To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causative agent of coronavirus disease (COVID-19), binds via ACE2 receptors, highly expressed in ciliated cells of the nasal epithelium. Micro-optical coherence tomography (μOCT) is a minimally invasive intranasal imaging technique that can determine functional dynamics of respiratory epithelia, enabling visualization and quantification of epithelial anatomy, ciliary motion, and mucus transport. We hypothesized that respiratory epithelial cell dysfunction in COVID-19 will manifest as reduced ciliated cell function and mucociliary abnormalities, features readily visualized by μOCT. Symptomatic outpatients aged ⩾18 years with SARS-CoV-2 were recruited within 14 days of symptom onset. μOCT imaging was conducted inside a custom-designed negative-pressure isolation booth to minimize the risk of virus transmission. Significant reduction in functional cilia, diminished ciliary beat frequency (CBF), and abnormal ciliary activity were evident. Other abnormalities included the presence of mucus rafts, denuded epithelium, and increased immune cell infiltration. Our results indicate that subjects with mild but symptomatic COVID-19 exhibit functional abnormalities of the respiratory mucosa, underscoring the importance of mucociliary health in viral illness and disease transmission. Ciliary imaging enables investigation of the early pathogenic mechanisms of COVID-19 and may be useful for evaluating disease progression and therapeutic response.
The upper respiratory tract has been implicated to be the gateway for replication and transmission of SARS-CoV-2, causative agent of COVID-19, thus necessitating better discernment of the functional pathophysiology of the ciliated nasal epithelium (1–4). In vitro literature suggests common respiratory viruses, including influenza, respiratory syncytial virus, rhinovirus, rhino-enterovirus, and coronaviruses, can each contribute to ciliary cell injury (5–7). Unlike SARS-CoV-2, in which axoneme loss, misorientation of the remaining basal bodies, and impaired mucociliary clearance have been documented (8–11), leading to replacement by differentiating precursor cells (1), in infections such as influenza and pertussis (5, 12, 13), inflammatory cytokine–mediated decreased ciliary activity and direct toxin-mediated ciliary damage have been postulated to be mechanisms contributing to ciliary injury. Intranasal μOCT has been used in patients with cystic fibrosis to characterize functional microanatomy and ascertain pathophysiology, including diminished airway surface liquid, periciliary layer depths, area of active ciliary beating termed percent ciliary coverage (pCC), CBF, and delayed mucociliary transport rate (MCT) (14). We hypothesized that respiratory epithelial cell dysfunction in COVID-19 will manifest as ciliary abnormalities and devised a method to isolate individuals with symptomatic COVID-19 to use intranasal μOCT imaging and characterize functional consequences of SARS-CoV-2 infection. Our results demonstrate profound ciliary loss in the nasal epithelium even in subjects with mild disease and shed light on mechanisms that may contribute to disease pathogenesis and probably predispose patients to secondary infections. Some of the results of these studies have been previously reported in preprint form (bioRxiv, 11 July 2022, http://www.biorxiv.org/content/10.1101/2022.07.08.499336v1). The study was approved by The Partners Institutional Review Board, Massachusetts General Hospital (protocol #2016P000272, isolation booth approved in AME #21, Aug 2020) and the University of Alabama at Birmingham Institutional Review Board (protocol #F160125001). All subjects provided written informed consent before participation.
Results
Thirty-six subjects with known COVID-19 positivity were screened between October 2020 and April 2021. Thirteen subjects with RT-PCR–confirmed COVID-19 were enrolled and included in the final analysis compared with eight healthy control subjects. Mean age of the subjects in the COVID-19 group was 35 years; 54% were female and 69% were White (Table 1). Fatigue was the most commonly reported symptom; median viral load was 4.28 log10 copies/ml (see Table E1 in the data supplement). No clinical progression of disease was found on long-term follow-up. μOCT imaging was conducted in an isolation benchtop box (Figure 1A) on all subjects with COVID-19 within 14 days of symptom onset to coincide with the peak infectivity period (average, 10.2 ± 2.2 d).
