Abstract
Polyphosphate kinase (Ppk) catalyzes the formation of polyphosphate from ATP. We cloned the ppk gene (2,073 bp) from Acinetobacter sp. strain ADP1; this gene encodes a putative polypeptide of 78.6 kDa with extensive homology to polyphosphate kinase from Escherichia coli and other bacteria. Chromosomal disruption of ppk by inserting a transcriptionally fused lacZ does not affect growth under conditions of phosphate limitation or excess. β-Galactosidase activity expressed from the single-copy ppk::lacZ fusion is induced 5- to 15-fold by phosphate starvation. An increased amount of ppk transcript (2.2 kb) was detected when cells were grown at a limiting phosphate concentration. Primer extension analysis revealed a regulated promoter located upstream of a second, constitutive promoter. Potential similarities of this regulation with the effects of PhoB and PhoR of E. coli are discussed.
Polyphosphate is a linear polymer of orthophosphate residues linked by high-energy phosphoanhydride bonds. It is found in a wide variety of organisms, including bacteria, fungi, protozoa, plants, and mammals (23, 24). While this may suggest a fundamental physiological role for polyphosphate in life, no essential function has been identified. However, polyphosphate may have many different functions in various organisms and under different physiological conditions, as reviewed previously (22, 43); e.g., this polymer may contribute to survival in stationary phase (30), inhibition of RNA degradation (5), storage of phosphate and energy, substitution of ATP, chelation of ions (20), formation of cell capsule, regulation under stress (19, 30), and formation of channels for DNA entry into competent Escherichia coli cells (6, 32).
In E. coli, polyphosphate is remarkably uniform in length (about 750 residues). It is built up from ATP by polyphosphate kinase (Ppk) in a reversible and highly processive reaction from a phosphohistidyl-Ppk intermediate (25). Ppk is a homotetrameric protein that is associated with the outer membrane (1, 3) and is a component of the RNA degradosome (5). Depolymerization of polyphosphate occurs either by the reverse Ppk reaction leading to ATP formation from ADP or via hydrolysis by an exopolyphosphatase (Ppx) (2) or pppGpp hydrolase (GppA) (19). The ppk and ppx genes are located in the same operon (2). Disputed indications hint that the ppk ppx operon is regulated by the PhoBR system (18, 40). Some strains of Acinetobacter which occur predominantly in wastewater are known to accumulate polyphosphate (8). However, the microbial process of phosphate removal from wastewater is very slow, and improvements are necessary to support industrial application of this trait (22). Knowledge about the genes involved in polyphosphate synthesis and their regulation may support this task. Here we report the isolation and analysis of the ppk gene from Acinetobacter sp. strain ADP1 and its transcriptional induction by phosphate starvation. Since it is not known if ADP1 accumulates polyphosphate, it serves as a model for regulation of ppk transcription in Acinetobacter.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Wild-type Acinetobacter sp. strain ADP1 was formerly classified as Acinetobacter calcoaceticus ADP1 and is synonymously called Acinetobacter sp. strain BD413 (17, 38).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genetic marker(s) | Reference or source |
|---|---|---|
| Strains | ||
| Acinetobacter sp. strain ADP1 | Wild type | 17, 38 |
| E. coli DH5α | recA1 endA1 supE44 gyrA96 thi hsdR17(rK− mK−) relA1 Φ80ΔlacZΔM15 Δ(lacZYA-argF)U169 | 13 |
| Acinetobacter sp. strain WH386 | ORF1(ΔAflII-NdeI)::lacZ-Kmr | 10 |
| Acinetobacter sp. strain WH435 | ppk::lacZ-Kmr | This study |
| Plasmids | ||
| pBluescript II SK+ | Apr | Stratagene, La Jolla, Calif. |
| pKOK6.1 | Apr, Kmr, promoterless lacZ | 21 |
| pWH891 | Apr | 11 |
| pWH891SK5i | Apr | This study |
| pWH969 | Apr | This study |
| pWH891SK5ippk::lacZ-Kmr | Apr, Kmr | This study |
General methods.
E. coli and Acinetobacter sp. strain ADP1 were transformed as described previously (13, 29). Total DNA was prepared as described by Ausubel et al. (4). Small-scale preparations of plasmids were made by the boiling lysis method (16); large-scale preparations were made by using the Nucleobond Kit (Macherey-Nagel, Düren, Germany). Total RNA was isolated with the RNeasy Mini Kit from Qiagen (Hilden, Germany).
Media, growth conditions, and β-galactosidase assays.
