Abstract
Aging is associated with changes in circulating levels of various molecules, some of which remain undefined. We find that concentrations of circulating taurine decline with aging in mice, monkeys, and humans. A reversal of this decline through taurine supplementation increased the healthy lifespan in worms and mice, and healthspan in monkeys. Mechanistically, taurine reduced cellular senescence, protected against telomerase deficiency, suppressed mitochondrial dysfunction, decreased DNA damage, and attenuated inflammaging. In humans, lower taurine concentrations correlated with several age-related diseases, and increased after acute endurance exercise. Thus, taurine deficiency may be a driver of aging as its reversal increases healthspan in worms, rodents and primates and lifespan in worms and rodents. Clinical trials in humans seem warranted to test whether taurine deficiency might drive aging in humans.
One-Sentence Summary:
Taurine supplementation extends healthy lifespan
According to the World Population Prospects, the number of people aged 65 and above will increase from 1 in 11 in 2019 to 1 in 6 in 2050 (1). Whilst this is a success of modern medicine and of government policies, it is vital to ensure that the elderly also remain healthy, as this will increase the quality of life and reduce the costs associated with societal aging (2–5). Over the last two decades, efforts to identify anti-aging interventions that reduce morbidity and increase lifespan have intensified (2–11). This has led to the identification of healthy lifespan-increasing compounds such as rapamycin, metformin, nicotinamide adenine dinucleotide (NAD) precursors, and senolytics (2–6, 12).
Aging is a complex process that affects all organs (13, 14). The age-induced decline in organ functions involves several cell-autonomous events termed “hallmarks of aging.” The central hallmarks include genomic instability, deregulated nutrient sensing, mitochondrial dysfunction, stem cell exhaustion, and accumulation of senescent cells (13). Aging associated decline in organ functions also results from changes in the concentrations of endogenous metabolites, hormones, and micronutrients in blood (15–17). However, it is unclear whether these changes are passengers or drivers of aging. If a molecule in blood is a driver of aging, then a correction to its youthful levels would delay aging and increase healthy lifespan.
Taurine (2-aminoethanesulfonic acid), a semi-essential micronutrient, is one of the most abundant amino acids found in organisms across eukaryotic phyla (18–22). In mammalian cells, taurine is produced from cysteine by the action of cysteine sulfinic acid decarboxylase (Csad) (20). Taurine can also be obtained from the diet and is taken up by cells through taurine transporters (20). Taurine deficiency during early life causes functional impairments in skeletal muscle, eye, and the central nervous system (23–26) related to aging-associated disorders. Moreover, concentrations of taurine and its metabolites decline in some tissues with age, and acute taurine supplementation in young animals enhances the functions of several organs (27–35). Given the decline in taurine abundance during aging and its known health effects, we aimed to find out whether taurine deficiency is a driver of aging and affects healthy lifespan.
Results:
Decline of serum concentrations of taurine with age in mice, monkeys, and humans
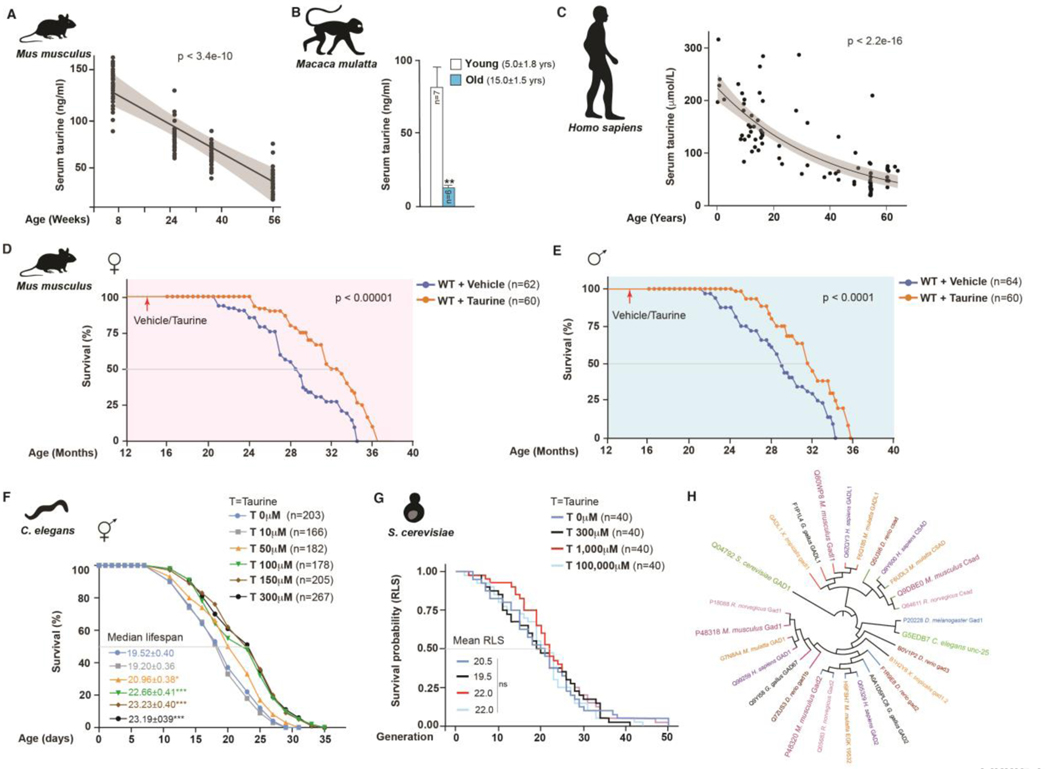
To comprehensively study whether taurine abundance influences healthy lifespan, we measured blood taurine concentrations at different ages in mice, monkeys, and humans. In C57Bl/6J wild-type (WT) mice, serum taurine concentrations declined from 132.3±14.2 ng/mL at 4 weeks to 40.2±7.1 ng/mL at 56 weeks, correlating negatively with age (slope = −25.7; p < 3.4e-10) (Fig. 1A). In 15-year-old monkeys, serum taurine concentrations were 85% lower than in 5-year-old monkeys (Fig. 1B). Likewise, taurine concentrations in elderly humans were decreased by more than 80%, compared with the concentration in serum of younger individuals (Fig. 1C).
Fig. 1. Taurine deficiency is a driver of aging in evolutionarily divergent species.
(A-C) Serum taurine levels in female mice at different ages (A), in young (5-year-old) and old (15-year-old) female monkeys (B), and in humans at different ages (C). (D-E) Lifespan assay of middle-aged (14-month-old) wild-type (WT) female (D) and male (E) C57Bl/6J mice orally fed taurine (T, 1000 mg/kg BW/day) at 10:00 h till the end of life. (F) Lifespan assay of wild-type nematodes that were fed diet supplemented with different concentrations of taurine (0, 10, 50, 100, 150, and 300 μM). (G) Replicative lifespan (RLS) assay in yeast cultured on YPD plates with different concentrations of taurine (0, 300, 1000, and 100,000 μM). (H) Phylogenetic analysis of taurine biosynthesis enzymes in eukaryotes. Statistical analysis: The OASIS software (http://sbi.postech.ac.kr/oasis) was used for calculating p-values using a log rank test (the Mantel–Cox method) in mice and worm experiments. Wilcoxon rank-sum test was used by for calculating p-values in yeast RLS assays. N is represented within panels. All values are mean ± SEM. p ≤ 0.0001****, p ≤ 0.001***, p ≤ 0.01**, and p ≤ 0.05* are versus WT or control.
Taurine supplementation increased lifespan of mice
To determine whether the observed drop in taurine concentration contributes to aging, we orally administered control solution or taurine at 1000 mg/kg body weight (BW) (T1000), once daily at 10:00 h, to 14-month-old (middle-aged) C57Bl/6J WT female and male mice, until the end of their life. The dose and frequency of taurine administration was selected based on a pilot study, which showed that when given once daily to middle-aged WT mice, this regimen increased the peak blood taurine concentrations to baseline concentrations in young (4-week-old) mice (See supplementary materials and methods, and Fig. S1 # A through D for a description of these studies). Regardless of their sex, taurine-fed mice survived longer than control mice (Fig. 1D and E). The median lifespan increase was 10 to 12%, and life expectancy at 28 months increased by 18 to 25% (Fig. 1D and E). Median lifespan estimates for control female and male mice were consistent in two independent cohorts (females: 871 to 885 days; males: 785 to 815 days). In these experiments, both control and taurine-fed mice had ad libitum access to the same chow (Teklad Irradiated 18% protein and 6% fat diet-2918). Thus, the improved survival of taurine-fed mice was not a consequence of low survival of control animals or differences in diet. Collectively, these results indicate that taurine deficiency is a driver of aging in mice as its reversal increases lifespan.
