Abstract
Among many types of wearable sensors, MOFs-based wearable sensors have recently been explored in both commercialization and research. There has been much effort in various aspects of the development of MOF-based wearable sensors including but not limited to miniaturization, size control, safety, improvements in conformal and flexible features, improvements in the analytical performance and long-term storage of these devices. Recent progress in the design and deployment of MOFs-based wearable sensors are covered in this paper, as are the remaining obstacles and prospects. This work also highlights the enormous potential for synergistic effects of MOFs used in combination with other nanomaterials for healthcare applications and raise attention toward the economic aspect and market diffusion of MOFs-based wearable sensors.
Keywords: flexible materials, Health monitoring applications, iontophoresis, metal organic framework, wearable sensor
Graphical abstract

Highlights
-
•
Review article on metal organic framework (MOFs)-bsed wearable sensors.
-
•
The advantages of using MOFs as sensing platform and their healthcare applications are discussed.
-
•
The use of MOFs combinations with other materials in the production of electrochemical sensors is also discussed.
-
•
MOF-basedsensors manufacturing, achievements, market diffusion and related limitations are described in depth.
-
•
Challenges and prospects using MOFs-based wearable sensors have been proposed.
1. Introduction
Wearable sensors have recently gained a lot of interest due to their exceptional performances and numerous benefits. The market demand for these technologies has rapidly expanded from $20 billion in 2016 to $80 billion in 2022 [1] because of their real-world applications that require quick, accurate detections. They have been utilized for a wide range of tasks including virus detection, cancer early diagnosis and monitoring, and pulse rate analysis [[2], [3], [4], [5], [6]].
In addition to sensing of various physiological variations, wearable sensors should be able to detect biomarkers specific to diseases at very early stages of their emergence, then it can help accurate diagnosis of diseases like DNA damage [7], Alzheimer's [8], and cancer [9,10]. For this purpose, numerous various nanomaterials have been developed as sensing elements since the sensing matrix is the most crucial component of any sensors. There has been much improvement in the sensitivity and selectivity of the sensors. the most commonly used nanomaterials for sensing purposes include nanostructures like carbon-based materials [11,12], MXenes [13], conducting polymers like molecularly imprinted polymers (MIPs) [14], metals and metal oxides, and most recently metal organic frameworks (MOFs) [[15], [16], [17], [18]].
Metal-organic frameworks (MOFs), commonly referred as porous coordination polymers (PCPs), are created when metal ions or clusters and organic bridging ligands are coordinated [19,20]. Extended infinite networks are created by the coordination of metal ions/clusters with ligands [21]. So far, various metal ions and organic ligands have been explored. As a result, a variety of MOF types haven been demonstrated with outstanding properties [[22], [23], [24]]. MOFs have wide structural variety and tunable physicochemical features since there are so many possible metal and ligand combinations. MOFs are benefited from the inherent advantages of both flexible organic materials and hard inorganic elements. Moreover, MOFs' distinct architectures can result in extremely high Langmuir surface area (>10,000 m2 g−1) [25]. Hence, MOFs have been used for desalination, water treatment, and pollution remediation due to their adjustable pores and high porosity [19,26,27], energy related applications [28], catalysis, and sensing applications [20,29,30]. Furthermore, MOFs can be frequently coupled with other nanostructures to create effective MOF-based hybrids for the development of sensors and biosensors because of their physicochemical alterations and tunable chemical functionalization [[21], [31]]. For example, Recently, SARS-CoV-2 and other viral diseases have been detected using MOF-based sensors [32,33].
This review aims to present the most recent uses of MOFs in wearable sensors for biomedical applications. While illustrative examples of these sensors are presented, basic architectures and operating principles of wearable sensors based on MOFs are reviewed. The key features of using MOFs as sensing platforms are highlighted. In addition, an outlook about the opportunities of these materials in the field of sensing is also provided along with an overview of the current challenges in the development of MOF-based wearable sensors for glucose detection as an example.
2. Research procedure
We conducted a Google Scholar search to identify references containing information on the MOFs, electrochemical (bio)sensing, wearable sensors, health diagnosis, commercialization and economic aspect of MOFs-based wearable sensors. We used single or combinatorial searches on keywords such as “MOFs”, “commercialization”, “health diagnosis”, “economic aspect”, “safety”, “glucose”, “biocompatibility”, etc. For each identified paper, we then reviewed the references therein to determine if additional relevant references could be identified. The initial search revealed more than 150 papers, from which 130 were selected for consideration in the present review to cover most recent progress in the design and deployment of MOFs-based wearable sensors are covered in this paper, as are the remaining obstacles and prospects. This work also highlights the enormous potential for synergistic effects of MOFs used in combination with other nanomaterials for healthcare applications and raise attention toward the economic aspect and market diffusion of MOFs-based wearable sensors (Scheme 1).
Scheme 1.
Research strategy and review over flow.
3. Integration of MOFs in sensing device
The sensing matrix should be integrated into the working electrode of the sensor for electrochemical sensor applications (Table 1), and it should be exceptionally sensitive to the target analyte [17,34,35]. A wide range of processes, including printing, electrodeposition, laser ablation, and drop-casting, can be used for integrating the sensing matrix into the electrode. As a promising sensing matrix, MOFs have gained huge interest for a variety of sensing devices.
Table 1.
Most recent wearable sensor based on MOFs for health-care applications.
| Article title | Authors and Publication date | MOF | Application and Analytical Performance | Outcomes |
|---|---|---|---|---|
| Design and fabrication of novel flexible sensor based on 2D Ni-MOF nanosheets as a preliminary step toward wearable sensor for onsite Ni (II) ions detection in biological and environmental samples. | Elashery et al., 2022 [18] | Ni-MOF Metal: Ni Ligand: benzenedicarboxylic acid: (BDC)) |
Determination of Nickel ions in biological and environmental samples. LR = 1.0 × 10−5–1.0 × 10−1 mol L−1. LOD = 2.7 × 10−6 mol L−1. |
|
| Highly Stretchable Wearable Electrochemical Sensor Based on Ni–Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection | Shu et al., 2021 [23] | Ni–Co-MOF Metal: Ni–Co. Ligand: benzenedicarboxylic acid: (BDC)) |
Detection of glucose in sweat Sensitivity = 425.9 μA mM−1·cm−2. LR: 10 μM–0.66 mM. |
|
| MOF-derived porous Ni/C material for high-performance hybrid nanogenerator and self-powered wearable sensor | Gui et al., 2023 [36] | Ni-MOF Metal: Ni–Co Ligand: Terephthalic acid |
Motion monitoring |
|
| Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates | Shahrokhian et al., 2020 [37] | Co3 (BTC) 2 MOFs Metal: Co Ligand: 1,3,5-benzene tricarboxylic acid (H3 BTC, C9H 6O 6))) |
Glucose detection in human blood LR = 1 μM - 0.33 mM LOD = 0.33 μM. |
|
| Smartphone light-driven zinc porphyrinic MOF nanosheets-based enzyme-free wearable photoelectrochemical sensor for continuous sweat vitamin C detection | Yan et al., 2023 [38] | Zn-MOF Metal: Zn Ligand: 4,4′-biphenyldicarboxylic acid (BPDC)) |
Detection of vitamin C in human sweat. LR = 10–1100 Μm. LOD = 3.61 μM. |
|
| A highly flexible Ni–Co MOF nanosheet coated Au/PDMS film based wearable electrochemical sensor for continuous human sweat glucose monitoring | Shu et al., 2018 [17] | Ni–Co-MOF Metal: Ni–Co Ligand: Terephthalic acid (PTA)) |
Glucose Detection in sweat LR = 20 μM–790 μM. Sensitivity = 205.1 μA mM−1 cm−2. |
|
| Fluorescent wearable platform for sweat Cl ‐ analysis and logic smart‐device fabrication based on color adjustable lanthanide MOFs |
Xu et al., 2013 [35] | DUT‐101 was synthesized by using biphenyl‐4,4′‐dicarboxylic acid (H 2bpdc) with Tb(NO 3) 3 ∙6H 2 O | Cl− ions detection in sweat LOD = 0.1 mM |
|
| Chiral MOF Derived Wearable Logic Sensor for Intuitive Discrimination of Physiologically Active Enantiomer | Yang et al., 2023 [39] | Chiral γ-cyclodextrin metal-organic framework (CDMOF) | Lactate enantiomers |
|
| Ultra-thin 2D bimetallic MOF nanosheets for highly sensitive and stable detection of glucose in sweat for dancer | Mao et al., 2023 [27] | The NiMn-MOF Metal: Ni–Mn Ligand: 1,3,5 Benzenetricarboxylic acid (H3BTC) |
Glucose Detection in sweat Sensitivity = 1576 μA mM−1 cm−2. LR = 0–0.205 mM. LOD, 0.28 μM. |
|
| A wearable sweat electrochemical aptasensor based on the Ni–Co MOF nanosheet-decorated CNTs/PU film for monitoring of stress biomarker | Su et al., 2023 [24] | Ni–Co-MOF Metal: Ni–Co Ligand: diamino terephthalic acid (C8H6O4), |
Cortisol detection LR = 0.1–100 ng/mL. LOD = 0.032 ng/mL. |
|
The particular structure of MOFs helps enhance the sensors' sensitivity and selectivity, both of which are regarded as essential characteristics of any sensors. For instance, an MOF nanostructure that contains manganese has been solvothermally created. With a detection limit estimated to be around 0.12 ppb, the synthesized nanohybrid demonstrated excellent water-stability and high affinity of nitrogen atoms on the frameworks to Cd2+. This indicates the excellent selectivity and anti-interference capacity of the synthesized MOF based composite [40]. Shahrokhian et al. [37], reported a quick and simple three-step synthesis process to coat the GCE active area by Co3(BTC)2 MOFs to produce a sensitive and selective matrix for glucose identification in human blood. The modified electrode showed two wide linear ranges of 1–0.33 mM and 0.33–1.38 mM with high sensitivity of 1792 mA/cm2 and 1002 mA/cm2, respectively [37]. However, it is necessary to always optimize the effects of the electrolyte content, current density, and conductive salt on the synthesis of MOF films [41].