E. coli was grown at 37°C, and Acinetobacter strains were grown at 28°C. E. coli cultures for preparation of DNA were grown in LB medium with ampicillin (100 mg/liter) or kanamycin (30 mg/liter). Acinetobacter sp. strains WH386 and WH435 were grown with kanamycin (10 mg/liter).
The minimal medium used in these studies consisted of 50 mM 3-[N-morpholino]-2-hydroxypropanesulfonic acid (MOPSO), 5 mM N-tris[hydroxymethyl]methylglycine (Tricine; pH 7.0), 10 mM KCl, 20 mM succinate, 15 mM NH4Cl, and 7.5 mM Na2SO4, supplemented with metal 44 solution (7). Phosphate was added as Na2HPO4-NaH2PO4 (pH 7.0) to final concentrations of 10 and 100 μM (phosphate starvation) or 1 mM (phosphate excess).
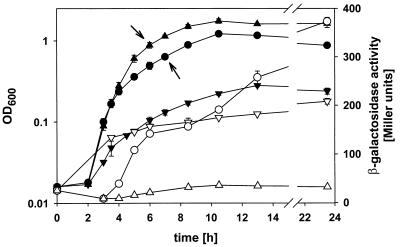
Overnight cultures were grown for 20 h in minimal medium with 1 mM phosphate, washed twice in minimal medium lacking phosphate, and used to inoculate 70 ml of minimal medium containing 10 μM, 100 μM, or 1 mM phosphate to an optical density at 600 nm (OD600) of 0.02. The OD600 was monitored over 24 h, and samples for RNA preparation and β-galactosidase assays were collected at times indicated in the respective results. Samples representing 1 ml of culture at an OD600 of 0.52 were centrifuged, and the cell pellets were frozen at −20°C for up to 3 days for β-galactosidase assays. The cell pellets were suspended in 1.3 ml of LB medium, the OD600 was determined, and 200 μl was used as described by Miller (28). The data (OD600 and Miller units) given in Fig. 3 were determined from three independent cultures. β-Galactosidase activities of frozen cells are identical to those obtained from fresh cells.
FIG. 3.
lacZ expression of Acinetobacter sp. strain WH435 (ppk::lacZ) grown in minimal medium with different phosphate concentrations. Cultures used for inoculation were grown in minimal medium with 1 mM phosphate. The OD600s (closed symbols) and β-galactosidase activities (open symbols) for cultures grown in medium supplemented with 10 μM (▾ and ▿), 100 μM (• and ○), and 1 mM (▴ and ▵) phosphate are shown. The means and standard deviations of three independently grown cultures are shown. Growth of ADP1 was identical to that WH435, but no β-galactosidase activity was demonstrated. Arrows indicate the samples used for preparation of RNA from ADP1 cultures for Northern blot and primer extension.
DNA sequence analysis.
Nucleotide sequences were determined on both strands by the dideoxy chain termination method (34) with Sequenase (U.S. Biochemicals, Cleveland, Ohio) and [α-32P]dATP or [α-35S]dATP. The Thermo Sequenase radiolabeled terminator sequencing kit with [α-33P]dideoxynucleoside triphosphates (Amersham, Buckinghamshire, United Kingdom) was used for sequencing reactions performed in connection with primer extension. Sequences were analyzed by using the UWGCG software package (9). Database searches were done by using the services offered by the National Center for Biotechnology Information (http://www.ncbi.nlm.nhi.gov).
Southern hybridization.
Restricted genomic DNA (8 μg) or plasmid DNA (10 ng) was run on a 1% agarose gel and blotted onto a nylon membrane (36). The probe was prepared by nick translation with biotin-7-dATP, and a Photogene kit was used for detection of signals. The membrane, nick translation, and Photogene kit were obtained from Gibco BRL (Gaithersburg, Md.).
Northern hybridization.
Total RNA (10 μg per lane) was run on a 1.3% agarose gel containing 6% formaldehyde and blotted onto a positively charged nylon membrane (Porablot NY Plus; Macherey-Nagel) by capillary transfer as described by Sambrook et al. (33). The ppk-specific probe was prepared by PCR with [α-32P]dATP amplifying a fragment from nucleotide 10775 to 11214 (see Fig. 1). The hybridization procedure was performed in accordance with the recommendations of the membrane manufacturer. Radioactivity was determined with a PhosphorImager (Fujifilm, BAS-1500).
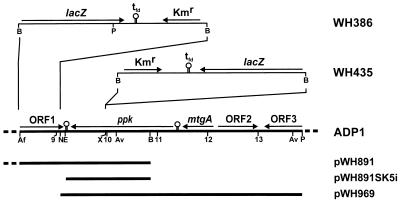
FIG. 1.