Taurine supplementation increased lifespan of worms but not yeast
The taurine biosynthetic pathway is evolutionarily conserved among multicellular eukaryotes (21, 36). To find out whether taurine also affects aging in species other than mice, we conducted taurine supplementation experiments in lower species. First, we tested the effect of taurine in worms, which also exhibit age-associated decline in taurine (37). Taurine supplementation significantly extended both median and maximum lifespans of C. elegans in a dose-dependent manner (Fig. 1F). Longevity, calculated using the median lifespan of untreated and taurine-treated worms, was extended by 10 to 23% in worms treated with higher taurine concentrations in four independent worm cohorts, and in two independent laboratories (University of Washington, USA, and National Institute of Immunology, India) (Fig. 1F and S1 # E through G). We also investigated the effect of taurine on replicative lifespan (RLS) in budding yeast, S. cerevisiae, a unicellular eukaryote. In contrast to mice and worms, taurine supplementation did not affect RLS (38) of yeast cultured on nutrient-rich yeast extract–peptone–dextrose (YPD) plates or on a synthetic medium (Fig. 1G and S1 # H through J). These results may be explained by organismal differences in taurine metabolism. For example, the taurine metabolism enzymes yeast glutamate decarboxylase (GAD) and mammalian CSAD, diverged early during evolution (Fig. 1H) (39). Thus, although taurine may not affect the RLS in unicellular eukaryotes, its effect on lifespan is conserved in invertebrates and mammals.
Taurine supplementation increased healthspan in aged wild-type female mice
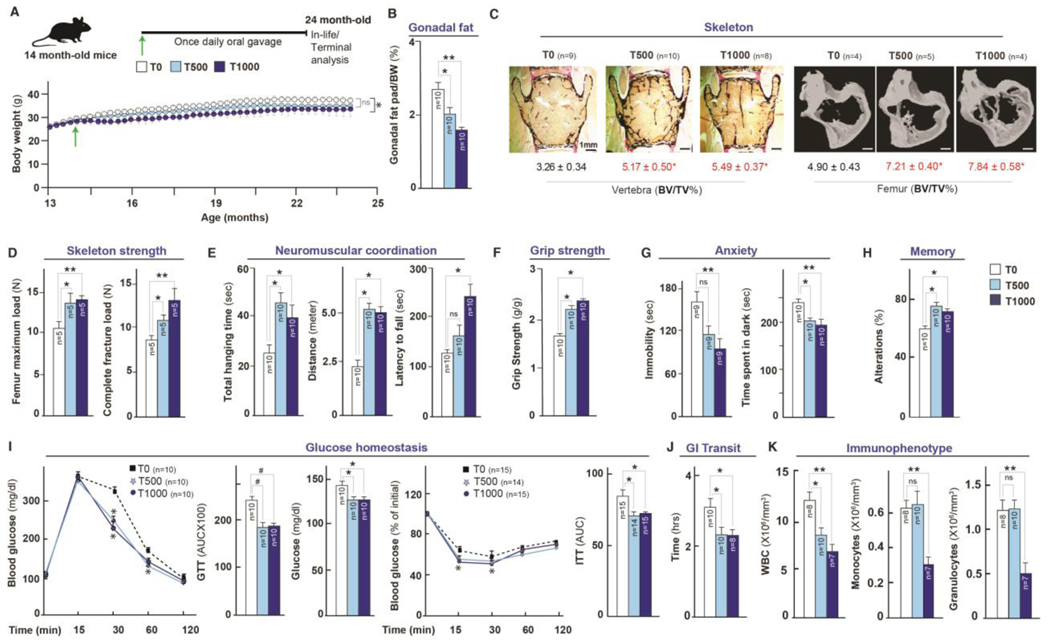
A meaningful anti-aging therapy should improve healthspan (2–5, 40). To assess the effects of taurine supplementation on healthspan, we orally administered taurine at 500 (T500) and 1000 (T1000) mg/kg BW/day to female mice, once daily, starting at the age of 14 months, for 10 to 12 months, and analyzed the health of bone, muscle, brain, pancreas, fat, gut and immune system through functional assays or tissue analysis of deceased animals (Fig. S2A).
Reduced age-associated body weight gain and improved bone mass in female mice treated with taurine
Taurine treatment suppressed age-associated body weight gain by ~10% in T1000 group compared to controls (Fig. 2A). Fat pad weight/BW% was dose-dependently reduced in taurine-treated mice (Fig. 2B). Taurine-administered mice did not differ in body length and food consumption (in weight-stable mice) or suffered obvious toxic effects (as evidenced by blinded histopathological scoring of tissue sections by a trained histopathologist) in multiple tissues compared to controls (Fig. S2 # B through D). Bone structure analysis through histology and micro-computed tomography (μCT) showed that taurine treatment increased bone mass (bone volume over total volume %) in both the spine and femur compared with that in controls (Fig. 2C). A three-point bending test showed that femur maximal load and stiffness—two surrogates of bone quality—improved in taurine-treated mice compared to controls (Fig. 2D). Taurine also cured osteoporosis and suppressed ovariectomy-induced body weight gain in a rodent model of menopause (Fig. S2 # E through G). This latter evidence indicates that effect of taurine on health parameters in females might be linked to its effect on body weight in other conditions of aging, such as menopause.
Fig. 2. Taurine supplementation increases healthspan in aged mice.
(A-K) Changes in body weight (A), Fat % (B), bone structure, and strength parameters in spine and femur (C-D), neuromuscular and muscle strength (E-F, rotarod, wire hang, and grip-strength tests), anxiety (G, tail suspension and dark-light tests), memory (H, Y maze test), pancreas function (I, glucose and insulin tolerance tests), gastrointestinal (GI) transit (J, oral carmine dye test), and immunophenotyping (K, immune cell parameters in blood) in 24-month-old wild-type C57Bl/6J female mice orally fed, once daily with taurine (0, 500 or 1000 mg/kg BW/day) from middle-age (14 months). Statistical analysis was performed using Graph Pad Prism 7. Data were considered statistically significant at p ≤ 0.05 using the Student’s t-test, one-way or two-way ANOVA. n is represented within panels. All values are mean ± SEM. ns, not significant. p ≤ 0.001***, p ≤ 0.01**, and p ≤ 0.05* are versus WT or control.
Increased muscle endurance, coordination, and strength in taurine-treated female mice
Analysis of the effect of taurine treatment on neuromuscular functions showed that total hanging time and distance run in the rotarod test was increased in the T500 and T1000 groups, whereas latency to fall in the wire hang test was increased in the T1000 group (Fig. 2E). Grip strength tests revealed that both doses of taurine increased muscle strength compared to controls (Fig. 2F).
Reduced depression-like behavior and anxiety, and enhanced exploratory behavior and memory in taurine-treated female mice
Increased anxiety and decreased exploration are common age-induced behavioral changes (41). In the tail suspension test (42), taurine-treated mice showed less depression-like behavior compared to controls (Fig. 2G). The light–dark box test (43) revealed that taurine-treated mice spent less time in the dark area, which is indicative of lesser anxiety (Fig. 2G). The Y maze test (44) showed that taurine-treated mice had higher natural curiosity for exploration compared to controls (Fig. 2H).
Improved glucose homeostasis and gastrointestinal transit time in taurine-treated female mice
Analysis of glucose homeostasis using an intraperitoneal glucose tolerance test showed that taurine-treated mice metabolized oral glucose more efficiently than controls, and had lower glucose concentrations in ad libitum-fed mice (Fig. 2I). Likewise, taurine-treated mice had improved insulin sensitivity in the insulin tolerance test (Fig. 2I). These improvements in glucose homeostasis might be a consequence of the reduced adiposity in taurine-treated mice. Gastrointestinal (GI) transit time increases with age (45). Analysis of intestinal transit time using non-absorbable red carmine dye administered by oral gavage (46) showed a faster transit in taurine-treated mice, which could contribute to the observed weight loss in these mice (Fig. 2J).
Ameliorated myeloid-leukocyte prominence in taurine-treated aged female mice
Aging alters immune cell numbers in the blood resulting in increased susceptibility to infection (47). A complete blood count (CBC) showed that taurine treatment decreased the number of white blood cells (WBCs), monocytes, and granulocytes, but not red blood cells (Fig. 2K and S2H). Although there was no difference in the efficacy of T500 and T1000 doses on the WBC numbers, the number of monocytes and granulocytes was only decreased at T1000 (Fig. 2K). These results show that myeloid-leukocyte prominence associated with aging-related inflammatory states is ameliorated by high-dose taurine treatment.
