Abstract
Background and Objectives
To compare mortality and causes of death in scoliotic children with cerebral palsy (CP) with and without scoliosis surgery.
Methods
National population-based registries were searched for children with CP and scoliosis with and without surgery for scoliosis and were analyzed for comorbidities, mortality, and causes of death.
Results
Two hundred thirty-six had not been operated and 238 had been operated on for scoliosis during the median follow-up of 17.8 (interquartile range [IQR] 11.7–25.7) and 23.0 (IQR 18.4–28.2) years, respectively. Both groups had similar comorbidities. During the follow-up, mortality was higher in the nonsurgically treated group than in the surgically treated group (n = 38/236, 16% and 8.7 per 1,000 follow-up years vs n = 29/238, 12% and 5.3 per 1,000 follow-up years, p = 0.047). In patients with nonsurgical treatment, the cause of death was respiratory in 76.3% (29/38) and 37.9% (11/29) in patients with surgical treatment of scoliosis (6.6 and 2.0 per 1,000 follow-up years, p = 0.002). Neurologic causes of death were more common in surgically treated patients than in nonsurgically treated patients, 44.8% (13/29) and 15.8% (6/38), respectively (3.0 and 1.1 per 1,000 follow-up years, p = 0.009).
Discussion
Surgical treatment of scoliosis associates to reduced mortality because of respiratory causes in children with CP and scoliosis.
Classification of Evidence
This study provides Class IV evidence of the effects of spinal fusion on mortality of children with severe scoliosis due to CP.
Introduction
Scoliosis is common in nonambulatory individuals with cerebral palsy (CP).1 The risk for scoliosis increases with age and increasing neurologic disability. Severe scoliosis exceeding 40° is observed at 20 years of age in 8% of Gross Motor Function Classification System (GMFCS) III, 35% at GMFCS IV, and 75% at GMFCS V.2,3 GMFCS has been reported to be a better predictor for the development of scoliosis than CP-subtype (e.g., spastic, or hypotonic) when estimating the risk of scoliosis on individual level.4
Severe scoliosis associates with abdominal and thoracic crowding, leading to reduced pulmonary function and the risk of pneumonia.5 In CP scoliosis, curves exceeding 40° are considered progressive, necessitating treatment.1,6
Spinal fusion is indicated for 50%–75% of children with CP in CMFCS IV and V and scoliosis.4 Scoliosis surgery aims to prevent curve progression, improve comfort, optimize sitting balance, and facilitate care. Collectively, surgical treatment leads to improved quality of life.7 Although surgery has an overall beneficial effect on the quality of life, it poses significant risks with major postoperative complication rate of 36% and spine-related reoperation rate of 14%.8 These are exacerbated in patients with multiple comorbidities.9 Mean predicted survival after spinal fusion for scoliosis in individuals with CP is 11 years 2 months.10 We aimed to describe mortality and causes of death after scoliosis surgery in individuals with CP compared with nonoperatively treated population of individuals with CP and scoliosis.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study has approval of ethical review board of Helsinki University Hospital and approval of Finnish Institute of Health and Welfare (THL) and Statistics Finland for use of their register data in this study.
Data Sources, Design, and Study Cohort
The study is based on the data of the medical birth register, the care register for health care, and the cause-of-death register maintained by the THL and Statistics Finland. These registers receive information based on a legally compulsory notifications on all health care units in our country. Multiple investigations have validated the accuracy and high coverage of these data.11,12
Selection of Cases and Controls
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), was used during our study period from 1996 to 2021. For our study, the register was searched with CP and scoliosis to identify study population. ICD codes for comorbidities and causes of deaths were compared as indicated in Tables 1 and 2. Procedures were identified according to Nordic Medico-Statistical Committee classification from 1997 to 2021 (Table 1).
Table 1.
