Abstract
Background and Objectives
Studies on the association between proton pump inhibitor (PPI) use and dementia report mixed results and do not examine the impact of cumulative PPI use. We evaluated the associations between current and cumulative PPI use and risk of incident dementia in the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
These analyses used participants from a community-based cohort (ARIC) from the time of enrollment (1987–1989) through 2017. PPI use was assessed through visual medication inventory at clinic visits 1 (1987–1989) to 5 (2011–2013) and reported annually in study phone calls (2006–2011). This study uses ARIC visit 5 as baseline because this was the first visit in which PPI use was common. PPI use was examined 2 ways: current use at visit 5 and duration of use before visit 5 (from visit 1 to 2011, exposure categories: 0 day, 1 day–2.8 years, 2.8–4.4 years, >4.4 years). The outcome was incident dementia after visit 5. Cox proportional hazard models were used, adjusted for demographics, comorbid conditions, and other medication use.
Results
A total of 5,712 dementia-free participants at visit 5 (mean age 75.4 ± 5.1 years; 22% Black race; 58% female) were included in our analysis. The median follow-up was 5.5 years. The minimum cumulative PPI use was 112 days, and the maximum use was 20.3 years. There were 585 cases of incident dementia identified during follow-up. Participants using PPIs at visit 5 were not at a significantly higher risk of developing dementia during subsequent follow-up than those not using PPIs (hazard ratio (HR): 1.1 [95% confidence interval (CI) 0.9–1.3]). Those who used PPIs for >4.4 cumulative years before visit 5 were at 33% higher risk of developing dementia during follow-up (HR: 1.3 [95% CI 1.0–1.8]) than those reporting no use. Associations were not significant for lesser durations of PPI use.
Discussion
Future studies are needed to understand possible pathways between cumulative PPI use and the development of dementia.
Classification of Evidence
This study provides Class III evidence that the use of prescribed PPIs for >4.4 years by individuals aged 45 years and older is associated with a higher incidence of newly diagnosed dementia.
Introduction
Proton pump inhibitors (PPIs), available through prescription and over the counter, are currently the first-line therapy for the short-term treatment (4–8 weeks) of gastroesophageal reflux disease (GERD) and peptic ulcers.1-4 In a study based on US emergency department visits, PPI use increased from 4% to 9% from 2002 to 2009.5 PPIs were dispensed more than 115 million times in 2016.6 In addition, up to 63% of PPI prescriptions did not have a documented gastrointestinal diagnosis and may have been inappropriately prescribed.5,7 Long-term use of PPIs has not been approved; nevertheless, chronic PPI use is common.8
Chronic PPI use has been linked to numerous health conditions such as stroke, cardiovascular disease, chronic kidney disease, and dementia.1,2 Previous studies on the relationship between PPI use and dementia report mixed results.9-15 Two recent meta-analyses report no association among 7 case-control/cohort studies (pooled HR: 1.10 [95% CI 0.88–1.37])16 and 8 cohort studies (pooled risk ratio (RR): 1.17 [95% CI 0.95–1.44]).17 However, precision was somewhat poor, and the heterogeneity of the included studies was high (I2 = 95%–96%).16,17 Furthermore, there were numerous limitations including suboptimal generalizability. Study participants were predominantly White or Asian. Another limitation was that in approximately half the studies, dementia diagnosis was defined entirely by ICD codes, and these are known to have poor sensitivity. Finally, a majority of the studies did not account for intraindividual variation in PPI use over follow-up, but instead defined the exposure as short-term or any use of PPIs during follow-up. Examining long-term or cumulative exposure to PPIs may be necessary due to the long latency period of dementia. Our primary research hypotheses tested in this study are whether current and greater cumulative exposure to prescribed PPIs would be associated with a higher risk of incident dementia in the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study Population and Design
The ARIC study is a population-based cohort of adults recruited from 4 US communities: Washington County, MD; Forsyth County, NC; selected suburbs of Minneapolis, MN; and Jackson, MS. In 1987–1989 (visit 1), the ARIC study recruited and examined 15,792 men and women aged 45–64 years, who predominantly identified as Black or White. Participants attended multiple in-person clinic visits: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), visit 5 (2011–2013), visit 6 (2016–2017), and visit 7 (2018–2019). Participants were also contacted annually by telephone from study baseline until 2011 and then twice-yearly thereafter.18,19
ARIC visit 5 (2011–2013), attended by 6,538 participants, was the first study visit in which PPI use was common. Therefore, ARIC visit 5 served as the baseline visit for this study. All participants who were dementia-free at visit 5 were included for analysis (n = 347 cases with prevalent dementia). Participants who attended visit 5 before the 2011 annual phone call were excluded from analysis (n = 30) due to complications of calculating cumulative exposure. Participants missing covariates of interest were excluded. A total of 5,712 participants were included for the current PPI use and cumulative exposure to PPI analyses (Figure).