On the other hand, Naghian et al. [42], revealed a straightforward electrochemical detection technique based on the modification of a screen-printed carbon electrode (SPCE) by drop-casting a metal-organic framework (MOF) with extremely high porosity, a sizable surface area, and good thermal and chemical durability on its surface. The suggested method assisted in creating a selective, sensitive, and cost-effective sensor that performed well on actual samples like blood and urine [42]. Similarly, Fernandez and coauthors made an effort to investigate the performance of 2D printers to produce composites made of ionic liquid (IL) and MOF that function as thin film sensors. In order to achieve this, the MOF is solvothermally produced and then impregnated with the IL. The IL/MOF systems are then tested on a specially designed gas flow equipment after being sprayed into a 2D manufactured silver capacitive circuit [43]. These materials provide infinite options for improving the prepared sensors’ analytical performance to detect the intended analyte and expand the field of wearable sensors. MOFs can be synthesized via laser ablation for use in sensing applications. In a fantastic work, a revolutionary method for creating MOF composites (Eu2O3@[Zn2(1,4-ndc)2dabco) by pulsed laser ablation in flowing liquid) was described. The composite Eu2O3@[Zn2(1,4-ndc)2dabco] has a BET specific surface area of 1087 m2/g, and physical and chemical analysis of the produced MOF revealed that the nanoparticles of the framework have an average size of 3.08 nm and exhibited well designed and uniform distribution throughout the crystal [44].
4. MOFs in wearable electrochemical sensors
Due to their advantages, such as quick and accurate detection, wearable sensors have recently gained a lot of attention. Indeed, as shown in Table 2, several objectives have been achieved but several issues need to be addressed in the nearest future to improve the applicability of MOFs-based wearable sensors. They also show tremendous promise for use in health care applications, such as the diagnosis and monitoring of diseases [45]. However, due to the majority of wearable sensors that are currently in use primarily recording physical activity and/or vital signs, their usage in healthcare is still limited. Therefore, several attempts must be made to resolve this issue. Wearable biosensors have been extensively used in numerous studies recently to examine biochemical and biological analytes in order to broaden the uses of such devices in human health diagnostics [46,47]. Even though practically all clinical tests now involve human blood, blood collection is still unpleasant and might spread diseases if the equipment is not properly cleaned [48]. On the other hand, sample storage is still a difficult problem that restricts long-term and continuous monitoring as well as transportation. In order to avoid problems with blood samples and to assure long-term accurate health diagnosis, future wearable sensors should be designed to analyze sweat, tears, saliva, or even sweat samples.
Table 2.
Most recent advances in MOFs-based wearable sensors and related limitations.
| MOFs-based wearable sensors | Achievements | Obstacles and Limitations |
|
|
4.1. Properties of the MOF-based wearable sensors
Given its unique properties, MOFs may be seen in this context as an emerging material for sensitive wearable sensors and biosensors [17,49]. The following are the reasons why MOFs should be employed in wearable biosensors. First of all, some MOFs have electrocatalytic activity toward the water-splitting reaction [50], which enhances the detection selectivity because they don't reduce energy consumption only, but MOFs can also determine the reaction pathway in a significant way. Second, several metal-MOF combinations exhibit remarkable electrocatalytic activity that is even competitive with those of these metals [51]. Third, nanomaterials that can have a synergistic influence on the sensing reaction can be accommodated on the MOF using it as a scaffold [52].
4.2. Insights on the recent applications of MOFs and conductive materials
MOFs have great porosity, flexibility, and surface area ratio, but their low conductivity makes it difficult to use them in sensing applications. Regarding this, numerous works have developed a variety of pairings of MOFs with more conductive materials like metals and carbon-based materials like graphite, reduced graphene oxide, and CNTs, including both SWCNTs and MWCNTs. Such combinations increase the conductivity and flexibility of MOFs, making them potentially effective composite materials for flexible electronics and bioelectronics [[53], [54], [55]].
An excellent study in this context described a unique, flexible, wearable device made of highly conductive MWCNTs and MOFs (ZIF-67-Co and MIL-88-Fe). According to the study, after being coupled with MWCNTs to form organic-inorganic fibers, the synthesized MOFs were properly post-treated. The synthesized materials are expected great potential in electrochemical sensing and also in energy storage applications due to their high surface area, presence of Co/Fe elements, and unique structural features (such as dodecahedral morphology). As shown in Fig. 1, MWCNT sheets that were aligned and combined with the MOFs (Fig. 1a) had controlled dimensions were taken from the arrays and placed one on top of the other as can be seen in Fig. 1b–d. Pre-fabricated MOFs, such as ZIF-67-Co and MIL-88-Fe, were injected into the stacked MWCNT sheets using an easy drop-casting technique as a proof of concept. The required amount of MOFs was tweaked by adjusting the volume of the MOFs/ethanol suspension [56].
Fig. 1.
Synthesis of MOFs based composites (a) and their characterization using SEM (b), mapping (c) and Raman spectra (d). Reproduced with permission from ACS [56].
Similarly, A palladium-based metal organic framework (Pd@ZIF-67)-based sweat glucose wearable sensor has been created. The synthesized Pd@ZIF-67 was processed using a water splitting reaction to preserve the required alkaline environment for efficiently detecting glucose. During the pretreatment procedure, Pd worked as a reducing agent of H2, ensuring a stable and selective detection platform. For wearable perspiration sensing, the wearable sensor was attached to a sweatband. With analytical results remaining consistent for two months, the proposed Pd@ZIF-67 could be a viable long-term solution for sensitive and stable glucose detection in future non-invasive, painless, and practical glucose tests for self-monitoring of diabetes (Fig. 2) [57]. SEM analysis revealed that ZIF-67 had a polyhedral structure with a relatively smooth surface, and Pd@ZIF-67 exhibited a similar polyhedral structure but with a relatively rough surface (Fig. 2a and b) while the TEM image (Fig. 2c) shows that the Pd NPs were encapsulated in ZIF-67. More detailed structure information about the Pd NPs can be found in the HRTEM image (Fig. 2d). From the image, the lattice spacing of fringes on the Pd NP was found to be about 0.248 nm, which indicates face-centered cubic Pd facets (0.246 nm) and that the Pd NPs were grown along the (111) facet direction.38 Characteristic peaks of C, N, Co, and Pd were observed from the EDS of the Pd@ZIF-67 particle (Fig. 2e). The valence state of each element in the Pd@ZIF-67 NPs was examined by XPS as shown in Fig. 2f.
Fig. 2.
Palladium-based metal organic framework (Pd@ZIF-67)-based sweat glucose wearable sensor. Scanning electron micrographs of (a) ZIF-67 and (b) Pd@ ZIF-67. (c) Transmission electron micrographs of Pd@ZIF-67. (d) High resolution transmission electron micrographs of the Pd NPs in Pd@ZIF-67. (e) Energy-dispersive spectrum of Pd@ZIF-67. (f) X-ray photoelectron spectrum of Pd@ZIF-67.Reproduced with permission from ACS [57].
4.3. MOF-based self-powered flexible piezoelectric sensors
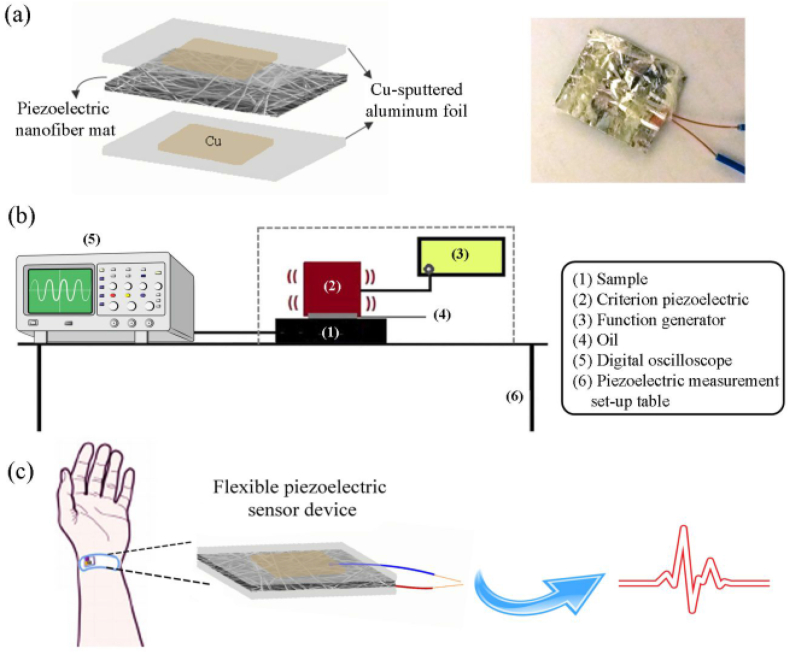
It is urgent to use self-powered flexible piezoelectric sensors since the old bioanalytical tools systems have numerous shortcomings caused by their inflexible shape and high power consumption. A novel flexible wrist pulse sensor has been reported in this regard [58]. The MOFs employed (UiO-66 nanoparticles) were made using a solvothermal procedure to give them a more crystalline nature. The nanoparticles made were then electrospun at the same time as the Nafion PVDF solution. This leads to develop flexible membranes with a high output voltage and excellent sensitivity (Fig. 3). The fabricated sensor was produced using a PVDF nanofiber combined with the MOF NPs and Cu-sputtered aluminum foil (Fig. 3a). For measuring (Fig. 3b), a criterion piezoelectric, function generator, and an oscilloscope was used to construct the experimental setup for the in-situ detection of radial artery pulse signals (Fig. 3c).
