Abstract
Small cell lung cancer (SCLC) is a neuroendocrine tumor with a high degree of malignancy. Due to limited treatment options, patients with SCLC have a poor prognosis. We have found, however, that intravenously administered octreotide (Oct) armed with astatine-211 ([211At]SAB-Oct) is effective against a somatostatin receptor 2 (SSTR2)-positive SCLC tumor in SCLC tumor-bearing BALB/c nude mice. In biodistribution analysis, [211At]SAB-Oct achieved the highest concentration in the SCLC tumors up to 3 h after injection as time proceeded. A single intravenous injection of [211At]SAB-Oct (370 kBq) was sufficient to suppress SSTR2-positive SCLC tumor growth in treated mice by inducing DNA double-strand breaks. Additionally, a multitreatment course (370 kBq followed by twice doses of 370 kBq for a total of 1110 kBq) inhibited the growth of the tumor compared to the untreated control group without significant off-target toxicity. Surprisingly, we found that [211At]SAB-Oct could up-regulate the expressions of calreticulin and major histocompatibility complex I (MHC-I) on the tumor cell membrane surface, suggesting that α-particle internal irradiation may activate an endogenous antitumor immune response through the regulation of immune cells in the tumor microenvironment, which could synergically enhance the efficacy of immunotherapy. We conclude that [211At]SAB-Oct is a potential new therapeutic option for SSTR2-positive SCLC.
Keywords: small cell lung cancer, somatostatin receptor 2, astatine-211, octreotide, immune response
1. Introduction
Although small cell lung cancer (SCLC) is highly sensitive to chemotherapeutic agents at the beginning of treatment, it is characterized by rapid recurrence, early widespread metastasis, and poor prognosis.1,2 As the basic treatment of SCLC, systemic chemotherapy has reached the therapeutic plateau, and more effective treatment plans for SCLC are urgently needed.3,4 Highly expressed somatostatin receptor (SSTR) on the surface of most SCLC cells provides an important target for nuclear medicine polypeptide-receptor-mediated radionuclide therapy (PRRT). PRRT targeting SSTR offers hope for patients with advanced SCLC who have lost the opportunity for surgery and failed to respond to other therapies.5−7
Octreotide is an octopeptide synthesized by the artificial modification of natural somatostatin. The peptide sequence is D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-Ol (Disulfide Bridge Cys2-Cys7).8 D-type amino acid is introduced into the structure of octreotide, which enhances the ability of resisting enzyme degradation, and the action time in vivo can be up to 2 h.9 Most octreotide-inhibited tumor cells have one or more SSTRs on the surface, and SSTR2 is the most common.10 The conjugate of octreotide and radionuclide can specifically bind to SSTR2 and enter tumor cells through endocytosis for targeted internal radiation therapy.11−13 In the early stage, 90Y was labeled on octreotide to play a therapeutic role.14,15 In recent years, researchers have found that the radiation energy and half-life of 177Lu, 225Ac, and 213Bi therapeutic nuclides are more suitable for tumor therapy than 90Y, which is mainly used for the treatment of large tumors.16−18
177Lu can release β-rays for therapeutic use, and its maximum β-ray tissue penetration is about 2 mm, which is suitable for small tumors.19 Although the effectiveness of β-nuclides in targeted tumor therapy has been demonstrated, their use in the treatment of diffuse micrometastases has been greatly limited. α-nuclide targeting therapy is expected to be a therapeutic method for this type of tumor.20,21 α-nuclides are helium nuclei in nature, and their LET values are 50–230 keV/μm. The relative biological effects of α-nuclides are 3–7 times that of β-nuclides, which are shown as irreparable breaks of DNA double strands during mitosis or redistribution. The general radiation range is 28–100 μm, equivalent to the diameter of 6–8 eukaryotic cells (10–50 μm), which can significantly increase cell mortality rate per unit absorbed dose, reduce bone marrow toxicity, and limit overexposure to radiation.22,23
However, unlike conventional drugs and toxins, which kill only directly targeted cells, α-nuclides are unique in their ability to induce radiation-induced bystander effects or cross-fire effects. As a result, adjacent tumor cells may be destroyed even if they do not possess the specific tumor-associated receptor, enzyme, or antigen.24 Moreover, the sensitivity of hypoxic cells to alpha ray is similar to that of normal oxygen cells, which avoids the tolerance of tumor heterogeneity to radiotherapy and overcomes the deficiency of traditional radiotherapy and chemotherapy.25,26 Furthermore, systemically administered targeted internal irradiation therapy combines molecular-specific cell recognition with ionizing radiation’s antitumor properties, possibly eliminating the primary tumor site as well as cancer that has spread throughout the body, including populations of malignant cells that are not detectable with diagnostic imaging.27−29
Currently, the mostly promising α-emitters are 211At, 223Ra, 213Bi, and 225Ac.30−33225Ac produces multiple daughter emitters, which may release from targeted carriers, leading to serious side effects caused by accidental irradiation of nontargeted tissues. The half-life of 213Bi is only 46 min, which is not sufficient for drug preparation to achieve its clinical application34; the low stability of 223Ra after chelation limits its application.35211At releases only one α particle, which helps reduce the off-target effects. Therefore, 211At is the most promising α-emitter for clinical translation in nuclear medicine.
We successfully synthesized the octreotide SPC conjugate in the early stage,36 which showed a good targeted therapeutic effect in the experimental study on non-small cell lung cancer (NSCLC). In the following study, we further synthesized the new octreotide ATE conjugate N-succinimidyl-3-[211At]astatobenzoate-Oct ([211At]SAB-Oct), aiming to evaluate the growth inhibition and targeting effect of the conjugate on SCLC cell H446 and search for new treatments for SCLC.
2. Materials and Methods
2.1. Peptide and Reagents
Octreotide (i.e., Oct) was purchased from GL Biochem (Shanghai, China, Co). N-succinimidyl-3-(trimethylstannyl)-benzoate (i.e., m-MeATE, 98%) was purchased from Toronto Research Chemicals.
N-chlorosuccinimide (NCS, 98%) was purchased from Acros (Belgium). Sephadex G-10 was purchased from GE Healthcare. The chemical reagents used were all analytical or chromatography grade.
2.2. 211At Production and Radiolabeling
Astatine-211 was produced via the 209Bi (α, 2n)211At nuclear reaction by irradiating an internal bismuth target with α-particles accelerated to 28 MeV using a CS-30 cyclotron at Sichuan University.37,38 The single separation yield of 211At was 550–740 MBq, and the radioactivity was detected by dose calibrator (CRC-15R, American. Inc.).
The radiolabeling of Oct with 211At was carried out using a previously described direct astatination procedure.36 Briefly, Oct (5 mg/mL) was conjugated to m-MeATE (26 mmol/L in DMSO) in 0.1 mol/L phosphate-buffered saline (PBS, pH 7.6). The mixture was incubated at room temperature for 2 h. The conjugate was subsequently isolated in PBS using a Sep-pak C18 solid phase extraction column (WAT020805, American. Inc.), and then adjusted to pH 5.5 by the addition of acetic acid prior to 211At labeling. HPLC was performed to confirm the identity of the product of the Oct-m-MeATE conjugate. The Oct-m-MeATE conjugate was added to Na211At (∼370 MBq, in methanol) solution with 20 μL of 20 mg/mL NCS in methanol solution. The reaction mixture was incubated at RT for 5 min. Finally, the labeling reaction was terminated by the addition of 20 μL of 40 mg/mL Na2S2O5 aqueous solution. The final product [211At]SAB-Oct was isolated in PBS using a Sephadex G-10 column and verified by instant TLC (3 mm CHR, GE Whatman, Mobile phase: MeOH/CHCl3 = 1:4; Final product: Rf = 0.1–0.2; free 211At: Rf = 0.9–1.0) in a γ-counter (FH463B, China National Nuclear Corporation, Beijing, China).
2.3. In Vitro Stability, Cellular Uptake, and Cytotoxicity
The in vitro stability of [211At]SAB-Oct was evaluated by incubating it in mouse serum at room temperature. Stability analysis was performed by iTLC at 1, 3, 6, 12, and 24 h, respectively.