Genetic organization of Acinetobacter sp. strain ADP1 DNA downstream of rubA (located at 6 kbp). Numbers refer to the kilobase-pair scale of the sequence in the EMBL database (accession no. Z46863), with 0 kbp representing the BamHI site of pWH891. The inserts cloned on pWH891, pWH891SK5i, and pWH969 are shown as bars. Putative transcriptional terminator sequences are indicated by stem-loop structures (○). For WH435 and WH386, the lacZ-Kmr cassette from pKOK6.1 is inserted on the chromosome into the XcmI and AflII-NdeI sites, respectively. Abbreviations: Af, AflII; Av, AvaII; B, BamHI; E, EcoRV; N, NdeI; P, PvuII; X, XcmI; lacZ, gene encoding β-galactosidase; tfd, transcriptional terminator sequence of phage fd; Kmr, kanamycin resistance gene; ppk, gene encoding the putative polyphosphate kinase; mtgA, gene encoding the putative monofunctional transglycosylase.
Primer extension.
Primer extension reactions were performed as described previously (42). Total RNA (15 μg) was incubated for 5 min at 80°C and hybridized for 5 min with the 5′-end-labeled primer (35 fmol) at 37°C. The reaction mixtures containing 9 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) were incubated for 45 min at 37°C. After treatment with RNase A and denaturation of the cDNA, half of the volume was loaded on the gel and analyzed with a PhosphorImager. The sequence of the ppk-specific primer is 5′-CTGGTGGTGTTGTCATCGC-3′.
Nucleotide sequence accession number.
The 13,879-bp nucleotide sequence of Acinetobacter sp. strain ADP1 DNA cloned on pWH891 and pWH969 has been deposited in the EMBL database under accession no. Z46863.
RESULTS
Cloning of ppk from Acinetobacter sp. strain ADP1.
Recently, we reported the cloning of a 10.8-kbp genomic BamHI fragment from Acinetobacter sp. strain ADP1 harboring a putative periplasmic Mn superoxide dismutase located upstream of the rubredoxin-encoding gene rubA on plasmid pWH891 (Fig. 1) (11). For analysis of the sequence neighboring the BamHI site downstream of rubA, we subcloned the 1.7-kbp EcoRV-BamHI fragment from pWH891 into pBluescript II SK+, cut with EcoRV, resulting in plasmid pWH891SK5i. Sequence analysis revealed the 3′-terminal part of an open reading frame (ORF) with homology to genes encoding polyphosphate kinases (ppk) (Fig. 1). For cloning of the complete ppk gene by a chromosome walking strategy, we used strain WH386. WH386 is a derivative of ADP1 in which a 736-bp AflII-NdeI fragment is replaced on the chromosome by a 4.73-kbp lacZ-Kmr cassette from pKOK6.1 (Fig. 1). Genomic DNA from WH386 was digested with PvuII and ligated with pBluescript II SK+, linearized with EcoRV. The ligation mixture was transformed to E. coli DH5α, and clones were selected on LB plates with kanamycin. The resulting plasmid was called pWH969. It contains a 6.5-kbp PvuII insert consisting of 4.85 kbp of genomic ADP1 DNA and 1.65 kbp from pKOK6.1 including the Kmr gene (Fig. 1). The nucleotide sequence of this insert from BamHI to PvuII was determined. AvaII restriction sites deduced from the sequence (Fig. 1) were used in Southern hybridization. A 3.5-kbp AvaII fragment hybridized to the probe in chromosomal DNA and pWH969, indicating that the insert of pWH969 corresponds to a contiguous part of ADP1 genomic DNA.
Sequence analysis of pWH969.
The part of pWH969 from NdeI to PvuII (Fig. 1) consists of 4,872 bp with 40% GC, which is typical for Acinetobacter (15). Sequence analysis revealed the complete ORF encoding a putative polyphosphate kinase (ppk) preceded by three ORFs (mtgA, ORF2, and ORF3 [partial]) (Fig. 1). Stem-loop structures which may act as transcriptional termination sequences were found downstream of mtgA and ppk (Fig. 1; see Fig. 5B). The codon frequency in each ORF reflects the codon usage of Acinetobacter (41).
