Abstract
Introduction
Lack of viral suppression (VS) among pregnant and breastfeeding women living with HIV poses challenges for maternal and infant health, and viral load (VL) monitoring via centralized laboratory systems faces many barriers. We aimed to determine the impact of point‐of‐care (POC) VL and targeted drug resistance mutation (DRM) testing in improving VS among pregnant and postpartum women on antiretroviral therapy.
Methods
We conducted a pre/post‐intervention prospective cohort study among 820 pregnant women accessing HIV care at five public‐sector facilities in western Kenya from 2019 to 2022. The pre‐intervention or “control” group consisted of standard‐of‐care (SOC) centralized VL testing every 6 months and the post‐intervention or “intervention” group consisted of a combined strategy of POC VL every 3 months, targeted DRM testing, and clinical management support. The primary outcome was VS (VL ≤1000 copies/ml) at 6 months postpartum; secondary outcomes included uptake and turnaround times for VL testing and sustained VS.
Results
At 6 months postpartum, 321/328 (98%) of participants in the intervention group and 339/347 (98%) in the control group achieved VS (aRR 1.00, 95% confidence interval [CI] 0.98, 1.02). When assessing VS using a threshold of <40 copies/ml, VS proportions were lower overall (90−91%) but remained similar between groups. Among women with viraemia (VL>1000 copies/ml) who underwent successful DRM testing in the intervention group, all (46/46, 100%) had some DRMs and 20 (43%) had major DRMs (of which 80% were nucleos(t)ide reverse transcriptase inhibitor mutations). POC VL testing uptake was high (>89%) throughout pregnancy, delivery, and postpartum periods, with a median turnaround time of 1 day (IQR 1, 4) for POC VL in the intervention group and 7 days (IQR 5, 9) for SOC VL in the control group. Sustained VS throughout follow‐up was similar between groups with either POC or SOC VL testing (90−91% for <1000 copies/ml, 62–70% for <40 copies/ml).
Conclusions
Our combined strategy markedly decreased turnaround time but did not increase VS rates, which were already very high, or sustained VS among pregnant and postpartum women living with HIV. Further research on how best to utilize POC VL and DRM testing is needed to optimize sustained VS among this population.
Keywords: HIV, pregnant and postpartum women, antiretroviral therapy (ART), point‐of‐care (POC) testing, viral load, drug resistance mutations (DRMs)
1. INTRODUCTION
Globally, an estimated 1.3 million pregnant women were living with HIV in 2021 [1]. Given the widespread scale‐up of universal antiretroviral therapy (ART) initiation for all individuals, an estimated 70–95% of pregnant women are on ART [2]. However, women do not always achieve or maintain viral suppression (VS), with 22–30% of women having at least one episode of viral load (VL) >1000 copies/ml during pregnancy or postpartum periods [3, 4]. Maternal HIV VL is the leading determinant of vertical transmission of HIV [5, 6], and lack of VS during critical periods of pregnancy and breastfeeding poses serious challenges to eliminating vertical transmission.
Routine VL monitoring while on ART is recommended in low‐ and middle‐income countries (LMICs) [7], but implementation is incomplete. In Kenya, vertical transmission rates decreased from 11.5% in 2017 to 8.9% in 2020, but the country remains short of its target of <5% [8, 9]. Current Kenya guidelines recommend VL monitoring at the first antenatal care (ANC) visit if already on ART or at 6 months post‐ART initiation for newly diagnosed women, and every 6 months postpartum while breastfeeding [10]. While estimates for repeat testing are largely lacking, 86% of all estimated people on ART have undergone at least one VL test in Kenya [11, 12]. VL testing occurs via centralized laboratory testing, which includes several challenges, such as long turnaround times and high costs of transporting samples [10, 13]. Point‐of‐care (POC), or even near POC, VL assessments have been shown to be feasible, accurate, and less expensive than laboratory‐based VL assays [14−18]. Kenya has a nationwide POC tuberculosis testing platform using GeneXpert technology which has been used to pilot HIV early infant diagnosis and VL testing [19−21]. However, the clinical impact of POC VL has been mixed [22−26], and the feasibility of its use during the dynamic periods of pregnancy and postpartum remains unclear.
While improved HIV VL monitoring would enhance the detection of viraemia (VL>1000 copies/ml), a variety of underlying causes exist for the lack of VS among pregnant and postpartum women, including HIV drug resistance mutations (DRMs) [27]. According to the 2021 WHO report on HIV DRMs, non‐nucleos(t)ide reverse transcriptase inhibitors (NNRTIs) resistance has reached a critical level (>10%) in five African countries [28]. With increasing rates of DRMs in LMICs, HIV DRMs could jeopardize the attainment of the global targets for HIV among pregnant women, which have additional implications for transmitted resistance to infants [7]. Nearly half of HIV‐infected infants have transmitted DRMs to one or more NNRTIs [29]. Current challenges in DRM monitoring in Kenya include pre‐consultation with centralized committees, extremely delayed turn‐around times, and testing only for those failing second‐ or third‐line regimens [15, 30−32]. Incorporating DRM testing into clinical decision‐making in LMICs has increased saliency, yet many questions remain on how to implement this testing programmatically and for which priority populations [33, 34].
We conducted the Opt4Mamas study to evaluate if a combined strategy of higher frequency POC VL with targeted DRM testing and clinical decision support could improve VS rates among pregnant and postpartum women on ART in Kenya. We hypothesized that our combined strategy would facilitate earlier and more appropriate clinical decision‐making, resulting in improved treatment outcomes.
2. METHODS
2.1. Study design and procedures
We conducted an open‐label, pre/post‐intervention (or intervention/control) prospective cohort study, enrolling pregnant women living with HIV during their ANC care and followed them through 6 months postpartum in five public‐sector HIV treatment facilities in Kenya from February 2019 to November 2022 (Figure 1). The pre‐intervention cohort served as the “control” group, receiving standard‐of‐care (SOC), which consisted of centralized VL testing approximately every 6 months, from all five facilities. The post‐intervention cohort served as the “intervention” group, receiving a combined strategy of POC VL every 3 months, targeted DRM testing, and clinical management support, also from all five facilities. HIV DRM testing was performed at the two accredited centralized laboratories in Kenya using Sanger sequencing for participants in the intervention group with VL ≥ 1000 copies/ml. Follow‐up of the control cohort overlapped in calendar time with enrolment of the intervention cohort (control group enrolment started on 26 February 2019 and follow‐up lasted until 22 April 2021; intervention group enrolment started on 7 October 2019 and follow‐up lasted until 31 December 2021). We chose the study facilities to leverage existing POC technologies, specifically the GeneXpert platform, and for geographical reach for study staff based in Kisumu, Kenya. Details on study procedures are found in Supplementary Text and Figure 1.
Figure 1.
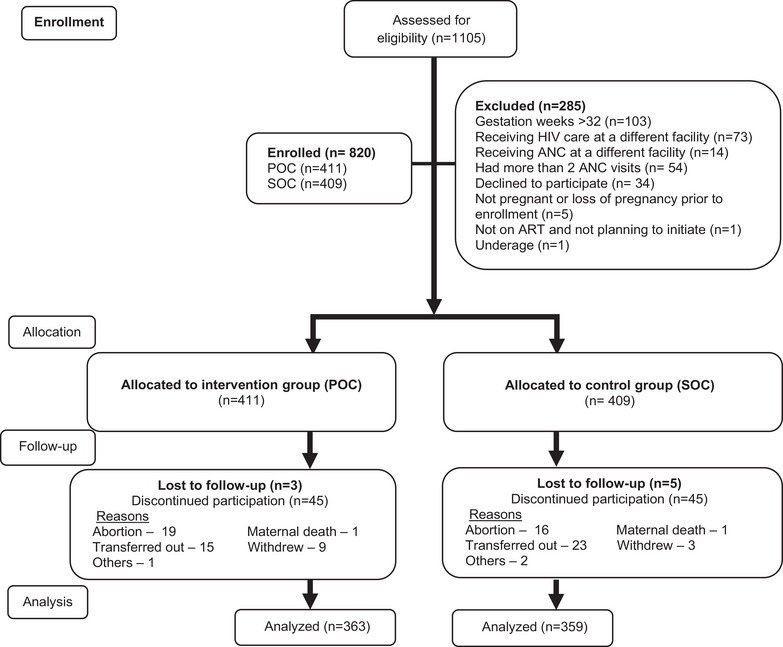
Flow diagram of study participants in the Opt4Mamas study, February 2019–December 2021.