The human SCLC cell line H446 and the human NSCLC cell line A549 were purchased from Cellcook Biotech (Guangzhou, China). Cells were cultured in RPMI-1640 media supplemented with 10% fetal calf serum (Sigma) and 1% PEST (penicillin 100 IU mL–1 and streptomycin 100 g mL–1). Cells were incubated at 37 °C in incubators with humidified air equilibrated at 5% CO2.
Cellular uptake of [211At]SAB-Oct was evaluated in both the H446 and A549 cell lines in an identical fashion. Briefly, cell suspension was prepared in PBS at a concentration of 5 × 106 in low-bind Eppendorf tubes. Radioactivity (3.7 kBq) was added to each tube, and the cells were incubated at 37 °C for 1, 3, and 6 h (in triplicates) at moderate speed to prevent cell settling. At each time point, cells were centrifuged, and the supernatant was aspirated from cell pellets, which was followed by 2 additional rinses with ice-cold PBS. The supernatants were combined with the PBS wash and counted using a gamma counter. Cell pellets were then collected and counted for radioactivity. Nonspecific binding was determined by adding Oct (10 mg/mL) as a blocking agent in parallel.
In vitro cytotoxicity of [211At]SAB-Oct was evaluated in comparison with PBS, unlabeled Oct (positive control), and free 211At (negative control), using both H446 and A549 cell lines. Briefly, cells (5–10 × 103/well) were seeded into 96-well plates 24 h before the experiment. Cells were incubated with cell culture media containing 1–5 μL of PBS, Oct (100 μg), free 211At (0.37, 1.85 kBq), or [211At]SAB-Oct (0.37, 1.85 kBq) at 37 °C for 24 h. After that, the media was removed, and the cells were washed twice with PBS. Fresh media (100 μL) containing 10 μL enhanced cell counting kit-8 solution was then added to each well, and the cells were incubated for 4 h.
2.4. DNA Damage of [211At]SAB-Oct Using γH2AX Imaging
H446 and A549 cells were plated in 6-well plates (5 × 104/well). Cells were treated with PBS, Oct, free 211At, and [211At]SAB-Oct at four different doses (1.85, 3.7, 18.5, and 37 kBq) for 2 h and recovered in a fresh medium for 24 h. The cells were then washed, fixed, permeated, and stained with a mouse anti-γH2AX antibody (JBW-301 clone, 1:800).
2.5. Western Blot Analysis
Western blotting was performed as described in our previous work.39 Cancer cells of H446 and A549 at the logarithmic growth phase, as well as H446 and A549 incubated with PBS, Oct, free 211At, and [211At]SAB-Oct at four different doses (1.85, 3.7, 18.5, and 37 kBq) for 2 h and recovered in fresh medium for 24 h were lysed on ice for 30 min with RIPA. The BCA Protein Assay Kit was used to calculate the quantity of protein. Equal amounts of proteins were denaturized, fractionated using 10% SDS-polyacrylamide gel electrophoresis, and then transferred to PVDF membranes. The membranes were then incubated with primary antibodies (SSTR2 antibody and γ-H2AX antibody) at 4 °C for 12 h after being blocked with 5% milk for 1 h. The protein blotting was treated with chemiluminescent (ECL) substrates after being incubated with the proper secondary antibody, and the expression levels of the proteins were then shown using an ECL detection apparatus.
2.6. Animal Experiments
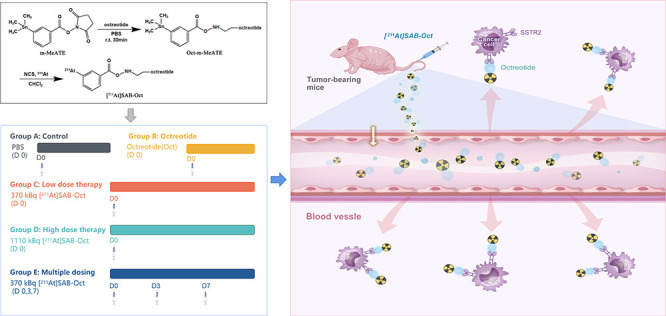
All animal experiments were approved by the Animal Welfare Ethics Committee of Shanghai Tenth People’s Hospital with an approval number (ID: SHDSYY-2021–3429–3711). The SCLC tumor-bearing BALB/c nude mice model was established by subcutaneously implanting H446 cells (1 × 107 cells in 0.2 mL PBS) into the left shoulder of BALB/c nude mice at 4–5 weeks of age (20 ± 1 g, both male) (Dossy Experimental Animals, Chengdu, China).
2.7. Biodistribution
The biodistributions of free 211At (1110 kBq) and [211At]SAB-Oct (1110 kBq) were evaluated in parallel using the BALB/c nude mice bearing subcutaneous SCLC tumors (described above, n = 4 in each group). Mice were injected with radioactivities via the tail vein and sacrificed at 1, 3, 6, 12, and 24 h. Tumor, main tissues, and whole blood were collected and weighted, and a gamma counter was used to measure 211At’s activity. Data were presented in percent injected dose per gram of tissue (%ID/g), except uptake in thyroid, which was presented in injected dose per organ (%ID/organ).
2.8. Therapeutic Efficacy of [211At]SAB-Oct
The SCLC tumor-bearing BALB/c nude mice were established as described above and randomized into 5 groups 2 weeks after tumor inoculation. Mice were injected via the tail vein with PBS (Group A), 5 mg/kg of octreotide (B), 370 kBq of [211At]SAB-Oct (C), 1110 kBq of [211At]SAB-Oct (D), and 3 doses of 370 kBq of [211At]SAB-Oct on Day 0, 3, and 7 (E). Mice were monitored for 30 days by measuring tumor size and body weight. Hematological indexes were detected on day 14, day 28 after the injection of Groups A,D,E, and liver and kidney function indexes were carried out on day 28 after the injection of Groups A–E. After 30 days of treatment, the random mice from each group were sacrificed, and tumors and other major organs (liver, spleen, kidney, stomach, and lung) on the mice were surgically excised, fixed with 10% (v/v) formalin, and embedded in paraffin, and stained with hematoxylin and eosin (H&E).40
2.9. Immunofluorescence and Immunohistochemistry
γ-H2AX was detected to evaluate DNA damage in vivo by immunofluorescence staining. Immunohistochemical staining of MHC-I and calreticulin was performed according to the manufacturer’s instruction.
2.10. Statistical Analysis
Data were presented as mean ± standard deviation (s.d). Comparisons of the values between two groups used an unpaired t test. Tumor volumes were analyzed by one-way ANOVA. A P-value of <0.05 was considered significant.
3. Results
3.1. Radiochemical Analyses of Radiolabeled Oct
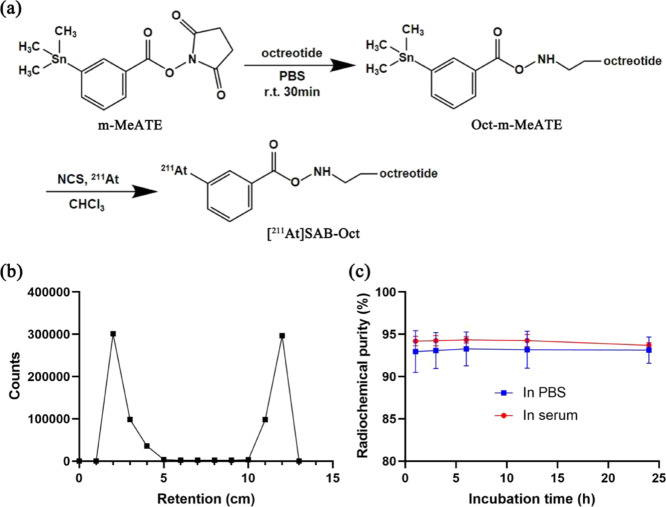
The tin precursor for radiolabeling of 211At, m-MeATE, was conjugated to Oct on the N-terminus d-phenylalanine (Figure 1a). HPLC results showed that the peaks of octreotide, m-MeATE, and Oct-m-MeATE conjugate correspond to 2.28, 2.76, and 3.13 min, respectively (Figure S1).