Demographics, Comorbidities, and Treatments in Patient Groupsa
| Demographics, comorbidities, and treatments | Unoperated children with CP and scoliosis (n = 236) | Operated children with CP and scoliosis (n = 238) | p Valueb |
| Follow-up, y, mean ± SD | 18.6 ± 8.1 | 22.8 ± 6.6 | <0.001 |
| Age at diagnosis of CP, y, mean ± SD | 3.0 ± 3.6 | 4.2 ± 4.8 | 0.002 |
| Age at diagnosis of scoliosis, y, mean ± SD | 12.1 ± 5.2 | 12.4 ± 4.0 | 0.346 |
| Age at death, y, mean ± SD | 16.3 ± 7.4 | 19.8 ± 5.8 | 0.026 |
| Birth weight, g, mean ± SD | 2,825 ± 1,064 | 2,807 ± 995 | 0.849 |
| Length of gestation, d, mean ± SD | 259 ± 34 | 258 ± 32 | 0.742 |
| Treatment of scoliosis | |||
| Brace treatment | 18.6 (23) | 38.7 (39) | 0.227 |
| Posterior surgery | N/A | 98.3 (234) | N/A |
| Anterior and posterior surgery | N/A | 1.7 (4) | N/A |
| Preoperative or intraoperative halo-traction | N/A | 10.5 (25) | N/A |
| Subtype of CP | |||
| Spastic quadriplegic CP | 21.2 (50) | 24.4 (58) | 0.984 |
| Spastic diplegic CP | 17.8 (42) | 16.8 (40) | 0.497 |
| Spastic hemiplegic CP | 15.7 (37) | 12.2 (29) | 0.158 |
| Dyskinetic CP | 16.1 (38) | 14.3 (34) | 0.902 |
| Atactic CP | 5.9 (14) | 6.3 (15) | 0.852 |
| Other CP | 11 (26) | 11.8 (28) | 0.187 |
| Undefined CP | 12.3 (29) | 14.3 (30) | 0.043 |
| Developmental disability | |||
| Mild developmental disability | 17.8 (42) | 16.0 (38) | 0.120 |
| Moderate developmental disability | 10.2 (24) | 13.0 (31) | 0.854 |
| Severe developmental disability | 19.1 (45) | 29.8 (71) | 0.103 |
| Neurologic comorbidities | |||
| Epilepsy | 68.6 (162) | 68.9 (164) | 0.065 |
| Surgery for hydrocephalus | 41.1 (97) | 40.3 (96) | 0.865 |
| Intrathecal baclofen pump | 1.7 (4) | 4.2 (10) | 0.225 |
| Sleep apnea | 4.7 (11) | 7.1 (17) | 0.547 |
| Respiratory comorbidities | |||
| Pneumonia | 47.9 (113) | 54.2 (129) | 0.527 |
| Asthma | 24.6 (58) | 20.2 (48) | 0.033 |
| Dependence on respirator | 1.3 (3) | 0.4 (1) | 0.221 |
| Chronic respiratory failure | 3.4 (8) | 5.9 (14) | 0.421 |
| Tracheostomy | 2.1 (5) | 4.6 (11) | 0.131 |
| Gastroenterologic comorbidities | |||
| Dysphagia | 10.6 (25) | 14.7 (35) | 0.629 |
| Gastroesophageal reflux | 20.3 (48) | 26.5 (63) | 0.666 |
| Gastrostomy | 32.2 (76) | 34.9 (83) | 0.427 |
| Fundoplication | 5.9 (14) | 3.4 (8) | 0.070 |
| Other comorbidities | |||
| Osteoporotic fracture | 2.5 (6) | 8.8 (21) | 0.016 |
| Malnutrition | 3.4 (8) | 7.1 (17) | 0.184 |
Abbreviations: CP = cerebral palsy; N/A = not applicable.
Values given in percentage and numbers of patients.
Comparison between unoperated and operated patients.
Table 2.
Documented Causes of Deatha
| Cause of death | Deceased unoperated children with CP and scoliosis (n = 38) | Deceased operated children with CP and scoliosis (n = 29) | p Valueb |
| Neurologic causes (all combined) | 15.8 (7) | 44.8 (13) | 0.009 |
| Epilepsy | 5.2 (2) | 10.3 (3) | 0.433 |
| Anoxic brain damage because of inhalation of gastric contents or foreign body | 0 (0) | 17.2 (5) | 0.008 |
| Anoxic brain damage, not classified | 0 (0) | 10.3 (3) | 0.042 |
| CP or unspecified disorder of brain | 13.1 (5) | 13.8 (4) | 0.940 |
| Respiratory causes (all combined) | 76.3 (29) | 37.9 (11) | 0.002 |
| Pneumonia or pneumonitis | 71.0 (27) | 37.9 (11) | 0.007 |
| ARDS or respiratory failure | 5.3 (2) | 0 (0) | 0.210 |
| Other medical causes, accidents or poisoning | 5.3 (2) | 10.3 (3) | 0.433 |
Abbreviations: ARDS = acute respiratory distress syndrome; CP = cerebral palsy.