2.2. Study setting and population
The study was conducted in low‐resource, high‐HIV burden public sector facilities in Kisumu County, western Kenya, with one of the highest prevalence of HIV infections in Kenya (Table S1). Comprehensive HIV care and treatment services were provided per Kenyan ART guidelines by facility staff, including ART for all persons diagnosed with HIV [10]. First‐line ART regimens among adults during the study period included combinations of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) lamivudine and tenofovir with either (1) NNRTI efavirenz or (2) integrase inhibitors (INSTIs) dolutegravir, and protease inhibitors (PIs)‐containing ART, for example with atazanavir/ritonavir, were considered alternative first‐line or second‐line regimens; a wider transition for patients on non‐dolutegravir first‐ or second‐line regimens to dolutegravir‐containing ART occurred in 2019 [10].
2.3. Study eligibility
We enrolled women presenting at ANC meeting the following inclusion criteria: (1) adult (>17 years of age) pregnant female either already known or newly diagnosed with HIV; (2) at 32 weeks or less of gestational age; (3) with first ANC visit; (4) planning to return to the same facility for the remainder of her pregnancy and postpartum care; and (5) on ART or planning to initiate ART within 1 week. Women on third or salvage ART regimens (due to the higher complexity of managing viraemia [VL>1000 copies/ml] for such individuals) or receiving ANC or HIV care at another facility were excluded.
2.4. Variables
Our primary outcome was VS (defined as VL <1000 copies/ml, as per country guidelines) by POC VL testing at 6 months postpartum (defined as 48 weeks +/− 16 weeks, a wide window intended to maximize our ability to obtain a VS measurement in spite of COVID interruptions). If a POC VL test was not available at 6 months postpartum, any available SOC test in the same window was used. Our secondary outcomes included VS defined by lower VL cutoffs and a set of process outcomes, such as uptake and turnaround time of VL testing results. We define major, minor, and accessory classifications for HIV drug resistance according to the Stanford HIV Resistance Database [35]. We define sustained VS (SVS) as having VS at all intervals through the 6‐month postpartum visit among participants with at least two study intervals tested and with the respective VL testing modality for the group (i.e. POC VL for intervention or SOC VL for control group).
The primary exposure was control versus intervention group. Participant clinical and socio‐demographic information as well as household characteristics (e.g. household commodities and food insecurity) were collected from all participants at enrolment. Other information, such as psychosocial and behavioural data (e.g. self‐reported adherence), were collected at every study visit.
2.5. Statistical analysis
Power for the study was for comparing the proportion of women with VS 6 months postpartum in the control versus intervention groups. Based on historical facility data, we expected approximately 75% VS in the control group. We estimated that 270 women per group would provide 80% power to detect an increase of 10% post‐intervention VS (i.e. 75% vs. 85%) using a chi‐squared test with α = 0.05. We aimed to screen 350 women in each group to account for an anticipated 25% of available women not enrolling, transferring out to other facilities, experiencing pregnancy loss or lost to follow‐up at 6 months postpartum. In February 2020, we generated additional power calculations given higher‐than‐expected baseline VS. We estimated that with 410 women per group and 90.5% VS in the control group, we would have approximately 80% power to detect a 50% decrease in the proportion unsuppressed (i.e. 9.5% vs. 4.7% unsuppressed).
We compared descriptive statistics for participant baseline characteristics by group using chi‐squared tests for categorical and t‐tests for continuous variables. We describe VS by group at enrolment (any blood draw 0–90 days prior to enrolment), 3 and 6 months after enrolment, delivery, and 3 and 6 months postpartum (any blood draw +/− 6 weeks within visit target except for the 3‐month visit which additionally included blood draws from day after enrolment to 6 weeks after the 3‐month visit). The primary analysis compared the observed proportion of women with VS at 6 months postpartum (primary outcome) in the control versus intervention groups using a modified Poisson regression model with robust standard error estimation, adjusting for facility, to obtain the adjusted relative risk (aRR) [36]. An a priori sensitivity analysis defined VS as VL<40 copies/ml and a post hoc analysis as VL<400 copies/ml. Further post hoc sensitivity analyses included: (a) adjusting for enrolment characteristics differing (α<0.05) by group, in case of confounding; and (b) using inverse probability weighting, to address missing outcomes. We also conducted a post hoc secondary analysis evaluating the effect of the intervention on SVS.
3. RESULTS
3.1. Enrolment characteristics
A total of 1105 women were assessed for study eligibility, of which 285 (26%) were excluded (Figure 1). Eight hundred and twenty women were enrolled, with 411 and 409 allocated to the intervention and control groups, respectively. Retention to study visit at 6 months postpartum was 88% and 92% and primary outcome ascertained for 80% and 85% of participants in the intervention and control groups, respectively, and two maternal deaths occurred unrelated to the study.
Median maternal age at enrolment was 29 years (interquartile range [IQR] 24, 33), gestational age was 19 weeks (IQR 13, 25), gravida was 3 (IQR 2, 4), parity was 2 (IQR 1, 3), 423 (52%) had achieved a secondary education and 696 (85%) were married (Table 1). The median CD4 cell count was 523 (IQR 370, 708.5) and 628 (76.6%) were WHO Stage I or II. Overall, 622 (76%) participants were on NNRTI‐, 71 (8.7%) on PI‐ and 80 (10%) on integrase‐containing ART at enrolment, and the median time on ART was 4.0 years (IQR 1.2, 6.3). Among 771 (94%) of the participants with a VL documented in the 24 months prior to enrolment, 672 (82%) had VS.
Table 1.
Characteristics at enrolment for enrolled participants in the Opt4Mamas study, February 2019–December 2021
| Variable | Total study group (n=820) | Intervention group (POC VL; n=411) | Control group (SOC VL; n=409) | p‐Value a |
|---|---|---|---|---|
| Participant characteristics | ||||
| Maternal age (median, IQR) | 29 (24, 33) | 29 (24, 33) | 29 (24, 33) | 0.641 |
| Gestational age in weeks (median, IQR) | 19 (13, 25) | 18 (14, 24) | 20 (12, 26) | 0.1886 |
| Gravida (i.e. number of pregnancies) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.9447 |
| Parity (i.e. number of live births) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.9045 |
| Age at ART initiation in years (median, IQR) | 24 (21, 28) | 24 (21, 28) | 24 (21, 28) | 0.1053 |
| Time on ART in years (median, IQR) | 3 (1, 6) | 4 (1, 6) | 3 (1, 6) | 0.5614 |
| ART regimen n (%) | <0.0001 | |||
| NNRTI‐containing | 622 (75.9%) | 272 (66.2%) | 350 (85.6%) | |
| PI‐containing | 71 (8.7%) | 39 (9.5%) | 32 (7.8%) | |
| Integrase‐containing | 80 (9.8%) | 70 (17.0%) | 10 (2.4%) | |
| Missing | 47 (5.7%) | 30 (7.3%) | 17 (4.2%) | |
| CD4 count, most recent recorded within prior 2 years or on day of enrolment n (%) | 0.8492 | |||
| 0−200 | 14 (1.7%) | 6 (1.5%) | 8 (2.0%) | |
| 201−500 | 82 (10.0%) | 29 (7.1%) | 53 (13.0%) | |
| 501+ | 107 (13.0%) | 38 (9.3%) | 69 (17.0%) | |
| Missing | 617 (75.2%) | 338 (82.2%) | 279 (68.2%) | |
| Median CD4, IQR | 523 (370, 708.5) | 523 (366, 719) | 523 (381.3, 706) | 0.9484 |
