported a crucial role for HIF1α in the control of several key factors during sepsis, including the negative regulator of inflammation IRAK-M, the NLRP3 inflammasome activation and the IC ligand PD-L1.10,11,44,45 Thus, to explore whether the SIGLEC5 gene was a direct target of HIF1α in human monocytes, we searched for potential HIF1α binding sites, known as Hypoxia Response Elements (HRE), in the proximal promoter region of human SIGLEC5 using bioinformatics approaches. As Fig. 1e shows, four potential HREs containing the consensus sequence (5′-ACGTG-3′) were found; three of them were identified within the SIGLEC5 promoter and the last one close to the transcript region. Then, by a chromatin immunoprecipitation (ChIP) assay, we demonstrated HIF1α binds to three of the reported HREs in the promoter region of SIGLEC5 in human monocytes (Fig. 1f). Along these lines, human HIF1α-transfected monocytes exhibited high expression of SIGLEC5 at both mRNA (Fig. 1g) and protein levels (Fig. 1h). Eventually, SIGLEC5 expression on LPS-stimulated monocytes was reduced in the presence of PX-478 (a potent HIF1α inhibitor)46 (Fig. 1i and Supplementary Figure S1b). These results indicate that myeloid circulating cells induce SIGLEC5 expression upon LPS stimulation in a HIF1α-dependent manner.
SIGLEC5 displays IC ligand hallmarks
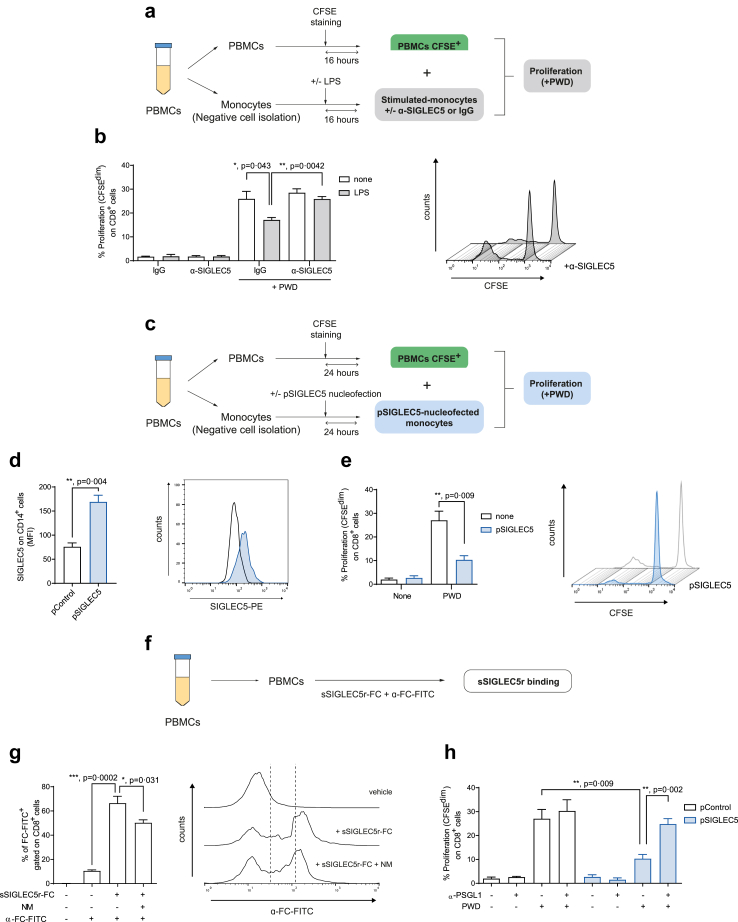
To study the potential immunomodulatory effect of SIGLEC5 on T cells, we stimulated CD4+ and CD8+ T cells with the classical mitogen-lectin pokeweed (PWD) in the presence of unstimulated or LPS-primed autologous monocytes from healthy volunteers (HVs) (Fig. 2a). We observed that LPS-stimulated monocytes from HVs reduced both CD8+ and CD4+ T cell proliferation (Fig. 2b and Supplementary Figure S2a). Besides, this effect was reverted in the presence of a SIGLEC5 blocking antibody in CD8+ (Fig. 2b) but not in CD4+ T cells (Supplementary Figure S2a). Moreover, an important reduction of CD8+ (Fig. 2c–e) but not CD4+ T cell (Supplementary Figure S2b) proliferation was observed when monocytes were transfected with a SIGLEC5 expression plasmid and co-cultured with autologous lymphocytes in presence of PWD. This data supports a specific role for SIGLEC5 in restraining the proliferation of CD8+ but not CD4+ T cells.
Fig. 2.
SIGLEC5 on monocytes impairs CD8+T cell proliferation, which is restored after blocking the SIGLEC5/PSGL1 axis. (a) Scheme of the proliferation assay of monocytes stimulated of not with LPS for 16 h in presence of a blocking antibody against SIGLEC5 (α-SIGLEC5) or an unspecific IgG, and then co-cultured with autologous lymphocytes stimulated or not with PWD (2.5 μg/mL) for 5 days. (b) Proliferation levels (CFSEdim) of CD8+ cells from HVs, stimulated or not with PWD and co-cultured for 5 days with autologous monocytes pre-challenged (grey filled columns) or not (white columns) with 10 ng/mL of LPS for 16 h. In some indicated conditions, a blocking antibody against SIGLEC5 (α-SIGLEC5) or an unspecific IgG was added (n = 3, left panel). Representative histogram overlay of proliferation levels (CFSEdim) of PWD-stimulated CD8+ cells from HVs, and co-cultured for 5 days with autologous monocytes pre-challenged with 10 ng/mL of LPS for 16 h, in presence or not of a blocking antibody against SIGLEC5, α-SIGLEC5 (right panel). (c) Scheme of the proliferation assay of monocytes nucleofected or not with a SIGLEC5 expression vector (pSIGLEC5) and then co-cultured with autologous lymphocytes stimulated or not with PWD (2.5 μg/mL) for 5 days. (d) MFI of SIGLEC5 on CD14+ cells 16 h after nucleofection with either an expression vector of SIGLEC5, (pSIGLEC5), or a control vector, pControl (n = 4) (left panel). Representative histogram overlay of SIGLEC5 expression on CD14+ cells 16 h after nucleofection with either an expression vector of SIGLEC5, pSIGLEC5 (blue histogram), or a control vector, pControl (white histogram) (right panel). (e) Proliferation levels (CFSEdim) of CD8+ cells from HV, stimulated or not with PWD and co-cultured for 5 days with autologous monocytes pre-nucleofected (blue filled column) or not (white column) with an expression vector of SIGLEC5 (pSIGLEC5) (n = 4) (left panel). Representative histogram overlay of proliferation levels (CFSEdim) of PWD-stimulated CD8+ cells from HVs, and co-cultured for 5 days with autologous monocytes pre-nucleofected (blue filled line) or not (white line) with pSIGLEC5 (right panel). (f) Scheme of the binding assay of recombinant sSIGLEC5r-FC to CD8+ T cells. (g) Quantification of sSIGLEC5r (sSIGLEC5r-FC) binding to CD8+ T cells from HVs pre-treated or not with Neuraminidase (NM, 0.01 U/mL) for 24 h (n = 6). Binding was revealed with α-FC-FITC and analyzed by flow cytometry, using the secondary antibody alone as control (left panel). Representative histogram overlay of sSIGLEC5r-FC binding to CD8+ cells from HVs pre-treated or not with NM (0.01 U/mL) for 24 h and unspecific control using the antibody α-FC-FITC only (vehicle, right panel). (h) Proliferation levels (CFSEdim) of CD8+ T cells from HVs in the presence or not of PWD and co-cultured for 5 days with autologous monocytes pre-nucleofected with an expression vector of SIGLEC5 (pSIGLEC5, blue filled columns) or an empty vector (pControl, white columns) (n = 6). In some conditions, a blocking antibody against PSGL1 (α-PSGL1) was added. Data shown as mean ± SEM. (b, d, e, g, and h) Paired t-test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
Since this activity resembles IC hallmarks, we performed a multiple sequence alignment analysis of the Ig-like V-type domain between SIGLEC5 and other known proteins postulated as ICs or IC ligands. As Supplementary Figure S3 illustrates, human SIGLEC5 and these proteins share a canonical Ig-like V-type ICs domain in its primary structure with pairwise identities and similarities that range from 14 to 25% and from 22 to 38%, respectively. This homology was even higher than that exhibited amongst ICs or IC ligands such as the B7 family of proteins, supporting the role of SIGLEC5 as IC or IC ligand.18,47,48
We next studied how SIGLEC5 interacts with T-cells (Fig. 2f). Fig. 2g shows the specific binding of the recombinant soluble SIGLEC5-FC protein (sSIGLEC5r-FC) to CD8+ T cells. Curiously, this binding was reduced in presence of neuraminidase (NM), a glycoside-hydrolase enzyme that cleaves the glycosidic linkage of neuraminic acids (Fig. 2g). It should be recalled that PSGL1 has been reported as a receptor for SIGLEC5 on T cells.33 In this context, a blocking antibody against P-Selectin Glycoprotein Ligand 1 (PSGL1) reverted CD8+ T cell proliferation when these cells were co-cultured with monocytes previously transfected in order to overexpress SIGLEC5 (Fig. 2h); however, this effect was not observed in CD4+ T cells (Supplementary Figure S2c). These results suggest that SIGLEC5 is acting as IC through PSGL1 engagement on CD8+ T cells.
Soluble SIGLEC5 specifically impairs CD8+, but not CD4+ T cell proliferation
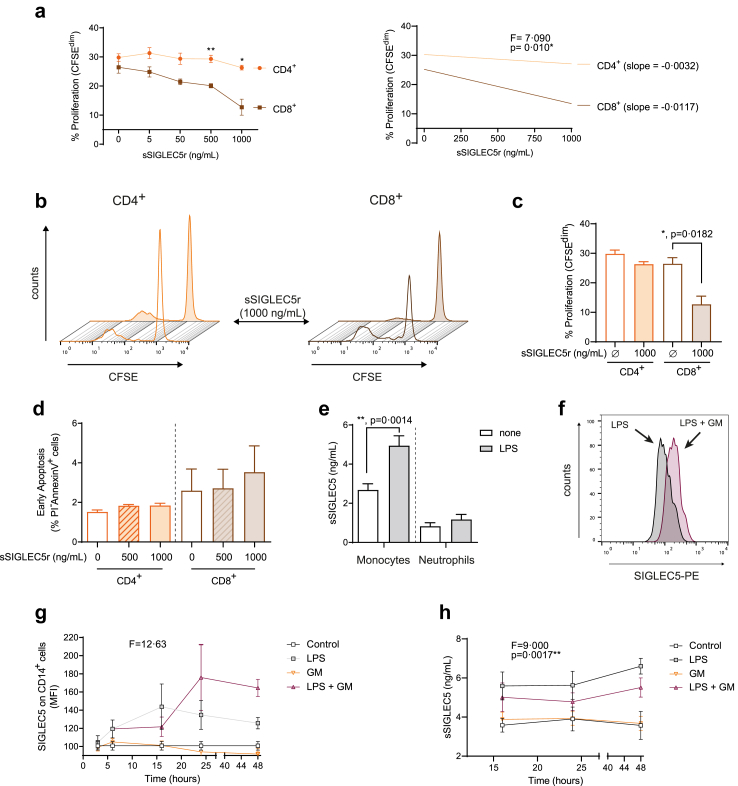
We extended the study of the modulation exerted by SIGLEC5 on the T cell response by looking at the potential role played by the soluble form of SIGLEC5 (sSIGLEC5) in this context. Thus, a soluble recombinant protein (sSIGLEC5r) reduced CD8+ but not CD4+ T cell proliferation in vitro in a dose-dependent manner (Fig. 3a). These differences increased in a sSIGLEC5r-dependent manner (Fig. 3a and c). Remarkably, we found no significant differences in apoptosis of any of the T cell subsets in these experiments (Fig. 3d). Note that sSIGLEC5 levels were higher in monocytes than neutrophils upon the same experimental conditions, both in steady-state (none) and after LPS stimulation, which reinforced our focus on monocytes (Fig. 3e).
Fig. 3.