Table 1.
Demographic and Clinical Baseline Characteristics
| Subject Characteristics | Subjects with COVID-19 (n = 13) | Healthy Control Subjects (n = 8) |
|---|---|---|
| Age, yr (±SD) | 35 ± 8.4 | 31 ± 6. 9 |
| Sex, female, n (%) | 7 (54) | 4 (50) |
| Race: White/Black/Asian, n | 9/3/1 | 6/2/0 |
| Smoking status, n | 0 | 0 |
| BMI, kg/m2 (±SD) | 41.2 ± 18.1 | — |
| Healthcare workers, n | 4 | 0 |
| Illness duration, d (±SD)* | 10.15 ± 2.2 | N/A |
Definition of abbreviations: BMI = body mass index (weight in kilograms divided by the square of the height in meters); COVID-19 = coronavirus disease; N/A = not applicable.
Illness duration was defined as days from symptom onset to imaging.
Figure 1.
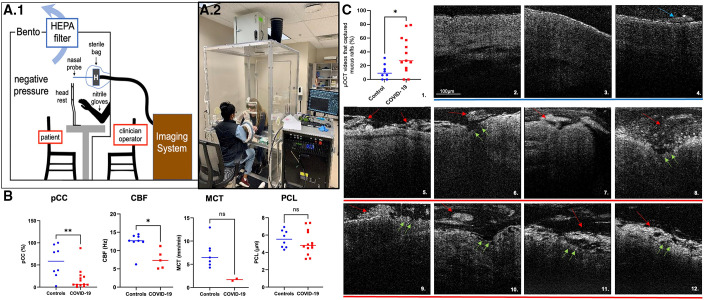
Micro-optical coherence tomography (μOCT) imaging and results. (A) Coronavirus disease (COVID-19) study subject isolation booth and demonstration photograph. (A.1) The personal protection booth is a closed negative-pressure HEPA filtration unit, with glove port and intercom communication access. (A.2) Imaging was performed with the subject seated inside the box with chin rested on the ophthalmologic headrest and operator interfacing with the subject from the exterior through the glove ports. (B) Demonstrates that functional microanatomical measurements are abnormal in COVID-19. Scatter plots of pCC, CBF, periciliary layer depth (PCL), and MCT measurements of healthy control subjects (blue) compared with subjects with COVID-19 (red) are shown. Each data point represents the mean measurement per individual; bars indicate means. Comparison of data by Mann-Whitney test: *P < 0.05 and **P < 0.01. (C) Demonstrates multiple μOCT imaging abnormalities in subjects with COVID-19. (C.1) Scatter plot with increased prevalence of mucus rafts in patients with COVID-19 are shown (11.6% ± 10.9% healthy control subjects [blue dots] vs. 35.9% ± 26.7% patients with COVID-19 [red dots]; P = 0.0282.) Each data point represents the mean measurement of percentage of μOCT videos with mucus rafts identified per subject. Bars indicate means; comparison of data by Mann-Whitney test: *P < 0.05. Healthy control subjects (C.2–C.4) with uniform PCL layer, preserved epithelium, and minimal mucus accumulation (blue arrows), versus subjects with COVID-19 (C.5–C.12) with larger and more readily apparent mucus rafts (red arrows), denuded epithelium, and loss of ciliary coverage (green arrows). CBF = ciliary beat frequency; HEPA = high efficiency particulate air; MCT = mucociliary transport rate; pCC = percent ciliary coverage.
COVID-19 causes mucus accumulation and disruption of nasal epithelium.
μOCT imaging readily identified mucus accumulation, denuded epithelium, and increased immune cell infiltration in subjects with COVID-19. Mucus secretion is stimulated by acute infection and has the propensity to become congealed into mucus plaques under pathological situations, which are quantified as mucus rafts in our study. Mucus rafts were delineated from the underlying epithelium through the slight separation (dark region in μOCT images) that exists between them. In the case of rafts that were adherent to the epithelium, the presence of large, abrupt extensions on the epithelial lining were used to distinguish them from denuded epithelium. A significantly greater number of mucus rafts were seen in COVID-19 (percentage mucus rafts per subject, 11.6% ± 10.9% controls vs. 35.9% ± 26.7% COVID-19; P = 0.0282) and frequently associated with disruption of the underlying epithelium, whereas in healthy control subjects the continuity of the underlying nasal epithelium was preserved, indicating that mucus hypersecretion was associated with underlying epithelial injury in COVID-19 (Figures 1C and E2).