Figure. Flowchart of Participants in the Atherosclerosis Risk in Communities Study, 2011–2017.
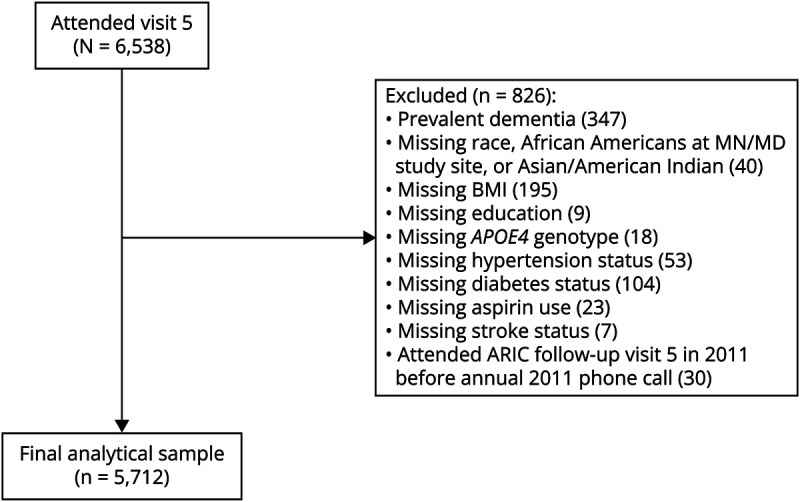
APOE4 = Apolipoprotein E4; BMI = body mass index.
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Boards of the collaborating institutions approved ARIC, and all participants gave written informed consent.
Measurement of Exposure
Participants were asked to bring to in-person clinic visits all over the counter and prescription medications used during the preceding 2 weeks. Medications were inventoried by trained staff. PPI use was also ascertained from annual phone calls (contact years 2006–2011) during which participants were asked to assemble all current medications and “read the names of all the medications prescribed by a doctor.” Over-the-counter medications not prescribed by a doctor were excluded. The first PPI drug became available over the counter in 2003.
Two exposures of PPIs were evaluated. Current exposure to PPIs was defined as use of PPIs at visit 5. Those who were not using PPIs at visit 5 served as the reference group. Cumulative exposure to PPIs was defined as years of use from date of visit 1 examination to date of the annual phone call in 2011. If a participant was taking a PPI at a visit or call, they were assumed to be taking it until the next visit. If information was missing for a given call or visit, the last observation carried forward method was used. Cumulative PPI use was the sum of all PPI use from visit 1 (pre-FDA approval of PPIs) to 2011 (before ascertainment at visit 5). Participants who had never used PPIs before visit 5, and did not use PPIs at the time of visit 5, served as the reference group. The remaining observations were categorized into tertiles of cumulative PPI use. Thirty participants were excluded from analyses because they had participated in visit 5 in 2011 and did not have their annual follow-up call before their visit. For ease of defining the population, we excluded these 30 participants from both analyses of PPI exposures.
Histamine2 receptor antagonists (H2RAs) are an alternative class of medication used to treat GERD and other gastric acid–related disorders. In a secondary analyses, we examined H2RA use as an active comparator. Assessment of H2RAs was similar to that of PPIs, including both through in-person medication inventory and regular phone calls. H2RAs were categorized into the same tertiles of cumulative exposure as PPIs for consistency.
Ascertainment of Outcome
Following a previous ARIC study,20 incident dementia was ascertained using information from 3 sources: (1) for those who completed in-person examinations at visits 6 (2016–2017) and 7 (2018–2019), dementia status was ascertained through neuropsychological examination, (2) twice-yearly participants were called by telephone and the Six-Item Screener was conducted, with follow-up proxy completion of the AD8 screening tool when appropriate, and (3) surveillance through hospital discharge codes and death records, which were gathered as part of the cohort's standard procedures. Suspected cases with dementia were adjudicated by a panel of physicians and neuropsychologists who classified both dementia status (yes/no) and estimated date at onset.