Figure 1.
Synthesis and analysis of [211At]SAB-Oct. (a) Synthetic scheme for [211At]SAB-Oct; (b) ITLC chromatogram of [211At]SAB-Oct; (c) stability over time of [211At]SAB-Oct in PBS and mouse serum. Data points are the average of three independent samples, and error bars represent mean ± s.d.
The purified conjugate was used for radiolabeling without further purification. Radiolabeling with 211At was conducted with 38–47% radiochemical yield (RCY) with greater than 90% radiochemical purity RCP (Figure 1b). The radiolabeled conjugate [211At]SAB-Oct inhibited good in vitro stability in mouse serum with 93% RCP after 24 h of incubation (Figure 1c).
3.2. Cellular Uptake of [211At]SAB-Oct in SSTR2-Positive Cell Lines
The H446 and A549 cell lines were reported with high levels of expression of SSTR2, which was validated upon receipt by a Western blot experiment using GAPDH as an internal standard (Figure 2a). A significantly higher expression level was seen with the H446 cell line, and hence H446 was used as a positive control while the A549, with a minimum expression of SSTR2, was used as a negative control.
Figure 2.
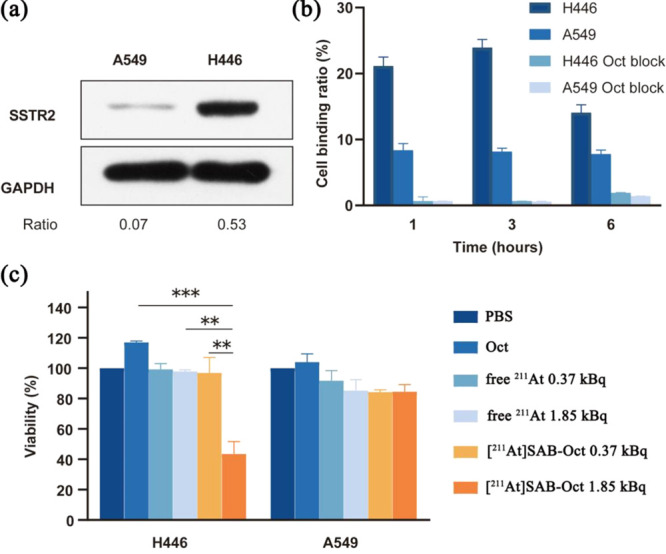
Cell-binding analysis in vitro by [211At]SAB-Oct and subsequent cell death. (a) SSTR2 expression in H446 and A549 cells. Load control was performed using GAPDH. The images below show the ratio of SSTR2 to GAPDH intensity; (b) the binding ratio of [211At]SAB-Oct to H446 and A549 cells with and without Oct block. Each experiment was conducted in triplicate (n = 3) in three independent experiments; (c) analysis of viability of cells treated for 24 h with PBS control, unlabeled Oct, free 211At (0.37 or 1.85 kBq), or[211At]SAB-Oct (0.37 or 1.85 kBq). There were three independent experiments conducted in triplicate (n = 3). Error bars represent mean ± s.d. (**P < 0.01 and ***P < 0.001).
Our next step was to determine if [211At]SAB-Oct binds to the H446 and A549 cells. In comparison to A549 cells, the [211At]SAB-Oct binding ratio to H446 cells was significantly higher. The binding ratio of [211At]SAB-Oct to H446 cells was markedly decreased by 100-fold excesses of unlabeled Oct, clearly indicating the specificity of this binding (Figure 2b).
In addition, we measured the viability of H446 and A549 cells after 24 h of exposure to [211At]SAB-Oct. In any case, survival was not affected by treatment with PBS, Oct, or free 211At. [211At]SAB-Oct doses of 1.85 kBq, however, effectively killed H446 cells and reduced their viability. According to the quantitative cell viability data, 1.85 kBq dose of [211At]SAB-Oct significantly reduced H446 cell numbers compared with unlabeled octreotide (***P < 0.001), 1.85 kBq dose of free 211At (**P < 0.01) (Figure 2c).
3.3. Biodistribution of [211At]SAB-Oct Conjugates in Tumor-Bearing Mice
The biodistributions of free 211At and [211At]SAB-Oct in H446 tumor-bearing mice are summarized in Figure 3. The biodistribution analysis revealed that injected free 211At was more likely to be absorbed mainly in the spleen and lung at 1 h, stomach and liver at 2 h, and then the uptake was gradually reduced within 24 h, the tumors remained at a low level of uptake for 1 to 24 h, almost similar to that of the muscle (Figure 3a).
Figure 3.
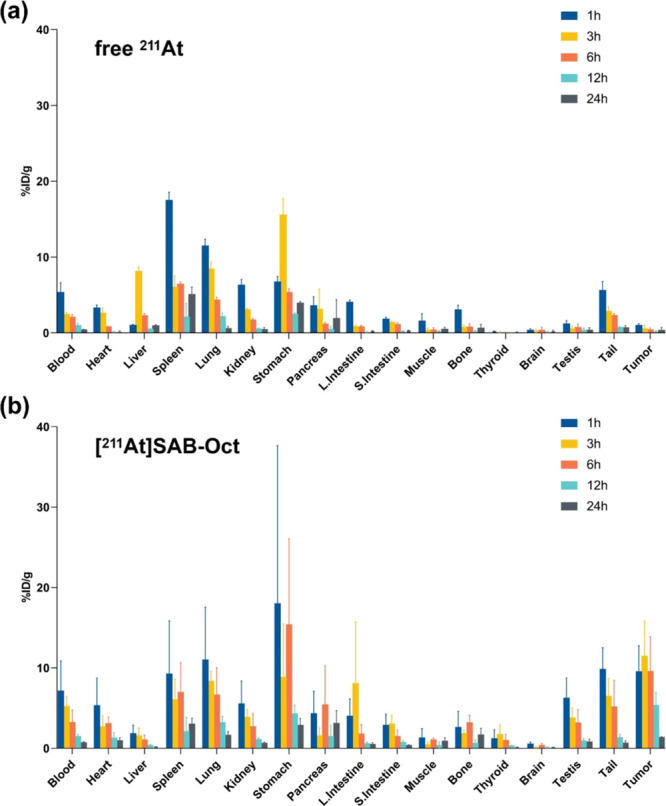
Biodistribution of [211At]SAB-Oct in SCLC tumor-bearing BALB/c nude mice. Tumor and organ uptake of 211At (%ID/g) at 1, 3, 6, 12, and 24 h after i.v. injection of (a) free 211At (1110 kBq) or (b) [211At]SAB-Oct (1110 kBq). Each time point involved four mice. Error bars represent mean ± s.d.
The stomach showed the highest uptake (17.99 ± 19.65% ID/g) of [211At]SAB-Oct in all tissues after 1 h postinjection, and as 24 h progressed, it decreased to (2.87 ± 0.86% ID/g). The maximum tumor uptake of [211At]SAB-Oct was (11.46 ± 4.35% ID/g) at 3 h postinjection. Besides, [211At]SAB-Oct showed physiologically high uptake in the lung and intestines. It was observed that free 211At or [211At]SAB-Oct always showed low radioactivity uptake in the thyroid. Low levels of radioactivity were observed in all tissues after 24 h (Figure 3b).
3.4. DNA Damage Is Induced in Tumor Cells by [211At]SAB-Oct
To uncover how [211At]SAB-Oct kills tumor cells, we determined the γH2AX foci caused by DNA damage in vitro.
Treatment of cells with [211At]SAB-Oct for 24 h showed clear clusters of γH2AX foci, but those treated with PBS or unlabeled octreotide showed little evidence of clusters. In H446 cells treated with [211At]SAB-Oct (18.5 and 37 kBq) compared to PBS in the same group, the level of γH2AX revealed a distinct dose-dependent increase(****P < 0.001). In A549 cells treated with [211At]SAB-Oct (37 kBq) compared to PBS in the same group, the level of γH2AX was slightly higher (***P < 0.001), but still much lower than in H446 (***P < 0.001) (Figure 4a,b). The γH2AX expression was then investigated reasonably by Western blotting test. γH2AX protein was visually increased after incubation with [211At]SAB-Oct (1.85, 3.7, 18.5, or 37 kBq) in H446 cells. In the A549 group, the protein expression of γH2AX was slightly expressed only when the dose reached 18.5 or 37 kBq (Figure 4c).