| WHO Clinical Stage, most recent recorded within prior 2 years or on day of enrolment n (%) | 0.289 | |||
| I or II | 628 (76.6%) | 266 (64.7%) | 362 (88.5%) | |
| III or IV | 64 (7.8%) | 32(8.0%) | 32 (7.8%) | |
| Not indicated or missing |
128 (15.6%) |
113 (27.5%) |
15 (3.7%) |
|
| Viral suppression (<1000 copies/ml via SOC), as closest VL prior to 2 years to or on day of enrolment n (%) | 0.4918 | |||
| Yes | 672 (82.0%) | 326 (79.3%) | 346 (84.6%) | |
| No | 99 (12.0%) | 41 (10.0%) | 58 (14.2%) | |
| Missing | 49 (6.0%) | 44 (10.7%) | 5 (1.2%) | |
| Socio‐demographic characteristics | ||||
| Highest education attained n (%) | 0.9103 | |||
| No education | 14 (1.7%) | 8 (2.0%) | 6 (1.5%) | |
| Primary | 390 (47.6%) | 196 (47.7%) | 194 (47.4%) | |
| Secondary | 303 (37.0%) | 153 (37.2%) | 150 (36.7%) | |
| Higher | 113 (13.8%) | 54 (13.1%) | 59 (14.4%) | |
| Marital status n (%) b | 0.7685 | |||
| Married | 696 (84.9%) | 346 (84.2%) | 350 (85.6%) | |
| Not married | 122 (14.9%) | 63 (15.3%) | 59 (14.4%) | |
| Missing | 2 (0.2%) | 2 (0.5%) | 0 | |
| Household or partner characteristics | ||||
| HIV status of primary sexual partner n (%) | 0.652 | |||
| Positive | 442 (53.9%) | 222 (54.0%) | 220 (53.8%) | |
| Negative | 238 (29.0%) | 123 (29.9%) | 115 (28.1%) | |
| Unknown | 139 (17.0%) | 65 (15.8%) | 74 (18.1%) | |
| Missing | 1 (0.1%) | 1 (0.2%) | 0 | |
| Having household commodities n (%) | ||||
| Electricity | 528 (64.4%) | 270 (65.7%) | 258 (63.1%) | 0.5653 |
| Radio | 641 (78.1%) | 313 (76.2%) | 328 (80.2%) | 0.2793 |
| Television | 457 (55.7%) | 224 (54.5%) | 233 (57.0%) | 0.8051 |
| Phone | 757 (92.3%) | 369 (89.8%) | 388 (94.9%) | 0.0120 |
| Kind of floor c | 555 (67.7%) | 277 (67.7%) | 278 (68.1%) | 0.9403 |
| More than one room | 580 (70.7%) | 274 (66.7%) | 306 (74.8%) | 0.0204 |
| Firewood/plant waste | 423 (51.6%) | 223 (54.3%) | 200 (48.9%) | 0.2808 |
| Reporting food insecurity n (%) | 0.0514 | |||
| None 0 | 284 (34.6%) | 158 (38.4%) | 126 (30.8%) | |
| Mild 1−9 | 355 (43.3%) | 159 (38.7%) | 196 (47.9%) | |
| Moderate 10−18 | 169 (20.6%) | 87 (21.2%) | 82 (20.0%) | |
| Severe 19−27 | 11 (1.3%) | 6 (1.5%) | 5 (1.2%) | |
| Missing | 1 (0.1%) | 1 (0.2%) | ||
Abbreviations: ART, antiretroviral therapy; IQR, inter quartile range; POC, point‐of‐care; SOC, standard‐of‐care; VL, viral load.
p‐Values estimated by Fisher's exact test for categorical variables and Wilcoxon test for continuous variables.
Various marital status categories include married and cohabiting, married but not co‐habitating, not married but co‐habitating. Not married status categories include single, widowed, separated or divorced.
Primary kind of floor of the main house can be carpet, cement, tile, earth/dung or others.
More women in the intervention group versus control group were on INSTI‐containing ART (17% vs. 2.4%, respectively, p<0.001) and reported no food insecurity (38% vs. 31%, respectively, p = 0.05). Facility characteristics, ART regimen changes, VS during the course of the study and delivery outcomes can be found in Tables S1–S4, respectively.
The proportion of women with VS at enrolment in the intervention group was 343/379 (90%) by POC VL testing, and 256/269 (95%) in the control group by SOC VL testing (Table 2). Among those in the intervention group who also had SOC VL testing, VS was 152/157 (97%).
Table 2.
Effect of the intervention on viral suppression proportions by time and varying threshold of VL cutoffs among the Opt4Mamas study participants (n=820), February 2019−December 2021
| Unadjusted model a | Adjusted model b | IPW model c | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group (n=411) | Control group (n=409) |
Risk ratio (RR, 95% CI) Risk difference (RD, 95% CI) |
p‐value for each |
Risk ratio (RR, 95% CI) Risk difference (RD, 95% CI) |
p‐value for each |
Risk ratio (RR, 95% CI) Risk difference (RD, 95% CI) |
p‐value for each | |
| Viral suppression <1000 copies/ml and baseline subgroups | ||||||||
| Viral suppression <1000 copies/ml by testing interval d | ||||||||
| Enrolment | 343/379 (90.2%) | 256/269 (95.2%) | − | − | ||||
| 3 months | 95/98 (97.0%) | 103/108 (95.4%) | − | − | ||||
| 6 months | 21/22 (95.5%) | 60/60 (100%) | − | − | ||||
| Delivery | 218/225 (96.9%) | 127/133 (95.5%) | − | − | ||||
| 3 months postpartum | 182/186 (97.8%) | 144/150 (96.0%) | − | − | ||||
| 6 months postpartum | 208/212 (98.1%) | 169/176 (96.0%) | − | − | ||||
|
Primary outcome e (6 months postpartum +/− 16 weeks) |
321/328 (97.9%) | 339/347 (97.7%) |
Primary analysis: RR: 1.04 (0.36, 3.02) RD: 0.2% (−.2.1%, 2.4%) |
0.941 0.880 |
RR: 1.0 (0.98, 1.03) RD: 0.2% (−.2.1%, 2.4%) |
0.962 0.880 |
RR: 1.00 (0.98, 1.02) RD: −0.05% (−2.1%, 2.0%) |
0.653 0.962 |
| Baseline subgroups | ||||||||
| ART regimen | ||||||||
| NNRTI‐containing | 202/206 (98.1%) | 293/296 (99.0%) |
RR: 1.00 RD: −1.0% (−3.1%, 1.3%) |
0.409 0.020 |
RR: 1.00 RD: −0.8% (−2.8%, 1.2%) |
0.425 0.022 |
||
| PI‐containing | 33/35 (94.3%) | 25/30 (83.3%) |
RR: 0.86 (0.76, 0.98) RD: 11.0% (−4.4%, 26.3%) |
0.163 0.745 |
RR: 0.90 (0.83, 0.99) RD: 9.7% (−4.7%, 24.0%) |
0.186 0.839 |
||
| Integrase‐containing | 62/63 (98.4%) | 7/7 (100%) |
RR: 0.99 (0.95, 1.04) RD: −1.6% (−4.7%, 1.5%) |
0.313 |
RR: 1.00 (0.97, 1.03) RD: −1.3% (−3.9%, 1.2%) |
0.314 | ||
| Food insecurity | ||||||||
| None | 123/124 (99.0%) | 100/101 (99.0%) |
RR: 1.00 RD: 0.2% (−2.3%, 2.7%) |
0.885 |
RR: 1.00 RD: 0.2% (−2.0%, 2.3%) |
0.863 | ||
| Mild | 121/125 (97.0%) | 169/172 (98.0%) |
RR: 0.99 (0.96, 1.02) RD: −1.5% (−5.1%, 2.2%) |
0.389 0.435 |
RR: 0.99 (0.97, 1.01) RD: −1.3% (−4.6%, 2.0%) |
0.185 0.447 |
||
| Moderate | 72/74 (97.0%) | 67/70 (95.7%) |
RR: 0.97 (0.93, 1.01 RD: 1.6 (−4.4%, 7.6%) |
0.142 0.606 |
RR: 0.98 (0.95, 1.01) RD: 2.6% (−2.9%, 8.0%) |
0.215 0.357 |
||
| Severe | 5/5 (100%) | 3/4 (75.0%) |
RR: 0.86 (0.63, 1.17) RD: 25% (−17.4%, 67.4%) |
0.323 0.248 |
RR: 0.90 (0.73, 1.10) RD: 25.0% (−17.3%, 67.4%) |
0.282 0.248 |
||
| Viral suppression <400 copies/ml by testing interval d | ||||||||
| Enrolment | 333/379 (87.9%) | 251/269 (93.3%) | − | − | ||||
| 3 months | 95/98 (97.0%) | 102/108 (94.4%) | − | − | ||||
| 6 months | 20/22 (91.0%) | 59/60 (98.3%) | − | − | ||||
| Delivery | 217/225 (96.4%) | 124/133 (93.2%) | − | − | ||||
| 3 months postpartum | 181/186 (97.3%) | 142/150 (94.7%) | − | − | ||||
| 6 months postpartum | 208/212 (98.1%) | 167/175 (95.4%) | − | − | ||||
|
Parallel to primary e (6 months postpartum +/− 16 weeks) |
321/328 (97.9%) | 339/347 (97.7%) |
RR: 1.04 (0.36,3.02) RD: 0.2% (−.2.1%, 2.4%) |
0.9410.880 |
RR: 1. 00 (0.96,1.04) RD: 0.2% (−.2.1%, 2.4%) |
0.962 0.880 |
RR: 1.00 (0.98, 1.03) RD: −0.05% (−2.1%, 2.0%) |
0.835 0.987 |
| Viral suppression <40 copies/ml by testing interval d | ||||||||
| Enrolment | 294/379 (77.6%) | 210/269 (71.1%) | ||||||
| 3 months after enrolment | 82/98 (83.7%) |
78/108 (72.2%) |
− | − | ||||
| 6 months after enrolment | 18/22 (81.8%) | 43/60 (71.7%) | − | − | ||||
| Delivery | 198/225 (88.0%) | 95/133 (71.4%) | − | − | ||||
| 3 months postpartum | 166/186 (89.2%) | 115/150 (76.7%) | − | − | ||||
| 6 months postpartum | 189/212 (89.2%) | 144/176 (81.8%) | − | − | ||||
|
Parallel to primary e (6 months postpartum +/− 16 weeks) |
297/328 (90.5%) | 311/347 (89.6%) |
RR: 1.01 (0.59,1.71) RD: 1.0% (−3.6%, 5.4%) |
0.976 0.688 |
RR: 1.01 (0.96,1.10) RD: 1.0% (−3.6%, 5.4%) |
0.732 0.688 |
RR: 1.01 (0.95, 1.06) RD: 0.5% (−3.8%, 4.6%) |
0.837 0.805 |
Abbreviations: CI, confidence interval; ml, millilitre; POC, point‐of‐care; Q1, quartile one (25%); Q3, quartile three (75%); RD, risk difference; RR, relative risk; SOC, standard‐of‐care; VL, viral load.