Improved healthspan metrics in middle-aged male WT mice after taurine administration
To assess whether taurine affects healthspan of male mice, as it does in female mice, we treated 14-month-old WT male mice with or without T1000 for 8 to 16 weeks and measured fat, bone, muscle, pancreas, and brain health (Fig. S3A). Taurine did not affect body weight gain in males up to 16 weeks but significantly reduced fat pad weight/BW% compared to controls (Fig. S3 # B and C). To identify the cause of the reduced adiposity of taurine-treated mice, we analyzed energy expenditure. Taurine-treated mice consumed more oxygen, generated more carbon dioxide, and had higher respiratory exchange ratios (RER) and energy expenditures even though their total activity was decreased compared to that of controls (Fig. S3 # D through H). Taurine-treated male mice also showed greater muscle strength, neuromuscular co-ordination, bone density, glucose tolerance, and memory, and reduced anxiety compared with controls (Figs. S3 # I through N).
In summary, taurine supplementation improved the function of every organ investigated in middle-aged female and male mice, and likely increased overall healthspan.
Effects of taurine on cellular mechanisms in increasing healthy lifespan
What are the mechanisms through which taurine affects cellular functions to increase healthy lifespan? To address this question, we performed an RNA-sequencing analysis in taurine-deficient and control osteoblasts. These bone-forming cells were chosen because they abundantly express a taurine transporter (Slc6a6), whose deletion impairs differentiation and function of mutant cells in culture and in mice (Fig. S4 # A through E). Conversely, numbers and function of WT osteoblasts were increased by taurine treatment in vitro and in vivo. (Fig. S4 # A through E). RNA-Seq analysis (48) of taurine-deficient osteoblasts showed that the top biological functions identified in the gene set enrichment analysis (GSEA) are related to aging mechanisms (13) such as telomere function, oxidative stress, immune system function, protein translation, and stem cell maintenance (S4 # F through M and 3A). A search for the term “aging” in the GSEA pathways output showed significant alterations in six gene signatures (See table S1 for details). All six signatures showed expected direction of change (up or down-regulation) for a pro-aging effect (Fig. S4N). Together, these results imply that taurine deficiency generates an aging-related transcriptomic signature in cells.
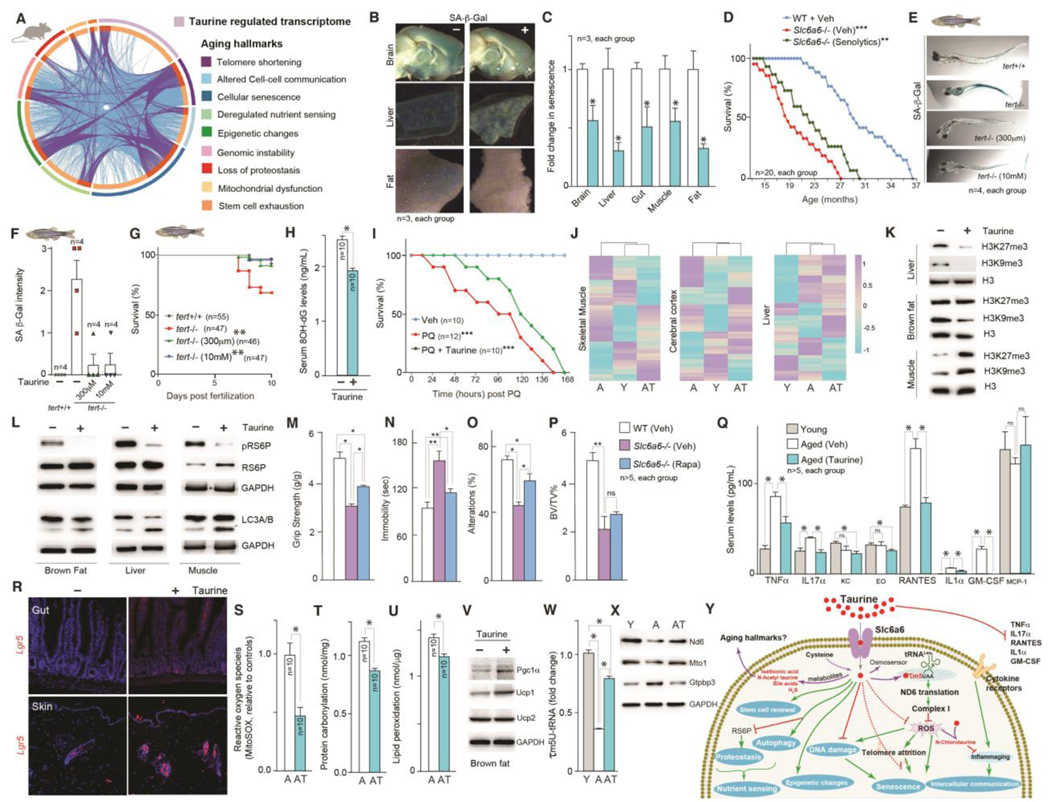
Fig. 3. Taurine regulation of healthy lifespan is associated with alterations in multiple aging hallmarks.
(A) Circos plot representing a comparative analysis of taurine-deficient transcriptome with the core gene signatures of nine aging hallmarks. (B-C) Senescence-associated beta-galactosidase (SA-β-Gal) staining (blue-stained cells) (B) and relative quantification of staining (C) in tissues collected from mice with or without taurine supplementation. (D) Lifespan assay of congenitally taurine-deficient (Slc6a6-/-) mice and littermate controls that received either vehicle or senolytics (dasatinib [D] + quercetin [Q]) bi-weekly till the end of their life. (E-G) SA-β-Gal staining photomicrographs (E), relative quantification of staining (F), and survival analysis (G) of telomerase deficient [tert-/-(G2)] zebrafish embryos with or without taurine supplementation (300 μm or 10 mM) from 2 days post-fertilization. (H) Serum 8-OH-dG concentrations in vehicle- or taurine-treated mice. (I) Kaplan–Meier survival curves for mice following paraquat, with or without prior taurine supplementation (T1000, for 1 month). (J-K) Comparative DNA methylation levels of 2045 age-related CpG sites in the muscle, cerebral cortex, and liver (J) and changes in histone H3K27me3, H3K9me3, and H3 levels in the liver, brown fat, and muscle (K) of 4-month-old WT (Young, Y), 16-month-old vehicle-treated WT (Aged, A), and 16-month-old taurine-treated WT (Aged-Taurine, AT) mice. (L) Changes in phospho-ribosomal S6 protein (pRS6P) and LC3A/B levels in the brown fat, liver, and muscle of vehicle- or taurine-treated aged mice. (M-P) Changes in muscle function (M, grip-strength test), anxiety (N, tail suspension test), memory (O, Y maze test), and bone mass (P, BV/TV %) in 6-month-old congenitally taurine-deficient (Slc6a6-/-) mice and littermate controls that received either vehicle or rapamycin (once-daily, for 6 weeks). (Q) Serum levels of various cytokines in young, aged, and aged mice treated with taurine. (R) In situ hybridization analysis of Lgr5 expression in the gut and skin (R), levels of mitochondrial ROS (superoxide anion radicals, MitoSOX assay) in skeletal muscle mitochondria (S), protein carbonyl levels in the liver (T), lipid peroxidation levels in the liver (U), Pgc1α, Ucp1, and Ucp2 levels in the brown fat (V) of aged mice treated without or with taurine. (W-X) Changes in 5-taurinomethylUridine (τm5U) tRNA modification (W), and Nd6, Mto1, and Gtpbp3 protein levels in the liver (X) of young, aged, and aged mice treated with taurine. (Y) Schematic representation of the effect of taurine and taurine-derived biomolecules (in red) on classical hallmarks of aging. n ≥ 6 mice in each group. Western blots are representative of at least three independent biological replicates. Statistical analysis: For panels D, G, I, the OASIS software (http://sbi.postech.ac.kr/oasis) was used for calculating p-values using a log rank test (the Mantel–Cox method). For other panels, statistical analysis was performed using Graph Pad Prism 7 employing Student’s t-test, one-way or two-way ANOVA. All values are mean ± SEM. ns, not significant. p ≤ 0.0001****, p ≤ 0.001***, p ≤ 0.01**, and p ≤ 0.05* are versus WT or control.