Other Covariates
Covariates in this analysis were assessed at ARIC visit 5 (baseline) unless otherwise stated and included educational attainment (ARIC visit 1), sex, age, smoking status, race, and ARIC center. Use of antihypertensives, H2RAs, anticholinergics, aspirin, and vitamin B12 use were assessed through in-person medication inventory at visit 5. Body mass index (BMI; in kg/m2) was computed from weight and standing height. BMI was categorized into underweight/normal weight, overweight, and obese categories. Very few individuals were in the underweight BMI category. Sitting blood pressure was measured 3 times using a random-zero sphygmomanometer after a 5-minute rest, and the average of the final 2 measurements was used. Hypertension was defined as current use of antihypertensive medication, systolic blood pressure (BP) > 140 mm Hg, or diastolic BP > 90 mm Hg. Diabetes was defined as treatment for diabetes, a fasting glucose level of ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, and/or a self-reported history of a physician diagnosis of diabetes. APOE4 genotyping has been previously described.21 APOE4 was coded as either present, not present, or missing if the participant requested not to have genotyping performed.
Statistical Analysis
Baseline covariates were examined by cumulative PPI exposure categories. Cox proportional hazard models estimated the association of PPI use and risk of incident dementia. We examined PPI exposure as current PPI use and by categories of cumulative PPI use. Follow-up time began at visit 5 and continued until incident dementia, death, loss to follow-up, or end of the follow-up period (December 31, 2017), whichever came first. Models for current PPI use adjusted for the following: model 1: age, sex, race center, education, and APOE4 genotype; model 2: model 1 + body mass index; and model 3: model 2 + current H2RA use, current aspirin use, diabetes mellitus, stroke, and hypertension. Model 3 was not adjusted for anticholinergic use due to small cell sizes. Interaction terms for age, sex, and BMI with PPI use were added to the fully adjusted models to evaluate whether these characteristics modified the association. We also examined whether the addition of participant B12 medication use to model 3 changed our observed results.
Secondary analyses used H2RA use as an active comparator. This design compared PPI use with H2RA use, another drug commonly used for the same indication. The analysis included only individuals who were using either PPIs or H2RAs. The same general methods were used as in the primary analyses. For the current use analysis, the exposure was defined as PPI use at visit 5 compared with use of H2RAs at visit 5. For the cumulative use analysis, due to less frequent use of H2RAs, the exposure was defined as any use of PPIs before visit 5 compared with any use of H2RAs before visit 5. Sensitivity analyses assessed the individual time lengths (i.e., >4.4 cumulative years of PPI use vs > 4.4 cumulative years of H2RA use). Analyses were conducted in RStudio version 3.6.1.22
Data Availability
Qualified researchers may obtain access to ARIC study data through the NIH National Heart, Lung, and Blood Institute (NHLBI) BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/).
Results
A total of 6,538 participants attended visit 5. Of them, 796 participants were excluded from the current PPI use analysis for the following reasons: prevalent dementia at visit 5 [n = 347], were of Black race at MN or MD study site or were Asian or Native American [n = 40; excluded because of small numbers], missing data at visit 5, including BMI [n = 195], education [n = 9], APOE4 genotype [n = 18], hypertension status [n = 53], diabetes status [n = 104], aspirin use [n = 23], and stroke status [n = 7]. An additional 30 participants were excluded from the cumulative PPI use analysis for having visit 5 before the 2011 annual phone call, due to complications in calculating cumulative exposure when this happened. These participants would have had their exposure calculated after the baseline visit (visit 5). For consistency, 5,712 participants were used for the cumulative PPI use and current PPI use analyses.