Risk ratios, confidence intervals and p‐values generated by Poisson regression modified with robust standard error estimation, adjusting for facility only. p‐Values indicate statistical significance of the effect of the intervention on viral suppression of study participants.
Risk ratios, confidence intervals and p‐values generated by Poisson regression modified with robust standard error estimation, adjusting for facility, baseline ART regimen and baseline food insecurity. p‐Values indicate statistical significance of the effect of the intervention on viral suppression of study participants.
Risk ratios, confidence intervals and p‐values generated by Poisson regression modified with robust standard error estimation, adjusting for inverse probability weighting for missing outcomes. p‐Values indicate statistical significance of the effect of the intervention on viral suppression of study participants.
For intervention group participants, POC VL testing was intended to be performed at each study visit, which were targeted for every 12 weeks. For both intervention and control group participants, SOC VL testing was performed at each facility, which generally would be expected to be every 6 months. We used chart review as well as review of the Kenya Ministry of Health's NASCOP HIV VL Database to find available regular clinic VL testing both prior to enrolment and during the study. We assigned such available SOC VL test results and from POC VL testing to the visit window into which the blood draw fell (the target date +/− 6 weeks) except for baseline (0 months) which included up to 90 days prior to enrolment. Thus, we assigned the VL results as 0 months if blood was drawn 0−90 days prior to enrolment, 3 months if 1 day to 18 weeks after enrolment, 6 months if 18 weeks + 1 day to 30 weeks after enrolment, delivery if 2 weeks before to 6 weeks after the date of delivery, 3 months postpartum if 6 weeks + 1 day to 18 weeks after date of delivery and 6 months postpartum if 18 weeks + 1 day to 30 weeks after date of delivery. If more than one POC or SOC VL test result was available in any interval, the one closest to the target study visit date was used. If a POC VL test was not available at 6 months postpartum, any available SOC test in the same window was used; this occurred in 7/328 (2.1%) and 120/347 (34.6%) of instances for the primary outcome of 6 months postpartum VL assessment in the intervention and control groups, respectively.
Primary outcome of viral suppression of participants with samples collected at 6 months +/− 16 weeks (56−280 days) after delivery were assigned as the 6‐month postpartum VL. Our protocol defined endpoint was POC testing at 6 months postpartum for both intervention and control groups; however, if POC testing was not available in the interval 224−448 days from date of delivery, the SOC VL test result was used if available.
3.2. Primary outcome of VS proportions
At 6 months postpartum, 321/328 (98%) of participants in the intervention group and 339/347 (98%) in the control group achieved VS by POC VL testing (aRR 1.00, 95% confidence interval [CI] 0.98, 1.02; Table 2). When using a lower threshold of <400, results were almost identical; using <40 copies/ml, the VS proportion at 6 months postpartum was lower than seen with the higher thresholds but still similar by group. Findings from the sensitivity analyses, adjusting for differing enrolment characteristics between the groups and inverse probability weighting analysis, were similar (Table 2).
3.3. DRM testing, resistance identified and ART change recommendations
In the intervention group, from enrolment up until 6 months postpartum, we identified 54 episodes of VL>1000 copies/ml, among 48 (11.7%) participants (Table 3). In the intervention group, 52 DRM tests were requested, of which 46 (88%) were successfully conducted (six samples failed to amplify) and all identified at least one DRM (K103N [n = 12, 28%] and M184V [n = 10, 22%] were most commonly detected mutations). Our Clinical Management Committee recommended that 6 (12%) of the 48 women undergo an ART change, of which all six (100%) had an ART change documented by 6 months postpartum. In contrast, we recorded 37 episodes of VL>1000 copies/ml, among 29 (7%) control participants with no DRM tests requested in this group.
Table 3.
Description of drug resistance testing up until 6 months postpartum of study follow‐up and outcomes by study group among Opt4Mamas study participants (n=820), February 2019−December 2021 a
| Variable | Intervention group (POC VL; n=411) | Control group (SOC VL; n=409) |
|---|---|---|
| Episodes of viraemia (can be more than one per participant) | ||
| Episodes of viraemia (> 1000 copies/ml) a | 54 b | 37 c |
| Number of DRM test requested | 52 | 0 |
| Number of DRM test performed successfully | 46/52 (88%) d | – |
| Turnaround time from time DRM tests requested to results returned to study staff (in days), median (IQR) | 22 (16, 30) | – |
| Any DRM identified | 46/46 (100%) | – |
| Any major DRMs identified e | 20/46 (43%) | – |
| Any resistance type by HIV drug classes f | ||
| NRTI | 46/46 (100%) | – |
| NNRTI | 46/46 (100%) | – |
| PI | 46/46 (100%) | – |
| Major resistance type by HIV drug classes | ||
| NRTI | 11/20 (55%) | – |
| NNRTI | 16/20 (80%) | – |
| PI | 2/20 (10%) | – |
| ART change recommended per each DRM test successfully conducted | 6/52 (12%) | – |
| Recommended ART change made by 6 months postpartum | 6/6 (100%) | – |
Abbreviations: ART, antiretroviral therapy; DRM, drug resistance mutation; IQR, interquartile range; NNRTI, non‐nucleos(t)ide reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; POC, point‐of‐care; SOC, standard‐of‐care; VL, viral load.
From date of study enrolment through any time point prior to the 6 months postpartum study visit (e.g. data exclude results obtained as part of postpartum 6 study visit).
Forty‐eight participants had a total of 54 viraemic episodes detected (5 of these 48 [10%] participants had repeat viraemic episodes). Of these 54 samples, we did not request DRM testing for two because of insufficient sample, resulting in our requesting DRM test for 52 samples.
Twenty‐nine participants had a total of 37 viraemic episodes detected.
Out of 52 samples where we requested DRM test, six (12%) samples failed to amplify.
We define major classification for HIV drug resistance according to the Stanford HIV Resistance Database.