Suppression of senescence by taurine
A network analysis of taurine-regulated genes showed that senescence-associated secretory phenotype (SASP) genes, such as p16 and p21, which encode inhibitors of cyclin-dependent kinases and promote cell cycle arrest, formed the highest number of genetic interactions (Fig. S4O). Consistent with the idea that taurine suppresses senescence, irradiation-induced increase in senescence-associated beta-galactosidase (SA β-Gal) staining in osteoblasts cultured with taurine was about one fourth of that in cells cultured without taurine (Fig. S4P). In neuronal culture experiments, taurine supplementation increased neuronal survival after treatment with paraquat, a DNA damaging agent that induces senescence (49) (Fig. S4Q). Moreover, taurine supplementation decreased age-associated increase in senescence in mice (Fig. 3 # B, C, and S5A). To test whether taurine deficiency causes accumulation of senescent cells, we used mice lacking the taurine transporter Slc6a6 (23). Lack of Slc6a6 compromises taurine entry into embryonic cells, rendering embryos taurine-deficient. The phenotypes observed postnatally in 0.5- to 3-month-old Slc6a6 mutant mice (23), could be due to taurine deficiency affecting these phenotypes during development or postnatally (hereinafter, we refer to these mice as congenitally taurine-deficient mice). Adult Slc6a6-/- mice showed accelerated aging-related phenotypes, including decreased bone density, poor neuromuscular coordination, compromised muscle strength, increased anxiety, and decreased memory (Fig. S5 # C through L). Analysis of bone, muscle, brain, fat and liver showed increased senescence in taurine-deficient mice compared to controls (Fig. S5 # A and B). To investigate whether accumulation of senescent cells in these organs contributes to the compromised healthspan of taurine-deficient mice, we treated 8-month-old Slc6a6-/- mice with or without a combination of senolytics, dasatinib (D) (50) and quercetin (Q), bi-monthly for 4 months. Relative to controls, D+Q-treated Slc6a6-/- mice had lower abundance of SASP markers (Fig. S5M). D+Q treatment also improved bone-, muscle-, anxiety- and memory-related parameters in Slc6a6-/- mice (Fig. S5 # N through Q). Taurine-deficient mice had shorter lives than WT mice, and, the median lifespan of mutant mice that received senolytic treatment until the end of their life increased by ~21% (Fig. 3D). The finding that senolytic treatment did not rescue shorter lifespan of taurine-deficient mice suggests that taurine also affects other factors besides senescence. We, therefore, assessed molecular and cellular features of other aging hallmarks in taurine-supplemented middle-aged mice, and taurine-deficient mice.
Taurine suppressed adverse consequences of telomerase deficiency
Replication-based telomere shortening triggers cellular senescence and affects aging (51). Taurine supplementation in mice or zebrafish or its deficiency in mice did not affect telomerase gene expression (Fig. S5 # R and S). To investigate whether taurine affects telomerase deficiency-induced deterioration in organismal health, we used a zebrafish model of telomerase deficiency (52). tert-/-(G2) fish show an increase in senescence and ~40% of them die within 10 days post-fertilization (dpf) (52). Supplementing the medium used for tert-/-(G2) fish with taurine, starting 2 dpf, suppressed senescence (Fig. 3 # E and F). Moreover, at 300 μM and 10 mM taurine rescued the lethality in tert-/-(G2) zebrafish embryos (Fig. 3G).
Taurine suppressed DNA damage and improved survival of mice after oxidative DNA damage
Aging is associated with genomic DNA lesions in multiple cell types (53). Taurine supplementation reduced serum 8-hydroxydeoxyguanosine (8-OH-dG) abundance, a measure of oxidative DNA damage (54), in aged mice (Fig. 3H). Conversely, DNA damage (measured as abundance of phospho-γ-H2A histone family member X [H2Ax]) was increased in the muscle of taurine-deficient mice (Fig. S5T). In a paraquat model of DNA damage-induced lethality, mice administered with paraquat without prior taurine supplementation died within 150 h, but mice treated with taurine lived slightly longer (Fig. 3I). Thus, taurine supplementation suppressed DNA damage and improved survival of mice after oxidative DNA damage.
Taurine affects epigenetic changes in the genome
Methylation at CpG sites and in histones changes with age and affects the state of chromatin, which affects DNA packaging and gene expression (55, 56). We, therefore, analyzed changes in methylation of 2045 CpG sites and measured two histone modifications (histone 3 lysine 9 trimethylation [H3K9me3] and histone 3 lysine 27 trimethylation [H3K27me3]) in multiple tissues obtained from middle-aged mice with or without taurine supplementation and compared them with those in young mice. Clustering analysis showed that the CpG methylation pattern in the muscle and cerebral cortex of taurine-treated old mice was more similar to that in young mice than to untreated old mice (Fig. 3J). However, the pattern in liver from taurine-supplemented mice was more similar to that in old mice than in young mice (Fig. 3J). Conversely, muscles from taurine-deficient mice showed changes in the amount of CpG site methylation, and the DNA methylation pattern of muscles in 70-week-old mutant mice was similar to that of 206-week-old WT mice (Fig. S5U). Taurine treatment decreased the abundance of H3K9me3 in brown fat and liver but increased it in skeletal muscle; H3K27me3 abundance was suppressed in the liver, increased in muscle, and unaffected in brown fat (Fig. 3K). The varied changes in DNA and histone methylation indicate that taurine may affect chromatin conformation, which could contribute to altered transcription during aging.
Taurine modulated nutrient sensing and proteostasis pathways
Aging cells have reduced ability to sense nutrients and maintain proteostasis (57). We assessed changes in nutrient sensing by measuring the phosphorylation of ribosomal S6 protein (RS6P), a key regulator of ribosomal function, and proteostasis by measuring changes in abundance ratio of isoforms A and B of the light chain 3 (LC3A/B), an autophagy marker. Taurine supplementation significantly decreased phosphorylation of RS6P in the liver, brown fat, and skeletal muscle (Fig. 3L). Phosphorylation of RS6P was increased in muscle of taurine-deficient mice (Fig. S5V). Taurine-supplemented mice had more autophagy (as judged by LC3A/B abundance) in the liver, brown fat, and skeletal muscle, whereas it was decreased in taurine-deficient mice (Fig. 3L and S5V). To test whether an increase in phosphorylation of RS6P and a decrease in autophagy contribute to the compromised healthspan in taurine-deficient mice, we treated Slc6a6-/- mice with or without rapamycin [8 mg/kg BW, once-daily, i.p. (58) for 6 weeks], which inhibits phosphorylation of RS6P and increases autophagy. Compared with control mice, rapamycin-treated taurine-deficient mice showed improved muscle-, anxiety-, and memory-related parameters, but not bone mass (Fig. 3 # M through P). Thus, effects of taurine supplementation on nutrient sensing and proteostasis pathways contribute to its beneficial effects on several health parameters.
Taurine effects on inflammatory cytokines
Intercellular communication is compromised with age (59). One example is the accumulation of proinflammatory and other cytokines (59). Serum concentrations of tumor necrosis factor alpha (TNFμ), interleukin 17 alpha (IL17μ), regulated upon activation, normal T cell expressed and presumably secreted (RANTES), interleukin 1 alpha (IL1μ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were increased in middle-aged mice compared to young mice, but taurine-treated middle-aged mice had amounts of these cytokines similar to those in young control animals (Fig. 3Q). These results, together with the observation that the ratio of myeloid cells to lymphoid cells was significantly decreased in taurine-supplemented mice (Fig. 2K), indicates that sustained taurine concentrations help prevent to proinflammatory state observed during aging.
Positive effects of taurine on health of stem cells or their renewal
Aging reduces the ability of tissues to regenerate after injury. This is linked to defects in tissue-specific stem cells (60). We analyzed changes in the number of stem cell populations through staining for leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), a wingless-related integration site (Wnt) target gene expressed in the stem or progenitor cells (61), in the gut epithelium and hair follicles obtained from middle-aged mice supplemented with or without taurine. The number of Lgr5+ cells in these two tissues was increased by taurine supplementation (Fig. 3R). Conversely, the number of Lgr5+ cells in the gut epithelium and hair follicles was decreased in taurine-deficient mice compared to controls (Fig. S5W). Thus, taurine supplementation may increase the regenerative capacity of some tissues by increasing the number of resident stem cells.
Taurine promotion of mitochondrial health
Compromised mitochondrial biogenesis and oxidative capacity leads to progressive accumulation of reactive oxygen species (ROS)-mediated damage that contributes to aging (62). ROS accumulation in mitochondria isolated from the muscle of taurine-treated middle-aged mice was decreased compared to controls (Fig. 3S), whereas it was increased in taurine-deficient mice (Fig. S6A). Measurement of lipid peroxidation and protein carbonylation, two indirect markers of ROS-induced molecular damage, in the liver showed a decrease (by ~22% and ~11%, respectively) in taurine-supplemented mice compared to controls (Fig. 3 # T and U). Assessment of abundance of peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (Pgc1α), a key regulator of mitochondrial biogenesis, and uncoupling protein 1 (Ucp1), which uncouples mitochondrial fuel oxidation and respiration from ATP production (63), in brown fat showed increased amounts in taurine-treated middle-aged mice, and their abundance was decreased in taurine-deficient mice (Fig. 3V and S6B). These results indicate that taurine promotion of mitochondrial homeostasis may contribute to its effect on health.