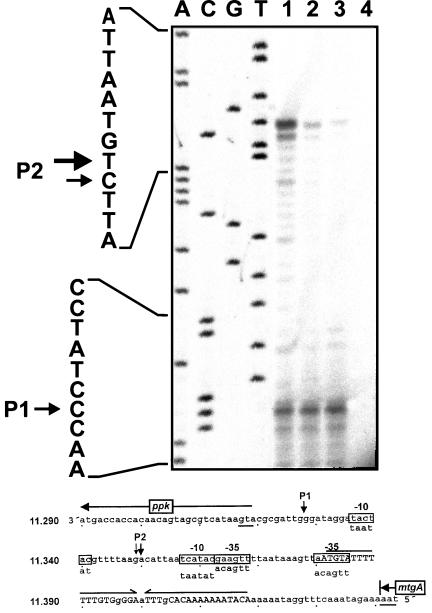
FIG. 5.
Mapping of the 5′ start site of ppk mRNA by primer extension. (Upper panel) Autoradiograph. RNA was prepared from ADP1 cells growing in minimal medium with 100 μM (lane 1) or 1 mM (lane 2) phosphate or growing exponentially in LB medium (lane 3). The primer extension control without RNA is also shown (lane 4). Lanes A, C, G, and T show the sequencing products obtained with the same primer. The sequence is shown on the left. Arrows indicate the start sites of transcription (P1 and P2). (Lower panel) Sequence interpretation. The sequence of the coding strand upstream of ppk is shown. The nucleotide positions are given on the left in accordance with the diagram shown in Fig. 1. The start codon of ppk and stop codon of mtgA are underlined, and the direction of translation is indicated by arrows. The putative transcriptional terminator sequence of mtgA is marked by inverted arrows, and the bases matching the inverted repeat are indicated by uppercase letters. The start sites of transcription (P1 and P2) are indicated by downward arrows, and the sequences with the highest similarity to E. coli ς70 promoter sequences (−10, −35) are boxed.
ORF2 and ORF3 show no striking correspondence to any protein of known function. The protein encoded by mtgA (224 amino acids; 26.7 kDa) is homologous to monofunctional biosynthetic peptidoglycan transglycosylases from Klebsiella pneumoniae (42% identical amino acids; GenBank accession no. Z54198), Neisseria gonorrhoeae (41% identity; GenBank accession no. U82700), Haemophilus influenzae (41% identity; SwissProt accession no. P44890), and E. coli (39% identity; SwissProt accession no. P46022). The members of this class of proteins have high similarity to the N-terminal transglycosylase domain of class A high-Mr penicillin-binding proteins (12), lacking the C-terminal transpeptidase. They are characterized by at least two positively charged amino acids near the N terminus followed by a stretch of about 20 hydrophobic amino acids but no AlaXAla site for cleavage by leader peptidase. Although no biochemical data are available, monofunctional biosynthetic peptidoglycan transglycosylases are believed to be anchored in the cytoplasmic membrane by a noncleaved signal sequence like class A high-Mr penicillin-binding proteins and to have a specific function in peptidoglycan biosynthesis (37).
The ppk gene of Acinetobacter has the capacity to encode a protein of 691 amino acids with a deduced molecular size of 78.6 kDa. The sequence of the putative gene product has high similarity to those of the Ppk proteins from Klebsiella aerogenes and E. coli, which have been characterized biochemically, and to six other putative Ppk proteins. No significant matches of the ppk sequence were found in the complete genomes of Mycoplasma pneumoniae, Mycoplasma genitalium, Saccharomyces cerevisiae, and H. influenzae.
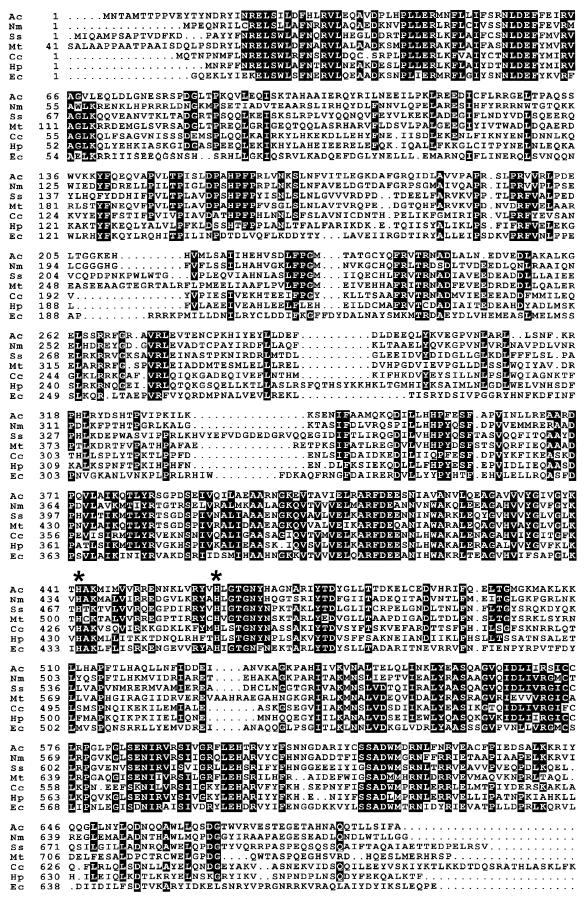
The entire sequences of the Ppk variants are homologous (Fig. 2). Highly conserved regions are located between amino acids 20 and 69 (21 of 50 amino acids strictly conserved), 367 and 468 (26 of 102 strictly conserved), and 556 and 630 (25 of 75 strictly conserved). Two histidine residues necessary for ppk activity in E. coli (His442 and His461 in the Acinetobacter sequence) are strictly conserved throughout the family (Fig. 2) (25). Therefore, we suppose that ppk encodes a functional Ppk of Acinetobacter sp. strain ADP1.