COVID-19 leads to severe loss of ciliary coverage, reduces CBF, and causes irregular ciliary beat pattern.
Subjects with COVID-19 had severely reduced ciliary coverage (pCC, 57.4% ± 36.11% controls vs. 18.1% ± 23.62% COVID-19; P = 0.009); however, no direct correlation between pCC and viral load at the time of imaging (r = 0.41) or duration of illness (r = 0.002) was found (Figure E1). Because of the frequent areas with complete absence of cilia, it was challenging to ascribe the motion of motile particles in the majority of the subjects with COVID-19. Wherever quantification was possible, although not statistically significant, mucociliary transport rates showed a pronounced reduction in the COVID-19 cohort (7.2 ± 2.9 mm/min controls, n = 7 vs. 1.6 ± 0.3 mm/min COVID-19, n = 2; P = 0.056; Figure 1B). μOCT demonstrated severely diminished CBF in patients with COVID-19 (12.32 ± 2.58 Hz controls vs. 7.57 ± 2.56 Hz COVID-19; P = 0.011). Qualitative ciliary waveform analysis in healthy subjects detected regular, rhythmic patterns of relatively consistent frequency and amplitude, whereas subjects with COVID-19 showed strikingly irregular beat patterns with erratic amplitudes (Figure E3).
Discussion
Structural integrity and coordinated movement of cilia are imperative for optimal mucociliary clearance, the primary defense mechanism of the respiratory tract (15). Clinical presentation of COVID-19 illness from asymptomatic to pneumonia and respiratory failure occurs through a variety of mechanisms, including cytopathic cellular effects and viral-mediated cytokine responses (16). Viral loads have failed to demonstrate a consistent prognostic potential for severity of illness (17, 18), highlighting the possibility that functional parameters may hold promise for disease stratification. We report, for the first time, in vivo evidence of extensive loss of motile cilia, accompanied by derangements in residual ciliary beating and loss of coordinated motion in patients with acute COVID-19. Despite including only subjects with mild illness, we found severe ciliated cell abnormalities and functional deficiencies in the mucociliary apparatus when imaged within the peak infectivity period.
These results are complementary to studies that have reported ciliary injury as a common pathological feature in various COVID-19 models (1, 8, 11, 19, 20). Several in vitro and in vivo studies have demonstrated proclivity for the SARS-CoV-2 virus to ciliated cells, evidenced by prominent ciliary abnormalities (8–10) and impaired mucociliary clearance (11, 20) in these model systems. In vitro evidence implicates ubiquitin-mediated loss of ciliary proteins as causative of ciliary dysfunction with SARS-CoV-2 infection (21). We observed extensive loss of motile cilia, and this was accompanied by derangements in residual ciliary beating and loss of coordinated motion. Prominent cellular inflammation of the overlying mucus with adherent mucus rafts was also apparent. These features are likely to contribute to the delayed mucociliary clearance that has been demonstrated in vitro. In μOCT studies of excised trachea in Syrian hamsters, we documented severe decrements in MCT that accompanied ciliary loss; these studies benefited from laboratory conditions conducive to the extensive survey of epithelium, where MCT could be more readily measured (20). Of note, findings in patients with COVID-19 contrasted with μOCT imaging of patients with cystic fibrosis, in whom significantly reduced MCT rates were observed and correlated to the depletion of the periciliary liquid layer and the presence of hyper-viscous mucus. The pathogenesis underlying several respiratory virus–induced mucus hypersecretions has been attributed to inflammatory cytokine and chemokine overproduction. Similarly, infection with SARS-CoV-2 is known to activate the inflammatory response, with an increase in respiratory mucosal secretions (22). Qualitative evidence of epithelial injury and increased inflammatory cell count as observed in our COVID-19 cohort indirectly supports this mechanism of mucus hypersecretion in COVID-19.