Figure 4.
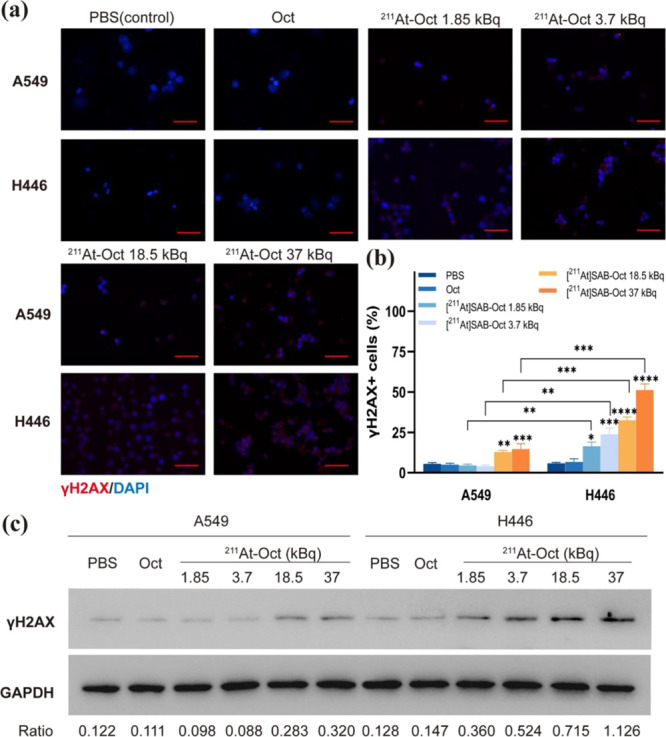
Images and quantitative analyses of γH2AX foci in cells following [211At]SAB-Oct treatment. (a) Immunofluorescence images of γH2AX foci in A549 or H446 cells incubated with PBS, Oct or [211At]SAB-Oct (1.85, 3.7, 18.5, or 37 kBq). Scale bar = 50 μm; (b) quantification of the γH2AX expression level of different groups. There were three independent experiments conducted in triplicate (n = 3). All data represent mean ± s.d. (*P < 0.01, **P < 0.01, ***P < 0.001, and ****P < 0.001); (c) Western blot analysis of γH2AX expression in A549 and NCI-446 cells incubated with PBS, Oct, or [211At]SAB-Oct (1.85, 3.7, 18.5, or 37 kBq). Load control was performed using GAPDH. The images below provide ratios of γH2AX to the GAPDH band intensity.
3.5. Therapeutic Efficacy of [211At]SAB-Oct against SCLC Tumors in the Mice
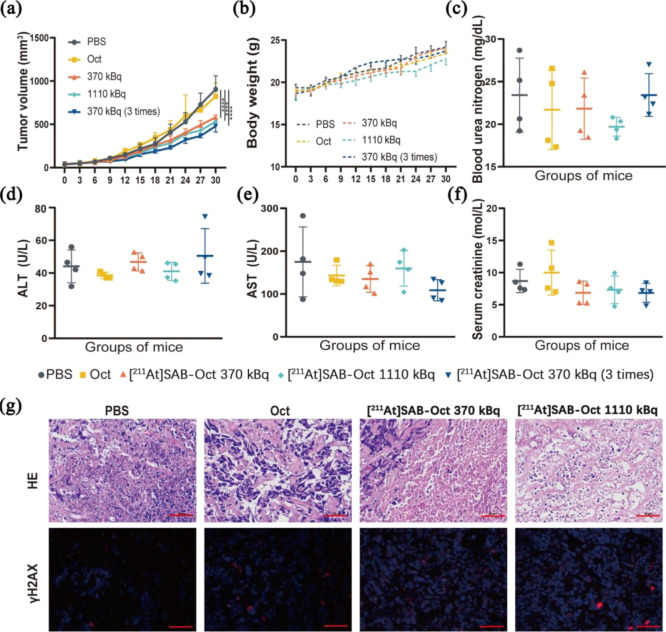
SCLC tumor-bearing mice were divided into 5 groups. Mice received a single injection of PBS (group A), Oct (group B), [211At]SAB-Oct 370 kBq (group C), [211At]SAB-Oct 1110 kBq (group D), and 3 times [211At]SAB-Oct with each time 370 kBq given on days 0, 3, and 7 (group E). Figure 5a shows the therapeutic effect of [211At]SAB-Oct on SCLC tumors. Compared with the PBS control group, tumor growth was significantly inhibited in group C, group D with a single injection, and group E.
Figure 5.
Therapeutic results of [211At]SAB-Oct in SCLC tumor-bearing BALB/c nude mice. (a) Time-dependent tumor volume variations of SCLC tumor-bearing mice experiencing corresponding treatments in different groups. Error bars represent mean ± s.d. (n = 6–10).(****P < 0.0001); (b) body weight variations of SCLC tumor-bearing BALB/c nude mice during treatment. (n = 6–10); (c–f) liver and kidney function monitoring of the BALB/c nude mice at day 28 postinjection with PBS, unlabeled Oct, 370 kBq, 1110 kBq or multiple dosing [211At]SAB-Oct. Error bars represent mean ± s.d; (g) images of H&E and γH2AX foci in SCLC tumors at day 30 postinjection with PBS, unlabeled octreotide (Oct), 370 or 1110 kBq of [211At]SAB-Oct. Scale bar = 50 μm.
Blood urea nitrogen (Bun) and serum creatinine (Cr) levels are measures of renal function. On day 28, the difference in Bun and Cr concentrations were not significant (P > 0.05) between mice injected with different treatment, but for mice injected with [211At]SAB-Oct 1110 kBq only a trend of decreased Bun values was observed (P > 0.05) (Figure 5c,f). Liver function is indicated by ALT and AST levels. The difference in ALT and AST concentrations were not significant (P > 0.05) between each group, AST was lower in mice of group E than in control mice on day 28 (P > 0.05) (Figure 5d,e). According to values for normal blood biochemical levels in BALB/c nude mice listed by Wuhan Saywell Biotechnology Co. Ltd., all concentrations were still within a physiological range, so impaired kidney or liver function cannot be inferred from the obtained results.
Compared with PBS control and unlabeled octreotide treated groups, we found that [211At]SAB-Oct 1110 kBq acquired the highest cell necrosis, including chromatic agglutination, karyopyknosis, and nuclear fragmentation in H&E assay. Clusters of γH2AX foci were still visible in the dissected tumors of SCLC model mice on day 30 after 1110 kBq [211At]SAB-Oct injection (Figure 5g).
3.6. Expression of MHC-I and Calreticulin on the Surface of Tumor Cells Was Up-Regulated by Intranuclide Irradiation
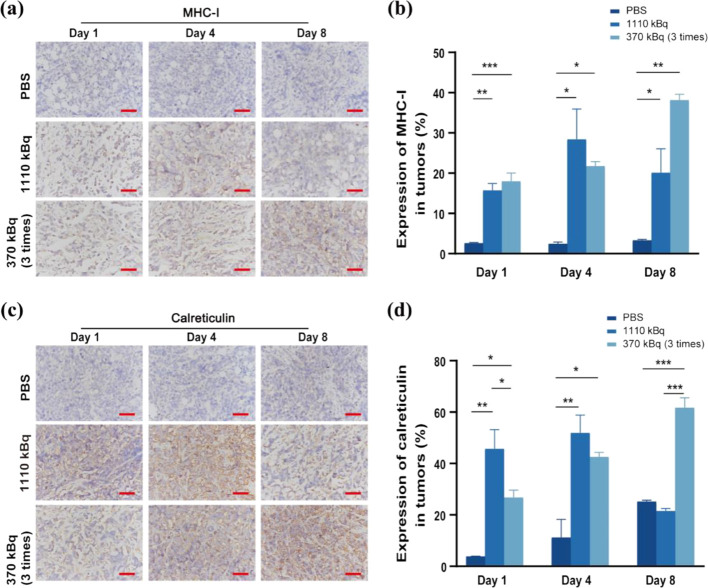
We studied the capability of 211At to stimulate antitumor immunity against SCLC. Major histocompatibility complex class I (MHC-I) expression level and calreticulin expression level in SCLC tumors were examined on day 1, 4, and 8 postvarious treatments.