The most commonly detected DRM by HIV drug class included: (1) NRTI—M184V (n=10 DRM tests; detected in 22% of all DRM tests resulted), K70R/Q (7; 15%), and one (2%) each of K65R, D67N, L74I, V75M and K219R; (2) NNRTI—K103N (n=12; 28%), V108I (3; 7%), P225H (3; 7%), G190A/S (2; 4%), E138A/G (2; 4%), K238T (2; 4%), and one (2%) each of K101E, V106I and Y181C; and (3) PI—L89M (32; 70%), I13V (28; 61%), and one (2%) each of L24I, L33F, K43T, M46L, I54V and V82A.
3.4. Secondary outcomes of VL testing uptake, turnaround time and infant testing
Of the participants attending each study visit in the intervention group, 100%, 51%, 43%, 90%, 75% and 94% had a POC VL conducted at 0, 3 and 6 months after enrolment, delivery, 3 and 6 months postpartum, respectively (Table 4); the 3‐ and 6‐month after enrolment visits were heavily impacted by COVID‐19‐related restrictions in 2020. Among participants in the control group, 66%, 27%, 15%, 0%, 38% and 42% had a SOC VL conducted at 0, 3 or 6 months after enrolment, delivery, and at 3 and 6 months postpartum, respectively.
Table 4.
Process measures regarding point‐of‐care viral load and drug resistance testing among Opt4Mamas study participants (n=820), February 2019−December 2021
| Intervention group (POC VL; n=411) | Control group (SOC VL; n=409) | |
|---|---|---|
| Participants attending the study visit among those expected a | ||
| 0 months | 411/411 (100%) | 409/409 (100%) |
| 3 months | 283/296 (96%) | 288/301 (96%) |
| 6 months | 53/70 (76%) | 78/85 (92%) |
| Delivery | 345/380 (91%) | 367/376 (98%) |
| Postpartum 3 | 276/373 (74%) | 342/368 (93%) |
| Postpartum 6 | 321/364 (88%) | 333/361 (92%) |
| Subtotal from 0 to postpartum 6 | 1689/1894 (89%) | 1817/1900 (96%) |
| Postpartum 9+ | 34/2148 (2%) | 288/2071 (14%) |
| Total from 0 to postpartum 24 | 1723/4042 (43%) | 2105/3971 (53%) |
| POC VL test conducted b for intended study visit among participants attending the study visit | ||
| 0 months | 409/411 (99.5%) | |
| 3 months | 143/283 (50.5%) | |
| 6 months | 23/53 (43.4%) | |
| Delivery | 309/345 (89.6%) | 16/367 (4.6%) |
| Postpartum 3 | 206/276 (74.6%) | |
| Postpartum 6 | 301/321 (93.8%) | 255/333 (76.6%) |
| Subtotal from 0 to postpartum 6 | 1391/1689 (82.4%) | 271/700 (38.7%) |
| Postpartum 9+ | 28/34 (82.4%) | |
| Total from 0 to postpartum 24 | 1419/1723 (82.4%) | 271/700 (38.7%) |
| SOC VL test conducted b within testing interval among participants regardless of attending the study visit | ||
| 0 months | 156/411 (38%) | 268/409 (65.5%) |
| 3 months | 40/411 (9.7%) | 111/409 (27.1%) |
| 6 months | 10/411 (2.4%) | 60/409 (14.7%) |
| Delivery | ||
| Postpartum 3 | 26/411 (6.3%) | 146/409 (35.7%) |
| Postpartum 6 | 17/411 (4.1%) | 173/409 (42.3%) |
| Subtotal from 0 to postpartum 6 | 249/2055 (12.11%) | 758/2045 (37.1%) |
| Postpartum 9 + months | 16/1233 (1.3%) | 289/1636 (17.7%) |
| Total from 0 to 24 months | 265/3288 (8.1%) | 1047/3681 (28.4%) |
| Either POC for intervention group or SOC for control group VL test returned to participant/caregiver, and within 24 hours of blood draw b | ||
| 0 months | 391/409 (95.6%), 334/409 (81.7%) | Data not available |
| 3 months | 131/143 (91.6%), 106/143 (74.1%) | Data not available |
| 6 months | 19/23 (82.6%), 16/23 (69.6%) | Data not available |
| Delivery | 281/309 (91.0%), 245/309 (79.3%) | Data not available |
| Postpartum 3 | 192/206 (93.2%), 156/206 (75.7%) | Data not available |
| Postpartum 6 | 291/301(96.7%), 93/301 (30.9%) | Data not available |
| Subtotal from 0 to postpartum 6 | 1305/1391 (93.8%), 494/816 (60.5%) | Data not available |
| Postpartum 9 + months | 28/28 (100%), 15/26 (57.7%) | Data not available |
| Total from 0 to 24 months | 1333/1419 (94%), 509/842 (60.5%) | Data not available |
| POC VL test returned to participant, and within 24 hours of blood draw | ||
| 0 months | 391/409 (95.6%), 334/409 (81.7%) | |
| 3 months | 131/143 (91.6%), 106/143 (74.1%) | |
| 6 months | 19/23 (82.6%), 16/23 (69.6%) | |
| Delivery | 281/309 (91.0%), 245/309 (79.3%) | 10/16 (62.5%), 9/16 (56.3%) |
| Postpartum 3 | 192/206 (93.2%), 156/206 (75.7%) | |
| Postpartum 6 | 291/301 (96.7%), 93/301 (30.9%) | 237/255 (93.0%), 189/255 (74.1%) |
| Subtotal from 0 to postpartum 6 | 1305/1391 (93.8%), 494/816 (60.5%) | 247/271 (91.1%), 198/205 (96.6%) |
| Postpartum 9+ | 28/28 (100%), 15/26 (57.7%) | |
| Total from 0 to 24 months | 1333/1419 (94%), 509/842 (60.5%) | 247/271 (91.1%), 198/205 (96.6%) |
| Either POC or SOC VL test returned to provider, and within 24 hours of blood draw c | ||
| 0 months | 391/409 (95.6%), 331/391 (84.7%) | 268/268 (100%) |
| 3 months | 132/143 (92.3%), 107/132 (81.1%) | 111/111 (100%) |
| 6 months | 19/23 (82.6%), 16/19 (84.2%) | 60/60 (100%) |
| Delivery | 281/309 (90.1%), 242/281 (86.1%) | |
| Postpartum 3 | 192/206 (93.2%), 153/192 (79.7%) | 146/146 (100%) |
| Postpartum 6 | 291/301 (96.7%), 93/291 (32.0%) d | 173/173 (100%) |
| Subtotal from 0 to postpartum 6 | 1306/1391 (93.9%), 942/1306 (72.1%) | 758/758 (100%) |
| Postpartum 9 + months | 28/ 28 (100%), 15/28 (53.6%) | 289/289 (100%) |
| Total from 0 to 24 months | 1334/1419 (94%), 957/1334 (71.7%) | 1047/1047 (100%) |
| Number of POC VL test returned to provider, and within 24 hours of blood draw | ||
| 0 months | 391/409 (95.6%), 331/391 (84.7%) | |
| 3 months | 132/143 (92.3%), 107/132 (81.1%) | |
| 6 months | 19/23 (82.6%), 16/19 (84.2%) | |
| Delivery | 281/309 (90.1%), 242/281 (86.1%) | 10/16 (62.5%), 9/10 (90.0%) |
| Postpartum 3 | 192/206 (93.2%), 153/192 (79.7%) | |
| Postpartum 6 | 291/301 (96.7%), 93/291 (32.0%) d | 237/255 (93.0%), 184/237 (77.6%) |
| Subtotal from 0 to postpartum 6 | 1306/1391 (93.9%), 942/1306 (72.1%) | 247/271 (91.1%), 193/247 (78.1%) |
| Postpartum 9 + months | 28/ 28 (100%), 15/28 (53.6%) | |
| Total from 0 to 24 months | 1334/1419 (94%), 957/1334 (71.7%) | 247/271 (91.1%), 193/247 (78.1%) |
| Median (IQR) turnaround time in days for VL requested (from sample collection to result return to provider), by POC VL testing for intervention group and SOC VL testing for control group | 1 (1, 4) | 7 (5, 9) |
| Number of VL tests from enrolment to 6 months postpartum (POC VL for intervention group, SOC VL for control group) | ||
| At least one | 406 (98.8%) | 393 (96.1%) |
| At least two | 358 (87.1%) | 351 (85.8%) |
| Median (IQR) | 4 (2, 5) | 3 (2, 3) |
| Number of VL tests from enrolment to delivery (POC VL for intervention group, SOC VL for control group) | ||
| At least one | 401 (97.6%) | 369 (90.2%) |
| At least two | 305 (74.3%) | 187 (45.7%) |
| Median (IQR) | 2 (2, 3) | 2 (1, 2) |
| Number of VL tests from delivery to 6 months postpartum (POC VL for intervention group, SOC VL for control group) | ||
| At least one | 343 (83.5%) | 355 (86.8%) |
| At least two | 238 (58.0%) | 183 (44.7%) |
| Median (IQR) | 2 (1, 3) | 2 (1, 2) |
Abbreviations: IQR, interquartile range; PI, protease inhibitor; POC, point‐of‐care; SOC, standard‐of‐care; VL, viral load.