Soluble SIGLEC5 impairs CD8+T cell proliferation. (a) Proliferation levels (percentage of CFSEdim cells) (left panel) and linear regression (right panel) of PWD-stimulated CD4+ (orange) and CD8+ (mocha) cells from HVs in presence of different concentrations of human recombinant sSIGLEC5r for 5 days (n = 5). (b) Representative histograms overlays of the proliferating CFSEdim PWD-stimulated CD4+ (left panel) and CD8+ (right panel) cells from HVs in presence (filled line) or not (clear line) of human sSIGLEC5r for 5 days. (c) Proliferation levels (percentage of CFSEdim cells) of PWD-stimulated CD4+ (orange) and CD8+ (mocha) T cells from HVs in presence or not of the highest concentration of sSIGLEC5r used (1000 ng/mL) for 5 days (n = 5). (d) Percentage of apoptotic (PI–/AnnexinV+) CD8+ T cells isolated from HV and incubated (filled column) or not (clear column) with sSIGLEC5r for 24 h at 500 and 1000 ng/mL (n = 5). (e) Soluble SIGLEC5 (sSIGLEC5) levels in supernatants of monocytes (n = 14) and neutrophils (n = 6) treated (grey filled column) or not (white column) with 10 ng/mL of LPS for 16 h. (f) Representative histogram overlay of SIGLEC5 expression on LPS-stimulated CD14+ cells in absence (grey filled) or presence (wine filled) of a pan-inhibitor of metalloproteinases (GM6001) added 6 h before the LPS stimulation for 16 h. (g) MFI of SIGLEC5 on CD14+ cells from HVs exposed to LPS (grey), GM6001 (orange), and combinations (wine) for the indicated times (n = 3). (h) sSIGLEC5 levels in the supernatants of monocytes from HVs exposed to LPS (grey), GM6001 (orange) and combinations (wine) for indicated times (n = 3). A control without any stimulation is represented in white. Data shown as mean ± SEM. (a, left panel) Two-way ANOVA test (∗∗p < 0.01). (a, right panel) Spearman linear regression test (∗p < 0.05). (c–e) Paired t-test (∗p < 0.05; ∗∗p < 0.01). (g and h) One-way ANOVA test (∗∗p < 0.01; ∗∗∗p < 0.001).
Eventually, as Fig. 3f–h illustrate, the presence of the pan-metalloproteinase inhibitor (GM6001) increased and stabilized SIGLEC5 expression on the surface of monocytes and reduced sSIGLEC5 levels in supernatants, pointing to enzymatic cleavage by metalloproteinases as the molecular mechanism for the generation of sSIGLEC5, as described for other membrane-anchored proteins such as TREM-1.49
SIGLEC5 reduces survival in mouse models of sepsis
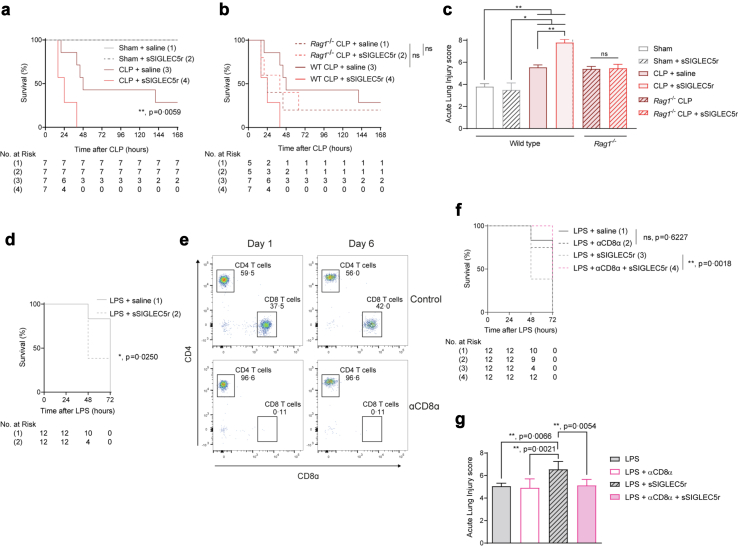
To better understand the role of SIGLEC5 in response to infection, two in vivo mouse models were assessed. First, we performed a cecal ligation and puncture (CLP) protocol where wild-type (WT) and Rag1−/− mice were simultaneously administered with either sSIGLEC5 or saline intraperitoneally. Compared with CLP mice injected with saline, CLP mice treated with sSIGLEC5r displayed a reduced survival (χ2 = 7.591, p = 0.0059, Hazard ratio CLP + sSIGLEC5r vs. CLP + saline of 3.521; 95% CI, 1.005–12.34, Fig. 4a). However, this effect was not observed in Rag1−/− mice, which lack mature B and T cells, where sSIGLEC5r administration did not influence on the survival rate (χ2 = 0.064, p = 0.801 (non-significant), Hazard ratio Rag1−/− CLP + sSIGLEC5r vs. Rag1−/− CLP + saline of 0.844; 95% CI, 0.210–3.382, Fig. 4b). No statistically significant differences were found in survival rates between wild-type and Rag1−/− mice after CLP model (χ2 = 0.910, p = 0.340 (non-significant), Hazard ratio WT CLP + saline vs. Rag1−/− CLP + saline of 1.830; 95% CI, 0.442–7.569, Fig. 4b). This suggests that sSIGLEC5r suppressive effect is dependent on adaptive response, even though this immunity was not required for the CLP model.
Fig. 4.
SIGLEC5 reduces survival in endotoxemia mouse models in a CD8α-dependent manner. (a) Kaplan–Meier estimation of survival from CLP-mice injected or not with sSIGLEC5r (χ2 = 33.20, p < 0.0001 for all four groups comparison: Sham, Sham + sSIGLEC5r, CLP + saline and CLP + sSIGLEC5r; χ2 = 7.591, p = 0.0059, hazard ratio [HR] CLP plus sSIGLEC5r vs. CLP groups comparison of 8.302; 95% CI, 1.842–37.42) (n = 7 per group). (b) Kaplan–Meier estimation of survival from CLP-WT or CLP-Rag1−/− mice injected or not with sSIGLEC5r (χ2 = 0.064, p = n.s., Hazard ratio [HR] Rag1−/− CLP vs. Rag1−/− CLP + sSIGLEC5 of 0.828, 95% CI, 0.191–3.582) (n = 5 per group in WT mice and n = 7 per group in Rag1−/− mice). (c) Acute lung injury evaluated on Haematoxylin/Eosin-stained lung sections of Sham or CLP-mice in the background wild-type or Rag1−/− injected or not with sSIGLEC5r (n = 7 per group). (d) Kaplan–Meier estimates of survival from mice injected with sSIGLEC5r and/or LPS (χ2 = 5.026, p = 0.0250, HR of 6.019, 95% CI, 1.253–28.91) (n = 12 per group). (e) Flow cytometry-gating strategy for CD4+ and CD8α+ T cells in blood from control and CD8α-depleted mice at day 1 and 6 after injection of a depleting CD8α antibody (20 μg/mice). (f) Kaplan–Meier estimates of survival from mice injected with LPS (20 mg/kg) with or without CD8α depletion (χ = 0.242, p = 0.6227, HR of 0.920, 95% CI, 0.413–2.049) or injected with LPS and sSIGLEC5r with or without CD8α depletion (χ2 = 9.731, p = 0.0018, HR of 14.21, 95% CI, 2.681–75.29) (n = 12 per group). (g) Acute lung injury evaluated on Haematoxylin/Eosin stained lung sections of the endotoxemia mouse model with LPS injection (20 mg/mL), CD8α-depleted or not, and injected or not with sSIGLEC5r (n = 12 per group). (a, b, and d–f) Kaplan–Meier estimation of survival test (∗p < 0.05; ∗∗p < 0.01). (c and g) Data shown as mean ± SEM. Paired t-test (ns, non-significant; ∗∗p < 0.01).