In the PBS group, the expression of MHC-I and calreticulin in tumor tissues was always decreased on day 1, 4, and 8 after the first administration. In the 1110 kBq administration group, the expression of MHC-I and calreticulin showed a trend of first increasing and then decreasing over time. Time-dependent increases in MHC-I and calreticulin expression were observed in the multiple-dosing group. On day 8, MHC-I and calreticulin expression reached their highest levels (***P < 0.001, **P < 0.01, respectively) (Figure 6a–d).
Figure 6.
MHC-I and calreticulin expression level in SCLC tumor postvarious treatments at days 1, 4, 8. (a) MHC-I expression level at day 1,4,8 postinjection with PBS, 1110 kBq or multiple dosing [211At]SAB-Oct. Scale bar = 50 μm; (b) quantification of the MHC-I expression level of different groups; (c) calreticulin expression level at 30 days postinjection with PBS, 1110 kBq or multiple dosing [211At]SAB-Oct. Scale bar = 50 μm; (d) quantification of the calreticulin expression level of different groups. There were three independent experiments conducted in triplicate (n = 3). All data represent mean ± s.d. (**P < 0.01 and ***P < 0.001).
3.7. Adverse Effects of Injected [211At]SAB-Oct in Mice Model
3.7.1. Body Weight
The body weight of mice was measured to determine the radioactive drug’s toxicity. Although these weights tended to be lower in mice receiving 1110 kBq doses, there was no statistically significant weight change in any treatment group during the observation period (P > 0.05) (Figure 5b).
3.7.2. Assessment of Potential Impairment of Blood Cells in SCLC Tumor-Bearing Mice
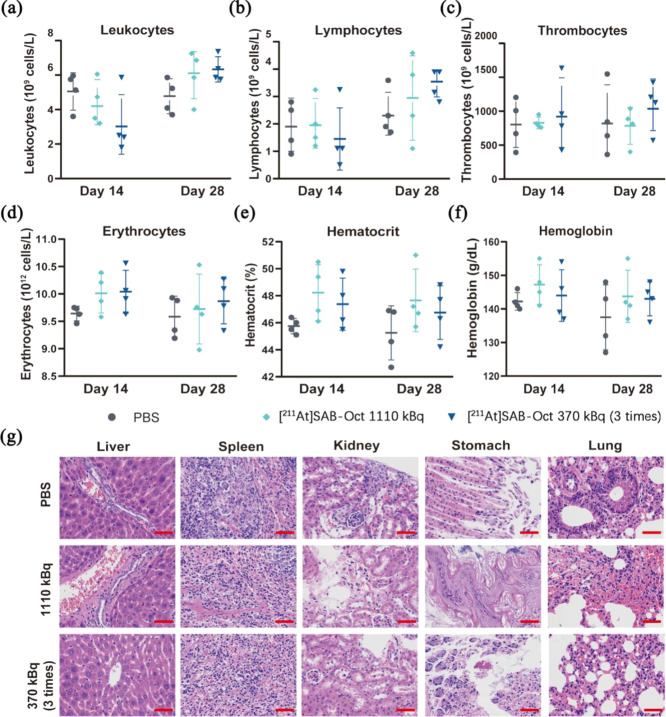
To assess the potential adverse effects of [211At]SAB-Oct in SCLC tumor-bearing mice, we monitored whether any changes in blood cells occurred in the treated mice. Blood cells monitoring the BALB/c nude tumor-bearing mice at day 14 and day 28 postinjection with 1110 kBq or multiple dosing [211At]SAB-Oct, which revealed no significant change compared with each group (Figure 7a–f). No leukocytopenia was found in the [211At]SAB-Oct 1110 kBq group on day 28. The blood plasma parameters were in the physiological range for all mice, which suggests that impaired blood cells are unlikely to be evident from the obtained results.
Figure 7.
Side effects in SCLC tumor-bearing BALB/c nude mice treated with [211At]SAB-Oct. Blood cell counts of SCLC tumor-bearing BALB/c nude mice (n = 4) on day 14 and day 28 after PBS, 1110 kBq or multiple dosing [211At]SAB-Oct treatments. (a) Leukocyte counts; (b) lymphocyte counts; (c) thrombocyte counts; (d) erythrocyte counts; (e) hematocrit; (f) hemoglobin concentrations; (g) analysis of major organs in different treatment groups at day 30 postinjection with PBS, 1110 kBq or multiple dosing group. Scale bar = 50 μm.
3.7.3. Radiation Effects on Nontargeted Tissues
According to the experimental results of the previous in vivo distribution, we carried out H&E staining analysis for nontargeted tissues that may potentially have ionizing radiation damage in groups A, D, and E.
H&E sections showed all groups’ tissue liver lobule structure was clear. Occasionally, a few neutrophils gathered in the hepatic sinusoids. Slight bile duct hyperplasia was observed in a few portal areas in these three groups. No obvious morphological difference was observed. Sections of spleen from groups D and E showed white pulp injury, reduced lymphocyte numbers, and more necrotic lymphocytes than those from group A. Kidney sections from groups D and E showed a few newly born renal tubular cells were observed in the local capsule of the tissue, with light cytoplasm and obvious nuclei and nucleoli. The brush border of a few renal tubular epithelial cells was slightly exfoliated. The interstitial inflammatory cells were scattered and infiltrated. In the sections of groups D and E, mild exfoliation of extensive mucosal epithelium was observed in the gastric gland area, with a disordered arrangement of local glands and more exfoliated glands. Lung H&E sections from groups A, D, and E showed no obvious abnormalities observed in the morphology and structure of tissue bronchus. Mild inflammatory cell infiltration was observed in about 50% of the alveolar walls in three groups (Figure 7g).
4. Discussion
In spite of the proliferation of chemotherapy and targeted treatment options for SCLC, the prognosis of SCLC patients remains dismal. As shown in our current study, [211At]SAB-Oct inhibits tumor growth as well as improves the antitumor immune response in vivo and in vitro in SSTR2-positive SCLC cells.
According to a previous study, we set various concentration doses to conduct the in vitro study.41 Analyses of in vitro cytotoxicity revealed that [211At]SAB-Oct specifically killed SSTR2-positive SCLC cells but not low-level SSTR2 A549 cells. The free 211At did not affect either type of cell. In light of the fact that radiolabeled octreotide is internalized into the cells it binds to,42,43 it appears that [211At]SAB-Oct exerts its cytotoxic effects when it is bound and internalized by targeted cells. Choosing the right targeting strategy is crucial for improving the cell-killing effect of α-particle emitters.
Our in vivo biodistribution experiments showed that the tumor reached the highest aggregation of [211At]SAB-Oct at the third hour after the administration, which was consistent with the half-life of the octreotide.9,22 Although the uptake of [211At]SAB-Oct in various organs decreased gradually with time delay compared with the in vivo distribution of free 211At, the gastrointestinal tract showed a relatively higher uptake earlier after administration, which may be partly related to the relatively high SSTR2 expression in the digestive tract.44−46
Based on our current analysis, we also found that α-PRRT with [211At]SAB-Oct suppressed tumor growth in SCLC tumor-bearing BALB/c nude mice. In our SCLC mice, we discovered that [211At]SAB-Oct led to extensive DNA DSBs in SSTR2-positive SCLC cells, while it was not in SSTR2-low-expression A549 cells. Octreotide without labeling equal in protein content to that of the labeled octreotide could not lead to DSBs in SCLC cells. Accordingly, our hypothesis is that [211At]SAB-Oct particles efficiently caused irreparable DSBs in SCLC cells and hence reduced the tumor size.