Our findings are limited by the small sample size, and although we captured a variety of functional deficits in subjects with mild disease, their course was self-limited, thus restricting our ability to discern disease progression or risks of future complications. MCT measurements require visualization of ciliated epithelium to define trackable mucus particles, limiting the number of cases in which this could be accurately measured, thus reducing optimal data extraction. Because serial imaging through the disease course or the ability to obtain invasive biopsies were not feasible at the time this study was conducted, we are unable to comment on the longitudinal progression of functional and microanatomical changes and their histopathological correlations.
Our study highlights the use of μOCT imaging in the investigation of early pathogenic mechanisms of COVID-19. Because asymptomatic individuals are also known to propagate the virus, it seems highly likely that substantial ciliated cell injury may be occurring in these individuals. These findings thus corroborate the need to further explore the role of targeting ciliated cells early in the disease process for mitigation of SARS-CoV-2 transmission and emphasize applicability of ciliary imaging in other viral respiratory disorders.
Participants
Symptomatic outpatients aged ⩾18 years with COVID-19 were recruited within 7 days of symptom onset. Detailed clinical histories, including risk factors and COVID-19 symptoms, were obtained. Long-term follow-up, including clinical outcomes at 21 days, were collected.
Design of the Full-Body Personal Protection Booth, Imaging Procedure, and μOCT Imaging Analysis
The customized negative-pressure full-body personal protection booth was designed and fabricated to isolate study subjects from study staff for COVID-19 prevention. The μOCT probe was maneuvered into the inferior meatus region while visualizing real-time images and acquiring data from approximately five discrete sites at the turbinate and floor of each nare per previously published methods (14). Data were deidentified and interpreted by a team blinded to the condition. μOCT outcomes in normal control subjects obtained in this study were similar and not statistically different from mean values of a combined analysis obtained from different studies including controls from cystic fibrosis and chronic sinusitis (N = 34; combined control means vs. COVID-19 control subjects: periciliary layer depths, 5.95 vs. 5.8 mm; CBF, 14.85 vs. 12.32 Hz; MCT, 8.5 vs. 7.2 mm/min). Detailed methods and statistical analysis are available in the data supplement.
Acknowledgments
Acknowledgment
The authors thank the participants enrolled in this study and their families.
Footnotes
Supported by National Institutes of Health grants P30DK072482, R35 HL135816, and R35 HL135816-04S1 (S.M.R.); National Institutes of Health grant UL1TR003096 (University of Alabama at Birmingham); Cystic Fibrosis Foundation grants ROWE19RO, ROWE17XX1, and TEARNE16XX0; institutional resources from the University of Alabama at Birmingham COVID-19 Research Award; and the Mike and Sue Hazard Family and the Remondi Family Foundations (G.J.T.).