Through haematoxylin/eosin staining, we corroborated a substantial lung injury caused by CLP in both WT and Rag1−/− mice reaching comparable damage. The lung insult worsened when mice were additionally injected with sSIGLEC5r in WT but not Rag1−/− mice (Fig. 4c and Supplementary Figure S4a and b). Together, these findings suggested that sSIGLEC5 modulates the severity of sepsis relying on lymphocytes.
In addition, mortality was also evaluated in the context of endotoxin challenge using lipopolysaccharide (LPS) from E. coli. Fig. 4d shows that mice treated simultaneously with LPS and sSIGLEC5r died earlier than those challenged only with LPS (χ2 = 5.432, p = 0.019, Hazard ratio LPS + sSIGLEC5r vs. LPS + saline of 7.123; 95% CI, 1.366–37.13). However, this effect was reversed in mice administered with a CD8α-depleting antibody, considering that there was not differences between LPS + saline vs. LPS + αCD8α (Fig. 4e and f). Moreover, haematoxylin/eosin staining of lungs sections showed that sSIGLEC5r increased acute lung injury caused by LPS (Fig. 4g and Supplementary Figure S4c), which regressed in CD8α-depleted mice. These results highlight the impact of sSIGLEC5 on the mortality of sepsis and its dependence on CD8+ T cells.
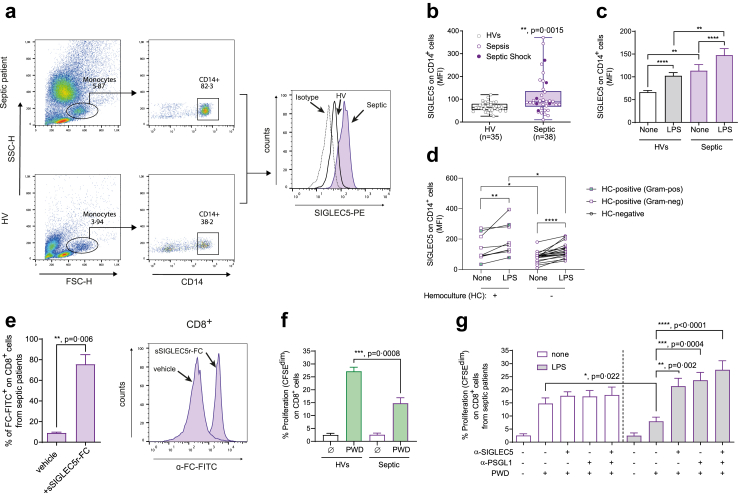
Monocyte from septic patients modulate CD8+ T cell proliferation thought SIGLEC5
To explore the impact of these results in patients with sepsis, we evaluated SIGLEC5 expression on blood monocytes from septic patients at the time of admission and prior to any treatment to compare it with healthy volunteers (HVs). Septic patients (sepsis or septic shock) exhibited higher SIGLEC5 expression on their circulating monocytes than HVs (Fig. 5a and b), which could be further increased after ex vivo LPS stimulation (Fig. 5c). Interestingly, septic patients with positive bacterial haemocultures showed higher expressions of SIGLEC5 on CD14+ cells than those with negative bacterial haemocultures. Moreover, even in those haemoculture-positive septic patients, the ex vivo LPS stimulation further increase SIGLEC5 expression (Fig. 5d). As expected, SIGLEC5 not only binds CD8+ T cells from HVs (Fig. 2g) but also those from septic patients (Fig. 5e). Accordingly, CD8+ lymphocytes from septic patients showed an impaired proliferation after PWD stimulation compared to HVs (Fig. 5f). This diminished CD8+ T cell proliferation was even more accused when monocytes were stimulated ex vivo with LPS, which was significantly reverted in presence of either anti-SIGLEC5 or anti-PSGL1 blocking antibodies (Fig. 5g). Despite CD4+ cells from septic patients bound sSIGLEC5r-FC, their proliferation was not affected (Supplementary Figure S2d and e).
Fig. 5.
Septic patients overexpress SIGLEC5 on monocytes, and blockade of the SIGLEC5/PSGL1 axis restores CD8+T cell proliferation. (a) Flow cytometry gating strategy for CD14+ cells from both HVs and septic patient (left panels). Representative histogram overlay of SIGLEC5 expression on CD14+ cells from a HVs (white line) and a septic patient (violet filled line); a dotted line was used as isotype control (right panel). (b) MFI of SIGLEC5 on CD14+ cells from HVs (grey clear box, n = 35) and septic patients (violet filled box, n = 38). (c) SIGLEC5 expression on monocytes from HVs (grey) and septic patients (violet) challenged or not with 10 ng/mL of LPS for 16 h. (d) SIGLEC5 expression on monocytes from hemoculture-positive (n = 9) and –negative (n = 19) septic patients challenged or not with 10 ng/mL of LPS for 16 h (Violet dots: Hemoculture-negative, violet squares: Hemoculture-positive with gram-negative bacteria, violet squares green filled: Hemoculture-positive with gram-positive bacteria). (e) sSIGLEC5r-FC binding quantification on CD8+ T cells from patients with sepsis (n = 4) (left panel) and a representative histogram overlay (right panel). (f) Proliferation levels (CFSEdim) of CD8+ T cells from HVs and patients with sepsis in the presence (green filled column) or not (white column) of PWD for 5 days. (g) Proliferation levels (CFSEdim) of CD8+ T cells from patients with sepsis in the presence or not of PWD and co-cultured for 5 days with autologous monocytes pre-challenged (grey filled column) or not (white column) with 10 ng/mL of LPS for 16 h in the presence of anti-SIGLEC5 blocking antibody and/or anti-PSGL1 blocking antibody (n = 6). Data shown as mean ± SEM. (b–d and f) Unpaired t-test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001). (c–e and g) Paired t-test (∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
SIGLEC5 is a biomarker of prognosis in septic patients
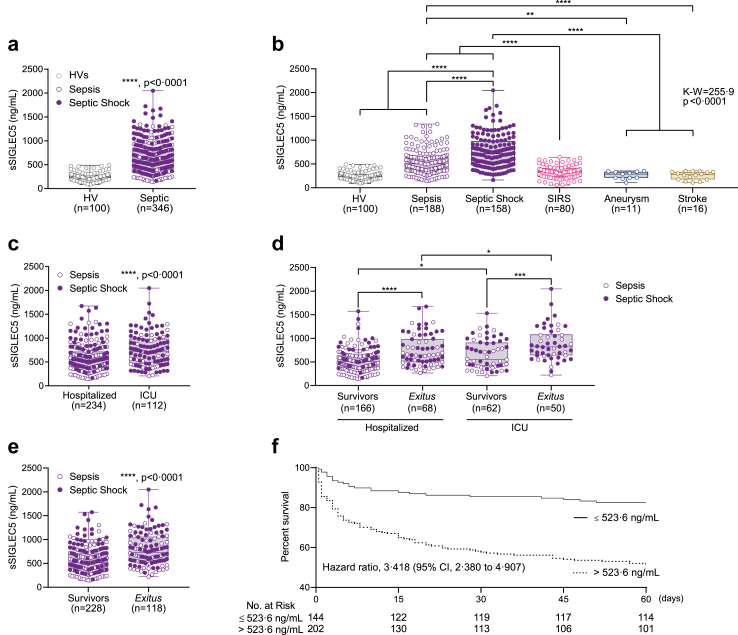
Considering the observed role for SIGLEC5 in monocytes from septic patients, and the presence of its soluble form cleaved from the membrane by metalloproteinases (Fig. 3), we sought to investigate the performance of sSIGLEC5 as a clinical biomarker. Thus, a total of 425 patients with systemic inflammatory response criteria were studied. Patients were classified as septic (44%), septic-shock (37.2%) and non-infectious SIRS (18.8%) according to their clinical parameters and severity.34,35 They were followed up for 60 days to establish their survivor or exitus condition (Table 1). As controls, HVs and also patients suffering aneurysm or stroke were assessed (Supplementary Table S1).