Based on our current experiments, it is clear that a single injection of [211At]SAB-Oct 1110 kBq is similar to the efficiency of a multiple injection but the same amount (370 kBq × 3) to SCLC tumors. Besides, we also found that 211At irradiation of SCLC tumors in vivo induced the release of MHC-I and calreticulin, considering that PRRT can not only directly cause DNA DSBs to play an antitumor role, but also change the tumor cell phenotype and microenvironment, thus inducing or regulating antitumor immune responses.47
The most common adverse reactions of PRRT are nephrotoxicity and myeloid toxicity.48,49 Nephrotoxicity, usually manifested by renal dysfunction, is a major factor affecting treatment and can be combined with the use of amino acids (lysine and arginine) to protect the kidney.50 In animal studies, enalapril maleate was used to protect the kidneys and reduce the risk of adverse reactions by speeding up drug metabolism with a diuretic.51 In our current experiments, leukocytopenia or weight loss were not observed in mice injected with [211At]SAB-Oct at 1110 kBq doses, which was consistent with a previous study.52 Additionally, 1110 kBq [211At]SAB-Oct did not significantly alter liver or kidney biochemical function in mice.
The positive expression of SSTR2 in SCLC allowed us to develop a novel SSTR2-targeting radiopharmaceutical. Earlier, 177Lu-dotatate has been approved by the FDA for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). It has been shown to inhibit SSTR2-positive tumors and has emerged as a powerful alternative to octreotide in the treatment of neuroendocrine tumors.53,54177Lu has a short range of β-rays and little effect on normal tissues while targeting tumors.55−57 We speculate, however, that α-rays have a shorter range and theoretically cause less radiation damage to normal tissues around tumors under the premise of precise targeting. Since β-particle-labeled somatostatin analogues have achieved better therapeutic results, α-particle ones should be more effective.
However, there are still some limitations to this research. For example, slight deastatination was observed in the spleen, lung, and stomach, which means it is necessary to introduce other substituent groups to improve the stability. In addition, the tumor volume and body weight were detected for only 30 days. They should be further observed in future studies.
5. Conclusions
We successfully constructed [211At]SAB-Oct with ideal radiochemical purity and radio stability. Cell experiments showed that the viability of H446 was inhibited after the treatment of [211At]SAB-Oct. The in vivo study further validated the treatment effect without causing damage to the major organs. More significantly, [211At]SAB-Oct induced immunogenic cell death, indicating that it could promote antitumor responses. These properties of [211At]SAB-Oct suggested its potential use as a novel adjuvant therapeutic method in conjunction with immunotherapy for the treatment of SCLC.
Data Availability Statement
Not applicable.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.3c00427.
HPLC chromatograms of a mixture of octreotide, m-MeATE, and Oct-m-MeATE conjugate (PDF)
Author Contributions
S.Q. and Y.Y. contributed equally to the work. Conceptualization, S.Q., Y.Y. (Yuanyou Yang) and F.Y.; methodology, S.Q., Y.Y. (Yuanyou Yang) and J.Z.; formal analysis, S.Q., Y.Y.(Yuzhen Yin) and J.Z, investigation Y.Y. (Yuzhen Yin), W.L. H.Z., X.F. and M.Y.; writing—original draft preparation, S.Q. and Y.Y. (Yuanyou Yang); writing—review and editing, S.Q., J.Z. and F.Y.; visualization, S.Q., W.L., H.Z., X.F. and M.Y.; supervision, F.Y. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Natural Science Foundation of China (82071956), and the Shanghai Anti-Cancer Association (SACA-CY22C05).
The authors declare no competing financial interest.
Notes
Ethics approval and consent to participate for all animals were provided by the animal center of Shanghai Tenth People’s Hospital, and all animal experiments were approved by Animal Welfare Ethics Committee of Shanghai Tenth People’s Hospital with an approval number (SHDSYY-2021–3429–3711).
Supplementary Material
References
- Apar K.; Ganti M.; Billy W.; Michael B.; Collin B.; Anne C.; Thomas A.; Christopher D. Small Cell Lung Cancer, Version 2.2022. J. Natl. Compr. Canc. Netw. 2021, 19, 1441–1464. 10.6004/jnccn.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin C. M.; Brambilla E.; Faivre-Finn C.; Sage J. Small-cell lung cancer. Nat. Rev. Dis Primers 2021, 7 (1), 3. 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart J.; Waqar S.; Mix M. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 661–670. 10.1200/JCO.21.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq S.; Kim S. Y.; de Oliveira Monteiro; Novaes J.; Cheng H. Update 2021: Management of Small Cell Lung Cancer. Lung 2021, 199 (6), 579–587. 10.1007/s00408-021-00486-y. [DOI] [PubMed] [Google Scholar]
- Puliani G.; Chiefari A.; Mormando M.; Bianchini M.; Lauretta R.; Appetecchia M. New Insights in PRRT: Lessons From 2021. Front. Endocrinol. (Lausanne) 2022, 13, 861434 10.3389/fendo.2022.861434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G.; Mete O.; Uccella S.; Basturk O.; La Rosa S.; Brosens L. A. A.; Ezzat S.; de Herder W. W.; Klimstra D. S.; Papotti M.; Asa S. L. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33 (1), 115–154. 10.1007/s12022-022-09708-2. [DOI] [PubMed] [Google Scholar]
- Fonti R.; Panico M.; Pellegrino S.; Pulcrano A.; Vastarella L.; Torbati A.; Giuliano M. Heterogeneity of SSTR2 Expression Assessed by 68Ga-DOTATOC PET/CT Using Coefficient of Variation in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2022, 63, 1509–1514. 10.2967/jnumed.121.262928. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G.; Affleck D.; Schottelius M.; Wester H.; Friedman H.; Zalutsky M. R. Synthesis and Evaluation of Glycosylated Octreotate Analogues Labeled with Radioiodine and 211At via a Tin Precursor. Bioconjugate Chem. 2006, 17, 195–203. 10.1021/bc0502560. [DOI] [PubMed] [Google Scholar]
- Chen L. N.; Wang W. W.; Dong Y. J.; Shen D. D.; Guo J.; Yu X.; Qin J.; Ji S. Y.; Zhang H.; Shen Q.; He Q.; Yang B.; Zhang Y.; Li Q.; Mao C. Structures of the endogenous peptide- and selective non-peptide agonist-bound SSTR2 signaling complexes. Cell Res. 2022, 32 (8), 785–788. 10.1038/s41422-022-00669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani M.; Mansi R.; Nicolas G. P.; Wild D. Radiolabeled Somatostatin Analogs-A Continuously Evolving Class of Radiopharmaceuticals. Cancers (Basel) 2022, 14 (5), 1172. 10.3390/cancers14051172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangler C.; Beyer L.; Bartenstein P.; Wangler B.; Schirrmacher R.; Lindner S. Favorable SSTR subtype selectivity of SiTATE: new momentum for clinical [18F]SiTATE PET. EJNMMI Radiopharm. Chem. 2022, 7 (1), 22. 10.1186/s41181-022-00176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose J. M.; Wosniack J.; Iking J.; Staniszewska M.; Zarrad F.; Trajkovic-Arsic M.; et al. Administration Routes for SSTR-/PSMA- and FAP-Directed Theranostic Radioligands in Mice. J. Nucl. Med. 2022, 63, 1357–1363. 10.2967/jnumed.121.263453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. D.; Rangarajan V.; Puranik A. D.; Agrawal A.; Shah S.; Sahay A.; Purandare N. C. Sporadic Cerebellar Hemangioblastoma With Strong SSTR Expression on 68Ga-DOTANOC PET/CT. Clin. Nucl. Med. 2023, 48, e28–e30. 10.1097/RLU.0000000000004456. [DOI] [PubMed] [Google Scholar]