We next investigated how taurine affects cellular mechanisms during aging (24). One pool of cytosolic taurine is actively transported into mitochondria where it is conjugated to the uridine residue at the wobble position of tRNALeu(UUA), forming 5-taurinomethyluridine-tRNALeu(UUA) (τm5U-tRNA) (64). τm5U modification is specific to mitochondrial tRNAs (64), and promotes the translation of electron transport chain complex I subunit, NADH-ubiquinone oxidoreductase chain 6 protein (ND6) (64). We, therefore, measured whether τm5U tRNA modification changed during aging in mice. The τm5U content of tRNAs was reduced by >60% in aged liver compared to young liver, and taurine supplementation prevented this decline to only about 20% (Fig. 3W and S6C). Consistent with the role of τm5U-tRNALeu in promoting the translation of ND6, amounts of this protein were decreased in aged mice compared to young mice, and were increased by taurine supplementation (Fig. 3X and S6D). Taurine supplementation, however, did not affect translation of nuclear DNA-encoded mitochondrial OXPHOS proteins in aged mice (Fig. S6E). We conducted experiments on worms to test whether regulation of organismal health by taurine requires complex I activity. Taurine increased the motility of control worms, indicative of better health status (65), but failed to do so in rotenone-treated worms (Fig. S6F), suggesting that a mechanism whereby taurine promotes health is by increasing the mitochondrial complex I activity.
The aforementioned analyses of molecular and cellular features of aging hallmarks show that during aging taurine supplementation may impart health benefits by affecting such features in various cells or tissues (Fig. 3Y).
Lower circulating taurine and its metabolites in humans are associated with multiple age-associated pathologies, and their abundance increases after an exercise regimen
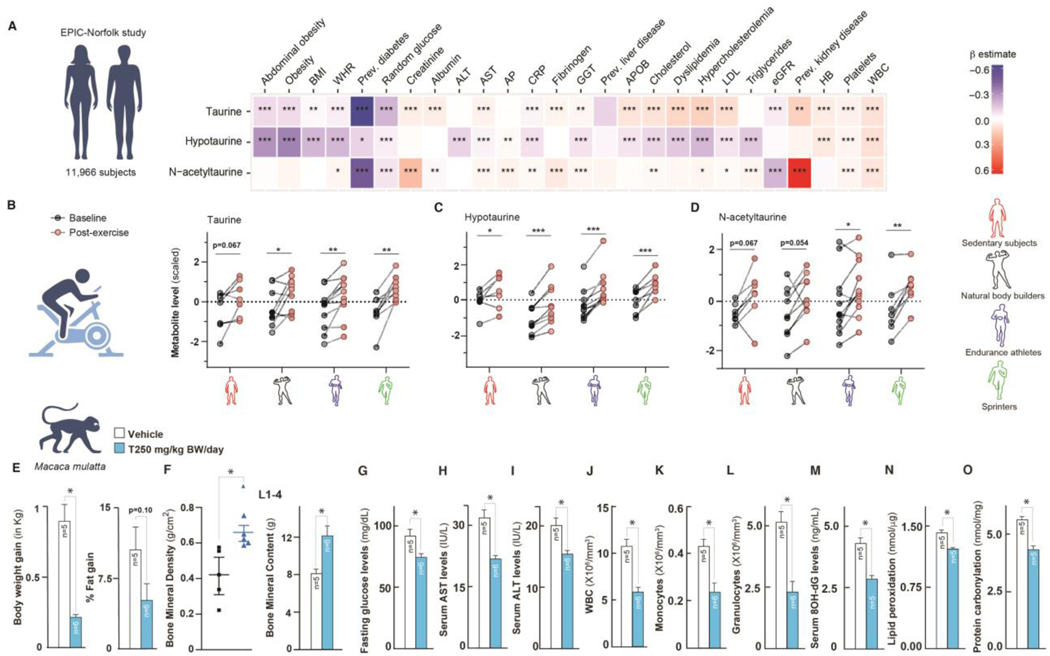
To determine whether blood levels of taurine pathway metabolites (taurine, hypotaurine, and N-acetyltaurine) are associated with health variables in humans, we performed an association analysis of circulating taurine metabolite levels with >50 clinical risk factors in 11,966 subjects of the EPIC-Norfolk study (Fig. S7 # A and B) (66). We found that higher blood taurine and hypotaurine levels were associated with lower body mass index (BMI) and waist-to-hip ratio (WHR), and less abdominal obesity (Fig. 4A). Furthermore, higher levels of taurine metabolites associated with a lower prevalence of type 2 diabetes and lower glucose levels (Fig. 4A). Also, higher taurine and hypotaurine levels were associated with lower levels of the inflammation marker, C-reactive protein (CRP). For liver- and lipid-related traits such as aspartate aminotransferase (AST) and blood cholesterol, we found positive associations with taurine levels but negative associations with those of its precursor hypotaurine (Fig. 4A). Blood cell parameters like hemoglobin, platelets, and WBC count correlated positively with the three taurine metabolites (Fig. 4A). Association does not establish causation, but these results are consistent with taurine deficiency contributing to human aging.
Fig. 4. Taurine pathway affects healthspan in primates.
(A) Heatmap showing the results from linear regression models for assessing the associations between clinical risk factors and taurine-related metabolites (taurine, hypotaurine, and N-acetyltaurine) in blood from 11,966 subjects in the EPIC-Norfolk study. Effect size and direction of these associations are given by the β-estimates resulting from these regression models. A negative β-estimate (blue color) indicates an inverse association, where higher levels of a metabolite correlated with lower levels of a clinical parameter. A positive β-estimate (red color) indicates a positive association, where higher levels of a metabolite correlated with higher levels of a clinical parameter. For example, as shown in blue, higher levels of taurine correlated with lower prevalence of type 2 diabetes. Taurine-related metabolites were measured using an untargeted metabolomics approach (Metabolon HD4 platform). Data were extracted from the open-access web server (https://omicscience.org/apps/mwasdisease/). BMI, Body mass index; WHR, waist-to-hip ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; CRP, C-reactive protein, APOB, apolipoprotein B; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; HB, hemoglobin; WBC, white blood cell count. (B-D) Serum taurine (B), hypotaurine (C), and N-acetyltaurine (D) levels at fasted rest (=baseline) and 5 minutes after a maximum graded exercise test (= post-exercise) in three groups of competitive athletes and healthy sedentary subjects. Metabolite levels are provided as z-scores, i.e., relative to the mean of measured levels with mean = 0 and standard deviation = 1. (E-O) Body weight gain in kilogram and % fat gain (E), bone mineral density and content in Lumber 1–4 (F, bone), fasting glucose levels (G, pancreas function), serum AST and ALT levels (H-I, liver dysfunction markers), WBC/monocyte/granulocyte numbers (J-L, immunophenotyping in blood), serum 8-OH-dG, lipid peroxide, and protein carbonyl levels (M-O, indirect markers of ROS-induced molecular damages) in 15-year-old monkeys orally fed once-daily with vehicle (T0) or taurine (T250) for 6 months. Statistical analysis: (A) Summary statistics, including standardized regression coefficients (β-estimates) and nominal p-values on a relevant subset of 26 clinical traits and three taurine-related metabolites were extracted from the web server. Regression coefficients and nominal p-values were plotted in a heatmap using R version 4.1.0. Statistical analysis for the exercise cohort (B-D): Differences between baseline and post-exercise metabolite levels were analyzed per subject group using a paired sample t-test. Batch corrections were done using R version 4.1.0; the graphs were prepared using GraphPad Prism. For other panels (E-O), statistical analysis was performed using Graph Pad Prism 7 employing the Student’s t-test, one-way or two-way ANOVA. All values are mean ± SEM. ns, not significant. p ≤ 0.0001****, p ≤ 0.001***, p ≤ 0.01**, and p ≤ 0.05* are versus WT or control.
We next investigated whether blood levels of taurine pathway metabolites respond to exercise, which improves many health- and aging-related variables (67, 68). Specifically, we analyzed concentrations of taurine pathway metabolites in serum before and after a graded exercise test in male athletes (sprinters, endurance runners, and natural bodybuilders), and sedentary individuals (Fig. S7C). Taurine levels significantly increased (1.16-fold) in response to a graded cycle exercise test in all the investigated athlete groups (pBodybuilding = 0.046, pEndurance = 0.0021, pSprint = 0.0017) (Fig. 4B), and tended to be higher in the sedentary subjects although the change was not significant (pSedentary = 0.067) (Fig. 4B). Levels of hypotaurine were significantly increased 1.36-fold in response to exercise in all subjects (Fig. 4C). Levels of N-acetyltaurine were significantly increased by 1.18-fold and 1.28-fold in endurance athletes (p = 0.027) and sprinters (p = 0.0016), respectively, and tended to be elevated in bodybuilders and sedentary subjects although the change was not significant (pBodybuilders = 0.054, pSedentary = 0.067) (Fig. 4D). These results are consistent with the idea that an increase in taurine and taurine-related metabolites might mediate some of the health benefits of exercise.