Higher levels of sSIGLEC5 were detected in plasma from septic patients (a multicentre cohort) compared with HVs (Fig. 6a). Moreover, once septic patients were further classified according to their severity into sepsis and septic shock,34 the latter presented increased levels sSIGLEC5 in their plasma than the former (Fig. 6b). Furthermore, we observed that the levels of sSIGLEC5 were also higher than those of patients with SIRS or non-infectious diseases such as aneurysm or stroke (Fig. 6b). On the one hand, when septic patients classified according to whether they required admission to the ICU, levels of sSIGLEC5 were higher in those patients requiring intensive care (Fig. 6c), with greater values in those patients with the worst outcome (Fig. 6d). On the other hand, septic patients were classified according to their evolution into survivors or exitus, observing higher levels of sSIGLEC5 in exitus than survivor patients (Fig. 6e). To differentiate between both outcomes, a receiver-operating characteristic (ROC) analysis of plasmatic sSIGLEC5 levels was performed (Supplementary Figure S5). The area under the curve (AUC) for exitus was 0.713 (95% confidence interval [CI], 0.656–0.769; p < 0.0001), while the AUC for their admission to ICU was 0.642 (95% confidence interval [CI], 0.577–0.706; p < 0.0001). The optimal cut-offs, estimated by Youden index, were 523.6 ng/mL and 663.2 ng/mL, respectively, indicating that patients with a circulating sSIGLEC5 level above both Youden indexes exhibit a poor outcome (Supplementary Figure S5a and b). We evaluated the predictive power of sSIGLEC5 in comparison to commonly used clinical indicators such as C-reactive protein and procalcitonin, in order to assess its advantage as an indicator of severity and mortality. Supplementary Figure S5c demonstrates that the predictive power of sSIGLEC5 is significantly higher than that of the other clinical indicators. In addition, sSIGLEC5 showed a weak positive correlation with lactate and moderate positive correlations with clinical prognosis indicators such as APACHE-II and SOFA scores (Supplementary Figure S5d). Kaplan–Meier survival curve showed a significant decrease in the survival of septic patients with levels of plasmatic sSIGLEC5 above the calculated cut-off (Hazard Ratio [HR] of 3.418, 95% CI, 2.380–4.907, p < 0.0001 by log-rank test, Fig. 6f). Collectively, these data illustrate that plasma concentrations of sSIGLEC5 increase in septic patients compared with non-infectious health conditions, while acting as a severity and exitus predictor in septic patients.
Fig. 6.
Soluble SIGLEC5 classified septic patients on admission. (a) Soluble SIGLEC5 (sSIGLEC5) levels in plasma from HVs (grey clear dots, n = 100) and septic patients (sepsis in violet clear dots and shock septic in purple filled dots, n = 346). (b) sSIGLEC5 levels in plasma from HVs (n = 100) and patients with sepsis (n = 188), septic shock (n = 158), SIRS (n = 80), aneurysm (n = 11) and stroke (n = 16). (c) sSIGLEC5 levels in plasma from septic patients classified according to their hospitalization (n = 234) or their admission to ICU (n = 112). (d) sSIGLEC5 levels in plasma from septic patients hospitalized survivors (n = 166), hospitalized exitus (n = 68), admitted in ICU survivors (n = 62) and admitted in ICU exitus (n = 50). (e) sSIGLEC5 levels in plasma from patients with sepsis (violet clear dots) and septic shock (purple filled dots) classified according to their outcome after 60 days as Survivors (n = 228) and Exitus (n = 118). (f) Septic patients were dichotomized according to the optimal cut-off, estimated by Youden index for plasmatic sSIGLEC5 concentration to be 523.6 ng/mL. Kaplan–Meier survival curves from diagnosis to day 60 according to baseline plasmatic sSIGLEC5 (χ2 = 33.20, p < 0.0001) are shown. Data shown as mean ± SEM. (a and c–e) Unpaired t-test (∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001). (b) One-way ANOVA test (∗∗∗∗p < 0.0001). (f) Kaplan–Meier estimation of survival from septic patients according Youden index (∗∗∗∗p < 0.0001).
Discussion
We report SIGLEC5 is mainly expressed in myeloid cells, including neutrophils, monocytes, dendritic cells and at low levels in B cells, as it has been previously described in the Human Atlas Protein database43 and some other reports.50,51 However, SIGLEC5 expression is only upregulated on circulating neutrophils and monocytes when they are stimulated with LPS from E. coli, simulating an encounter with Gram-negative bacteria. This data matched the high levels of SIGLEC5 expression on circulating monocytes during sepsis, suggesting a critical role in this clinical condition. Nevertheless, neutrophils express the highest levels of SIGLEC5, being the most frequent cell type in circulation and their numbers increased during sepsis.52 Note that, although the basal expression of SIGLEC5 on neutrophils is higher than on monocytes, this relationship is reversed in the presence of LPS. Moreover, the interaction of monocytes with T cells during the antigen presentation and the regulation of T cell proliferation by them makes monocytes the best candidate for studying the role of SIGLEC5 in the context of infectious diseases.