Values given in percentage and numbers of patients.
Comparison between unoperated on and operated on patients with scoliosis.
Statistical Analysis
All outcomes were calculated per 100 children and per 1,000 follow-up years. Comparisons were made between cases and controls and tested with the test of relative proportion and Student t test. Five-year survival was analyzed for cases and controls by using the Kaplan–Meier survival analysis.
Data Availability
Individualized data cannot be shared because of legal reasons. Statistical data can be received from Finnish Social and Health Data Permit Authority.11
Results
We identified 4,571 children with CP from the registers, of which 474 had also been diagnosed with scoliosis and included in this study: 236 had not been operated on and 238 had been operated on for scoliosis.
Diagnosis of CP was established significantly later in surgically treated children with CP and scoliosis. Estimates for comorbidities such as birth weight and length of gestation were similar in both groups, except asthma was more common in nonsurgically treated patients and osteoporotic fractures were more common in surgically treated patients (Table 1).
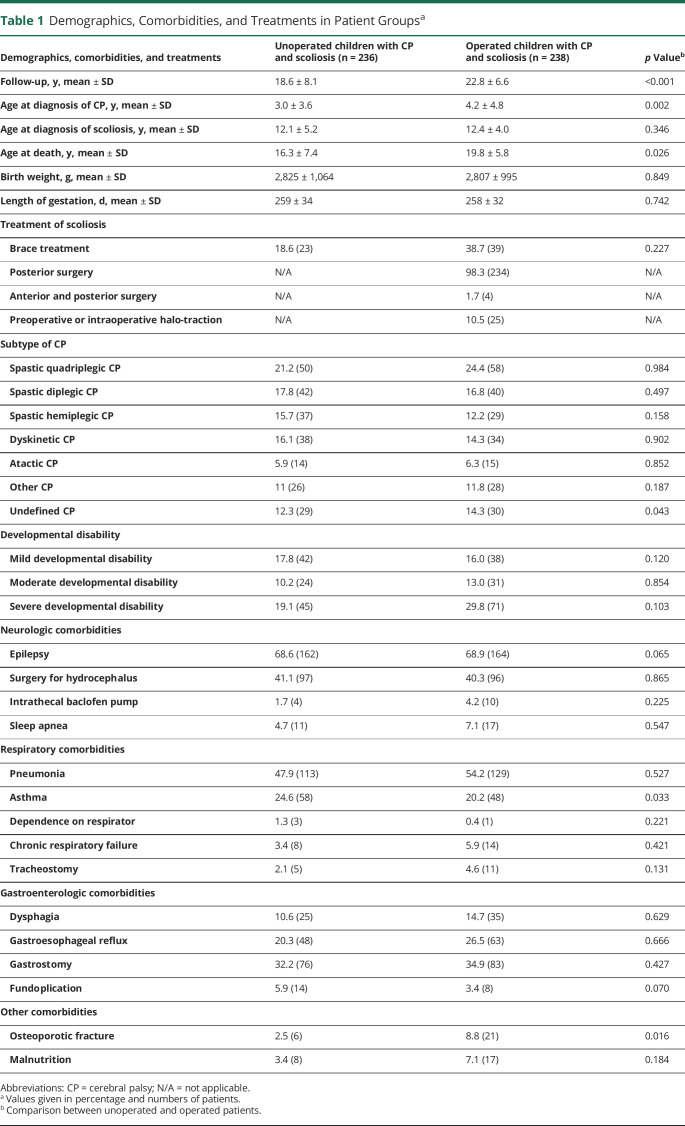
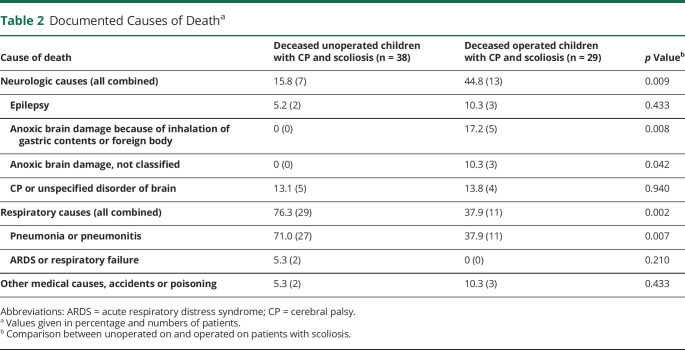
Observed mortality was significantly higher in the nonsurgically treated group than in the surgically treated group (n = 38/236, 16% and 8.7 per 1,000 follow-up years vs n = 29/238, 12% and 5.3 per 1,000 follow-up years, p = 0.047) (Figure, A). The survival difference between nonsurgical and surgical group was 12 percent units for children born 1987–1991 (72% vs 84%), whereas the difference was 5%–6% units for later cohorts, but none of these differences reached statistical significance (Figure, B). The cause of death was respiratory in 76.3% (29/38) in patients with nonsurgical treatment and 37.9% (11/29) in patients with surgical treatment of scoliosis (6.6 and 2.2 per 1,000 follow-up years p = 0.002). Neurologic causes of death were significantly more common in surgically treated patients than in nonsurgically treated patients, 44.8% (13/29) and 15.8% (6/38), respectively (3.0 and 1.1 per 1,000 follow-up years, p = 0.009). We further stratified neurologic causes of death in Table 2. This shows that increase in neurologic causes of death is caused by increase in anoxic brain damage (ICD-10 code G93.1), which was recorded in 8 cases, with an additional code for inhalation of gastric contents or foreign body in 5 cases (Table 2).
Figure. Observed Survival of Patients.
(A) Survival function showing the risk of death in months starting at the age of surgery (median 12.8, interquartile range 9.2–15.2) in surgically treated patients and at the age of diagnosis of scoliosis in nonsurgically treated patients (p = 0.047). (B) The survival difference between nonsurgical and surgical groups during different periods of the study.
Classification of Evidence