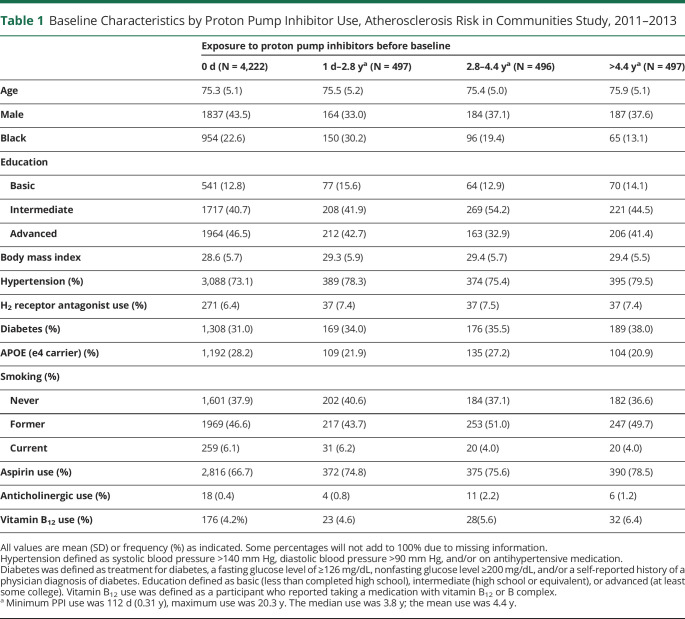
The median follow-up was 5.5 years. A total of 585 (10.2%) participants developed dementia over follow-up. There were 1,490 cumulative PPI users (26.1%) from visit 1 to 2011 and 1,450 current PPI users (25.4%) at visit 5. Cumulative PPI use ranged from 112 days to 20.3 years by 2011. (the median use was 3.8 years; interquartile range 2.2–5.1 years; mean use was 4.4 years) Baseline characteristics of cumulative PPI users and nonusers are summarized in Table 1. Current PPI users had a mean age of 75.6 ± 5.1 years, similar to 75.3 ± 5.1 years among nonusers (p > 0.05). Users were more likely to be female, of White race, have HTN and diabetes, and not have the APOE4 genotype compared with nonusers.
Table 1.
Baseline Characteristics by Proton Pump Inhibitor Use, Atherosclerosis Risk in Communities Study, 2011–2013
| Exposure to proton pump inhibitors before baseline | ||||
| 0 d (N = 4,222) | 1 d–2.8 ya (N = 497) | 2.8–4.4 ya (N = 496) | >4.4 ya (N = 497) | |
| Age | 75.3 (5.1) | 75.5 (5.2) | 75.4 (5.0) | 75.9 (5.1) |
| Male | 1837 (43.5) | 164 (33.0) | 184 (37.1) | 187 (37.6) |
| Black | 954 (22.6) | 150 (30.2) | 96 (19.4) | 65 (13.1) |
| Education | ||||
| Basic | 541 (12.8) | 77 (15.6) | 64 (12.9) | 70 (14.1) |
| Intermediate | 1717 (40.7) | 208 (41.9) | 269 (54.2) | 221 (44.5) |
| Advanced | 1964 (46.5) | 212 (42.7) | 163 (32.9) | 206 (41.4) |
| Body mass index | 28.6 (5.7) | 29.3 (5.9) | 29.4 (5.7) | 29.4 (5.5) |
| Hypertension (%) | 3,088 (73.1) | 389 (78.3) | 374 (75.4) | 395 (79.5) |
| H2 receptor antagonist use (%) | 271 (6.4) | 37 (7.4) | 37 (7.5) | 37 (7.4) |
| Diabetes (%) | 1,308 (31.0) | 169 (34.0) | 176 (35.5) | 189 (38.0) |
| APOE (e4 carrier) (%) | 1,192 (28.2) | 109 (21.9) | 135 (27.2) | 104 (20.9) |
| Smoking (%) | ||||
| Never | 1,601 (37.9) | 202 (40.6) | 184 (37.1) | 182 (36.6) |
| Former | 1969 (46.6) | 217 (43.7) | 253 (51.0) | 247 (49.7) |
| Current | 259 (6.1) | 31 (6.2) | 20 (4.0) | 20 (4.0) |
| Aspirin use (%) | 2,816 (66.7) | 372 (74.8) | 375 (75.6) | 390 (78.5) |
| Anticholinergic use (%) | 18 (0.4) | 4 (0.8) | 11 (2.2) | 6 (1.2) |
| Vitamin B12 use (%) | 176 (4.2%) | 23 (4.6) | 28(5.6) | 32 (6.4) |
All values are mean (SD) or frequency (%) as indicated. Some percentages will not add to 100% due to missing information.
Hypertension defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, and/or on antihypertensive medication.
Diabetes was defined as treatment for diabetes, a fasting glucose level of ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, and/or a self-reported history of a physician diagnosis of diabetes. Education defined as basic (less than completed high school), intermediate (high school or equivalent), or advanced (at least some college). Vitamin B12 use was defined as a participant who reported taking a medication with vitamin B12 or B complex.
Minimum PPI use was 112 d (0.31 y), maximum use was 20.3 y. The median use was 3.8 y; the mean use was 4.4 y.
Current use of PPIs at visit 5 was not associated with an increased risk of incident dementia in the minimally adjusted (hazard ratio (HR): 1.05 [95% CI 0.87–1.26]) or fully adjusted models (HR: 1.09 [95% CI 0.90–1.31]) (Table 2). H2RAs were not significantly associated with an increased risk in dementia in the fully adjusted model (HR: 1.30 [95% CI 0.97–1.75]).