We have previously demonstrated the involvement of HIF1α in the regulation of the innate and adaptive immune response during sepsis under hypoxic stress, where blood oxygen saturation and tissue perfusion can be compromised, controlling the transcription of a set of genes including IRAK-M, VEGFA, and MMP.10,11,44 Herein, we demonstrated the presence of three HREs in the human SIGLEC5 promoter, and our data confirmed that this gene upregulation in response to LPS is mediated by HIF1α. Furthermore, SIGLEC5 is cleaved by metalloproteinases, generating a soluble form of this protein, which is significantly upregulated in supernatants of LPS-stimulated monocytes but not in neutrophils.
By exploring the potential role of SIGLEC5 in the regulation of lymphocyte functionality during an infectious process, our findings indicated that the binding of SIGLEC5 to CD8+ cells is PSGL1-dependent. The latter had previously been described as an immunoglobulin-like cell adhesion molecule that promotes CD8+ T-cell exhaustion in a murine model of chronic virus infection.33 However, its ligand was unknown until recently.32 Our data suggest SIGLEC5 behaves as a ligand for PSGL1, whose binding relies on sialic acid motifs/residues. Moreover, blockade of the SIGLEC5/PSGL1 axis was able to revert the impaired CD8+ proliferation without affecting their apoptosis, suggesting this could be a potential pharmacological target in sepsis.
Previous work has focused on the role of SIGLEC5 expressed on lymphocytes, where its expression is triggered upon TCR activation and dampens immune activation.53 Here, in line with its structural similarities to well-known immunoglobulin-like cell adhesion molecules, we showed that CD8+ T lymphocyte proliferation could be modulated by sSIGLEC5 or membrane-bound SIGLEC5 on monocytes. Curiously, several authors have indicated that the number of these effector cytotoxic cells decreases in patients with septic-shock on admission.54 It is widely accepted that the composition of the naïve pathogen-specific CD8+ cell repertoire is essential for both the clearance of infection and the generation of memory CD8+ cells in response to pathogens.55 This is compatible with SIGLEC5 providing a negative signal for migration of leukocytes to the infected sites, contributing to the immunosuppression stage of sepsis.33 Thus, an impaired CD8+ T cell proliferation together with their low viability can compromise the recovery of septic patients, increasing their risk of suffering from secondary infections and death.
Regarding mouse models used, both endotoxemia and cecal ligation and puncture (CLP) are considered gold standards in sepsis research,56 with similar mortality rates, but with significant differences in the kinetics and magnitude of cytokine storms. In this context, exogenous sSIGLEC5r has been observed to increase mortality and acute lung injury in both CLP and mice administered with LPS from E. coli. Interestingly, when lymphocyte-deficient mice (Rag1−/−) were subjected to both CLP and injected with exogenous sSIGLEC5r, they did not exhibit any differences in mortality rate. Furthermore, CD8α depletion was found to protect mice administered with LPS and sSIGLEC5r, thus preventing the accelerated death observed in this model. Thus, it can be concluded that the impact of sSIGLEC5r on the mortality of endotoxin mouse models is dependent on the presence of lymphocytes, specifically on CD8α T cells.
The data from in vivo experiments suggest that the suppression induced by sSIGLEC5r depends on an adaptive immune response, although this response is not necessary for the CLP model. This concept has been observed in other inflammatory settings57 where the inhibition of a specific protein expressed in a particular population produces a suppressive effect, although the absence of this population does not have any observable effect.
It is important to note the absence of B and T cells (both CD4 and CD8) in Rag1−/−. We hypothesize that the effects of these populations on CLP-induced polymicrobial sepsis are offsetting one another. Our first CLP in vivo model supported the impact of sSIGLEC5 on adaptive immunity (Fig. 4a–c), leading us to develop a more precise and regulated in vivo model involving the administration of LPS and specific depletion of CD8α T cells (Fig. 4d–g). The survival rates between LPS + saline and LPS + anti-CD8α in this model were not significantly different (Fig. 4f), demonstrating the redundancy of CD8α T cells in LPS-induced mortality. However, this model also confirmed the specific targeting of sSIGLEC5r on these cells, as the inhibitory effect of sSIGLEC5r was eliminated when CD8α T cells were depleted (Fig. 4e–g).
Unfortunately, SIGLEC5 does not have a homolog in mice. Both potential candidates, SIGLEC-F and SIGLEC-E, have insufficient levels of identity with the human SIGLEC5. SIGLEC-F shares 45.3% of the sequence with human SIGLEC5, but is mainly expressed in eosinophils in mice, which implies that its functional counterpart is human SIGLEC8. In contrast, SIGLEC-E is thought to be a functional human paralogue of SIGLEC9, primarily expressed on NK cells, or SIGLEC12 due to its high homology with SIGLEC5 (52.4%). This means we are not able to use an anti-SIGLEC5 antibody in mice models of endotoxemia or CLP.
In vivo results support the specific effect observed in vitro on human cells, where monocyte-expressed SIGLEC5 or its soluble form is found to attenuate CD8+ T cell proliferation. Collectively, these outcomes suggest that SIGLEC5 could be functioning as an immunoregulatory ligand.
The relevance of all these experimental data was reflected in the clinic. Monocyte-bound and soluble SIGLEC5 levels in plasma are increased in septic patients but not in patients with non-infectious Systemic Inflammatory Response Syndrome (SIRS), aneurysm or stroke, or healthy donors. It is worth noting that non-infectious SIRS patients suffer from a pro-inflammatory phenotype that is not produced by any endotoxin, such as burn patients, trauma, or ischemia, which suggests that the SIGLEC5 induction is LPS-dependent or involves pathways with similar downstream signals. Interestingly, the expression of SIGLEC5 on CD14+ cells was markedly higher in septic patients with positive bacterial haemocultures than in those with negative bacterial haemocultures. Moreover, ex vivo LPS stimulation further augmented SIGLEC5 expression in the haemoculture-positive septic patients, implying that SIGLEC5 has a broad range of expression which spans from untreated healthy volunteers to LPS-treated septic patients, as shown in Fig. 4c. In addition, plasma levels of soluble SIGLEC5, generated by metalloproteinase shedding, are increased in septic patients compared to healthy volunteers (HVs), with higher levels in septic shock than sepsis patients. We emphasize that the above was demonstrated using a multicentre cohort of patients. It could additionally serve as a biomarker of sepsis evolution, being higher in patients with poor prognosis. Note that the soluble form of SIGLEC5 has been identified by ourselves as a prognosis indicator in some cancer contexts, confirming it can be used as plasmatic biomarker in several diseases with immune component associated.58,59
Along these lines, ROC analysis showed a robust AUC for sSIGLEC5 as predictor of requirement of admission to the ICU and as an exitus predictor in sepsis. Thereby, the optimal cut-off values, calculated based on the Youden index, revealed that sSIGLEC5 plasma levels on admission and before any treatment, indicated a poor prognosis of septic patients. These data could constitute a feasible score to incorporate into routine analysis of patients achieving sepsis criteria upon arrival in hospital admission.