Fig. 3.
(a) Flexible sensor architecture, (b) measurement set up, and (c) pulse signals recording using the prepared sensor. Reproduced with permission from ACS [58].
Combining MOFs with metals offers up new possibilities for MOFs in applications other than sensing. Due to ZnO's direct bandgap wide-bandgap semiconductor's superior optical and electrical properties, MOFs are expected to join with ZnO to produce type-II heterojunctions, which is helpful for effective charge separation. For instance, by layer-by-layer (LbL) growing Cu3(HHTP)2 on the surface of ZnO thin film, a novel composite based on ZnO and MOFs (Cu3(HHTP)2) has been generated to prepare type-II heterojunction UV photodetector [59]. The MOF layer's development cycles, variations in the power density of the light source, and light incidence direction were all considered when analyzing photodetection performance. The sensor exhibits 78.2 A/W response and 3.8109 Jones detectivity at 1 V. Our MOF device is therefore highly resistant to bending and fatigue, making it a good candidate for wearable photodetection [59].
4.4. Advances in MOFs sensitivity
Additionally, in the past 10 years, efforts have been made to increase the sensitivity of wearable pressure sensors so they can be used in a wider range of medical applications. In order to achieve fast and accurate health monitoring, wearable pressure sensors must be produced with high sensitivity, wide sensing range, excellent reproducibility and/or reusability, and with few restrictions and drawbacks related to small mechanical deformations such as physical motions of fingers. Recently, various skin wearable sensors have b een developed, however, often limited by air and moisture, which restrict their long-term applicability and make it too difficult to obtain tight adhesiveness to the skin [60,61].
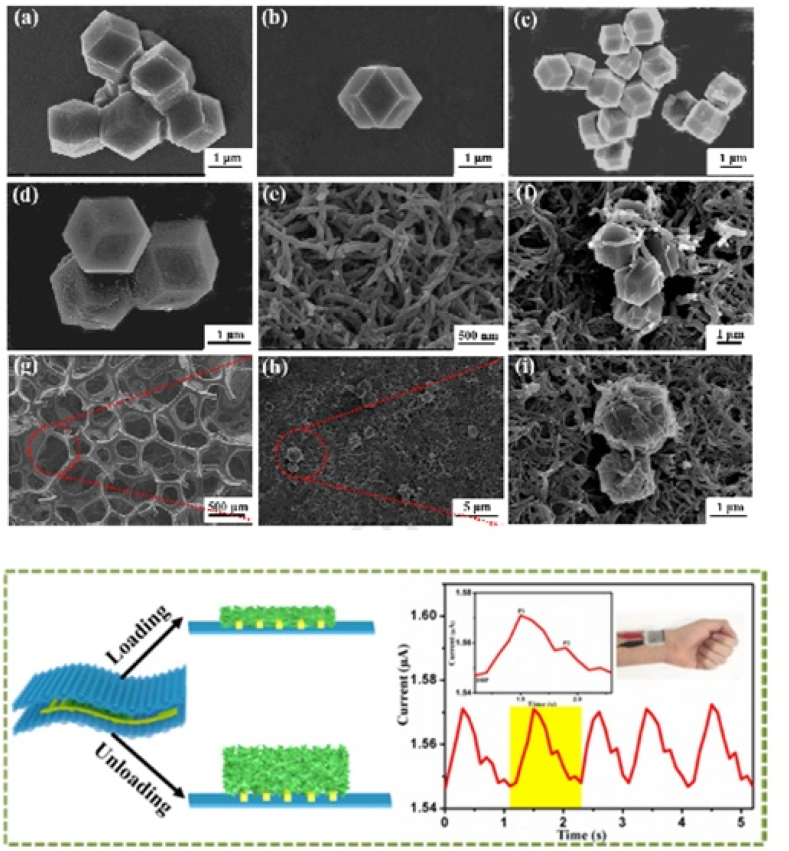
In this regard, a sandwiched C-MOF/PANIF@PU-based wearable pressure sensor has been fabricated (Fig. 4). C-MOF and PANIF are combined to create interconnected nanocomposites, which are then dipcoated onto PU sponges to create C-MOF/PANIF@PU with high specific surface areas, porous microstructures permeable to air and liquid, and reliable elasticity. The rhombic dodecahedron ZIF-8 crystals were synthesized in methanol solution (Fig. 4a and b), which were carbonized at 800 °C for 4 h to obtain the relatively stable C-MOFs (Fig. 4c and d) with high specific surface area, good electrical conductivity and mechanical stability. After mixing with PANIF (Fig. 4e), the interconnected nanocomposites of C-MOF/PANIF were obtained (Fig. 4f). After combination, the prepared conductive C-MOF/PANIF@ PU (Fig. 4g–i) had good compressibility and bend-ability [62]. The prepared sensor exhibited a wide range up to 60 kPa and provided excellent sensitivity and fast response, stable breathability, wireless biomonitoring, and a remarkable repeatability over 15000 cycles. The sensor can also be used to track both small and large human movements, including blood pressure and cheek occlusion (finger bending, and finger pressing) [62].
Fig. 4.
Sandwiched C-MOF/PANIF@PU based wearable pressure sensor. (a–b) SEM image of the ZIF-8, (c–d) SEM image of C-MOF, (e) SEM image of the PANIF, (f) SEM image of the C-MOF/PANIF, (g–i) SEM images of the C-MOF/PANIF@PU. Reproduced with permission from Elsevier [62].
Unfortunately, there aren't many research reporting MOF-based wearable sweat sensors in the literature so far. However, a number of research that were published in this field and offered some answers that ought to be taken into account in the near future to enhance MOFs' potential future utility in creating more precise wearable sensors. Sweat is a readily available, non-invasive fluid that can be drawn from a person's body during any physical activity, as well as on demand using thermal heating or a chemical induction process known as iontophoresis [63,64].
Sweat analysis is more advantageous than blood diagnosis. However, this method has a number of issues and restrictions that make it difficult to create wearable sweat sensors based on MOF. Four significant issues need to be resolved: (i) the inability to control sweat production rates; (ii) the impact of health circumstances, such as pregnancy, on sweat production; (iii) the limitations of iontophoresis; and (iv) the stretchable wearable heater that makes diagnosis uncomfortable and annoying. By regulating sweat generation and enhancing its storage and transportation, efforts should be made to develop MOF-based wearable sweat sensors [65].
For instance, because patients frequently detest such non-invasive approaches, producing perspiration through physical activity is not a desirable technique. Since excessive sweating makes it uncomfortable to take showers six times a day for a diabetic person. Accurate glucose detection requires diabetic patients to produce sweat during six times each day from breakfast to dinner [3,66]. Even a healthy individual won't ever feel at ease with such a diagnosis and won't be able to withstand six times as much physical activity to produce enough sweat to ensure continues glucose monitoring during the whole day.
Additionally, once perspiration starts with any physical activity, glucose concentrations decrease over time in sweat because it is quickly ejected from the sweat gland. The diluting effect brought on by an increase in sweat rate during an extensive exercise, is thought to be responsible for the lower levels of sweat glucose [67,68]. In addition to the increase in perspiration rate that causes glucose dilution during exercise, the rise in skin temperature will also have an impact on the activity of glucose oxidase, which must be taken into consideration to prevent overestimating the exact glucose concentration [69].
Despite similar patterns, different body areas sweat at different rates, which, as a result of the dilution effect, because varying glucose concentrations at any given time.
In conclusion, it is highly unlikely that sweat glucose monitoring that is accurate, real-time, continuous, and achieved through exercise sweat will be the best course of action, especially for diabetic persons who suffer from other serious diseases such as blood pressure and heart related disease (Fig. 5a and b). Furthermore, this strategy probably won't be the best for people who are aged, disabled, or pregnant.
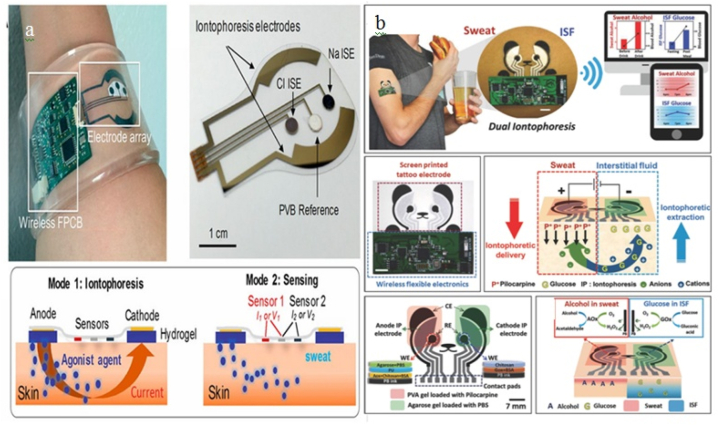
Fig. 5.
(a)Wearable sweat sensor for glucose detection. Reproduced with permission from PNAS [70]. (b)Wearable sweat sensor for sweat (iontophoresis) detection Reproduced with permission from Wiley [71].
Iontophoresis is a well-known technique for health monitoring and therapeutic medicines, particularly for sedentary individuals [72]. It works by administering a modest electrical current to each electrode to polarize electrode's surface. In the iontophoresis process, a sweat-inducing substance is iontophorized into the skin (sweat glands) to produce perspiration [73]. As a result, sweat can be created on-demand and without invasion at any convenient area on the body.
To collect local sweat effectively, the system should have embedded iontophoresis capabilities. The sensing area is sealed to collect sweat using an iontophoresis technique with the sweat-rate sensor mounted on the positive electrode [70]. Additionally, constant secretion rates greater than 100 nL/min/cm2 may be reached by carefully constructing the iontophoresis electrodes and improve sweat stimulation of the medication in the synthesized films. It was possible to extract enough perspiration for an accurate in-situ glucose analysis without endangering the individuals' epidermis or making them uncomfortable [70].