This study provides Class IV evidence of the effects of spinal fusion on mortality of children with severe scoliosis due to CP.
Discussion
In this population-based comparative cohort study of 2 similar groups of children with CP and scoliosis, surgical treatment associates with reduced mortality.
Scoliosis results in the deterioration of pulmonary function.1,5,13 The evidence of surgical treatment on pulmonary function in scoliosis has been studied in other patient populations with an evidence of slight increase of forced ventilatory capacity and reduction in loss of lung function because of underlying neuromuscular disorder and progressive scoliosis.13 Owing to difficulties in conducting pulmonary function testing in nonambulatory individuals with CP, data for this patient group are lacking. As an indirect measure of pulmonary function in children with CP, the incidence of pneumonia has been investigated. There are data supporting that the degree of scoliosis does not influence the incidence of pneumonia in this patient population.1 Nevertheless, early intervention by surgical treatment of scoliosis may prevent the development of recurrent pneumonia in children with CP.5 In this investigation, we did not observe benefit from surgery in pneumonia incidence reduction after surgery. Regardless of etiology, severe scoliosis increases mortality because of respiratory failure.1,14
Causes of death in 10- to 19-year-old individuals with CP has been described to be respiratory causes in 52% and neurologic causes 16%.14 In this study, the cause of death was respiratory cause in 76% in nonoperated on children with CP and scoliosis, which is significantly higher than in general CP-population and in individuals with CP, who have been operated on for scoliosis. Although the rate of pulmonary infections is similar in nonoperated on and operated on individuals with CP and scoliosis, the scoliosis operation may protect patients from dying to pulmonary infection. This indicates positive effect of scoliosis surgery on pulmonary function in patients with CP with scoliosis. We observed a significant increase in anoxic brain damage because of aspiration. Scoliosis surgery causes abrupt change in the relative position of abdominal organs. Previously difficulty in swallowing and slowing of gastric emptying has been observed after surgical treatment of scoliosis in children with CP.15 In individuals with CP, this may further increase the risk of inhaling food or gastric contents. According to our data, this may be a significant contributor to mortality in surgically treated individuals with CP.
To our knowledge, this is the first study to compare mortality in surgically treated and untreated individuals with CP and scoliosis. This investigation was conducted using high-quality, unselected population-based nationwide data, which provided sample size large enough to investigate the association of surgical treatment to mortality.