Table 2.
Hazard Ratios of Incident Dementia by Current Proton Pump Inhibitor Use: The Atherosclerosis Risk in Communities Study, 2011–2017
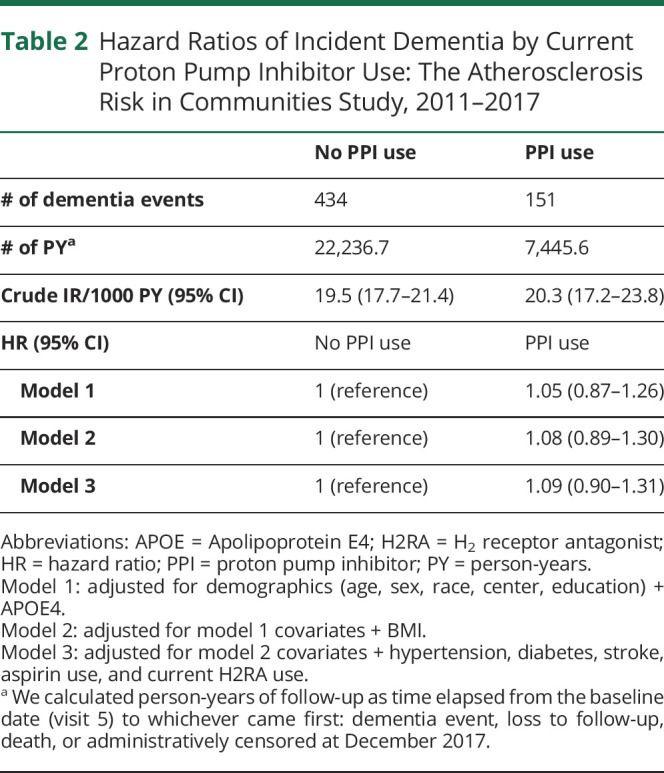
| No PPI use | PPI use | |
| # of dementia events | 434 | 151 |
| # of PYa | 22,236.7 | 7,445.6 |
| Crude IR/1000 PY (95% CI) | 19.5 (17.7–21.4) | 20.3 (17.2–23.8) |
| HR (95% CI) | No PPI use | PPI use |
| Model 1 | 1 (reference) | 1.05 (0.87–1.26) |
| Model 2 | 1 (reference) | 1.08 (0.89–1.30) |
| Model 3 | 1 (reference) | 1.09 (0.90–1.31) |
Abbreviations: APOE = Apolipoprotein E4; H2RA = H2 receptor antagonist; HR = hazard ratio; PPI = proton pump inhibitor; PY = person-years.
Model 1: adjusted for demographics (age, sex, race, center, education) + APOE4.
Model 2: adjusted for model 1 covariates + BMI.
Model 3: adjusted for model 2 covariates + hypertension, diabetes, stroke, aspirin use, and current H2RA use.
We calculated person-years of follow-up as time elapsed from the baseline date (visit 5) to whichever came first: dementia event, loss to follow-up, death, or administratively censored at December 2017.
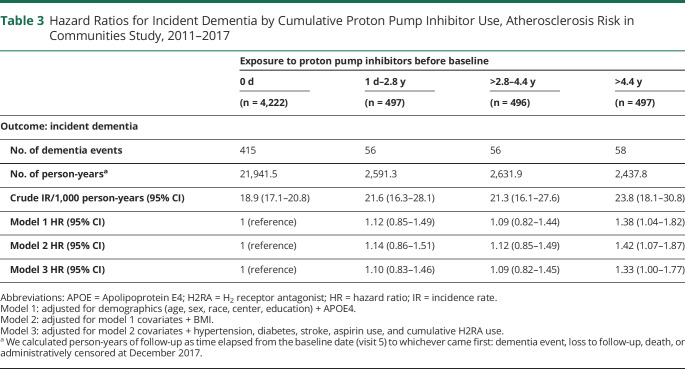
After minimal adjustment, participants who used PPIs for more than 4.4 cumulative years were at a 38% higher risk of developing dementia later in life (HR: 1.38 [95% CI 1.04–1.82]) compared with those who had never used PPIs (Table 3). The association persisted after full adjustment (HR: 1.33 [95% CI 1.00–1.77]). Short-term (1 day–2.8 cumulative years) and intermediate (>2.8–4.4 cumulative years) PPI users had a nonsignificant higher risk of developing dementia (Table 3). There were no significant interactions of cumulative PPI use by age, sex, or BMI category. The addition of participant B12 use as an explanatory covariate to model 3 did not change the results summarized in Tables 2 and 3.