Iontophoresis is essential in addressing the issues of an aging population and diabetic people with health issues and a lack of access to healthcare thanks to these benefits. While the chemical makeup of workout sweat is significant for uses in physiology and athletics, chemical iontophoresis-generated sweat is more beneficial for medical diagnostics [73,74]. Due to the quick sampling times and the ability to take measurements while sitting, which is more convenient than exercising [75]. Iontophoresis is the best method for extensive population monitoring since it may reduce the risks of exercise-induced hypoglycemia in diabetes individuals. Given that exercise causes the body to go through dynamic physiological changes whereas local sweat stimulation by iontophoresis essentially leaves the body in its resting state.
Iontophoresis, however, might be more appropriate for a single application as opposed to ongoing monitoring, as the repetitive administration of iontophoretic current at the same location could be damaging to the underlying skin. To protect the skin from operational damage and irritation, the wearable sensors should be prepared taking into account the current density and iontophoresis time. As a result of repetitive sweat induction of iontophoresis, the pH of the epidermis will vary due to ionic buildup at the sweat-sampling site. In order to minimize burns brought on by unfavorable pH effects, safeguard the epidermis, and solve this problem, buffered gel coatings may be utilized [71,76]. The Ag nanowire network can be employed as a very transparent and flexible heater for wearable electronics applications because of its better electrical conductivity at high aspect ratios [77]. According to Ko and his coworkers' research, the distinctive structure of the Ag nanowire network/PDMS could provide Joule heating with a quick thermal response, improved electrical stability to endure repeated mechanical stress, and a minimal variation in resistance [78]. Sweat can be produced on the curved and irregular surfaces of the human body thanks to the wearable stretchy heater's soft and thin qualities (e.g., chest, forehead, temple, forearm). The exposed layer of Ag nanowires is coated with gold (Au), a non-toxic, oxidation-resistant material, to produce sweat over an extended period of time utilizing wearable stretchy heaters [77,78]. As a result of the galvanic coating method used on au, the device's reliable, long-term sweat production for continuous sweat glucose monitoring provides improved electrical stability under sweating conditions.
However, if the stretchable wearable heater is made of a soft, thin, solid-layer patch, it will be difficult to wear and will not allow for adequate airflow. The solution to this issue is the use of super hydrophobic materials, which can introduce the sweat into the sensing system and keep the skin dry during the sweating process. This might be a useful method in the field of for non-invasive biosensing of glucose, however it requires power. The use of this method for in-situ sweat glucose monitoring has not been documented in any studies [73].
The market for electrochemical biosensors is being driven by diagnostic and health monitoring applications, notably the growing need for wearable sensors [79,80]. The cost-effective and scalable technology pushed by screen printed electrodes utilized on wearable sensing technology [81] owns the biggest market share of electrochemical systems. In terms of commercial applications, qualities like as adaptability, miniaturization, reliability, and device cost are critical. Several technological difficulties must be considered for the market, including robustness, dependable and fast response, and sensor fouling prevention [82,83]. Furthermore, regulatory agency approval by comparison with standardized analytical techniques is required [84].
MOF-based wearable sensors frequently include a biological component in the sensing matrix, such as enzymes and antibodies, which require special storage conditions. Although specific characteristics of commercially marketed glucometers' excellent storage stability are covered by patent, the capillary chamber where the enzyme is maintained along with mediators and stabilizers appears to play a significant role in it [85]. Despite the commercial success of electrochemical systems, several hurdles remain for applications that rely on long-term stability, as most strips can only be held for two years under optimum conditions [86]. There are several publications and patenting activities connected to the development of electrochemical biosensors for the detection of environmental pollutants, food toxins, and biomarkers, however they are rarely commercialized [87]. The majority of biosensing experiments have been performed under controlled settings and evaluated using buffer solutions deemed "fairly clean" rather than complex real-world materials.
It should be noted that approaches based on enzymatic inhibition can produce false positive results since various substances impede enzyme activity, reducing sensor selectivity and lowering its sensitivity [88]. The restrictions associated with natural enzymes have made the creation of commercially viable biosensors extremely difficult. The principal disadvantages are incompatibility with organic solvents, poor stability, the high expense of demanding extraction and purification operations, and the limited experimental settings of pH, temperature, and ionic strength [89]. The main issue impeding the commercialization of most electrochemical biosensors is their lack of robustness and reliability due to low long-term activity, repeatability, and matrix interference [90]. Another critical issue is enzyme immobilization. Enzymes undergo conformational changes on the electrode surface, leading in activity loss, which decreases biosensor sensitivity and short-term storage [91]. These concerns remain unresolved and necessitate extra efforts. To address the shortcomings of the biological recognition element, artificial enzymes and molecularly imprinted polymers have been developed to mimic enzymatic function or to improve particular binding affinity towards target analytes [92]. These elements provide exceptional opportunities for the development of new biomimetic electrochemical sensors.
Non-enzymatic biosensors based on biomimetic materials are the subject of significant research because they have the potential to overcome recurring stability difficulties, paving the path for mass production and commercialization of viable sensors [93]. In addition, advances in material science and micro-engineering have accelerated the development of wearable, simple-to-use, low-cost, and noninvasive biosensors. They are, however, largely employed for research, and the integration of biomimetic materials with commercial products is still in the works. Furthermore, biofouling is a significant challenge in market applications of these biomimetic materials incorporated into electrochemical sensing platforms [94]. Many diverse species may adsorb on the electrode surface, reducing sensor repeatability, because the electrodes are in close contact with complex matrices to perform the intended analysis. Biofouling is not just a challenge for biomimetic electrochemical sensors; enzymatic-based sensors face the same issue, necessitating ongoing attempts to solve it.
One of the major technological challenges is the gap between the innovative concepts brought by academic research and their integration with market needs, as well as the inaccessibility of biological samples, known as real-world samples, and limited experience on marketable devices. Collaboration between academies and companies could yield a viable solution to the need for more durable, stable, and low-cost sensors.
Table 3 highlights the advantages and disadvantages of MOFs, and provide a comparison of MOFs with other porous nanomaterials.
Table 3.
Advantageous and disadvantages of MOFs and other nanomaterials.
| Porous Materials | Composition | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| MOFs | Metal–organic framework (MOF) Organic ligands and their coordinated metal ions/ion clusters |
Ordered porous structure, biocompatibility, and ease of functional modification. | Targeting and potential biotoxicity. | [95,96] |
| COFs | Light elements (H, C, N, O, B) |
Large surface area, high thermal stability, good biocompatibility, and good biodegradability. | The synthesis condition is not mild enough, the preparation cost is high, and the structure is uncontrollable. | [97,98] |
| MIPs | Conductive polymers | Good biocompatibility and excellent selectivity | usually higher effort required for template cleavage after MIP synthesis | [99,100] |
| LDH | Metals and hydroxide molecules separated by exchangeable anions and water molecules | High conductivity | Complicated and expensive synthesis procedures | [101,102] |
| CNTs | A layer of carbon atoms that are bonded together in a hexagonal (honeycomb) mesh | High surface area Antifouling |
High cost synthesis and purification procedures | [103,104] |
| Graphene | Carbon atoms positioned in a hexagonal design | Excellent flexibility | Sophisticated synthesis procedures and may be toxic (e.g the use of hydrazine) | [105,106] |
| Mesoporous silica nanoparticles (MSN) | Silica (SiO2) | Huge loading capacity, controllable particle size and shape, suitability for easy functionalization, and biocompatibility. | Poor dispersibility and stability, prone to accumulation, and requires modification. A fully reversible lid is required to close the pore access. | [107,108] |
5. Limitations
The prospects presented by MOFs-based wearable sensors are limited in certain ways by several obstacles that have yet to be fully resolved. Ionic strength, temperature, and liquid-junction potentials can all be adjusted for or taken into consideration [109]. Leaching of membrane components, i.e., biocompatibility/toxicity, can be reduced by using membranes based on polymers [110] or perfluorocarbons; such membranes may increase membrane stability and hence the sensor's long-term usefulness.
The use of ordinary addition to overcome the matrix effect is incompatible with the wearable concept and will almost certainly increase the cost of the sensing platform for environmental analysis [111]. For wearable applications, sampling challenges, such as sweat evaporation [112], contribute to a delay in the sensor's commercialization. Finally, the practical implementation of wearable chemical sensors and deployed autonomous systems for environmental trace analysis necessitates significant efforts from mechanical and electronic engineers to solve other issues, such as passive pumping, sensor integration in fabric, microfluidic design, and wireless data transmission [113]. It should be also noted that the miniaturization of the MOFs-based sensors may raise the costs of production and may cause lower potential stability because of the smaller redox or double-layer capacitances, the leakage of membrane components, the adherence and the exfoliation of membranes and overall, a trade-off in decreasing the size of the electrode may exist [114]. It should also be noted that miniaturization of MOFs-based sensors may increase production costs and result in lower potential stability due to smaller redox or double-layer capacitances, membrane component leakage, membrane adherence and exfoliation [115], and overall, a trade-off in decreasing the size of the electrode may exist.
It appears that there aren't any convincing designs for inexpensive, mass-producible ion-selective microelectrodes that can be used for wearable electronics and environmental monitoring. Out-sensor computing consumes a lot of energy and adds a lot of latency. It is critical to create physical intelligent devices that allow near-sensor or in-sensor computing in order to solve this problem. Such a problem might be resolved by machine learning, which might also enhance the utilization of artificial e-skins with multiple wearable sensing nodes for gathering external information. On the other hand, a significant issue for everyday monitoring is the service life of wearable electromechanical sensors. Based on their self-power supply, chemical stability, wearing comfort, and mechanical durability, wearable electromechanical sensors will have an effect on long-term use. Self-repairing hydrogels and elastomers may open new avenues to overcome wearable sensors stability in the future. A unique wearable sensor needs to be built through material design and structural optimization in order to enable long-term monitoring and high-fidelity electrophysiological recording.