FIG. 2.
Alignment of Ppk from Acinetobacter sp. strain ADP1 (Ac; this work) with the putative Ppk proteins from N. meningitidis (Nm; GenBank accession no. U16262), Synechocystis sp. (Ss; GenBank accession no. D64005), M. tuberculosis (Mt; GenBank accession no. Z83018), Campylobacter coli (Cc; EMBL accession no. Y07620), and Helicobacter pylori (Hp; GenBank accession no. AE000609) and with the biochemically characterized Ppk from E. coli (Ec) (3). Amino acids are given in a one-letter code. White letters indicate that at least five of the seven compared residues were identical. Two histidine residues functionally important in E. coli Ppk are marked with asterisks (His442 and His461 in the Acinetobacter sequence).
Chromosomal disruption of ppk.
We inactivated the ppk gene on the chromosome by insertion of the lacZ-Kmr cassette from pKOK6.1, creating a single-copy transcriptional ppk::lacZ fusion. The promoterless lacZ gene is preceded by stop codons in all reading frames (27). pWH891SK5i was cut with XcmI, and the lacZ-Kmr cassette was excised from pKOK6.1 with BamHI. The protruding ends of both fragments were filled in with Klenow polymerase and ligated. The resulting plasmid, called pWH891SK5ippk::lacZ was cut with ApaI and SacII within the vector sequence to prevent integration of the circular plasmid, and the linear DNA was transformed into Acinetobacter sp. strain ADP1. Transformants were selected on LB plates with kanamycin (10 mg/liter), and integration of the cassette was confirmed by Southern hybridization (data not shown). The chromosomal organization of the resulting strain, called WH435, is shown in Fig. 1. No difference in growth rate between the wild type and the ppk mutant strain was found in LB or minimal medium under phosphate starvation or excess conditions (data not shown). Tolerance of Cd2+ ions was not affected by inactivation of ppk because WH435 and ADP1 tolerated 1 mM CdCl2, whereas 3 mM CdCl2 inhibited growth in the presence of 100 μM phosphate (data not shown). These results show that polyphosphate kinase is not essential in Acinetobacter sp. strain ADP1 and that it is not involved in heavy metal tolerance.
Induction of ppk transcription by phosphate starvation.
We used the single-copy ppk::lacZ fusion in WH435 to examine the regulation of ppk transcription by phosphate. The growth curves and lacZ expression data are shown in Fig. 3. There was no difference between the growth of WH435 and that of the wild type (data not shown), which grew to OD600s of 1.8, 1.2, and 0.3 in minimal medium with 1 mM, 100 μM, and 10 μM phosphate, respectively. This shows that a phosphate concentration of 100 μM limits growth under these conditions. With 1 mM phosphate, lacZ is expressed in WH435 at a nearly constant low level, ranging between 9 ± 0.4 and 36 ± 0.6 Miller units (mean ± standard deviation). In contrast, lacZ expression increased to 208 ± 6 Miller units when strain WH435 was grown in the presence of 10 μM phosphate, indicating a 5- to 15-fold induction from the 1 mM phosphate level. The low level of expression in cells grown with 1 mM phosphate, which is the same over the entire growth curve, clearly shows that expression is not induced in stationary phase but depends specifically on phosphate starvation. The graph of lacZ expression in cells grown in medium with 100 μM phosphate shows a biphasic curve, with a plateau at 150 Miller units at 6 to 9 h after inoculation and then an increase to 373 ± 6 Miller units in stationary phase. The first increase coincides with growth retardation and reaches the level also observed for cells grown in the presence of 10 μM phosphate (Fig. 3). Therefore, this increase may reflect the induction by phosphate starvation as a consequence of phosphate consumption during exponential growth. The second increase results in doubling of the β-galactosidase activity, which may be due to accumulation of β-galactosidase during stationary phase.
ppk constitutes a separate transcription unit.