Table 3.
Hazard Ratios for Incident Dementia by Cumulative Proton Pump Inhibitor Use, Atherosclerosis Risk in Communities Study, 2011–2017
| Exposure to proton pump inhibitors before baseline | ||||
| 0 d | 1 d–2.8 y | >2.8–4.4 y | >4.4 y | |
| (n = 4,222) | (n = 497) | (n = 496) | (n = 497) | |
| Outcome: incident dementia | ||||
| No. of dementia events | 415 | 56 | 56 | 58 |
| No. of person-yearsa | 21,941.5 | 2,591.3 | 2,631.9 | 2,437.8 |
| Crude IR/1,000 person-years (95% CI) | 18.9 (17.1–20.8) | 21.6 (16.3–28.1) | 21.3 (16.1–27.6) | 23.8 (18.1–30.8) |
| Model 1 HR (95% CI) | 1 (reference) | 1.12 (0.85–1.49) | 1.09 (0.82–1.44) | 1.38 (1.04–1.82) |
| Model 2 HR (95% CI) | 1 (reference) | 1.14 (0.86–1.51) | 1.12 (0.85–1.49) | 1.42 (1.07–1.87) |
| Model 3 HR (95% CI) | 1 (reference) | 1.10 (0.83–1.46) | 1.09 (0.82–1.45) | 1.33 (1.00–1.77) |
Abbreviations: APOE = Apolipoprotein E4; H2RA = H2 receptor antagonist; HR = hazard ratio; IR = incidence rate.
Model 1: adjusted for demographics (age, sex, race, center, education) + APOE4.
Model 2: adjusted for model 1 covariates + BMI.
Model 3: adjusted for model 2 covariates + hypertension, diabetes, stroke, aspirin use, and cumulative H2RA use.
We calculated person-years of follow-up as time elapsed from the baseline date (visit 5) to whichever came first: dementia event, loss to follow-up, death, or administratively censored at December 2017.
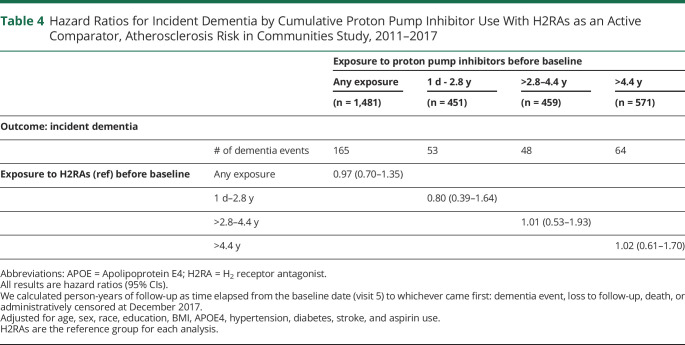
There was no significant difference between risk of dementia and PPIs when H2RAs were used as an active comparator for both cumulative (Table 4) and current exposure (HR: 0.83 [0.58–1.18])
Table 4.
Hazard Ratios for Incident Dementia by Cumulative Proton Pump Inhibitor Use With H2RAs as an Active Comparator, Atherosclerosis Risk in Communities Study, 2011–2017
| Exposure to proton pump inhibitors before baseline | |||||
| Any exposure | 1 d - 2.8 y | >2.8–4.4 y | >4.4 y | ||
| (n = 1,481) | (n = 451) | (n = 459) | (n = 571) | ||
| Outcome: incident dementia | |||||
| # of dementia events | 165 | 53 | 48 | 64 | |
| Exposure to H2RAs (ref) before baseline | Any exposure | 0.97 (0.70–1.35) | |||
| 1 d–2.8 y | 0.80 (0.39–1.64) | ||||
| >2.8–4.4 y | 1.01 (0.53–1.93) | ||||
| >4.4 y | 1.02 (0.61–1.70) | ||||
Abbreviations: APOE = Apolipoprotein E4; H2RA = H2 receptor antagonist.
All results are hazard ratios (95% CIs).
We calculated person-years of follow-up as time elapsed from the baseline date (visit 5) to whichever came first: dementia event, loss to follow-up, death, or administratively censored at December 2017.
Adjusted for age, sex, race, education, BMI, APOE4, hypertension, diabetes, stroke, and aspirin use.
H2RAs are the reference group for each analysis.