In conclusion, there is still a lot of work to be done in order to create wearable sensors based on MOFs that are affordable and dependable for research on untreated real samples.
6. Challenges and directions
6.1. Challenges
MOFs-based Wearable sensors are characterized by various challenges including usual sensing challenges such as pretreatment, stability and reproducibility, sensitivity challenges and real sample sensitivity along with MOFs wearability related challenges such as materials related challenges, Self-destroyed sensor-based challenges and consumers’ behavior toward such devices. These are also the major challenges in the fabrication of electrochemical biosensors.
-
1
Stability of MOFs-based wearable sensors is a major issue since they are relied on a biorecognition system that requires a stable environment for the biorecognition element such as enzymes especially for long-term storage.
-
2
The sensitivity, selectivity and LOD of wearable sensors are the key features of any wearable sensor and ideal biosensors must have a very low value of LOD and outstanding selectivity toward the studied analyte so that they can provide accurate data with negligible hysteresis at lowest interference.
-
3
Up-scaling wearable technology always confront reproducibility related challenges. To ensure accurate fabrication and marketing of MOFs-based wearable sensors they must have excellent reproducibility and repeatability, so similar sensors with similar features could be produced.
-
4
To be consider for human health diagnosis, MOF-based wearable sensor must be able to operate properly in a real sample such as saliva, blood, urine, sweat, body fluid, tears, etc. The real sample collection is itself a challenge; some factors need to be considered for collecting a real sample for detection.
-
5
Limitations due to MOFs morphology, safety and biodegradability for constructing highly performed wearable sensors are one of the most critical issues in developing MOFs-based wearable sensors for health diagnosis. MOFs-based sensors must be safe and have mechanical features so they can be compatible with the studied area (e.g. skin) without interfering the results causing no damage to the patient.
-
6
MOFs-based wearable sensors must be removed from the body once their mission is accomplished to avoid any side effects which require additional sophisticated procedures. Some health complications may appear if one of the component of the wearable sensor failed to be removed and/or in case of an in-vivo damage which may lead to serious in-vivo injuries during the diagnosis of a disease. Wearable sensors must be resistant to any mechanical and physicochemical changes to avoid any in-vivo issues.
-
7
In case of commercialization and scaling-up, production costs and customer behavior toward MOFs-based wearable sensor could be considered as a serious challenge. To get a rapid and efficient market diffusion, the customer needs to be convinced about the advantages of the product taking into account its cost, ease of operation with minimal efforts and product safety.
-
8
Several sensors require calibration before every use and incubation in conditioning solution. Such conditions are incompatible with wearable technology. Furthermore, many forms of sensors have the issue of drift that causes significant error in calculating analyte concentration. Therefore, these sensors need to be re-calibrated at regular intervals. Researchers are addressing this issue by developing calibration-free sensors
6.2. Solutions
-
1
The issue related to conformal contact between wearable electrochemical sensors and the human body can be resolved by matching the mechanical properties of the device with that of the human tissues. The human tissue is characterized as soft, stretchable and curvilinear with unique mechanical properties. In this regard, researcher must conduct more efforts in developing MOFs with outstanding mechanical properties including but not limited to curvilinearity, softness, flexibility and outstanding stretchability.
-
2
A combination of MOFs and silicon based materials could help in improving MOFs-based wearable sensor toward extreme mechanical and physichochemical changes such as pressure and temperature. Such innovative strategies must be explored by researchers to realize highly biocompatible and biodegradable chemical sensors that are eventually consumed by the body.
-
3
MOFs functionalization and their combinations with other materials such as graphene, reduced graphene oxide (rGO), carbon nanotubes (CNTs), metals and metal oxide nanoparticles may improve the stability of the biorecognition matrix and then, ensure long-term storage.
-
4
Developing cost-effective and eco-friendly synthesis procedures to prepare MOFs is highly recommended to ensure safe, biodegradable and cheap wearable sensors.
-
5
Fast response and Micro-nano MOFs-based wearable sensors can be disposed and removed easily and may improve their performance would involve fewer efforts.
-
6
The gap between research laboratory and hospitals should be bridged so more trails will conducted and MOFs-based wearable sensors can be tested in real-life circumstances, which will help in evaluating their performance and convincing people to use such tools to diagnosis their health.
-
7
To ensure up-scaling and excellent market diffusion of such sensors, more efforts should be conducted focusing on the key element for a successful up-scaling strategy including but not limited to production costs, stability and advertising.
-
8
MOFs-based wearable sensors should benefit from machine learning and artificial intelligence to overcome the aforementioned challenges. Such technologies may offer excellent opportunities to improve sensors analytical performance and improving their stability, controlling and miniaturizing their size.
7. Conclusion and future prospects
MOF based wearable sensors showed promising performance for future applications in human health care applications and disease diagnosis. However, these sensors must be sensitive, selective and stable to maintain long term performance. The design and architecture of the MOF-based sensing platform while being doped with other materials such as carbon based materials and metals nanoparticules which have high flexibility, stability, conductivity and trustworthy durability can be useful in the fabrication of high performance MOF-based wearable sensors. The development of high performance MOF-based sensors depends ongoing research and technological advances. Therefore, future researchers will likely focus on the following areas, as stated below.
-
•
Designing eco-friendly and more conductive MOF-based composites to create more stable and sensitive MOF-based sensing platforms that ensure the accuracy and linearity of the produced MOF-based wearable sensors.
-
•
To expand their applications in wearable sensing, MOFs-based sensing platform should be stretchable and flexible which require more efforts on improving their mechanical properties and their biocompatibility taking into account their costs of production.
-
•
To increase wearing comfort and skin affinity, MOF wearable sensors should be created using bionic structures rather than artificial glue.
-
•
Integration of 3D technologies in the fabrication of MF-based wearable sensors since such technologies helps in improving the long term stability and the reproducibility of the sensors, and may also help in the up-calling of these sensors.
-
•
The operational conditions of wearable sensors should be optimized to produce more adaptable MOF-based wearable sensors for each application and to meet a variety of end-user biological needs.
-
•
To produce more accurate and smart MOF-based wearable sensors, machine learning and artificial intelligence should be integrated during the process of production.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Funding statement
The publication of this article was funded by the Qatar National Library (QNL). The authors would like to acknowledge the library for supporting the publication of this article.
CRediT authorship contribution statement
Hicham Meskher: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. Samir Brahim Belhaouari: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. Fariborz Sharifianjazi: Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financialinterestsor personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Hicham Meskher, Email: h.meskher@univ-eltarf.dz.
Samir Brahim Belhaouari, Email: sbelhaouari@hbku.edu.qa.
References
- 1.Makar A.B., McMartin K.E., Palese M., et al. Tephly T.R. Formate assay in body fluids: application in methanol poisoning. Biochem. Med. juin 1975;13(2):117–126. doi: 10.1016/0006-2944(75)90147-7. [DOI] [PubMed] [Google Scholar]
- 2.Meskher H., Belhaouari S.B., Deshmukh K., Hussain C.M., Sharifianjazi F. A magnetite composite of molecularly imprinted polymer and reduced graphene oxide for sensitive and selective electrochemical detection of catechol in water and milk samples: an artificial neural network (ANN) application. J. Electrochem. Soc. Apr. 2023;170(4) doi: 10.1149/1945-7111/acc97c. [DOI] [Google Scholar]
- 3.Sharma A., Singh A., Gupta V., Sundramoorthy A.K., Arya S. Involvement of metal organic frameworks in wearable electrochemical sensor for efficient performance. Trends in Environmental Analytical Chemistry. Jun. 2023;38 doi: 10.1016/j.teac.2023.e00200. [DOI] [Google Scholar]