Taurine supplementation improved health parameters in middle-aged non-human primates
To test whether taurine has health and anti-aging effects in non-human primates, we fed aged rhesus monkeys (15 ± 1.5-years-old, equivalent to 45 to 50 years human age), control solution or taurine (250 mg/kg BW [T250], equivalent to T1000 in mice) at 10:00 h once daily for 6 months, and then measured the health variables (Fig. S7D). Prior to the start of taurine supplementation, body weight and bone density were not significantly different in the two groups of aged monkeys (Fig. S7 # E and F). Three-hour after oral feeding, serum taurine concentrations in taurine-fed monkeys were approximately twice (65.4 ± 10.1 ng/mL) compared to levels in controls (35.1 ± 7.3 ng/mL). Monkeys that received taurine gained 0.75 kg less body weight, and their fat % tended to be lower compared to controls (Fig. 4E). In-life dual-energy X-ray absorptiometry (DEXA) analysis after 6 months of taurine treatment showed that taurine increased bone density and content at the lumbar spine (L1–4) and legs, but not in the head compared to controls (Fig. 4F and S7 # G through H). Serum markers of bone formation (osteocalcin) increased, whereas those of resorption (C-terminal telopeptide of type 1 collagen [Ctx]) decreased approximately 16 weeks after the start of treatment; these levels were maintained until the end of the dosing period (Fig. S # 7 I through J). Taurine treatment reduced fasting blood glucose concentrations by 19% (Fig. 4G). Taurine also reduced the serum concentrations of liver damage markers, aspartate aminotransferase (AST) and alanine transaminase (ALT) by ~36% and 20%, respectively (Fig. 4 # H through I). Numbers of WBCs, monocytes, and granulocytes, which increase with age, were decreased by ~50% in taurine-treated monkeys compared to controls (Fig. 4 # J through L). Consistent with the beneficial effect of taurine on the mitochondrial health observed in worms and mice, indirect markers of ROS-induced molecular damage, DNA 8OH-dG, lipid peroxide, and protein carbonyl concentrations, were decreased by ~36%, 11%, and 20%, respectively, in the sera of taurine-supplemented monkeys (Fig. 4 # M through O). Thus, taurine has beneficial effects on most tested health parameters (body weight, bone, glucose, liver, and immunophenotype) in non-human primates.
Discussion:
Taurine abundance decreases in blood and tissues during aging. We find that a reversal of this decline through taurine supplementation increased markers of healthy lifespan in worms and mice, and healthspan in monkeys, identifying taurine deficiency as a driver of aging in these species. In mice, the effect of taurine supplementation on healthy lifespan was greater in females than in males, indicating that sex-specific pathways may mediate taurine action. The optimal dose of taurine to maximize its efficacy differed depending on the physiological functions tested possibly due to a wide variation in the uptake rate, synthesis, and metabolism of taurine in different biological fluids and tissues (24, 69–76).
Taurine appeared to impact all the established hallmarks of aging. Although we do not yet know the initial events that taurine elicits, we provide evidence for the suppressed taurinylation of mitochondrial tRNAs during aging in mitochondrial dysfunction, a prominent feature of aging. It is also possible that other taurine-derived biomolecules, besides (τm5U-tRNA), may directly or indirectly affect mitochondrial homeostasis or other aging features. Indeed, taurine contributes to production of several other biomolecules, depending on the cell type(s) that affect, or can potentially affect, aging (24). These molecules include N-chloro-taurine (77), hydrogen sulfide (H2S) (78), isethionic acid (24), N-acetyl taurine (79), and 5-taurinomethyl-2-thiouridine (τm5s2U)-tRNALys (24). We propose that a combination of taurine and taurine-derived biomolecules may delay aging by affecting various aging hallmarks in distinct cells and tissues.
The effects of taurine intervention on aging and congenital taurine deficiency in a mouse model are largely consistent, except for body weight accrual and glucose homeostasis (Fig. 2 and S5). The concentrations of taurine in serum and tissues of congenitally taurine-deficient mice are more severely reduced than in biological fluids and tissues of aged rodents and humans (23, 27, 80). However, in the liver the concentrations of τm5U, a downstream conjugate of taurine, was similarly affected. Thus, during early life, taurine appears to be essential for homeostasis in several organ systems and its deficiency during development may compromise these functions postnatally. Consistent with this hypothesis, organisms have 3- to 4-fold higher taurine concentration in embryonic than in adult tissues; moreover, taurine deficiency during development leads to growth retardation, blindness and osteoporosis (25, 81), and its supplementation during gestation increased bone mass postnatally (Fig. S5X). This role of taurine in embryonic tissues affecting postnatal phenotypes would be consistent with the theory of developmental origin of aging phenotypes (82, 83). It is possible that developmental or postnatal changes in taurine metabolism might affect the rate of aging during late life, and adjusting this endogenous machinery might extend healthy lifespan.
In humans, lower levels of taurine pathway metabolites were associated with multiple age-associated diseases, such as obesity, diabetes and inflammation (Fig. 4A). In the FinnGen database (Freeze R5, http://r2.finngen.fi/), polymorphisms in the taurine biosynthesis gene, CSAD, are associated with hypertension (Fig. S7K), and SLC6A6 mutations cause retinal degeneration and cardiomyopathy (26, 84). However, taurine supplementation in subjects with metabolic abnormalities does not affect BMI (85). Furthermore, our results, together with those of previous studies (86, 87), show that taurine concentrations increase in healthy men after acute endurance exercise, and following 24-weeks of exercise training in obese individuals. Whilst the mechanisms that increase the blood taurine concentration after exercise are unclear, it suggests that some of the health benefits of exercise may be explained by an increase in the blood taurine concentrations.
A limitation of our study is that we have not tested the effect of taurine in male monkeys, and our association studies in humans did not distinguish between sexes. Nevertheless, together with our supplementation studies in 15-year-old monkeys, these results suggest that an increase in taurine concentrations or its actions may have the potential to suppress the decline in biological functions during human aging.
Reversal of taurine deficiency during aging may be a promising anti-aging strategy. Given that taurine has no known toxic effects in humans (though rarely used in concentrations used here), can be administered orally, and affects all the major hallmarks of aging, human trials would be warranted to examine whether taurine supplementation increases healthy lifespan in humans.
Methods summary:
Lifespan analysis. Mice: Lifespan analysis was performed in middle-aged mice administered once-daily oral taurine supplementation with or without other interventions. Yeast: Replicative life span of yeast was assessed on nutrient-rich YPD plates or on a synthetic medium with or without taurine. Worms: Life span of worms was assessed on agar plates supplemented with or without taurine. Healthspan analysis: Functions and health of various organs in middle-aged mice and monkeys was assessed following taurine supplementation and included the following: body weight; fat pad weight; bone histology and μCT or DEXA measurements of bone; rotarod, wire-hang and grip strength tests of neuro-muscular strength; glucose and insulin tolerance tests of glucose homeostasis; tail suspension, light-dark box and Y maze tests of behavior; GI transit test; energy expenditure tests; and blood count of immune cells. Aging hallmarks were assessed in WT middle-aged mice supplemented with taurine, taurine-deficient mice, telomerase-deficient zebrafish, and worms. This analysis included assessment of senescence through SA-β-Gal staining, SASP markers, irradiation, and senolytic intervention in taurine-deficient mice; DNA damage assessment using molecular markers and paraquat-induced lethality assays; telomere function was assessed using telomerase expression in mice and zebrafishes, and telomerase-deficient zebrafishes; epigenetic changes were assessed based on CpG and histone methylations; nutrient sensing and proteostasis were assessed through phospho-RS6P measurements, autophagy marker analysis through LC3A/B abundance, and rapamycin intervention in taurine-deficient mice; mitochondrial function was assessed through ROS measurements; electron transport chain assessments, OXPHOS western blotting, and rotenone assay in worms; stem cells were assessed using Lgr5 in-situ hybridization; cytokine levels were measured in the blood. Human association analysis of taurine pathway metabolites with health variables was performed in individuals from the EPIC-Norfolk study. Effect size and direction of these associations are given by the β-estimates resulting from these regression models. A negative β-estimate (blue color) indicates an inverse association, where higher levels of a metabolite correlated with lower levels of a clinical parameter. A positive β-estimate (red color) indicates a positive association, where higher levels of a metabolite correlated with higher levels of a clinical parameter. Effect of exercise in humans on serum levels of taurine pathway metabolites was assessed before and after an endurance exercise test in athletes (sprinters, body builders, and marathon runners) and sedentary individuals. A detailed account of the methods and statistical analysis used in this study is provided in the supplementary materials.