- Wong T. Y.; Zhang K. S.; Gandhi R. T.; Collins Z. S.; O’Hara R.; Wang E. A.; Vaheesan K.; Matsuoka L.; Sze D. Y.; Kennedy A. S.; Brown D. B. Long-term outcomes following 90Y Radioembolization of neuroendocrine liver metastases: evaluation of the radiation-emitting SIR-spheres in non-resectable liver tumor (RESiN) registry. BMC Cancer 2022, 22 (1), 224. 10.1186/s12885-022-09302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober B.; Saracyn M.; Lubas A.; Kolodziej M.; Brodowska-Kania D.; Kapusta W.; Kaminski G. Hepatic complications of peptide receptor radionuclide therapy with Lutetium-177 and Yttrium-90 in patients with neuroendocrine neoplasm. Nucl. Med. Rev. Cent. East. Eur. 2022, 25 (1), 54–61. 10.5603/NMR.a2022.0016. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Watabe T.; Kaneda-Nakashima K.; Shirakami Y.; Naka S.; Ooe K.; Toyoshima A.; Nagata K.; Haberkorn U.; Kratochwil C.; Shinohara A.; Hatazawa J.; Giesel F. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (3), 871–880. 10.1007/s00259-021-05554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanal A. J.; Satapathy S.; Sood A.; Khosla D.; Mittal B. R. Subclinical Hypothyroidism After 225Ac-DOTATATE Therapy in a Case of Metastatic Neuroendocrine Tumor: Unknown Adverse Effect of PRRT. Clin. Nucl. Med. 2022, 47 (2), e184–e186. 10.1097/RLU.0000000000003893. [DOI] [PubMed] [Google Scholar]
- Simon M.; Jorgensen J. T.; Khare H. A.; Christensen C.; Nielsen C. H.; Kjaer A. Combination of [177Lu]Lu-DOTA-TATE Targeted Radionuclide Therapy and Photothermal Therapy as a Promising Approach for Cancer Treatment: In Vivo Studies in a Human Xenograft Mouse Model. Pharmaceutics 2022, 14 (6), 1284. 10.3390/pharmaceutics14061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahenkorah S.; Murce E.; Cawthorne C.; Ketchemen J. P.; Deroose C. M.; Cardinaels T.; Seimbille Y.; Fonge H.; Gsell W.; Bormans G.; Ooms M.; Cleeren F. 3p-C-NETA: A versatile and effective chelator for development of Al18F-labeled and therapeutic radiopharmaceuticals. Theranostics 2022, 12 (13), 5971–5985. 10.7150/thno.75336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra V.; Samanta M.; Makvandi M.; Groff D.; Martorano P.; Elias J.; Ranieri P.; Tsang M.; Hou C.; Li Y.; Pawel B.; Martinez D.; Vaidyanathan G.; Carlin S.; Pryma D. A.; Maris J. M. Preclinical Development of [211At]meta- astatobenzylguanidine ([211At]MABG) as an Alpha Particle Radiopharmaceutical Therapy for Neuroblastoma. Clin. Cancer Res. 2022, 28 (18), 4146–4157. 10.1158/1078-0432.CCR-22-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Kulkarni H.; Baum R. 225Ac-DOTATOC-Targeted Somatostatin Receptor alpha-Therapy in a Patient With Metastatic Neuroendocrine Tumor of the Thymus Refractory to beta-Radiation. Clin. Nucl. Med. 2021, 46 (12), 1030–1031. 10.1097/RLU.0000000000003792. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G.; Boskovitz A.; Shankar S.; Zalutsky M. R. Radioiodine and 211At-labeled guanidinomethyl halobenzoyl octreotate conjugates: potential peptide radiotherapeutics for somatostatin receptor-positive cancers. Peptides 2004, 25 (12), 2087–2097. 10.1016/j.peptides.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Meshaw R.; Zhao X. G.; Jannetti S.; Vaidyanathan G.; Zalutsky M. R. Effective Treatment of Human Breast Carcinoma Xenografts with Single-Dose (211)At-Labeled Anti-HER2 Single-Domain Antibody Fragment. J. Nucl. Med. 2023, 64 (1), 124–130. 10.2967/jnumed.122.264071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpassand E. S.; Tworowska I.; Esfandiari R.; Torgue J.; Hurt J.; Shafie A.; Nunez R. Targeted alpha-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63 (9), 1326–1333. 10.2967/jnumed.121.263230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky M.; Reardon D.; Pozzi O.; Vaidyanathan G.; Bigner D. Targeted α-Particle Radiotherapy with 211At-labeled Monoclonal Antibodies. Nucl. Med. Biol. 2007, 34, 779–785. 10.1016/j.nucmedbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballal S.; Yadav M. P.; Tripathi M.; Sahoo R. K.; Bal C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients receiving Concomitant (225)Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-world Scenario Management Based Long-term Outcome Study. J. Nucl. Med. 2022, jnumed.122.264043. 10.2967/jnumed.122.264043. [DOI] [PubMed] [Google Scholar]
- Bezzi C.; Mapelli P.; Presotto L.; Neri I.; Scifo P.; Savi A.; Bettinardi V.; Partelli S.; Gianolli L.; Falconi M.; Picchio M. Radiomics in pancreatic neuroendocrine tumors: methodological issues and clinical significance. Eur. J. Nucl. Med. Mol. Imaging 2021, 48 (12), 4002–4015. 10.1007/s00259-021-05338-8. [DOI] [PubMed] [Google Scholar]
- Liu W.; Ma H.; Li F.; Cai H.; Liang R.; Chen X.; Lan T.; Yang J.; Liao J.; Yang Y.; Liu N. PET imaging of VEGFR and integrins in glioma tumor xenografts using 89Zr labelled heterodimeric peptide. Bioorg. Med. Chem. 2022, 59, 116677 10.1016/j.bmc.2022.116677. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Tian Q.; Xu X.; Li P.; He S.; Chen J.; Yao B.; Zhang J.; Yang Z.; Song S. Enhanced antitumor immune responses via a new agent [131I]-labeled dual-target immunosuppressant. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 275–286. 10.1007/s00259-022-05986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Steen S.; Comstock M. L.; Orozco J. J.; Hamlin D. K.; Wilbur D. S.; Jones J. C.; Kenoyer A.; Nartea M. E.; Lin Y.; Miller B. W.; Gooley T. A.; Tuazon S. A.; Till B. G.; Gopal A. K.; Sandmaier B. M.; Press O. W.; Green D. J. The α-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood 2019, 134 (15), 1247–1256. 10.1182/blood.2019001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. J.; Corey E.; Guise T. A.; Gulley J. L.; Kevin Kelly W.; Quinn D. I.; Scholz A.; Sgouros G. Radium-223 mechanism of action: implications for use in treatment combinations. Nat. Rev. Urol. 2019, 16 (12), 745–756. 10.1038/s41585-019-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian A.; Ji N. T.; Song H.; Sgouros G. Mathematical Modeling of Preclinical Alpha-Emitter Radiopharmaceutical Therapy. Cancer Res. 2020, 80 (4), 868–876. 10.1158/0008-5472.CAN-19-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Li F.; Yin Y.; Liu N.; Zhu M.; Zhang H.; Liu W.; Yang M.; Qin S.; Fan X.; Yang Y.; Zhang K.; Yu F. Alpha radionuclide-chelated radioimmunotherapy promoters enable local radiotherapy/chemodynamic therapy to discourage cancer progression. Biomater. Res. 2022, 26 (1), 44. 10.1186/s40824-022-00290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafreshi N. K.; Pandya D. N.; Tichacek C. J.; Budzevich M. M.; Wang Z.; Reff J. N.; Engelman R. W.; Boulware D. C.; Chiappori A. A.; Strosberg J. R.; Ji H.; Wadas T. J.; El-Haddad G.; Morse D. L. Preclinical evaluation of [(225)Ac]Ac-DOTA-TATE for treatment of lung neuroendocrine neoplasms. Eur. J. Nucl. Med. Mol. Imaging 2021, 48 (11), 3408–3421. 10.1007/s00259-021-05315-1. [DOI] [PubMed] [Google Scholar]