We define participants attending the study visit as those completing study questionnaires, though not necessarily in‐person, among those expected to attend the study visit (i.e. retention in study).
VL tests were considered to have been conducted if a sample was collected for testing and sent for VL testing.
Because VL test results are only tracked by results released to the local laboratory in the Kenya Ministry of Health's NASCOP HIV VL database, we are not able to track how many VL tests were requested versus those finally resulted. Thus, the results returned are 100% for SOC.
Of note, our study encountered a 3‐month delay in being able to test our study samples via POC VL testing due to the global reagent shortages experienced during the COVID‐19 pandemic.
Of the POC VL tests conducted in the intervention group during the entire study period, 90% were returned to the participant, and ≥ 60% were returned within 24 hours of the blood draw (excluding the 6‐month postpartum visit in which only 31% were returned within 24 hours due to disruptions in the global supply of POC VL cartridges for GeneXpert systems; Table 4). Return of results to providers followed similar patterns. Neither the number of total VL test requests nor the turnaround time from sample collection to return of results to the women was available in the control group. From sample collection to result return to providers, the median turnaround time was 1 day (IQR 1, 4) for POC VL testing in the intervention group and 7 days (IQR 5, 9) for SOC VL testing in the control group.
Through the 6‐month postpartum visit, one and three infants tested HIV positive in the intervention and control groups, respectively (Table S5).
3.5. Secondary outcome of sustained VS
SVS was lower than VS point prevalence at 6 months postpartum but was still similar by group; 311/352 (88.4%) in intervention group and 332/366 (90.7%) in control group (aRR 0.98, 95% CI 0.93, 1.03) (Table 5). SVS measured at <400 copies/ml was 304/352 (86.4%) and 321/366 (87.7%; aRR 0.97, 95% CI 0.91, 1.02) and at <40 copies/ml was 246/352 (70.0%) and 226/366 (61.7%; aRR 1.00, 95% CI 0.89, 1.12) in the intervention and control groups, respectively.
Table 5.
Effect of the intervention on sustained a viral suppression for all study intervals through 6‐month postpartum visit for every participant with two or more VL test results in two separate study intervals, and varying threshold of VL cutoffs among the Opt4Mamas study participants (n=820), February 2019−December 2021
| Variable | Intervention (n=411) | Control (n=409) | Unadjusted RR b (95% CI) | p‐Value | Adjusted RR b (95% CI) | p‐Value |
|---|---|---|---|---|---|---|
| Viral suppression < 1000 copies/ml | 311/352 (88.4%) | 332/366 (90.7%) | 0.97 (0.92, 1.02) | 0.213 | 0.98 (0.93, 1.03) | 0.378 |
| Viral suppression < 400 copies/ml | 304/352 (86.4%) | 321/366 (87.7%) | 0.97 (0.91, 1.02) | 0.256 | 0.97 (0.91, 1.02) | 0.239 |
| Viral suppression < 40 copies/ml | 246/352 (70.0%) | 226/366 (61.7%) | 1.03 (0.92, 1.15) | 0.587 | 1.00 (0.89, 1.12) | 0.958 |
Abbreviations: POC, point‐of‐care; SOC, standard‐of‐care; VL, viral load.
Sustained viral suppression is defined as having viral load less than viral load cutoff in all viral load tests. For instance, for viral load cutoff <1000 copies/ml, a participant is sustained virally suppressed if the participant has viral load <1000 copies/ml in all viral load tests taken.
Risk ratio of intervention adjusted for number of tests.
4. DISCUSSION
In this prospective, intervention/control cohort study among women living with HIV on ART, we did not observe differences in VS between women receiving a combined intervention with POC with higher frequency VL testing every 3 months, targeted DRM testing, and clinical decision support, and control women, during pregnancy, delivery or the postpartum periods. Overall, we observed >90% VS at 6 months postpartum in this study at thresholds of VL <1000, <400 and <40 copies/ml. However, a sizeable proportion of women experienced viraemia during pregnancy and postpartum periods when considering all VL checks (10−38%), and major DRMs among these women may be important. POC VL and DRM testing was highly feasible in this setting, with rapid turnaround times, and resulted in a larger proportion of women undergoing these testing during pregnancy and postpartum periods.
We did not observe any associations between our combined intervention and VS among pregnant and postpartum women. Our failure to demonstrate efficacy may be due to biased sampling of women better engaged in care, inability to implement POC VL testing with optimal fidelity, overall improvements in VS among the women over time and/or the increased use of dolutegravir‐containing ART which is equally or more potent than efavirenz‐containing ART [37−39]. Nonetheless, when considering SVS throughout the pregnancy and postpartum periods, a sizeable number of women lacked VS at some point during pregnancy, as low as 62% when using the lowest VL threshold of <40 copies/ml. With maternal VL being the greatest predictor of vertical transmission, achieving VS among pregnant and postpartum women remains an enduring concern; vertical transmission rates were around 2% among mothers with VLs ranging from 40 to 1000 copies/ml [3]. In our study, few vertical transmissions were recorded and POC VL uptake was high. Efforts to eliminate vertical transmission will require rapid identification and intervention for pregnant and breastfeeding women with viraemia, and POC VL may still have a targeted role in achieving the elimination of vertical transmission.
The evidence base regarding the utility of POC VL testing in improving clinical outcomes is mixed. We saw no efficacy on VS in our parallel, randomized controlled trial in children [22]. A South African randomized controlled trial compared 3‐monthly POC VL testing to 6‐monthly SOC laboratory‐based VL testing among postpartum women living with HIV on first‐line ART, and found no significant difference in VL suppression rates [23]. Similarly, a study among Nigerian adults initiating ART reported that POC VL monitoring did not improve 12‐month VS, but it did improve retention and VS documentation and was favoured by the majority of patients and healthcare workers [24]. Other studies demonstrated some benefits to POC VL. A preliminary analysis suggests significant improvement in VS (7%) among pregnant/breastfeeding Ugandan women, children/adolescents (2–17 years), viraemic patients and patients overdue for VL who received POC VL [25]. A study that combined POC VL with a differentiated service delivery strategy resulted in enhanced VS by 10.3% and retention by 7.7% among South African adults living with HIV [26]. Ultimately, despite some studies showing no efficacy, enthusiasm for POC VL testing exists not only among patients and providers, but also among policymakers at the national and international levels [18, 32, 40]. Future research needs to help elucidate cost‐effective ways for when best to use POC VL testing, among whom and at what interval frequency.