Supplementary Material
Acknowledgments:
We thank Drs. Renn for histology, Liu for genotyping, Surender for monkey experiments, and Karsenty and Mahajan for facilities. VKY dedicates this study to his mother, Bhagwanti Devi for showing the path of perseverance.
Funding:
Nathan Shock Center of Excellence in the Basic Biology of Aging Project Grant (VKY),
NIH R01HD107574 (VKY),
Wellcome 098051 (VKY),
DFG 450149205-TRR333/1 (PB, HW),
NIH P30AG013280 (MK),
NIH T32AG066574 (MGK),
INCa, PLBIO21-228 (MGF),
SERB STR/2019/00064 (AM),
DBT BT/PR40325/BTIS/137/1/2020 (BKB),
Longevity Impetus Grant (AK),
The Academy of Finland Center of Excellence in Complex Disease Genetics Grant # s 312074, 336824, 352793 (AP),
The Sigrid Juselius Foundation (AP),
The Larry L. Hillblom Foundation Fellowship (MC),
VCA Fellowship MCRF21002 (BP),
DBT Ramalingaswamy Fellowship (VKY)
Footnotes
Competing interests: Columbia University has filed provisional patent applications for which VKY is an inventor. The remaining authors declare no competing interests.
Data and materials availability:
All data are available in the main text or the supplementary materials. The codes used for data analysis is stored publicly at github, stemangiola/singh_et_al_taurine_bone. Sequencing scaled counts have been deposited at 10.5281/zenodo.7700452.
References:
- 1.D. o. E. a. S. A. United Nations, Population Division, World populations ageing 2019, highlights. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf, (2019).
- 2.Mkrtchyan GV et al. , ARDD 2020: from aging mechanisms to interventions. Aging (Albany NY) 12, 24484–24503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gems D, Partridge L, Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75, 621–644 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. et al. , From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer K, Yankner BA, Slowing Down Aging. Cell Metab 26, 592–593 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Pan H, Finkel T, Key proteins and pathways that regulate lifespan. J Biol Chem 292, 6452–6460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haigis MC, Yankner BA, The aging stress response. Mol Cell 40, 333–344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rando TA, Chang HY, Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell 148, 46–57 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn EH, Greider CW, Szostak JW, Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12, 1133–1138 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Lamming DW, Ye L, Sabatini DM, Baur JA, Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 123, 980–989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabral WA et al. , Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome. Aging Cell 20, e13457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedernhofer LJ, Robbins PD, Senotherapeutics for healthy ageing. Nat Rev Drug Discov 17, 377 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkwood TB, Understanding the odd science of aging. Cell 120, 437–447 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Imai SI, Guarente L, It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2, 16017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y. et al. , The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1, e00065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asadi Shahmirzadi A. et al. , Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab 32, 447–456 e446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripps H, Shen W, Review: taurine: a “very essential” amino acid. Mol Vis 18, 2673–2686 (2012). [PMC free article] [PubMed] [Google Scholar]
- 19.Spriet LL, Whitfield J, Taurine and skeletal muscle function. Curr Opin Clin Nutr Metab Care 18, 96–101 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Lambert IH, Kristensen DM, Holm JB, Mortensen OH, Physiological role of taurine--from organism to organelle. Acta Physiol (Oxf) 213, 191–212 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Hebert A. et al. , New insights into sulfur metabolism in yeasts as revealed by studies of Yarrowia lipolytica. Appl Environ Microbiol 79, 1200–1211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiedemann L. FG, Einige neue Bestandtheile der Galle des Ochsen. . Ann. Physik. Chem. 9, 326–337 (1827). [Google Scholar]
- 23.Warskulat U. et al. , Phenotype of the taurine transporter knockout mouse. Methods Enzymol 428, 439–458 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen JG, Smith LH, Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48, 424–511 (1968). [DOI] [PubMed] [Google Scholar]
- 25.Hayes KC, Carey RE, Schmidt SY, Retinal degeneration associated with taurine deficiency in the cat. Science 188, 949–951 (1975). [DOI] [PubMed] [Google Scholar]
- 26.Preising MN et al. , Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J 33, 11507–11527 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Eppler B, Dawson R Jr., Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol 62, 29–39 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Stuerenburg HJ, Stangneth B, Schoser BG, Age related profiles of free amino acids in human skeletal muscle. Neuro Endocrinol Lett 27, 133–136 (2006). [PubMed] [Google Scholar]
- 29.Suarez LM, Munoz MD, Martin Del Rio R, Solis JM, Taurine content in different brain structures during ageing: effect on hippocampal synaptic plasticity. Amino Acids 48, 1199–1208 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura T. et al. , Age-related decline in the taurine content of the skin in rodents. Amino Acids 53, 429–434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carneiro EM et al. , Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem 20, 503–511 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Nishi Y, Usui T, Free amino acid concentrations in plasma, erythrocytes, granulocytes, and lymphocytes in umbilical cord blood, children, and adults. J Pediatr Gastroenterol Nutr 3, 432–439 (1984). [DOI] [PubMed] [Google Scholar]
- 33.Jeevanandam M, Young DH, Ramias L, Schiller WR, Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr 51, 1040–1045 (1990). [DOI] [PubMed] [Google Scholar]
- 34.Ames BN, Prolonging healthy aging: Longevity vitamins and proteins. Proc Natl Acad Sci U S A 115, 10836–10844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito T. et al. , Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS One 9, e107409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yilmaz LS et al. , Modeling tissue-relevant Caenorhabditis elegans metabolism at network, pathway, reaction, and metabolite levels. Mol Syst Biol 16, e9649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan QL et al. , Metabolomic signature associated with reproduction-regulated aging in Caenorhabditis elegans. Aging (Albany NY) 9, 447–474 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick MA et al. , A Comprehensive Analysis of Replicative Lifespan in 4,698 Single-Gene Deletion Strains Uncovers Conserved Mechanisms of Aging. Cell Metab 22, 895–906 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye-Rowley WS, Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 276, 244–250 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Olshansky SJ, From Lifespan to Healthspan. JAMA 320, 1323–1324 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Shoji H, Takao K, Hattori S, Miyakawa T, Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 9, 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steru L, Chermat R, Thierry B, Simon P, The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85, 367–370 (1985). [DOI] [PubMed] [Google Scholar]
- 43.Bourin M, Hascoet M, The mouse light/dark box test. Eur J Pharmacol 463, 55–65 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Pavlopoulos E. et al. , Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med 5, 200ra115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dey N. et al. , Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell 163, 95–107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yano JM et al. , Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho RH, Sieburg HB, Muller-Sieburg CE, A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangiola S, Molania R, Dong R, Doyle MA, Papenfuss AT, tidybulk: an R tidy framework for modular transcriptomic data analysis. Genome Biol 22, 42 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chinta SJ et al. , Cellular Senescence Is Induced by the Environmental Neurotoxin Paraquat and Contributes to Neuropathology Linked to Parkinson's Disease. Cell Rep 22, 930–940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi JS et al. , Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight 1, e90220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEachern MJ, Krauskopf A, Blackburn EH, Telomeres and their control. Annu Rev Genet 34, 331–358 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Henriques CM, Carneiro MC, Tenente IM, Jacinto A, Ferreira MG, Telomerase is required for zebrafish lifespan. PLoS Genet 9, e1003214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maslov AY et al. , DNA damage in normally and prematurely aged mice. Aging Cell 12, 467–477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton ML et al. , Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A 98, 10469–10474 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marioni RE et al. , DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michalak EM, Burr ML, Bannister AJ, Dawson MA, The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol 20, 573–589 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Bettedi L, Foukas LC, Growth factor, energy and nutrient sensing signalling pathways in metabolic ageing. Biogerontology 18, 913–929 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bitto A. et al. , Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrucci L, Fabbri E, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh J, Lee YD, Wagers AJ, Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med 20, 870–880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker N. et al. , Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Kauppila TES, Kauppila JHK, Larsson NG, Mammalian Mitochondria and Aging: An Update. Cell Metab 25, 57–71 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT, Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 106, 20405–20410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Suzuki T, Suzuki T, Wada T, Saigo K, Watanabe K, Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J 21, 6581–6589 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pierce-Shimomura JT et al. , Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A 105, 20982–20987 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pietzner M. et al. , Plasma metabolites to profile pathways in noncommunicable disease multimorbidity. Nat Med 27, 471–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warburton DE, Nicol CW, Bredin SS, Prescribing exercise as preventive therapy. CMAJ 174, 961–974 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen BK, Saltin B, Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25 Suppl 3, 1–72 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Bergstrom J, Furst P, Noree LO, Vinnars E, Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 36, 693–697 (1974). [DOI] [PubMed] [Google Scholar]