- 4.Meskher H., et al. A review on CNTs-based electrochemical sensors and biosensors: unique properties and potential applications. Crit. Rev. Anal. Chem. Feb. 2023:1–24. doi: 10.1080/10408347.2023.2171277. [DOI] [PubMed] [Google Scholar]
- 5.Singh A., et al. Recent advances in electrochemical biosensors: applications, challenges, and future scope. Biosensors. Sep. 2021;11(9):336. doi: 10.3390/bios11090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padha Bhavya, Verma S., Mahajan P., Sundramoorthy A.K., Arya S. Energy technology; Jun. 2023. An Insight into the Wearable Technologies Based on Novel Hybrid Piezoelectric‐Triboelectric Nanogenerators. [DOI] [Google Scholar]
- 7.Ghanbari K., Roshani M., Goicoechea H.C., Jalalvand A.R. Developing an elegant and integrated electrochemical-theoretical approach for detection of DNA damage induced by 4-nonylphenol. Heliyon. Oct. 2019;5(10) doi: 10.1016/j.heliyon.2019.e02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piau A., Wild K., Mattek N., et al. Kaye J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild alzheimer disease and implications for clinical care: systematic review. J. Med. Internet Res. 2019;21(8) doi: 10.2196/12785. août. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T., et al. Mechano-based transductive sensing for wearable healthcare. Small. 2018;14(11) doi: 10.1002/smll.201702933. mars. [DOI] [PubMed] [Google Scholar]
- 10.Meskher H., Achi F. Electrochemical sensing systems for the analysis of catechol and hydroquinone in the aquatic environments: a critical review. Crit. Rev. Anal. Chem. Aug. 2022:1–14. doi: 10.1080/10408347.2022.2114784. [DOI] [PubMed] [Google Scholar]
- 11.Elgazzar Elsayed, Attala Khaled, Atty S.A., Abdel-Raoof A.M. A screen printed methodology optimized by molecular dynamics simulation and Lean Six Sigma for the determination of xylometazoline in the presence of benzalkonium chloride in nasal drops. Talanta. May 2022;242 doi: 10.1016/j.talanta.2022.123321. 123321–123321. [DOI] [PubMed] [Google Scholar]
- 12.Mostafa A.E., et al. Computer-aided design of eco-friendly imprinted polymer decorated sensors augmented by self-validated ensemble modeling designs for the quantitation of drotaverine hydrochloride in dosage form and human plasma. J. AOAC Int. May 2023 doi: 10.1093/jaoacint/qsad049. [DOI] [PubMed] [Google Scholar]
- 13.Meskher H., Achi F., Ha S., Berregui B., Babanini F., Belkhalfa H. Sensitive rGO/MOF based electrochemical sensor for penta-chlorophenol detection: a novel artificial neural network (ANN) application. Sens. Diagn. 2022;1(5):1032–1043. doi: 10.1039/D2SD00100D. [DOI] [Google Scholar]
- 14.Mostafa A.E., et al. Miniaturized chip integrated ecological sensor for the quantitation of milnacipran hydrochloride in the presence of its impurities in dosage form and human plasma. J. Electrochem. Soc. Jul. 2023 doi: 10.1149/1945-7111/ace9fd. [DOI] [Google Scholar]
- 15.Meskher H., et al. Recent trends in carbon nanotube (CNT)-based biosensors for the fast and sensitive detection of human viruses: a critical review. Nanoscale Adv. 2023;5(4):992–1010. doi: 10.1016/j.biosx.2022.100284. 10.1039/D2NA00236A.electron. X, vol. 12, p. 100284, déc. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meskher H. A critical review about metal organic framework-based composites: potential applications and future perspective. Journal of Composites and Compounds. Mar. 2023;5(14):25–37. 10.52547.jcc.5.1.5. [Google Scholar]
- 17.Shu Y., et al. A highly flexible Ni–Co MOF nanosheet coated Au/PDMS film based wearable electrochemical sensor for continuous human sweat glucose monitoring. The Analyst. 2022;147(7):1440–1448. doi: 10.1039/D1AN02214H. [DOI] [PubMed] [Google Scholar]
- 18.Elashery S.E.A., Attia N.F., et al. Oh H. Design and fabrication of novel flexible sensor based on 2D Ni-MOF nanosheets as a preliminary step toward wearable sensor for onsite Ni (II) ions detection in biological and environmental samples. Anal. Chim. Acta. 2022;1197 doi: 10.1016/j.aca.2022.339518. mars. [DOI] [PubMed] [Google Scholar]
- 19.Ghasemzadeh Mohammad Ali, Mirhosseini-Eshkevari Boshra. Poly(acrylic acid)/Fe3O4 supported on MIL-100(Cr) MOF as a novel and magnetic nanocatalyst for the synthesis of Pyrido[2,3-d]Pyrimidines. Heliyon. Aug. 2022;8(8) doi: 10.1016/j.heliyon.2022.e10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.mahmoudi S., Chaichi Mohammad Javad, Shamsipur Mojtaba, Ome Leila Nazari, Samadi R. Modification of bimetal Zn/Mg MOF with nanoparticles Fe3O4 and Fe3O4@SiO2, investigation of the peroxidase-like activity of these compounds by calorimetry and fluorimetry methods. Heliyon. Feb. 2023;9(2) doi: 10.1016/j.heliyon.2023.e12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karimzadeh Z., Rahimpour E., Jouyban A. A follow-up study on ‘A sensitive determination of morphine in plasma using AuNPs@UiO-66/PVA hydrogel as an advanced optical scaffold. Heliyon. Apr. 2023;9(4) doi: 10.1016/j.heliyon.2023.e15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annamalai J., et al. Synthesis of various dimensional metal organic frameworks (MOFs) and their hybrid composites for emerging applications – a review. Chemosphere. Jul. 2022;298 doi: 10.1016/j.chemosphere.2022.134184. [DOI] [PubMed] [Google Scholar]
- 23.Shu Y., Su T., Lu Q., Shang Z., Xu Q., et al. Hu X. Highly stretchable wearable electrochemical sensor based on Ni-Co MOF nanosheet-decorated Ag/rGO/PU fiber for continuous sweat glucose detection. Anal. Chem. déc. 2021;93(48):16222–16230. doi: 10.1021/acs.analchem.1c04106. [DOI] [PubMed] [Google Scholar]
- 24.Singh A., Sharma A., Sundramoorthy A.K., Arya Quantifying ethanol in sweat with a wearable Al-doped NiO electrode and data analysis. IEEE Sensor. J. Jan. 2023;(1–1) doi: 10.1109/jsen.2023.3304978. [DOI] [Google Scholar]
- 25.Farha O.K., et al. Metal–organic framework materials with ultrahigh surface areas: is the sky the limit? J. Am. Chem. Soc. sept. 2012;134(36):15016–15021. doi: 10.1021/ja3055639. [DOI] [PubMed] [Google Scholar]
- 26.Wu T., et al. Application of QD-MOF composites for photocatalysis: energy production and environmental remediation. Coord. Chem. Rev. 2020;403 doi: 10.1016/j.ccr.2019.213097. janv. [DOI] [Google Scholar]
- 27.Mao Y., Chen T., Hu Y., et al. Son K. Ultra-thin 2D bimetallic MOF nanosheets for highly sensitive and stable detection of glucose in sweat for dancer. Discov. Nano. 2023;18(1):62. doi: 10.1186/s11671-023-03838-0. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y., et al. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. sept. 2022;307 doi: 10.1016/j.cis.2022.102732. [DOI] [PubMed] [Google Scholar]
- 29.Liu M., Mou J., Xu X., Zhang F., Xia J., et al. Wang Z. High-efficiency artificial enzyme cascade bio-platform based on MOF-derived bimetal nanocomposite for biosensing. Talanta. 2020;220 doi: 10.1016/j.talanta.2020.121374. déc. [DOI] [PubMed] [Google Scholar]
- 30.Yin S.-J., Chen H., Wang S., Wang Y., Yang F.-Q. Preparation of core-shell MOF@MOF nanoparticle as matrix for the analysis of rhubarb anthraquinones in plasma by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Heliyon. May 2023;9(5) doi: 10.1016/j.heliyon.2023.e16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraf M., Rajak R., et al. Mobin S.M. A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J. Mater. Chem. A. 2016;4(42):16432–16445. doi: 10.1039/C6TA06470A. [DOI] [Google Scholar]
- 32.Rahmati Z., et al. Roushani M. SARS-CoV-2 virus label-free electrochemical nanohybrid MIP-aptasensor based on Ni3(BTC)2 MOF as a high-performance surface substrate. Microchim. Acta. 2022;189(8):287. doi: 10.1007/s00604-022-05357-8. août. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botelho C.N., et al. Evaluation of a photoelectrochemical platform based on strontium titanate, sulfur doped carbon nitride and palladium nanoparticles for detection of SARS-CoV-2 spike glycoprotein S1. Biosens. Bioelectron. X. sept. 2022;11 doi: 10.1016/j.biosx.2022.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chokkareddy R., Redhi G.G., Karthick T. A lignin polymer nanocomposite based electrochemical sensor for the sensitive detection of chlorogenic acid in coffee samples. Heliyon. Mar. 2019;5(3) doi: 10.1016/j.heliyon.2019.e01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dakshayini B.S., et al. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. Jun. 2019;147:7–24. doi: 10.1016/j.microc.2019.02.061. [DOI] [Google Scholar]
- 36.Gui Y., He S., Wang Y., et al. Yang J. MOF-derived porous Ni/C material for high-performance hybrid nanogenerator and self-powered wearable sensor. Compos. Part Appl. Sci. Manuf. 2023;168 doi: 10.1016/j.compositesa.2023.107492. mai. [DOI] [Google Scholar]
- 37.Shahrokhian S., Ezzati M., et al. Hosseini H. Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates. Talanta. 2020;210 doi: 10.1016/j.talanta.2019.120696. avr. [DOI] [PubMed] [Google Scholar]
- 38.Yan T., et al. Smartphone light-driven zinc porphyrinic MOF nanosheets-based enzyme-free wearable photoelectrochemical sensor for continuous sweat vitamin C detection. Chem. Eng. J. 2023;455 doi: 10.1016/j.cej.2022.140779. janv. [DOI] [Google Scholar]