Author Contributions: K.V., H.M.L., G.M.S., G.J.T., and S.M.R. developed the study design. K.V., G.M.S., H.Y.H., J.D.W., K.M., K.B.S., and S.M.R. participated in experimental conduct and acquisition of data. K.V., H.M.L., A.B., C.M.F.-P., G.M.S., G.J.T., and S.M.R. performed data analysis. S.M.L., D.B.M., and H.M.P. performed virologic testing and analysis. K.R.O., P.C., S.F., D.M., G.J.T., and S.M.R. participated in the design and development of the personal protection booth. K.V., H.M.L., Q.L., and S.M.R. prepared the manuscript. All authors provided necessary revisions and approved a final draft before submission.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Ahn JH, Kim J, Hong SP, Choi SY, Yang MJ, Ju YS, et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest . 2021;131:e148517. doi: 10.1172/JCI148517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med . 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu CT, Lidsky PV, Xiao Y, Cheng R, Lee IT, Nakayama T, et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell . 2023;186:112–130.e20. doi: 10.1016/j.cell.2022.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell . 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Essaidi-Laziosi M, Brito F, Benaoudia S, Royston L, Cagno V, Fernandes-Rocha M, et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin Immunol . 2018;141:2074–2084. doi: 10.1016/j.jaci.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith CM, Kulkarni H, Radhakrishnan P, Rutman A, Bankart MJ, Williams G, et al. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J . 2014;43:485–496. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- 7. Wu NH, Yang W, Beineke A, Dijkman R, Matrosovich M, Baumgärtner W, et al. The differentiated airway epithelium infected by influenza viruses maintains the barrier function despite a dramatic loss of ciliated cells. Sci Rep . 2016;6:39668. doi: 10.1038/srep39668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu N, Wang W, Liu Z, Liang C, Wang W, Ye F, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun . 2020;11:3910. doi: 10.1038/s41467-020-17796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehre C. SARS-CoV-2 infection of airway cells. N Engl J Med . 2020;383:969. doi: 10.1056/NEJMicm2023328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijenhuis W, Damstra HGJ, van Grinsven EJ, Iwanski MK, Praest P, Soltani ZE, et al. 2021. https://www.biorxiv.org/content/10.1101/2021.08.05.455126v1
- 11. Robinot R, Hubert M, de Melo GD, Lazarini F, Bruel T, Smith N, et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun . 2021;12:4354. doi: 10.1038/s41467-021-24521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brand JD, Lazrak A, Trombley JE, Shei R-J, Adewale AT, Tipper JL, et al. Influenza-mediated reduction of lung epithelial ion channel activity leads to dysregulated pulmonary fluid homeostasis. JCI Insight . 2018;3:e123467. doi: 10.1172/jci.insight.123467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis . 1985;152:118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 14. Leung HM, Birket SE, Hyun C, Ford TN, Cui D, Solomon GM, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Sci Transl Med . 2019;11:eaav3505. doi: 10.1126/scitranslmed.aav3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest . 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridges JP, Vladar EK, Huang H, Mason RJ. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax. 2022;77:203–209. doi: 10.1136/thoraxjnl-2021-217561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stankiewicz Karita HC, Dong TQ, Johnston C, Neuzil KM, Paasche-Orlow MK, Kissinger PJ, et al. Trajectory of viral RNA load among persons with incident SARS-CoV-2 G614 infection (Wuhan strain) in association with COVID-19 symptom onset and severity. JAMA Netw Open . 2022;5:e2142796. doi: 10.1001/jamanetworkopen.2021.42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Argyropoulos KV, Serrano A, Hu J, Black M, Feng X, Shen G, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol . 2020;190:1881–1887. doi: 10.1016/j.ajpath.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajan A, Weaver AM, Aloisio GM, Jelinski J, Johnson HL, Venable SF, et al. The human nose organoid respiratory virus model: an ex vivo human challenge model to study respiratory syncytial virus (RSV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogenesis and evaluate therapeutics. mBio . 2021;13:e0351121. doi: 10.1128/mbio.03511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Q, Vijaykumar K, Phillips SE, Hussain SS, Huynh NV, Fernandez-Petty CM, et al. Mucociliary transport deficiency and disease progression in Syrian hamsters with SARS-CoV-2 infection. JCI Insight . 2023;8:e163962. doi: 10.1172/jci.insight.163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Liu C, Yang B, Zhang H, Jiao J, Zhang R, et al. SARS-CoV-2 ORF10 impairs cilia by enhancing CUL2ZYG11B activity. J Cell Biol . 2022;221:e202108015. doi: 10.1083/jcb.202108015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan MA, Khan ZA, Charles M, Pratap P, Naeem A, Siddiqui Z, et al. Cytokine storm and mucus hypersecretion in COVID-19: review of mechanisms. J Inflamm Res . 2021;14:175–189. doi: 10.2147/JIR.S271292. [DOI] [PMC free article] [PubMed] [Google Scholar]