Discussion
In this community-based cohort, participants with long-term cumulative (>4.4 years) use of PPIs from mid-to-late life had a modestly higher risk of dementia in late life compared with nonusers. Shorter-term use in midlife and current use in late life were not associated with increased risk of dementia in late life.
Previous cohort studies found mixed results when assessing the relationship between time-varying PPI use and risk of dementia or Alzheimer disease. Two heterogeneous meta-analyses report no significant association.16,17 The follow-up time of the included cohort studies was similar to the follow-up of our study (1.5–8.4 years). Time-varying PPI use in most of the studies was defined as users vs nonusers. In our analysis, the risk of dementia among current PPI users is in agreement with the results of these meta-analyses.
Few previous studies have taken into account the long latency of dementia. A Finnish nationwide nested case-control study found no association for risk of Alzheimer disease after a 3-year lag window (odds ratio [OR]: 1.03 [95% CI 1.00–1.05]).23 In addition, it found no association with ≥3 years of PPI use (OR: 0.99 [95% CI 0.94–1.04]).23 A US cohort study also found a null association between daily PPI dose dispensed in the previous 10 years and risk of dementia during follow-up (HR 1.13, 95% CI 0.82–1.56).10 Our study reports that long-term previous cumulative use (>4.4 years) was significantly associated with developing dementia. While we did not incorporate a long lag window, or define PPI dosage, we aimed to capture total use before baseline among a dementia-free population.
The underlying mechanism between PPIs and dementia has been postulated through 2 plausible pathways: vitamin B12 deficiency and impaired amyloid metabolism.24 A previous study25 reported a significant association between PPI use and low levels of vitamin B12. Low vitamin B12 levels are associated with a decline in cognition.26 We are unable to comment on participant B12 levels in our dataset. However, adjusting for participant baseline B12 use did not alter the relation between PPI use and dementia in our data. In experimental mice models, PPI use has been associated with an increase in β-amyloid levels in the brain.24,27 PPIs may modify the γ-secretase enzyme, which cleaves a precursor protein to β-amyloid. This contributes to the development of Alzheimer disease by increasing β-amyloid plaques within the brain.24 Another pathway is through vascular causes. PPIs have been implicated in the development of other health outcomes including stroke28,29 and chronic kidney disease (CKD).30,31 Studies report that CKD and stroke patients are at higher risk of developing dementia.32-37 The microbiota-gut-brain axis is another postulated mediating pathway between cumulative PPI use and cognitive outcomes. PPI use has been associated with significant changes to the gut microbiome possibly driven by hypochlorhydria.38 Specific gut microbiota changes reported with PPI use include an overall decrease in microbial diversity and dysbiosis or alterations in the composition of the microbiome. In turn, dysbiosis and decreased microbial diversity have been reported in patients with Alzheimer disease and in animal models of dementia.39,40 Specific pathways postulated to link gut dysbiosis and Alzheimer disease include neuroinflammation, oxidative stress, and triggering of amyloid-beta aggregation.39,40 A different consideration is the use of PPI as part of quadruple therapy for eradicating Helicobacter pylori infection. H pylori, a gastric pathogen, has been shown to be associated with dementia in both cohort and case-control studies, though all data are still observational.41-43 The diagnosis of H pylori in these studies is based on seropositivity, and the timing of H pylori infection and dementia onset is not well characterized. The duration of quadruple therapy is typically 14 days. Hence, PPI use in this context is short-term. Our data suggest an association with cumulative long-term use (>4.4 years). Furthermore, we lack information on the indications for PPI use in our dataset. It is possible that the relationship between PPI use and dementia is mediated by 1 or more of these pathways. Future studies assessing potential mediators in long-term cumulative PPI use and risk of dementia is warranted.
Active comparator designs reduce the risk of unmeasured confounding.44 In active comparator studies, the comparison group is another drug of interest instead of a “no-use” comparison group.44 This form of study design offers advantages such as reduced unmeasured confounding and has increased potential for overlapping characteristics between groups.44 As a secondary analysis, we examined the relationship between PPIs and dementia with H2RAs as an active comparator. Greater than 4.4 cumulative years of exposure to PPIs was not associated with greater risk when compared with use of H2RAs. Additionally, there was no association with shorter durations or current use. Few prior studies on the association of PPIs and risk of dementia have considered H2RAs.12 However, a previous study found that H2RAs but not PPIs increased the risk of cognitive impairment among Black participants.45 This study is in contrast with our findings and the hypothesized association.