- 39.Yang X., Yang Z., Shao R., Guan R., Dong S., et al. Xie M. Chiral MOF derived wearable logic sensor for intuitive discrimination of physiologically active enantiomer. Adv. Mater. 2023 doi: 10.1002/adma.202304046. juill. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Chu H., Zhang J., Ma W., Qin S., Gao L. ZnS:Eu @ZIF-8: selective formation of ZnS:Eu QDs within a zinc methylimidazole framework for chemical sensing applications. Heliyon. May 2023;9(5) doi: 10.1016/j.heliyon.2023.e16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H., Wang H., Chu T., Yu M., et al. Yang Y. An electrodeposited lanthanide MOF thin film as a luminescent sensor for carbonate detection in aqueous solution. J. Mater. Chem. C. 2014;2(41):8683–8690. doi: 10.1039/C4TC01551G. [DOI] [Google Scholar]
- 42.Naghian E., Marzi Khosrowshahi E., Sohouli E., Ahmadi F., Rahimi-Nasrabadi M., et al. Safarifard V. A new electrochemical sensor for the detection of fentanyl lethal drug by a screen-printed carbon electrode modified with the open-ended channels of Zn(ii)-MOF. New J. Chem. 2020;44(22):9271–9277. doi: 10.1039/D0NJ01322F. [DOI] [Google Scholar]
- 43.Fernandez E., Saiz P.G., Peřinka N., Wuttke S., et al. Fernández de Luis R. Printed capacitive sensors based on ionic liquid/metal‐organic framework composites for volatile organic compounds detection. Adv. Funct. Mater. 2021;31(25) doi: 10.1002/adfm.202010703. juin. [DOI] [Google Scholar]
- 44.Chen Q., et al. Metal organic frameworks composite Eu2O3@[Zn2(1,4-ndc)2dabco] synthesized by pulsed laser ablation in flowing liquid and its fluorescent sensing of fatty alcohol with different branch chains. Opt. Mater. 2020;105 doi: 10.1016/j.optmat.2020.109886. juill. [DOI] [Google Scholar]
- 45.Romero L.E., Chatterjee P., et al. Armentano R.L. An IoT approach for integration of computational intelligence and wearable sensors for Parkinson's disease diagnosis and monitoring. Health Technol. nov. 2016;6(3):167–172. doi: 10.1007/s12553-016-0148-0. [DOI] [Google Scholar]
- 46.Dervisevic M., Alba M., Prieto-Simon B., et al. Voelcker N.H. Skin in the diagnostics game: wearable biosensor nano- and microsystems for medical diagnostics. Nano Today. 2020;30 doi: 10.1016/j.nantod.2019.100828. févr. [DOI] [Google Scholar]
- 47.Verma D., et al. Internet of things (IoT) in nano-integrated wearable biosensor devices for healthcare applications. Biosens. Bioelectron. X. sept. 2022;11 doi: 10.1016/j.biosx.2022.100153. [DOI] [Google Scholar]
- 48.Brossart P., Keilholz U., Willhauck M., Scheibenbogen C., Möhler T., et al. Hunstein W. Hematogenous spread of malignant melanoma cells in different stages of disease. J. Invest. Dermatol. 1993;101(6):887–889. doi: 10.1111/1523-1747.ep12371713. déc. [DOI] [PubMed] [Google Scholar]
- 49.Li Z., Guo J., Wan Y., Qin Y., et al. Zhao M. Combining metal-organic frameworks (MOFs) and covalent-organic frameworks (COFs): emerging opportunities for new materials and applications. Nano Res. 2022;15(4):3514–3532. doi: 10.1007/s12274-021-3980-0. avr. [DOI] [Google Scholar]
- 50.Liang X., Zheng B., Chen L., Zhang J., Zhuang Z., et al. Chen B. MOF-derived formation of Ni 2 P–CoP bimetallic phosphides with strong interfacial effect toward electrocatalytic water splitting. ACS Appl. Mater. Interfaces. 2017;9(27):23222–23229. doi: 10.1021/acsami.7b06152. juill. [DOI] [PubMed] [Google Scholar]
- 51.Hussain I., et al. Zn–Co-MOF on solution-free CuO nanowires for flexible hybrid energy storage devices. Mater. Today Phys. 2022;23 doi: 10.1016/j.mtphys.2022.100655. mars. [DOI] [Google Scholar]
- 52.Zhang W., et al. Regulation of porosity in MOFs: a review on tunable scaffolds and related effects and advances in different applications. J. Environ. Chem. Eng. 2022;10(6) doi: 10.1016/j.jece.2022.108836. déc. [DOI] [Google Scholar]
- 53.Jabbari V., Veleta J.M., Zarei-Chaleshtori M., Gardea-Torresdey J., et al. Villagrán D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. nov. 2016;304:774–783. doi: 10.1016/j.cej.2016.06.034. [DOI] [Google Scholar]
- 54.Rahmanifar M.S., Hesari H., Noori A., Masoomi M.Y., Morsali A., et al. Mousavi M.F. A dual Ni/Co-MOF-reduced graphene oxide nanocomposite as a high performance supercapacitor electrode material. Electrochim. Acta. juin 2018;275:76–86. doi: 10.1016/j.electacta.2018.04.130. [DOI] [Google Scholar]
- 55.Zhang X., Xu Y., et al. Ye B. An efficient electrochemical glucose sensor based on porous nickel-based metal organic framework/carbon nanotubes composite (Ni-MOF/CNTs) J. Alloys Compd. oct. 2018;767:651–656. doi: 10.1016/j.jallcom.2018.07.175. [DOI] [Google Scholar]
- 56.Rui K., et al. Dual-function metal–organic framework-based wearable fibers for gas probing and energy storage. ACS Appl. Mater. Interfaces. 2018;10(3):2837–2842. doi: 10.1021/acsami.7b16761. janv. [DOI] [PubMed] [Google Scholar]
- 57.Zhu X., Yuan S., Ju Y., Yang J., Zhao C., et al. Liu H. Water splitting-assisted electrocatalytic oxidation of glucose with a metal–organic framework for wearable nonenzymatic perspiration sensing. Anal. Chem. 2019;91(16):10764–10771. doi: 10.1021/acs.analchem.9b02328. août. [DOI] [PubMed] [Google Scholar]
- 58.Moghadam B.H., Hasanzadeh M., et al. Simchi A. Self-powered wearable piezoelectric sensors based on polymer nanofiber–metal–organic framework nanoparticle composites for arterial pulse monitoring. ACS Appl. Nano Mater. sept. 2020;3(9):8742–8752. doi: 10.1021/acsanm.0c01551. [DOI] [Google Scholar]
- 59.Kang C., et al. Cu 3 (HHTP) 2 c‐MOF/ZnO ultrafast ultraviolet photodetector for wearable optoelectronics. Chem. Eur J. nov. 2022;28(64) doi: 10.1002/chem.202201705. [DOI] [PubMed] [Google Scholar]
- 60.Shakeriaski F., et al. Ghodrat M. Challenges and limitation of wearable sensors used in firefighters' protective clothing. J. Fire Sci. 2022;40(3):214–245. doi: 10.1177/07349041221079004. mai. [DOI] [Google Scholar]
- 61.Ma D., Wu X., Wang Y., Liao H., Wan P., et al. Zhang L. Wearable, antifreezing, and healable epidermal sensor assembled from long-lasting moist conductive nanocomposite organohydrogel. ACS Appl. Mater. Interfaces. nov. 2019;11(44):41701–41709. doi: 10.1021/acsami.9b15412. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Chao M., Wan P., et al. Zhang L. A wearable breathable pressure sensor from metal-organic framework derived nanocomposites for highly sensitive broad-range healthcare monitoring. Nano Energy. 2020;70 doi: 10.1016/j.nanoen.2020.104560. avr. [DOI] [Google Scholar]
- 63.Jagannath B., et al. An observational study demonstrating the measurement, characterization and validation of expression of calprotectin in human sweat through a sweat wearable. Biosens. Bioelectron. X. 2023;13 doi: 10.1016/j.biosx.2023.100314. mai. [DOI] [Google Scholar]
- 64.Khan A., Winder M., et al. Hossain G. Modified graphene-based nanocomposite material for smart textile biosensor to detect lactate from human sweat. Biosens. Bioelectron. X. 2022;10 doi: 10.1016/j.biosx.2021.100103. mai. [DOI] [Google Scholar]
- 65.Francis J., Stamper I., Heikenfeld J., et al. Gomez E.F. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip. 2019;19(1):178–185. doi: 10.1039/C8LC00968F. [DOI] [PubMed] [Google Scholar]
- 66.Zafar H., Channa A., Jeoti V., et al. Stojanović G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors. 2022;22(2):638. doi: 10.3390/s22020638. janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jajack A., Brothers M., Kasting G., et al. Heikenfeld J. Enhancing glucose flux into sweat by increasing paracellular permeability of the sweat gland. PLoS One. juill. 2018;13(7) doi: 10.1371/journal.pone.0200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klous L., de Ruiter C.J., Scherrer S., Gerrett N., et al. Daanen H.A.M. The (in)dependency of blood and sweat sodium, chloride, potassium, ammonia, lactate and glucose concentrations during submaximal exercise. Eur. J. Appl. Physiol. 2021;121(3):803–816. doi: 10.1007/s00421-020-04562-8. mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zahed M.A., et al. A nanoporous carbon‐MXene heterostructured nanocomposite‐based epidermal patch for real‐time biopotentials and sweat glucose monitoring. Adv. Funct. Mater. 2022;32(49) doi: 10.1002/adfm.202208344. déc. [DOI] [Google Scholar]
- 70.Emaminejad S., et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. mai 2017;114(18):4625–4630. doi: 10.1073/pnas.1701740114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J., et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv. Sci. oct. 2018;5(10) doi: 10.1002/advs.201800880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petraglia F., Ramazzina I., et al. Costantino C. Plantar fasciitis in athletes: diagnostic and treatment strategies. A systematic review. Muscle Ligaments Tendons J. 2019;7(1):107. doi: 10.32098/mltj.01.2017.14. janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khor S.M., Choi J., Won P., et al. Ko S.H. Challenges and strategies in developing an enzymatic wearable sweat glucose biosensor as a practical point-of-care monitoring tool for type II diabetes. Nanomaterials. 2022;12(2):221. doi: 10.3390/nano12020221. janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma A., Singh A., Gupta V., et al. Arya S. Advancements and future prospects of wearable sensing technology for healthcare applications. Sens. Diagn. 2022;1(3):387–404. doi: 10.1039/D2SD00005A. [DOI] [Google Scholar]