Therefore, we have reported an IC ligand, transcriptionally regulated by HIF1α, which could be therapeutically targeted in septic patients. Our results show SIGLEC5 is acting as an IC ligand on T cell lymphocytes through PSGL1, especially on CD8+ cells, which could be potentially blocked with anti-SIGLEC5, anti-PSGL1 antibodies or other small molecules. Furthermore, two mouse models of sepsis show that soluble SIGLEC5 decreases survival in a CD8α-dependent manner. In terms of clinical application, one or the first questions to emerge would be identifying which septic patients could benefit from the blockade of the SIGLEC5/PSGL1 axis. All in all, our data support that the determination of sSIGLEC5 at admission could be used as prognosis biomarker as well as the therapeutic potential of SIGLEC5/PSGL1 blocking strategies in sepsis.
Contributors
López-Collazo, E. performed the conceptualisation of the study. Lozano-Rodríguez, R. and Avendaño-Ortíz, J. performed the main experiments and investigation. Pelegrin, P., García-Palenciano, C., Ruiz-Rodríguez, J.C., Ferrer, R., González-López, J.J., Fàbrega, A., Martín-Quirós A., and Maroun-Eid, Ch. recruited the septic patients and followed-up them. Gutiérrez-Fernández, M. and Alonso-López, E. recruited patients with stroke. Cubillos-Zapata, C. contributed samples from patients with aneurysms. Lozano-Rodríguez, R., Montalbán-Hernández, K., Terrón-Arcos V., and Pérez de Diego R. performed specific in vitro experimental assays. Lozano-Rodríguez, R., del Fresno, C., Terrón-Arcos, V., and Avendaño-Ortíz, J. performed in vivo assays. Lozano-Rodríguez R. and Avendaño-Ortíz, J. performed statistical analyses. López-Collazo, E., del Fresno, C., Lozano-Rodríguez, R., Cueto, F.J., and Avendaño-Ortíz, J. discussed the results. López-Collazo, E. and Lozano-Rodríguez, R. wrote the manuscript. del Fresno, C., Cueto, F.J., Pérez de Diego, R., Pelegrin, P., and Fernández-Velasco, M. performed a critical review of the manuscript. All authors read and approved the final version of the manuscript. In addition, the authors declare no competing financial interests. Note that more than one author has accessed and verified the data, in this case: Lozano-Rodríguez, R., Avendaño-Ortíz, J., del Fresno, C., and López-Collazo, E.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of interests
We declare that none of the authors have any conflicts of interest with the content of this manuscript.
Acknowledgements
We acknowledge the Sepsis Bank of Vall d’Hebron University Hospital Biobank (Barcelona, Spain) and Virgen de la Arrixaca University Clinical Hospital (“Biobanco en Red de la Región de Murcia” Murcia, Spain) for plasma samples. Authors thank Emilio Llanos for his technical assistance.
This work was supported by grants from Instituto de Salud Carlos III (ISCIII) and “Fondos FEDER” to ELC (PIE15/00065, PI18/00148, PI14/01234, PI21/00869), to PP (20859/PI/18) and to CdF (PI21/01178), and received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowaska-Curie grant agreement to KMH (No. 713673; “laCaixa”). R.L.-R. was supported by “Predoctotales de formación en Investigación” (PFIS) grant FI19/00334 and J.A.-O. by Sara Borrell grant CD21/00059 from ISCIII. The Vall d'Hebron University Hospital and Vall d’Hebron Research Institute were supported by Plan Nacional de I+D+i 2013–2016, the ISCIII and Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0003)—co-financed by European Development Regional Fund “A way to achieve Europe”, and by the European Union’s Horizon 2020 Research and Innovation Program (JCRR, RF, JJGL, AF). Authors thank Emilio Llanos for his technical assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104841.
Appendix A. Supplementary data
References
- 1.Vincent J.-L., Marshall J.C., Ñamendys-Silva S.A., et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 2.Biswas S.K., Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss R.S., Coopersmith C.M., McDunn J.E., Ferguson T.A. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang B.M., Huang S.J., McLean A.S. Genome-wide transcription profiling of human sepsis: a systematic review. Crit Care. 2010;14 doi: 10.1186/cc9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boomer J.S., To K., Chang K.C., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachot A., Lepape A., Vey S., Bienvenu J., Mougin B., Monneret G. Systemic transcriptional analysis in survivor and non-survivor septic shock patients: a preliminary study. Immunol Lett. 2006;106:63–71. doi: 10.1016/j.imlet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Delano M.J., Ward P.A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Collazo E., Gómez-Piña V., Arnalich F. Understanding immune dysfunctions in sepsis patients. Crit Care. 2010;14:435. doi: 10.1186/cc9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen I.J., Sjaastad F.V., Griffith T.S., Badovinac V.P. Sepsis-induced T cell immunoparalysis: the Ins and outs of impaired T cell immunity. J Immunol. 2018;200:1543–1553. doi: 10.4049/jimmunol.1701618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avendaño-Ortiz J., Maroun-Eid C., Martín-Quirós A., et al. Oxygen saturation on admission is a predictive biomarker for PD-L1 expression on circulating monocytes and impaired immune response in patients with sepsis. Front Immunol. 2018;9:2008. doi: 10.3389/fimmu.2018.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avendaño-Ortiz J., Maroun-Eid C., Martín-Quirós A., et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α. J Infect Dis. 2018;217:393–404. doi: 10.1093/infdis/jix279. [DOI] [PubMed] [Google Scholar]
- 12.Tai H., Xing H., Xiang D., et al. Monocyte programmed death ligand-1, a predicator for 28-day mortality in septic patients. Am J Med Sci. 2018;355:362–367. doi: 10.1016/j.amjms.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J.K., Zhao Y., Singer M., Spencer J., Shankar-Hari M. Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis - pilot study. Crit Care. 2018;22:95. doi: 10.1186/s13054-018-2020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraschilla I., Pillai S. Viewing Siglecs through the lens of tumor immunology. Immunol Rev. 2017;276:178–191. doi: 10.1111/imr.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanczak M.A., Siddiqui S.S., Trefny M.P., et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest. 2018;128:4912–4923. doi: 10.1172/JCI120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lübbers J., Rodríguez E., van Kooyk Y. Modulation of immune tolerance via Siglec-sialic acid interactions. Front Immunol. 2018;9:2807. doi: 10.3389/fimmu.2018.02807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macauley M.S., Crocker P.R., Paulson J.C. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascolutti R., Sun X., Kao J., et al. Structure and dynamics of PD-L1 and an ultra-high-affinity PD-1 receptor mutant. Structure. 2016;24:1719–1728. doi: 10.1016/j.str.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y., Lan C., Ren D., Chen G.-Y. Induction of Siglec-1 by endotoxin tolerance suppresses the innate immune response by promoting TGF-β1 production. J Biol Chem. 2016;291:12370–12382. doi: 10.1074/jbc.M116.721258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y.-N., Cai X., Zhou H.-M., et al. Diagnostic and prognostic roles of soluble CD22 in patients with Gram-negative bacterial sepsis. Hepatobiliary Pancreat Dis Int. 2015;14:523–529. doi: 10.1016/S1499-3872(15)60394-0. [DOI] [PubMed] [Google Scholar]