Among women with viraemia in our study who underwent successful DRM testing, all had some DRMs and 43% had major DRMs (with NNRTI K103N and NRTI M184V being the most common). A study conducted in Sierra Leone showed K103N as the most frequent DRM, occurring in 20% of pregnant women, with M184V in 11% [41]. Among Kenyan pregnant women, 65% of viraemic women showed some DRMs [42]. Among women initiating ART, most of whom reported prior exposure to antiretrovirals for prevention of vertical transmission, the prevalence of DRM to NNRTIs was 14.6% [28]. INSTI resistance testing was unavailable in Kenya during the study period, and while greater than half of the women transitioned to INSTI‐containing regimens by the study end, we do not think the inclusion of INSTI resistance testing would have altered our overall findings, as the emergence of dolutegravir resistance with short exposures is unlikely [43]. However, the inclusion of INSTI resistance testing will become necessary as women become viraemic after a longer duration on dolutegravir‐containing regimens. In the current study, around one in eight women with a DRM test result required ART change; at a population level, this is a substantial number. Lack of drug resistance may be equally valuable for clinicians as this indicates that the regimen does not require a switch for resistance reasons and suggests other causes for viraemia. From our clinical case reviews, it appears that many women with viraemia face larger, psychosocial and behavioural challenges (manuscript forthcoming) which require additional interventions in combination with information provided by DRM testing. Thus, differentiated service delivery models need to be urgently developed to concentrate the needed extra resources for those women struggling with VS, including women on salvage regimens or not engage in care (noting such women were excluded from our study), during this dynamic period of their pregnancy care.
4.1. Limitations
Ours is the first study to combine VL with DRM testing in optimizing VS among pregnant and postpartum women; however, it has limitations. First, Opt4Mamas could not be pursued as a randomized clinical trial as a limitation of its funding source, but we believed a contemporaneous intervention/control study, which had overlapping cohorts in time, was the next best robust study design to pursue. Second, our combined strategy of multiple interventions precludes the determination of the success or failure of any one component of the strategy. Additionally, layering on POC VL testing on top of routine care, where providers were not prevented from ordering routine SOC VL testing, further limits the interpretation of our findings. Third, some spillover effects from intervention to control participants may have occurred. Though our intervention package was only offered to intervention group participants, it was the same providers caring for control group participants. We observed greater fidelity in conducting SOC VL tests in the control group than expected and, anecdotally, increased confidence in facility staff over time in managing women with viraemia. Our randomized trial in children faced this same issue [22]. Alternative study designs, such as facility‐level cluster randomization, may avoid potential spillover effects though would require greater resources. Fourth, we note that VS was assessed more frequently in the intervention versus control group, so, theoretically, the intervention group participants had a greater number of opportunities to act on their test results but also greater opportunities to detect viraemia. Ultimately, restrictions and reagent stockouts related to COVID‐19 greatly compromised our ability to conduct POC VL testing every 3 months or return results within 24 hours as planned. Thus, it is possible that intervention group participants did not receive sufficient POC VL testing to impact their outcomes. While the impacts of COVID‐19 may not be as significant in the future, it is likely that similar programmatic challenges will persist. Lastly, potential measurement bias in intention‐to‐treat estimates and selection bias due to missing outcome data (on approximately 15–20% of our enrolled participants) are limitations; however, our sensitivity analysis using inverse probability weighting substantiated our primary outcome analysis findings.
5. CONCLUSIONS
In a prospective cohort study with an intervention/control study design with the intervention consisting of a combined strategy of POC with higher frequency VL testing, targeted DRM testing and clinical decision‐making support versus SOC, we observed high rates of VS at 6 months postpartum in both groups and no difference between the intervention and control groups during pregnancy, at delivery or postpartum. Nonetheless, a sizeable number of women experienced viraemia, when considering SVS throughout pregnancy and postpartum periods, many of whom had major DRMs. POC VL uptake was high and DRM testing was feasible. Ultimately, it remains unclear what interventions pregnant/postpartum women with viraemia need to optimize VS, their health outcomes and help prevent vertical transmission.
COMPETING INTERESTS
The authors declare no competing interests.
AUTHORS’ CONTRIBUTIONS
Conceptualization—RCP, LLA, PO, IM and KKT.
Data curation—NY and KKT.
Access and verified data—NY, GWB and KKT.
Formal analysis—GWB, NY, KKT, RCP, LLA and PO.
Funding acquisition—RCP.
Investigation—RCP, LLA, PO, IM, KKT, JW, LK and EK.
Methodology—RCP, LLA, PO and KKT.
Project administration—EB, PO, KKT, SAH, BO, LK, EK, NY, RCP and LLA.
Resources—PO, KKT, JW, EK, FO, FO, BO, LK, EK, NY, LO, RCP and LLA.
Software—GWB, NY, KKT, BO and LK.
Supervision—EB, PO, KKT, JW, EK, FO, FO, BO, LK, EK, NY, LO, RCP, LLA and GCJ‐S.
Validation—N/A.
Visualization—GWB, NY, KKT and SAH.
Writing, original draft—RCP, LLA, PO, KKT, GWB and SAH.
Writing, review and editing—all coauthors.
Decision to submit manuscript—RCP, LLA, PO and KKT.
FUNDING
This work was supported by the National Institutes of Allergy and Infectious Diseases of the U.S. National Institutes of Health (NIH, CFAR NIA 2P30AI027757‐31 and R21 R21AI145450). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington Institute of Translational Health Sciences and supported by the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319).
DISCLAIMER
The funding sources or study sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
ETHICAL REVIEW STATEMENT
Ethical approval for this study has been obtained from the African Medical and Research Foundation (AMREF) and Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) Institutional Review Boards (IRBs) in Kenya, as well as the University of Washington and the University of Colorado Denver IRBs in the United States. All study procedures were performed in accordance with the Declaration of Helsinki.
Supporting information
Supplementary Text
Supplemental Figure 1
Supplemental Tables
ACKNOWLEDGEMENTS
We recognize the study participants, caregivers and facility staff participating in and supporting the Opt4Kids study. We acknowledge the support of the Kisumu County Health Management Team, Ministry of Health, and Family AIDS Care and Education Services. We also thank members of our Scientific Advisory Committee.
DATA AVAILABILITY STATEMENT
De‐identified participant data, data dictionary or other specified data sets may be made available to others requesting them upon communication with corresponding author, demonstration of appropriate ethic reviews and establishment of data sharing agreements. Study protocol, statistical analysis plan, informed consent forms, analysis code or other documents can be made available upon request with the corresponding author.
REFERENCES
- 1. The Global Health Observatory . World Health Organization data on the HIV response. https://www.who.int/data/gho/data/themes/hiv‐aids/data‐on‐the‐hiv‐aids‐response. Accessed 21 June 2023.
- 2. The World Bank UNAIDS estimates—antiretroviral therapy coverage for PMTCT (% of pregnant women living with HIV). https://data.worldbank.org/indicator/SH.HIV.PMTC.ZS. Accessed 21 June 2023.