- 75.Baker L.B. Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 2017;47(S1):111–128. doi: 10.1007/s40279-017-0691-5. mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao F., Fan S., Ghate D., Romanova S., Bronich T.K., et al. Zhao S. A hydrogel ionic circuit based high‐intensity iontophoresis device for intraocular macromolecule and nanoparticle delivery. Adv. Mater. 2022;34(5) doi: 10.1002/adma.202107315. févr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo Z., Sun C., Zhao J., Cai Z., et al. Ge F. Low‐voltage electrical heater based on one‐step fabrication of conductive Cu nanowire networks for application in wearable devices. Adv. Mater. Interfaces. 2021;8(3) doi: 10.1002/admi.202001695. févr. [DOI] [Google Scholar]
- 78.Hong S., et al. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015;27(32):4744–4751. doi: 10.1002/adma.201500917. août. [DOI] [PubMed] [Google Scholar]
- 79.Min J., Sempionatto J.R., Teymourian H., Wang J., Gao W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. Jan. 2021;172 doi: 10.1016/j.bios.2020.112750. [DOI] [PubMed] [Google Scholar]
- 80.Arakawa T., Dao D.V., Mitsubayashi K. Biosensors and chemical sensors for healthcare monitoring: a review. IEEJ Trans. Electr. Electron. Eng. Mar. 2022;17(5):626–636. doi: 10.1002/tee.23580. [DOI] [Google Scholar]
- 81.Ahmed Minhaz Uddin, et al. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. Jan. 2015:1–11. doi: 10.3109/07388551.2014.992387. [DOI] [PubMed] [Google Scholar]
- 82.Jimenez J., et al. Instrumentation and control of anaerobic digestion processes: a review and some research challenges. Rev. Environ. Sci. Biotechnol. Oct. 2015;14(4):615–648. doi: 10.1007/s11157-015-9382-6. [DOI] [Google Scholar]
- 83.Mayer M., Baeumner A.J. A megatrend challenging analytical chemistry: biosensor and chemosensor concepts ready for the internet of things. Chem. Rev. May 2019;119(13):7996–8027. doi: 10.1021/acs.chemrev.8b00719. [DOI] [PubMed] [Google Scholar]
- 84.Đorđević S., et al. Current Hurdles to the Translation of Nanomedicines from Bench to the Clinic. vol. 12. Jul. 2021. pp. 500–525. (Drug Delivery and Translational Research). 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koo W.-T., Jang J.-S., Kim I.-D. Metal-organic frameworks for chemiresistive sensors. Chem. Aug. 2019;5(8):1938–1963. doi: 10.1016/j.chempr.2019.04.013. [DOI] [Google Scholar]
- 86.Khor S.M., Choi J., Won P., Ko S.H. Challenges and strategies in developing an enzymatic wearable sweat glucose biosensor as a practical point-of-care monitoring tool for type II diabetes. Nanomaterials. Jan. 2022;12(2):221. doi: 10.3390/nano12020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bag P., et al. Recent development in synthesis of carbon dots from natural Resources and their applications in biomedicine and multi‐sensing platform. ChemistrySelect. Mar. 2021;6(11):2774–2789. doi: 10.1002/slct.202100468. [DOI] [Google Scholar]
- 88.Amirzehni Maliheh, Hassanzadeh J., Vahid B. Surface imprinted CoZn-bimetalic MOFs as selective colorimetric probe: application for detection of dimethoate. Sensors and Actuators B-chemical. Dec. 2020;325 doi: 10.1016/j.snb.2020.128768. 128768–128768. [DOI] [Google Scholar]
- 89.Pashazadeh-Panahi Paria, et al. Metal-organic frameworks conjugated with biomolecules as efficient platforms for development of biosensors. Trends Anal. Chem. Aug. 2021;141 doi: 10.1016/j.trac.2021.116285. 116285–116285. [DOI] [Google Scholar]
- 90.Abdullah M., Abdul Wahab Yasmin, Hossain M. Shamim, Hashem A., Johan Mohd Rafie. Electrochemical biosensors with Aptamer recognition layer for the diagnosis of pathogenic bacteria: barriers to commercialization and remediation. Trends Anal. Chem. Oct. 2021;145 doi: 10.1016/j.trac.2021.116458. 116458–116458. [DOI] [Google Scholar]
- 91.Bilal M., Anh Nguyen T., Iqbal H.M.N. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization – a paradigm shift in biocatalyst design. Coord. Chem. Rev. Nov. 2020;422 doi: 10.1016/j.ccr.2020.213475. [DOI] [Google Scholar]
- 92.Tian R., Li Y., Xu J., Hou C., Luo Q., Liu J. Recent development in the design of artificial enzymes through molecular imprinting technology. J. Mater. Chem. B. Jan. 2022;10(35):6590–6606. doi: 10.1039/d2tb00276k. [DOI] [PubMed] [Google Scholar]
- 93.Radhakrishnan S., Mathew M., Rout C.S. Microfluidic sensors based on two-dimensional materials for chemical and biological assessments. Materials Advances. 2022;3(4):1874–1904. doi: 10.1039/d1ma00929j. [DOI] [Google Scholar]
- 94.Sharma R., Geranpayehvaghei M., Ejeian F., Razmjou A., Asadnia M. Recent advances in polymeric nanostructured ion selective membranes for biomedical applications. Talanta. Dec. 2021;235 doi: 10.1016/j.talanta.2021.122815. [DOI] [PubMed] [Google Scholar]
- 95.Gutiérrez M., Zhang Y., Tan J.-C. Confinement of luminescent guests in metal–organic frameworks: understanding pathways from synthesis and multimodal characterization to potential applications of LG@MOF systems. Chem. Rev. Apr. 2022;122(11):10438–10483. doi: 10.1021/acs.chemrev.1c00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devaraj M., Sasikumar Y., Rajendran S., Ponce L.C. Review—metal organic framework based nanomaterials for electrochemical sensing of toxic heavy metal ions: progress and their prospects. J. Electrochem. Soc. Mar. 2021;168(3) doi: 10.1149/1945-7111/abec97. [DOI] [Google Scholar]
- 97.Xu K., Huang N. Chemical Research in Chinese Universities; Feb. 2022. Recent Advances of Covalent Organic Frameworks in Chemical Sensing. [DOI] [Google Scholar]
- 98.Zhao X., Pachfule P., Thomas A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021;50(12):6871–6913. doi: 10.1039/d0cs01569e. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez-Mozaz S., Lopez de Alda M.J., Barceló D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A. Jun. 2007;1152(1–2):97–115. doi: 10.1016/j.chroma.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 100.Ali G.K., Omer K.M. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Review. Talanta. Jan. 2022;236 doi: 10.1016/j.talanta.2021.122878. [DOI] [PubMed] [Google Scholar]
- 101.Yan L., Gonca Sevil, Zhu G., Zhang W., Chen X. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. Sep. 2019;7(37):5583–5601. doi: 10.1039/c9tb01312a. [DOI] [PubMed] [Google Scholar]
- 102.Baig N., Sajid M. Applications of layered double hydroxides based electrochemical sensors for determination of environmental pollutants: a review. Trends in Environmental Analytical Chemistry. Oct. 2017;16:1–15. doi: 10.1016/j.teac.2017.10.003. [DOI] [Google Scholar]
- 103.Zhou Y., Fang Y., Ramasamy R. Non-Covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors. Jan. 2019;19(2):392. doi: 10.3390/s19020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kolahdouz Mohammadreza, et al. Carbon-Related Materials: Graphene and Carbon Nanotubes in Semiconductor Applications and Design. Aug. 2022;13(8) doi: 10.3390/mi13081257. 1257–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piras A., Ehlert C., Gryn’ova G. Sensing and sensitivity: computational chemistry of graphene‐based sensors. WIREs Computational Molecular Science. Mar. 2021;11(5) doi: 10.1002/wcms.1526. [DOI] [Google Scholar]
- 106.Gupta Chatterjee S., Chatterjee S., Ray A.K., Chakraborty A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sensor. Actuator. B Chem. Dec. 2015;221:1170–1181. doi: 10.1016/j.snb.2015.07.070. [DOI] [Google Scholar]
- 107.Jafari S., Derakhshankhah H., Alaei L., Fattahi A., Varnamkhasti B.S., Saboury A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. Jan. 2019;109:1100–1111. doi: 10.1016/j.biopha.2018.10.167. [DOI] [PubMed] [Google Scholar]
- 108.Castillo R.R., Baeza A. Recent applications of the combination of mesoporous silica nanoparticles with nucleic acids: development of bioresponsive devices, carriers and sensors. Biomater. Sci. Jan. 2017;5(3):353–377. doi: 10.1039/c6bm00872k. [DOI] [PubMed] [Google Scholar]
- 109.Khalil I.E., Fonseca J., Reithofer M.R., Eder T., Min Chin Jia. Tackling orientation of metal-organic frameworks (MOFs): the quest to enhance MOF performance. Coord. Chem. Rev. Apr. 2023;481 doi: 10.1016/j.ccr.2023.215043. 215043–215043. [DOI] [Google Scholar]
- 110.Mourdikoudis Stefanos, Kostopoulou Athanasia, LaGrow A.P. Magnetic nanoparticle composites: synergistic effects and applications. Adv. Sci. May 2021;8(12) doi: 10.1002/advs.202004951. 2004951–2004951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo Y., et al. Technology Roadmap for Flexible Sensors. Mar. 2023;17(6):5211–5295. doi: 10.1021/acsnano.2c12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y., et al. Recent advancements in flexible and wearable sensors for biomedical and healthcare applications. J. Phys. D. Nov. 2021;55(13) doi: 10.1088/1361-6463/ac3c73. 134001–134001. [DOI] [Google Scholar]
- 113.Ibrahim N.F.A., et al. A comprehensive review of the recent developments in wearable sweat-sensing devices. Sensors. Oct. 2022;22(19):7670. doi: 10.3390/s22197670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ike I.S., Sigalas I., Iyuke S.E. Understanding performance limitation and suppression of leakage current or self-discharge in electrochemical capacitors: a review. Jan. 2016;18(2):661–680. doi: 10.1039/c5cp05459a. [DOI] [PubMed] [Google Scholar]
- 115.Hu M., et al. Electrochemical applications of ferrocene‐based coordination polymers. ChemPlusChem. Nov. 2020;85(11):2397–2418. doi: 10.1002/cplu.202000584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.