- 21.Gjertsson I., Nitschke L., Tarkowski A. The role of B cell CD22 expression in Staphylococcus aureus arthritis and sepsis. Microb Infect. 2004;6:377–382. doi: 10.1016/j.micinf.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Boyd C.R., Orr S.J., Spence S., et al. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J Immunol. 2009;183:7703–7709. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson H.N., Mills D.C., Jones H., et al. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel Flagellin-host interaction. J Infect Dis. 2014;210:1487–1498. doi: 10.1093/infdis/jiu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W., Han C., Xie B., et al. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Carlin A.F., Chang Y.-C., Areschoug T., et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206:1691–1699. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali S.R., Fong J.J., Carlin A.F., et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211:1231–1242. doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanaka M., Kato Y., Angata T., Narimatsu H. Deletion polymorphism of SIGLEC14 and its functional implications. Glycobiology. 2009;19:841–846. doi: 10.1093/glycob/cwp052. [DOI] [PubMed] [Google Scholar]
- 28.Cornish A.L., Freeman S., Forbes G., et al. Characterization of Siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. doi: 10.1182/blood.V92.6.2123. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman-Van der Linden E.C.M., Sjoberg E.R., Juneja L.R., Crocker P.R., Varki N., Varki A. Loss of N-glycolylneuraminic acid in human evolution. J Biol Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y.-C., Yu M.-M., Chai Y.-F., Shou S.-T. Sialic acids in the immune response during sepsis. Front Immunol. 2017;8:1601. doi: 10.3389/fimmu.2017.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong J.J., Sreedhara K., Deng L., et al. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J. 2015;34:2775–2788. doi: 10.15252/embj.201591407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinoco R., Carrette F., Barraza M.L., et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. 2016;44:1190–1203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepin M., Mezouar S., Pegon J., et al. Soluble Siglec-5 associates to PSGL-1 and displays anti-inflammatory activity. Sci Rep. 2016;6 doi: 10.1038/srep37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy M.M., Fink M.P., Marshall J.C., et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 36.del Fresno C., García-Rio F., Gómez-Piña V., et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol. 2009;182:6494–6507. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- 37.Jung S.-H. Stratified Fisher’s exact test and its sample size calculation: stratified Fisher’s exact test. Biom J. 2014;56:129–140. doi: 10.1002/bimj.201300048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cubillos-Zapata C., Hernández-Jiménez E., Toledano V., et al. NFκB2/p100 is a key factor for endotoxin tolerance in human monocytes: a demonstration using primary human monocytes from patients with sepsis. J Immunol. 2014;193:4195–4202. doi: 10.4049/jimmunol.1400721. [DOI] [PubMed] [Google Scholar]
- 39.Varshney S., Stanley P. Notch ligand binding assay using flow cytometry. Bio Protoc. 2017;7 doi: 10.21769/BioProtoc.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuenca A.G., Delano M.J., Kelly-Scumpia K.M., Moldawer L.L., Efron P.A. Cecal ligation and puncture. Curr Protoc Immunol. 2010;91:310–313. doi: 10.1002/0471142735.im1913s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matute-Bello G., Winn R.K., Jonas M., Chi E.Y., Martin T.R., Liles W.C. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratner B. The correlation coefficient: its values range between +1/−1, or do they? J Target Meas Anal Mark. 2009;17:139–142. doi: 10.1057/jt.2009.5. [DOI] [Google Scholar]
- 43.Monaco G., Lee B., Xu W., et al. RNA-seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 2019;26:1627–1640.e7. doi: 10.1016/j.celrep.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shalova I.N., Lim J.Y., Chittezhath M., et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity. 2015;42:484–498. doi: 10.1016/j.immuni.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-García J.J., Martínez-Banaclocha H., Angosto-Bazarra D., et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun. 2019;10:2711. doi: 10.1038/s41467-019-10626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao T., Ren H., Jia L., et al. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6:2250–2262. doi: 10.18632/oncotarget.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schönfeld D., Matschiner G., Chatwell L., et al. An engineered lipocalin specific for CTLA-4 reveals a combining site with structural and conformational features similar to antibodies. Proc Natl Acad Sci U S A. 2009;106:8198–8203. doi: 10.1073/pnas.0813399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lines J.L., Pantazi E., Mak J., et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-Piña V., Soares-Schanoski A., Rodríguez-Rojas A., et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 50.Lock K., Zhang J., Lu J., Lee S.H., Crocker P.R. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Crocker P.R., Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001;22:337–342. doi: 10.1016/S1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 52.Shen X., Cao K., Zhao Y., Du J. Targeting neutrophils in sepsis: from mechanism to translation. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.644270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vuchkovska A., Glanville D.G., Scurti G.M., et al. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology. 2022;166:238–248. doi: 10.1111/imm.13470. [DOI] [PubMed] [Google Scholar]
- 54.Danahy D.B., Strother R.K., Badovinac V.P., Griffith T.S. Clinical and experimental sepsis impairs CD8 T-cell-mediated immunity. Crit Rev Immunol. 2016;36:57–74. doi: 10.1615/CritRevImmunol.2016017098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Condotta S.A., Rai D., James B.R., Griffith T.S., Badovinac V.P. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seemann S., Zohles F., Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci. 2017;24:60. doi: 10.1186/s12929-017-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.del Fresno C., Saz-Leal P., Enamorado M., et al. DNGR-1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science. 2018;362:351–356. doi: 10.1126/science.aan8423. [DOI] [PubMed] [Google Scholar]
- 58.Montalbán-Hernández K., Cantero-Cid R., Lozano-Rodríguez R., et al. Soluble SIGLEC5: a new prognosis marker in colorectal cancer patients. Cancers. 2021;13:3896. doi: 10.3390/cancers13153896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montalbán-Hernández K., Casalvilla-Dueñas J.C., Cruz-Castellanos P., et al. Identification of sSIGLEC5 and sLAG3 as new relapse predictors in lung cancer. Biomedicines. 2022;10:1047. doi: 10.3390/biomedicines10051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.