There are several limitations in this investigation: a lack of GMFCS classification, antiepilepsy medication, and radiographs are major limitations, and we do not know how many patients have been deemed not fit for surgery. These leads to uncertainty in the homogeneity of nonsurgically and surgically treated cohorts.
According to this investigation, surgical treatment of scoliosis is associated with lower mortality in individuals with CP and scoliosis than nonsurgical treatment.
Acknowledgment
Prof. Leena Haataja, MD, PhD, Helena Mäenpää, MD, PhD, and Tiina Remes, MD, PhD, are acknowledged for their help and expert advice during the drafting and revision of this article.
Glossary
- CP
cerebral palsy
- GMFCS
Gross Motor Function Classification System
- ICD-10
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- THL
Finnish Institute for Health and Welfare
Appendix. Authors
| Name | Location | Contribution |
| Matti Ahonen, MD, PhD | Helsinki University Hospital, Finland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Ilkka Helenius, MD, PhD | Department of Orthopaedics and Traumatology, University of Helsinki and Helsinki University Hospital, Finland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Mika Gissler, DPhil | THL Finnish Institute for Health and Welfare, Helsinki, Finland | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Ira Jeglinsky-Kankainen, PhD | Department of Health and Welfare, Arcada University of Applied Sciences, Helsinki, Finland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by NordForsk (grant no. 82866).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351(9112):1687-1692. [DOI] [PubMed] [Google Scholar]
- 2.Hägglund G, Pettersson K, Czuba T, Persson-Bunke M, Rodby-Bousquet E. Incidence of scoliosis in cerebral palsy. Acta Orthop. 2018;89(4):443-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and validation of a gross motor function classification system for children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. [DOI] [PubMed] [Google Scholar]
- 4.Persson-Bunke M, Hägglund G, Lauge-Pedersen H, Wagner P, Westbom L. Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976). 2012;37(12):E708-E713. [DOI] [PubMed] [Google Scholar]
- 5.Keskinen H, Lukkarinen H, Korhonen K, Jalanko T, Koivusalo A, Helenius I. The lifetime risk of pneumonia in patients with neuromuscular scoliosis at a mean age of 21 years: the role of spinal deformity surgery. J Child Orthop. 2015;9(5):357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy JJ, D'Andrea LP, Betz RR, Clements D. Scoliosis in the child with cerebral palsy. J Am Acad Orthop Surg. 2006;14(6):367-375. [DOI] [PubMed] [Google Scholar]
- 7.Miyanji F, Nasto L, Sponseller P, et al. Assessing the risk-benefit ratio of scoliosis surgery in cerebral palsy: surgery is worth it. J Bone Joint Surg. 2018;100(7):556-563. [DOI] [PubMed] [Google Scholar]
- 8.Yaszay B, Bartley CE, Sponseller PD, et al. Major complications following surgical correction of spine deformity in 257 patients with cerebral palsy. Spine Deform. 2020;8(6):1305-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry JG, Glotzbecker M, Rodean J, et al. Comorbidities and complications of spinal fusion for scoliosis. Pediatrics. 2017;139(3):e20162574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsirikos AI, Chang WN, Dabney KW, Freeman M, Glutting J. Life expectancy in pediatric patients with cerebral palsy and neuromuscular scoliosis who underwent spinal fusion. Dev Med Child Neurol. 2003;45(10):677-682. [DOI] [PubMed] [Google Scholar]
- 11.Finnish Social and Health Data Permit Authority Findata. Updated June 19, 2023. Accessed June 19, 2023. findata.fi/en.
- 12.Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JH, Kim KS, Lee GH, Kim HS. Comparison of survival analysis between surgical and non-surgical treatments in Duchenne muscular dystrophy scoliosis. Spine J. 2020;20(11):1840-1849. [DOI] [PubMed] [Google Scholar]
- 14.Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Dev Med Child Neurol. 2015;57(8):762-767. [DOI] [PubMed] [Google Scholar]
- 15.Jalanko T, Helenius I, Pakarinen M, et al. Effects of surgical correction of neuromuscular scoliosis on gastric myoelectrical activity, emptying, and upper gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2014;58(1):38-45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individualized data cannot be shared because of legal reasons. Statistical data can be received from Finnish Social and Health Data Permit Authority.11