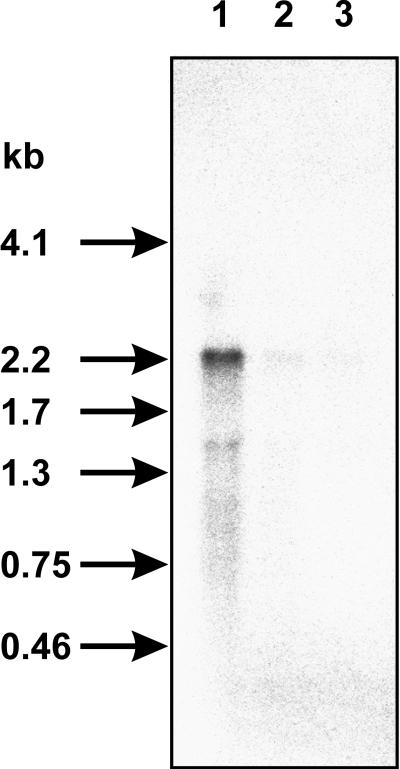
A putative transcriptional terminator sequence (5′-AAAAAAGGAGTCTTTAAAGACTCCTTTTTT-3′) is found downstream of the ppk gene. Therefore, the size of the ppk transcript should indicate whether ppk is a single transcription unit or if it is cotranscribed in an operon together with the preceding mtgA gene (Fig. 1). The ppk-specific probe was hybridized to 10 μg of RNA from ADP1 cultures (Fig. 3) and to 10 μg of RNA isolated from ADP1 growing exponentially in LB medium. A ppk-specific mRNA of 2.2 kb was detected only in RNA from cells grown under limiting phosphate conditions (Fig. 4). This confirms regulation of ppk expression by phosphate in the medium, as was also seen in the β-galactosidase assays (Fig. 3). The size of the ppk mRNA corresponds well with the size of the ppk gene (2,073 bp), indicating the presence of a promoter in front of the gene.
FIG. 4.
Northern blot analysis. Ten micrograms of total RNA was used for each lane, and the ppk transcript was detected by hybridization to a ppk-specific probe. RNA was prepared from ADP1 cells growing in minimal medium with 100 μM (lane 1) or 1 mM (lane 2) phosphate or growing exponentially in LB medium (lane 3). The positions of RNA molecular size marker bands are given in kilobases on the left.
ppk is transcribed from two promoters.
The start point of ppk transcription was determined by primer extension with a primer hybridizing within the coding region of ppk. The same RNA preparation was used for Northern blot analysis (see above) and primer extension. Two major products were detected (P1 and P2) (Fig. 5). The P1 band intensity is independent of the phosphate concentration in the culture medium. The P2 signal, however, is visible only when cells are grown under limiting phosphate conditions and disappears when an excess of phosphate is present. This reconfirms the transcriptional regulation of ppk by phosphate limitation. The deduced start points of transcription corresponding to P1 and P2 (Fig. 5, sequence interpretation) are preceded by potential −10 and −35 promoter sequences with four and five mismatches to the E. coli consensus sequence, respectively (14, 26). For nucleotides up to 300 bp upstream of P1 and P2, no sequences displaying similarity to ς54 promoters or pho boxes were found.
DISCUSSION
We cloned the ppk gene from Acinetobacter sp. strain ADP1 that encodes a putative protein with high similarity to the polyphosphate kinase (Ppk) of E. coli and homologous gene products of various gram-negative bacteria and Mycobacterium tuberculosis. We demonstrate that transcription of ppk is induced by phosphate starvation. This suggests an involvement of ppk in phosphorus metabolism.
In contrast to E. coli (2) and K. aerogenes (18), ppk in Acinetobacter sp. strain ADP1 is not followed by a ppx gene encoding an exopolyphosphatase. This is also the case for all the other bacteria containing a ppk gene, indicating that the organization of ppk and ppx in an operon is the exception found in E. coli and closely related organisms. Polyphosphate, on the other hand, is widely distributed in bacteria, fungi, protozoa, plants, and mammals (23, 24). However, no ppk homologous gene is found in S. cerevisiae, suggesting that polyphosphate synthesis in eucaryotes may require enzymes which are not related to the eubacterial Ppk. No ppk-homologous gene is found in the complete genomes of M. genitalium and M. pneumoniae, which may constitute exceptions because of their small size. H. influenzae does not contain ppk either, but a gene with high similarity to E. coli ppx is present (SwissProt accession no. P44828).