- Jurcic J. G. Targeted Alpha-Particle Therapy for Hematologic Malignancies. Semin. Nucl. Med. 2020, 50 (2), 152–161. 10.1053/j.semnuclmed.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Qin S.; Chai L.; Lu G.; Yang Y.; Cai H.; Yuan X.; Fan S.; Huang Q.; Yu F. Evaluation of astatine-211-labeled octreotide as a potential radiotherapeutic agent for NSCLC treatment. Bioorg. Med. Chem. 2018, 26 (5), 1086–1091. 10.1016/j.bmc.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Liu W.; Ma H.; Liang R.; Chen X.; Li H.; Lan T.; Yang J.; Liao J.; Qin Z.; Yang Y.; Liu N.; Li F. Targeted Alpha Therapy of Glioma Using 211At-Labeled Heterodimeric Peptide Targeting Both VEGFR and Integrins. Mol. Pharmaceutics 2022, 19 (9), 3206–3216. 10.1021/acs.molpharmaceut.2c00349. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Watabe T.; Kaneda-Nakashima K.; Shirakami Y.; Naka S.; Ooe K.; Toyoshima A.; Nagata K.; Haberkorn U.; Kratochwil C.; Shinohara A.; Hatazawa J.; Giesel F. Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (3), 871–880. 10.1007/s00259-021-05554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.; Liu J. B.; Wu Z. J.; Xie W. T.; Zhong X. J.; Hou L. K.; Wu W.; Lu H. M.; Jiang X. H.; Jiang J. J.; Cao Z. Y.; Cong G. J.; Shi M. X.; Jia C. Y.; Lu G. X.; Song Y. C.; Chai L.; Lv Z. W.; Wu C. Y.; Ma Y. S.; Fu D. Tumor suppressive microRNA-124a inhibits stemness and enhances gefitinib sensitivity of non-small cell lung cancer cells by targeting ubiquitin-specific protease 14. Cancer Lett. 2018, 427, 74–84. 10.1016/j.canlet.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Yang M.; Fan X.; Zhu M.; Yin Y.; Li H.; Chen J.; Qin S.; Zhang H.; Zhang K.; Yu F. Biomimetic radiosensitizers unlock radiogenetics for local interstitial radiotherapy to activate systematic immune responses and resist tumor metastasis. J. Nanobiotechnol. 2022, 20 (1), 103. 10.1186/s12951-022-01324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. K.; Morokoshi Y.; Nagatsu K.; Kamada T.; Hasegawa S. Locoregional therapy with α-emitting trastuzumab against peritoneal metastasis of human epidermal growth factor receptor 2-positive gastric cancer in mice. Cancer Sci. 2017, 108 (8), 1648–1656. 10.1111/cas.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. K.; Morokoshi Y.; Nagatsu K.; Kamada T.; Hasegawa S. Locoregional therapy with alpha-emitting trastuzumab against peritoneal metastasis of human epidermal growth factor receptor 2-positive gastric cancer in mice. Cancer Sci. 2017, 108 (8), 1648–1656. 10.1111/cas.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. K.; Sugyo A.; Tsuji A. B.; Morokoshi Y.; Minegishi K.; Nagatsu K.; Kanda H.; Harada Y.; Nagayama S.; Katagiri T.; Nakamura Y.; Higashi T.; Hasegawa S. α-particle therapy for synovial sarcoma in the mouse using an astatine-211-labeled antibody against frizzled homolog 10. Cancer Sci. 2018, 109 (7), 2302–2309. 10.1111/cas.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refardt J.; Hofland J.; Wild D.; Christ E. Molecular Imaging of Neuroendocrine Neoplasms. J. Clin. Endocrinol. Metab. 2022, 107 (7), e2662–e2670. 10.1210/clinem/dgac207. [DOI] [PubMed] [Google Scholar]
- Anzola L. K.; Rivera J. N.; Ramirez J. C.; Signore A.; Mut F. Molecular Imaging of Vulnerable Coronary Plaque with Radiolabeled Somatostatin Receptors (SSTR). J. Clin. Med. 2021, 10 (23), 5515. 10.3390/jcm10235515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustoulidou S.; Handula M.; de Ridder C.; Stuurman D.; Beekman S.; de Jong M.; Nonnekens J.; Seimbille Y. Synthesis and Evaluation of Two Long-Acting SSTR2 Antagonists for Radionuclide Therapy of Neuroendocrine Tumors. Pharmaceuticals (Basel, Switzerland) 2022, 15 (9), 1155. 10.3390/ph15091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Yang M.; Zhang J.; Yin Y.; Fan X.; Zhang Y.; Qin S.; Zhang H.; Yu F. Immunogenic Cell Death Induction by Ionizing Radiation. Front. Immunol. 2021, 12, 705361 10.3389/fimmu.2021.705361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda R.; Wakabayashi H.; Araki R.; Inaki A.; Nishimura R.; Ikawa Y.; Yoshimura K.; Murayama T.; Imai Y.; Funasaka T.; Wada T.; Kinuya S. Phase I/II clinical trial of high-dose [131I] meta-iodobenzylguanidine therapy for high-risk neuroblastoma preceding single myeloablative chemotherapy and haematopoietic stem cell transplantation. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (5), 1574–1583. 10.1007/s00259-021-05630-7. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Shi D.; Xu Z.; Gao Z.; Si Z.; Zhao Y.; Ye R.; Fu Z.; Fu W.; Yang T.; Xiu Y.; Lin Q.; Cheng D. 124I-Labeled Monoclonal Antibody and Fragment for the Noninvasive Evaluation of Tumor PD-L1 Expression In Vivo. Mol. Pharmaceutics 2022, 19 (10), 3551–3562. 10.1021/acs.molpharmaceut.2c00084. [DOI] [PubMed] [Google Scholar]
- Iikuni S.; Kamei I.; Ohara T.; Watanabe H.; Ono M. Development of an 111In-Labeled Glucagon-Like Peptide-1 Receptor-Targeting Exendin-4 Derivative that Exhibits Reduced Renal Uptake. Mol. Pharmaceutics 2022, 19 (3), 1019–1027. 10.1021/acs.molpharmaceut.2c00068. [DOI] [PubMed] [Google Scholar]
- Benesova M.; Guzik P.; Deberle L. M.; Busslinger S. D.; Landolt T.; Schibli R.; Muller C. Design and Evaluation of Novel Albumin-Binding Folate Radioconjugates: Systematic Approach of Varying the Linker Entities. Mol. Pharmaceutics 2022, 19 (3), 963–973. 10.1021/acs.molpharmaceut.1c00932. [DOI] [PubMed] [Google Scholar]
- Mielke C. H. Jr.; Gerich J. E.; Lorenzi M.; Tsalikian E.; Rodvien R.; Forsham P. H. The effect of somatostatin on coagulation and platelet function in man. N. Engl. J. Med. 1975, 293 (10), 480–483. 10.1056/NEJM197509042931005. [DOI] [PubMed] [Google Scholar]
- Strosberg J. R.; Caplin M. E.; Kunz P. L.; Ruszniewski P. B.; Bodei L.; Hendifar A.; Mittra E.; Wolin E. M.; Yao J. C.; Pavel M. E.; Grande E.; Van Cutsem E.; Seregni E.; Duarte H.; Gericke G.; Bartalotta A.; Mariani M. F.; Demange A.; Mutevelic S.; Krenning E. P. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22 (12), 1752–1763. 10.1016/S1470-2045(21)00572-6. [DOI] [PubMed] [Google Scholar]
- Exarchou K.; Stephens N. A.; Moore A. R.; Howes N. R.; Pritchard D. M. New Developments in Gastric Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2022, 24 (1), 77–88. 10.1007/s11912-021-01175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C.; Struthers H.; Winiger C.; Zhernosekov K.; Schibli R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. 10.2967/jnumed.112.107235. [DOI] [PubMed] [Google Scholar]
- Parghane R. V.; Bhandare M.; Chaudhari V.; Ostwal V.; Ramaswamy A.; Talole S.; Shrikhande S. V.; Basu S. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant 177Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2021, 62 (11), 1558–1563. 10.2967/jnumed.120.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschan V. J.; Borgna F.; Busslinger S. D.; Stirn M.; Rodriguez J. M. M.; Bernhardt P.; Schibli R.; Muller C. Preclinical investigations using [177Lu]Lu-Ibu-DAB-PSMA toward its clinical translation for radioligand therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49 (11), 3639–3650. 10.1007/s00259-022-05837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.