- 70.Wright CE, Tallan HH, Lin YY, Gaull GE, Taurine: biological update. Annu Rev Biochem 55, 427–453 (1986). [DOI] [PubMed] [Google Scholar]
- 71.Lambert IH, Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem Res 29, 27–63 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Holopainen I, Kontro P, Taurine and hypotaurine transport by a single system in cultured neuroblastoma cells. Acta Physiol Scand 122, 381–386 (1984). [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K. et al. , Taurine transporter in primary cultured neonatal rat heart cells: a comparison between cardiac myocytes and nonmyocytes. Biochem Pharmacol 65, 1181–1187 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Oja SS, Lehtinen I, Lahdesmaki P, Taurine transport rates between plasma and tissues in adult and 7-day-old mice. Q J Exp Physiol Cogn Med Sci 61, 133–143 (1976). [DOI] [PubMed] [Google Scholar]
- 75.Lee G, Lee H, Hong J, Lee SH, Jung BH, Quantitative profiling of bile acids in rat bile using ultrahigh-performance liquid chromatography-orbitrap mass spectrometry: Alteration of the bile acid composition with aging. J Chromatogr B Analyt Technol Biomed Life Sci 1031, 37–49 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Calvani R. et al. , Fecal and urinary NMR-based metabolomics unveil an aging signature in mice. Exp Gerontol 49, 5–11 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Barua M, Liu Y, Quinn MR, Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: decreased NF-kappaB activation and IkappaB kinase activity. J Immunol 167, 2275–2281 (2001). [DOI] [PubMed] [Google Scholar]
- 78.DiNicolantonio JJ, JH OK, McCarty MF, Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: a novel way to promote cardiovascular health. Open Heart 4, e000600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi X, Yao D, Chen C, Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided biochemical analysis. J Biol Chem 287, 6336–6349 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito T. et al. , Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J Biomed Sci 17 Suppl 1, S20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roman-Garcia P. et al. , Vitamin B(1)(2)-dependent taurine synthesis regulates growth and bone mass. J Clin Invest 124, 2988–3002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Magalhaes JP, Church GM, Genomes optimize reproduction: aging as a consequence of the developmental program. Physiology (Bethesda) 20, 252–259 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Shindyapina AV et al. , Rapamycin treatment during development extends lifespan and healthspan. bioRxiv, 2022.2002.2018.481092 (2022). [Google Scholar]
- 84.Ansar M. et al. , Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum Mol Genet 29, 618–623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guan L, Miao P, The effects of taurine supplementation on obesity, blood pressure and lipid profile: A meta-analysis of randomized controlled trials. Eur J Pharmacol 885, 173533 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Brennan AM et al. , Plasma Metabolite Profiles in Response to Chronic Exercise. Med Sci Sports Exerc 50, 1480–1486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuisinier C. et al. , Role of taurine in osmoregulation during endurance exercise. Eur J Appl Physiol 87, 489–495 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Sharan K, Lewis K, Furukawa T, Yadav VK, Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J Pineal Res 63, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh P. et al. , Maternal vitamin B12 in mice positively regulates bone, but not muscle mass and strength in post-weaning and mature offspring. Am J Physiol Regul Integr Comp Physiol, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yadav VK, Lakshmi G, Medhamurthy R, Prostaglandin F2alpha-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis: involvement of caspase-activated DNase. J Biol Chem 280, 10357–10367 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Lee MB et al. , A system to identify inhibitors of mTOR signaling using high-resolution growth analysis in Saccharomyces cerevisiae. Geroscience 39, 419–428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steffen KK, Kennedy BK, Kaeberlein M, Measuring replicative life span in the budding yeast. J Vis Exp, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beaupere C. et al. , Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc Natl Acad Sci U S A 115, 9586–9591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murakami CJ, Wall V, Basisty N, Kaeberlein M, Composition and acidification of the culture medium influences chronological aging similarly in vineyard and laboratory yeast. PLoS One 6, e24530 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee MB et al. , Pterocarpus marsupium extract extends replicative lifespan in budding yeast. Geroscience 43, 2595–2609 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olsen B, Murakami CJ, Kaeberlein M, YODA: software to facilitate high-throughput analysis of chronological life span, growth rate, and survival in budding yeast. BMC bioinformatics 11, 141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aartsma-Rus A, van Putten M, Assessing functional performance in the mdx mouse model. J Vis Exp, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen JJ et al. , Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 21, 6348–6361 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayorga AJ, Lucki I, Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 155, 110–112 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Oh CM et al. , Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun 6, 6794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parfitt AM et al. , Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2, 595–610 (1987). [DOI] [PubMed] [Google Scholar]
- 102.Lewis KE, Sharan K, Takumi T, Yadav VK, Skeletal Site-specific Changes in Bone Mass in a Genetic Mouse Model for Human 15q11–13 Duplication Seen in Autism. Sci Rep 7, 9902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P, Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14, 1167–1174 (1999). [DOI] [PubMed] [Google Scholar]
- 104.Feldkamp LA, Goldstein SA, Parfitt AM, Jesion G, Kleerekoper M, The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res 4, 3–11 (1989). [DOI] [PubMed] [Google Scholar]
- 105.Gundersen HJ, Boyce RW, Nyengaard JR, Odgaard A, The Conneulor: unbiased estimation of connectivity using physical disectors under projection. Bone 14, 217–222 (1993). [DOI] [PubMed] [Google Scholar]
- 106.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O, Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4, 1798–1806 (2009). [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Park C, Bennett C, Thornton M, Kim D, Rapid and accurate alignment of nucleotide conversion sequencing reads with HISAT-3N. Genome Res, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Putri GH, Anders S, Pyl PT, Pimanda JE, Zanini F, Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 38, 2943–2945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wickham H, Welcome to the Tidyverse. . Journal of Open Source Software 4, 1686, (2019). [Google Scholar]
- 110.Robinson MD, McCarthy DJ, Smyth GK, edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson MD, Oshlack A, A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou X, Lindsay H, Robinson MD, Robustly detecting differential expression in RNA sequencing data using observation weights. Nucleic Acids Res 42, e91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCarthy DJ, Smyth GK, Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics 25, 765–771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mangiola S, Thomas EA, Modrak M, Vehtari A, Papenfuss AT, Probabilistic outlier identification for RNA sequencing generalized linear models. NAR Genom Bioinform 3, lqab005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wickham H, ggplot2: Elegant Graphics for Data Analysis. . Springer International Publishing, (2016). [Google Scholar]
- 116.Mangiola SP, A. T. , tidyHeatmap: an R package for modular heatmap production based on tidy principles. Journal of Open Source Software 5, 2472 (2020). (2020). [Google Scholar]
- 117.Gu Z, Eils R, Schlesner M, Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Yu G, Wang LG, Han Y, He QY, clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hernandez-Segura A. et al. , Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 27, 2652–2660 e2654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hochberg Y, Benjamini Y, More powerful procedures for multiple significance testing. Stat Med 9, 811–818 (1990). [DOI] [PubMed] [Google Scholar]
- 121.Verlaan S. et al. , Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin Nutr 36, 267–274 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Rausser S. et al. , Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anand N. et al. , Dysregulated iron metabolism in C. elegans catp-6/ATP13A2 mutant impairs mitochondrial function. Neurobiol Dis 139, 104786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vanharanta M, Voutilainen S, Rissanen TH, Adlercreutz H, Salonen JT, Risk of cardiovascular disease-related and all-cause death according to serum concentrations of enterolactone: Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med 163, 1099–1104 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V, Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis 11, 321–334 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Buzkova J. et al. , Metabolomes of mitochondrial diseases and inclusion body myositis patients: treatment targets and biomarkers. EMBO Mol Med 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Day N. et al. , EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 80 Suppl 1, 95–103 (1999). [PubMed] [Google Scholar]
- 128.Schranner D. et al. , Physiological extremes of the human blood metabolome: A metabolomics analysis of highly glycolytic, oxidative, and anabolic athletes. Physiol Rep 9, e14885 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Percie du Sert N. et al. , The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol 177, 3617–3624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. The codes used for data analysis is stored publicly at github, stemangiola/singh_et_al_taurine_bone. Sequencing scaled counts have been deposited at 10.5281/zenodo.7700452.