We inactivated ppk in Acinetobacter sp. strain ADP1 by insertion of a lacZ-Kmr cassette, disrupting the amino acid sequence between the two His residues necessary for function in E. coli (Fig. 2). This shows that the ppk gene is not essential in Acinetobacter. The ppk mutant has no growth defect in complex or minimal media. In E. coli, tolerance to Cd2+ ions had been increased in a ppk ppx mutant by overexpression of ppk and ppx from a plasmid compared to the mutant overexpressing only ppk, indicating that polyphosphate turnover is required for heavy-metal resistance (20). A. calcoaceticus WH435 (ppk::lacZ) exhibits no increase in sensitivity to heavy-metal ions compared to that of the wild type. Currently, we have no experimental indications of the function of the putative Ppk in Acinetobacter sp. strain ADP1. However, considering the inducibility of ppk transcription by phosphate starvation, it seems unlikely that Ppk forms polyphosphate serving as phosphate storage in Acinetobacter. It may be speculated that Ppk provides phosphate by degradation of polyphosphate under conditions of phosphate limitation.
Transcription of ppk in Acinetobacter sp. strain ADP1 is induced 5- to 15-fold by phosphate starvation. Regulation by phosphate limitation is well known in E. coli, where PhoR, the sensorkinase, phosphorylates PhoB upon phosphate starvation. Phospho-PhoB, in turn, binds to the pho box [consensus sequence, CTGTCATA(T/A)A(T/A)CTGT(A/C)A(C/T)], which typically overlaps with the −35 promoter region, and induces transcription of about 30 genes of the pho regulon (40). There are indications that ppk in K. aerogenes is regulated by the phoBR system because expression of a ppk::lacZ fusion is induced 10-fold under phosphate starvation in the heterologous E. coli host but not in an E. coli phoB mutant (18). However, the significance of this observation was called into question because of the low-level induction (threefold) detected in K. aerogenes, the use of multicopy reporter constructs in E. coli, and the experimental setup leading to phosphate limitation (40).
No phoB or phoR genes have been cloned from any Acinetobacter strain to date. However, it seems probable that Acinetobacter contains genes with homology to phoBR. First, a promoter of the polyhydroxyalkanoic acid biosynthetic genes from Acinetobacter strain RA3849 was shown to be induced by phosphate starvation. Putative pho box sequences were found upstream of the −10 region, and heterologous regulation occurs in wild-type E. coli but not in an E. coli phoB mutant (35). Second, two phosphate uptake systems from Acinetobacter johnsonii 210A were characterized biochemically (39): a constitutive, low-affinity system and a high-affinity, phosphate-binding-protein-dependent system. The latter is induced by phosphate starvation. This resembles the situation in E. coli, where the high-affinity system is induced by phoBR (31). Taken together, these data suggest that phoBR-like genes probably exist in Acinetobacter and therefore PhoB may be a good candidate for mediating transcriptional induction of ppk in Acinetobacter sp. strain ADP1. We did not find a pho box-like sequence in front of ppk, indicating either that the putative Acinetobacter PhoB must differ from E. coli PhoB in DNA recognition or that a regulator without homology to PhoB may be involved.
ACKNOWLEDGMENT
This work was supported by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 2.Akiyama M, Crooke E, Kornberg A. An exopolyphosphatase of Escherichia coli. The enzyme and its ppx gene in a polyphosphate operon. J Biol Chem. 1993;268:633–639. [PubMed] [Google Scholar]
- 3.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. I to III. New York, N.Y: Greene Publishing Associates; 1994. [Google Scholar]
- 5.Blum E, Py B, Carpousis A J, Higgins C F. Polyphosphate kinase is a component of the Escherichia coli RNA degradosome. Mol Microbiol. 1997;26:387–398. doi: 10.1046/j.1365-2958.1997.5901947.x. [DOI] [PubMed] [Google Scholar]
- 6.Castuma C E, Huang R, Kornberg A, Reusch R N. Inorganic polyphosphates in the acquisition of competence in Escherichia coli. J Biol Chem. 1995;270:12980–12983. doi: 10.1074/jbc.270.22.12980. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1957;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 8.Deinema M H, Habets L H A, Scholten J, Turkstra E, Webers H A A M. The accumulation of polyphosphate in Acinetobacter spp. FEMS Microbiol Lett. 1980;9:275–279. [Google Scholar]
- 9.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geißdörfer, W. 1997. Unpublished data.