- 3. Myer L, Phillips T, Mcintyre J, Hsiao N‐Y, Petro G, Zerbe A, et al. HIV viraemia and mother‐to‐child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18(2):80–88. [DOI] [PubMed] [Google Scholar]
- 4. Boucoiran I, Albert AYK, Tulloch K, Wagner EC, Pick N, Van Schalkwyk J, et al. Human immunodeficiency virus viral load rebound near delivery in previously suppressed, combination antiretroviral therapy‐treated pregnant women. Obstet Gynecol. 2017;130(3):497–501. [DOI] [PubMed] [Google Scholar]
- 5. Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA, Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341(6):385–393. [DOI] [PubMed] [Google Scholar]
- 6. Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization Global Action Plan on HIV Drug Resistance. https://www.who.int/publications/i/item/978‐92‐4‐151284‐8. Accessed 21 June 2023
- 8. UNAIDS JUNP . Kenya‐HIV and AIDS estimates. https://www.unaids.org/en/regionscountries/countries/kenya. Accessed 21 June 2023
- 9. Ministry of Health NASCP . Preliminary KENPHIA 2018 Report. Nairobi, Kenya: Kenya Ministry of Health; 2020. [Google Scholar]
- 10. Ministry of Health NASCP . Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. http://cquin.icap.columbia.edu/wp‐content/uploads/2017/04/ICAP_CQUIN_Kenya‐ARV‐Guidelines‐2018‐Final_20thAug2018.pdf. Accessed 21 June 2023
- 11. Lecher SL, Fonjungo P, Ellenberger D, Toure CA, Alemnji G, Bowen N, et al. HIV viral load monitoring among patients receiving antiretroviral therapy—eight sub‐Saharan Africa countries, 2013–2018. MMWR Morb Mortal Wkly Rep. 2021;70(21):775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mwau M, Syeunda CA, Adhiambo M, Bwana P, Kithinji L, Mwende J, et al. Scale‐up of Kenya's national HIV viral load program: findings and lessons learned. PLoS One. 2018;13(1):e0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lecher S, Ellenberger D, Kim AA, Fonjungo PN, Agolory S, Borget MY, et al. Scale‐up of HIV viral load monitoring—seven sub‐Saharan African countries. MMWR Morb Mortal Wkly Rep. 2015;64(46):1287–1290. [DOI] [PubMed] [Google Scholar]
- 14. Kadima J, Patterson E, Mburu M, Blat C, Nyanduko M, Bukusi EA, et al. Adoption of routine virologic testing and predictors of virologic failure among HIV‐infected children on antiretroviral treatment in western Kenya. PLoS One. 2018;13(11):e0200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutstein SE, Golin CE, Wheeler SB, Kamwendo D, Hosseinipour MC, Weinberger M, et al. On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource‐limited settings. AIDS Care. 2016;28(1):5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simeon K, Sharma M, Dorward J, Naidoo J, Dlamini N, Moodley P, et al. Comparative cost analysis of point‐of‐care versus laboratory‐based testing to initiate and monitor HIV treatment in South Africa. PLoS One. 2019;14(10):e0223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bulterys MA, Oyaro P, Brown E, Yongo N, Karauki E, Wagude J, et al. Costs of point‐of‐care viral load testing for adults and children living with HIV in Kenya. Diagnostics. 2021;11(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meggi B, Bollinger T, Zitha A, Mudenyanga C, Vubil A, Mutsaka D, et al. Performance of a true point‐of‐care assay for HIV‐1/2 viral load measurement at antenatal and postpartum services. J Acquir Immune Defic Syndr. 2021;87(1):693–699. [DOI] [PubMed] [Google Scholar]
- 19. Sandbulte MR, Gautney BJ, Maloba M, Wexler C, Brown M, Mabachi N, et al. Infant HIV testing at birth using point‐of‐care and conventional HIV DNA PCR: an implementation feasibility pilot study in Kenya. Pilot Feasibility Stud. 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bwana P, Ageng'o J, Mwau M. Performance and usability of Cepheid GeneXpert HIV‐1 qualitative and quantitative assay in Kenya. PLoS One. 2019;14(3):e0213865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ministry of Health NASCP . National Point of Care Testing Implementation Roadmap in Kenya 2019 Edition. Nairobi, Kenya; 2019. [Google Scholar]
- 22. Patel RC, Oyaro P, Thomas KK, Wagude J, Mukui I, Brown E, et al. Point‐of‐care HIV viral load and targeted drug resistance mutation testing versus standard care for Kenyan children on antiretroviral therapy (Opt4Kids): an open‐label, randomised controlled trial. Lancet Child Adolesc Health. 2022;6(10):681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fairlie L, Sawry S, Pals S, Sherman G, Williamson D, Chivafa A, et al., “More frequent viral load testing, with point‐of‐care tests has no impact on viral suppression in postpartum HIV‐positive women in a randomized controlled trial in two clinics in Johannesburg, South Africa.” American Medical Education. International Workshop on HIV Pediatrics 2021 Abstract. Accessed 21 June 2023.. https://academicmedicaleducation.com/meeting/international‐workshop‐hiv‐pediatrics‐2021/abstract/more‐frequent‐viral‐load‐testing‐point [Google Scholar]
- 24. Chang C, Mitruka K, Olatunde B, Sule H, Dajel T, Zee A, et al. Clinical outcomes in a randomized controlled trial comparing point‐of‐care versus standard HIV viral load monitoring in Nigeria. Clinical Infectious Diseases. 2023; 76(3):e681‐e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jain VEA. RAPID‐VL intervention improves viral load ordering, results turnaround time and viral suppression: a cluster randomized trial in HIV clinics in Uganda. 11th International AIDS Society Conference on HIV Science. 2021.
- 26. Drain PK, Dorward J, Violette LR, Quame‐Amaglo J, Thomas KK, Samsunder N, et al. Point‐of‐care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open‐label, non‐inferiority, randomised controlled trial. Lancet HIV. 2020;7(4):e229–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pilgrim NA, Okal J, Matheka J, Mukui I, Kalibala S. Challenges to and opportunities for the adoption and routine use of early warning indicators to monitor pediatric HIV drug resistance in Kenya. BMC Pediatr. 2018;18(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization HIV Drug Resistance Mutation. https://www.who.int/publications/i/item/9789240038608. Accessed 21 June 2023
- 29. World Health Organization HIV Drug Resistance Key Facts. https://www.who.int/news‐room/fact‐sheets/detail/hiv‐drug‐resistance. Accessed 21 June 2023
- 30. Aghokeng AF, Monleau M, Eymard‐Duvernay S, Dagnra A, Kania D, Ngo‐Giang‐Huong N, et al. Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization public health approach in sub‐Saharan Africa and southeast Asia. Clin Infect Dis. 2014;58(1):99–109. [DOI] [PubMed] [Google Scholar]
- 31. Roberts T, Cohn J, Bonner K, Hargreaves S. Scale‐up of routine viral load testing in resource‐poor settings: current and future implementation challenges. Clinical Infectious Diseases. 2016.;62(8):1043‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frontiers MS. Making viral load routine: successes and challenges in the implementation of routine HIV viral load monitoring. 2017. www.msfaccess.org/makingviralloadroutine. Accessed 21 June 2023
- 33. Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV‐1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rhee S‐Y, Jordan MR, Raizes E, Chua A, Parkin N, Kantor R, et al. HIV‐1 drug resistance mutations: potential applications for point‐of‐care genotypic resistance testing. PLoS One. 2015;10(12):e0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stanford University HIV Drug Resistance Database. https://hivdb.stanford.edu. Accessed 21 June 2023
- 36. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 37. Romo ML, Edwards JK, Semeere AS, Musick BS, Urassa M, Odhiambo F, Diero L, et al. Viral load status before switching to dolutegravir‐containing antiretroviral therapy and associations with HIV treatment outcomes in sub‐Saharan Africa. Clinical Infectious Diseases. 2022;75(4):630‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calmy A, Tovar Sanchez T, Kouanfack C, Mpoudi‐Etame M, Leroy S, Perrineau S, et al. Dolutegravir‐based and low‐dose efavirenz‐based regimen for the initial treatment of HIV‐1 infection (NAMSAL): week 96 results from a two‐group, multicentre, randomised, open label, phase 3 non‐inferiority trial in Cameroon. Lancet HIV. 2020;7(10):e677–e687. [DOI] [PubMed] [Google Scholar]
- 39. Pepperrell T, Venter WDF, Mccann K, Bosch B, Tibbatts M, Woods J, et al. Participants on dolutegravir resuppress human immunodeficiency virus RNA after virologic failure: updated data from the ADVANCE Trial. Clin Infect Dis. 2021;73(4):e1008–e1010. [DOI] [PubMed] [Google Scholar]
- 40. Qian SRW, Hassan SA, Scallon AJ, Oyaro P, Brown E, Wagude J, et al. “After viral load testing, I get my results so I get to know which path my life is taking me”: qualitative insights on routine centralized and point‐of‐care viral load testing in western Kenya from the Opt4Kids and Opt4Mamas studies. BMC Health Serv Res. 2022;22(1):1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yendewa GA, Lakoh S, Yendewa SA, Bangura K, Tabernilla A, Patiño L, et al. Characterizing HIV‐1 genetic subtypes and drug resistance mutations among children, adolescents and pregnant women in Sierra Leone. Genes. 2021;12(9):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chohan BH, Ronen K, Khasimwa B, Matemo D, Osborn L, Unger JA, et al. Food insecurity, drug resistance and non‐disclosure are associated with virologic non‐suppression among HIV pregnant women on antiretroviral treatment. PLoS One. 2021;16(8):e0256249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Oosterhout JJ, Chipungu C, Nkhoma L, Kanise H, Hosseinipour MC, Sagno JB, et al. Dolutegravir resistance in Malawi's National HIV Treatment Program. Open Forum Infect Dis. 2022;9(5):ofac148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Supplemental Figure 1
Supplemental Tables
Data Availability Statement
De‐identified participant data, data dictionary or other specified data sets may be made available to others requesting them upon communication with corresponding author, demonstration of appropriate ethic reviews and establishment of data sharing agreements. Study protocol, statistical analysis plan, informed consent forms, analysis code or other documents can be made available upon request with the corresponding author.