- 11.Geißdörfer W, Ratajczak A, Hillen W. Nucleotide sequence of a putative periplasmic Mn superoxide dismutase from Acinetobacter calcoaceticus ADP1. Gene. 1997;186:305–308. doi: 10.1016/s0378-1119(96)00728-7. [DOI] [PubMed] [Google Scholar]
- 12.Ghuysen J M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen S D. Moraxella, Neisseria, Branhamella, and Acinetobacter. Annu Rev Microbiol. 1976;30:63–83. doi: 10.1146/annurev.mi.30.100176.000431. [DOI] [PubMed] [Google Scholar]
- 16.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 17.Juni E, Janik A. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum) J Bacteriol. 1969;98:281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato J, Yamamoto T, Yamada K, Ohtake H. Cloning, sequence and characterization of the polyphosphate kinase-encoding gene (ppk) of Klebsiella aerogenes. Gene. 1993;137:237–242. doi: 10.1016/0378-1119(93)90013-s. [DOI] [PubMed] [Google Scholar]
- 19.Keasling J D, Bertsch L, Kornberg A. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. Proc Natl Acad Sci USA. 1993;90:7029–7033. doi: 10.1073/pnas.90.15.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keasling J D, Hupf G A. Genetic manipulation of polyphosphate metabolism affects cadmium tolerance in Escherichia coli. Appl Environ Microbiol. 1996;62:743–746. doi: 10.1128/aem.62.2.743-746.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokotek W, Lotz W. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene. 1989;84:467–471. doi: 10.1016/0378-1119(89)90522-2. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulaev I S. The biochemistry of inorganic polyphosphates. New York, N.Y: John Wiley & Sons, Inc.; 1979. [Google Scholar]
- 24.Kulaev I S, Vagabov V M. Polyphosphate metabolism in microorganisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- 25.Kumble K D, Ahn K, Kornberg A. Phosphohistidyl active sites in polyphosphate kinase of Escherichia coli. Proc Natl Acad Sci USA. 1996;93:14391–14395. doi: 10.1073/pnas.93.25.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisser S, Margalit H. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 1993;21:1507–1516. doi: 10.1093/nar/21.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotz, W. 1997. Personal communication.
- 28.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 29.Palmen R, Vosman B, Buijsman P, Breek C K, Hellingwerf K J. Physiological characterization of natural transformation in Acinetobacter calcoaceticus. J Gen Microbiol. 1993;139:295–305. doi: 10.1099/00221287-139-2-295. [DOI] [PubMed] [Google Scholar]
- 30.Rao N N, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J Bacteriol. 1996;178:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao N N, Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990;4:1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 32.Reusch R N, Sadoff H L. Putative structure and functions of a poly-beta-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA. 1988;85:4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schembri M A, Bayly R C, Davies J K. Phosphate concentration regulates transcription of the Acinetobacter polyhydroxyalkanoic acid biosynthetic genes. J Bacteriol. 1995;177:4501–4507. doi: 10.1128/jb.177.15.4501-4507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 37.Spratt B G, Zhou J, Taylor M, Merrick M J. Monofunctional biosynthetic peptidoglycan transglycosylases. Mol Microbiol. 1996;19:639–640. doi: 10.1046/j.1365-2958.1996.442924.x. [DOI] [PubMed] [Google Scholar]
- 38.Strätz M, Mau M, Timmis K N. System to study horizontal gene exchange among microorganisms without cultivation of recipients. Mol Microbiol. 1996;22:207–215. doi: 10.1046/j.1365-2958.1996.00099.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Veen H W, Abee T, Kortstee G J, Konings W N, Zehnder A J. Characterization of two phosphate transport systems in Acinetobacter johnsonii 210A. J Bacteriol. 1993;175:200–206. doi: 10.1128/jb.175.1.200-206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1357–1381. [Google Scholar]
- 41.White P J, Hunter I S, Fewson C A. Codon usage in Acinetobacter structural genes. In: Towner K J, Bergogne-Berezin E, Fewson C A, editors. The biology of Acinetobacter. New York, N.Y: Plenum Press; 1991. pp. 251–257. [Google Scholar]
- 42.Williams J G, Mason P J. Hybridisation in the analysis of RNA. In: Hames B D, Higgins S J, editors. Nucleic acid hybridisation—a practical approach. Oxford, United Kingdom: IRL Press; 1985. pp. 139–160. [Google Scholar]
- 43.Wood H G, Clark J E. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]