Our study has several strengths. We have physician-adjudicated cases of dementia identified through ICD codes, screening, and cognitive functioning tests. We were able to capture PPI use since the beginning of the FDA approval for various PPI drugs. In addition, we assessed the association of PPI use and dementia among a community-based cohort of Black and White participants. Our study was more heterogeneous in race (22% Black race) compared with previous studies10,12 conducted within the United States. Finally, we adjusted for the APOE4 genotype.
We acknowledge the following limitations. While we were able to adjust for many potential confounders, residual confounding may still be an issue, and therefore causal inference is limited. We attempted to adjust for anticholinergic medication use due to the reported associations between anticholinergic medication use and cognitive impairment.46-48 We were, nevertheless, unable to adjust for anticholinergic use because <2% of the total sample used anticholinergics at visit 5. We could not continuously measure PPI use and used the last observation carried forward method to capture all use over a given year. Participants were called annually and may have ended and reinitiated use more than once throughout the contact year. Therefore, we may have misestimated our days of exposure. In addition, we cannot be certain that everyone in our control group did not take PPIs due their over-the-counter availability. ARIC participants were asked to not include over-the-counter medications unless they were prescribed by a doctor. Our study sample included only individuals in the United States who self-identified as Black or White and therefore may not be generalizable to other populations. We also excluded participants with missing covariate information on variables known to be associated with dementia risk including diabetes mellitus, hypertension, and BMI.49 We believe that these data are missing at random—hence, we do not expect that the results were biased by their absence. Nevertheless, these covariates are associated with the outcome of interest, and this is a limitation. Many ARIC participants died before visit 5, and this could be considered as a source of selection bias. We argue that our sample would be expected to be representative of living older adults.
In summary, we found a nonsignificant association between current use of PPIs and risk of dementia over a median 5.5 years of follow-up. However, long-term cumulative users of PPIs had a 33% increased risk of developing dementia in late life. Future studies should explore possible pathways or mediators between PPI use and the development of dementia.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CKD
chronic kidney disease
- GERD
gastroesophageal reflux disease
- H2RAs
Histamine2 receptor antagonists
- HR
hazard ratio
- NHLBI
National Heart, Lung, and Blood Institute
- PPIs
proton pump inhibitors
Appendix. Authors

| Name | Location | Contribution |
| Carin A. Northuis, MPH, PhD | University of Minnesota | Drafting/revision of the article for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Elizabeth J. Bell, MPH, PhD | Optum | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Pamela L. Lutsey, MPH, PhD | University of Minnesota | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Kristen M. George, MPH, PhD | University of California, Davis | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Rebecca F. Gottesman, MD, PhD | National Institute of Neurological Disorders and Stroke | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Tom H. Mosley, PhD | University of Mississippi Medical Center | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Eric A. Whitsel, MPH, MD | UNC | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Kamakshi Lakshminarayan, MD, PhD | University of Minnesota | Drafting/revision of the article for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The Atherosclerosis Risk in Communities Study is conducted as a collaborative study supported by NIH National Heart, Lung, and Blood Institute (NHLBI) contracts (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, and 75N92022D00005). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. This work was also supported by NHLBI training grant T32 HL007779 to C.A. Northuis and K24 HL159246 to P.L. Lutsey.
Disclosure
C.A. Northuis reports no disclosures relevant to the manuscript; E.J. Bell is employed by Optum, and she has received funding Novartis, Incyte, AstraZeneca, EMD Serono, Sandoz, Celgene, and Pfizer; P.L. Lutsey receives NIH funding; K.M. George reports no disclosures relevant to the manuscript; R.F. Gottesman is supported by the NIH intramural program; T.H. Mosley receives NIH funding; E.A. Whitsel reports no disclosures relevant to the manuscript; K. Lakshminarayan receives NIH funding. Go to Neurology.org/N for full disclosures.
References
- 1.Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. 2017;8(9):273-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2019;10:2042098618809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328. quiz 329. [DOI] [PubMed] [Google Scholar]
- 4.Farrell B, Pottie K, Thompson W, et al. . Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354-364. [PMC free article] [PubMed] [Google Scholar]
- 5.Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One. 2013;8(2):e56060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Total purchases by prescribed drug, United States, 2016. Medical Expenditure Panel Survey. 2016. Accessed January 2, 2022. datatools.ahrq.gov/meps-hc?type=tab&tab=mepshcpd. [Google Scholar]
- 7.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228-e234. [PubMed] [Google Scholar]
- 8.Wallerstedt SM, Fastbom J, Linke J, Vitols S. Long-term use of proton pump inhibitors and prevalence of disease- and drug-related reasons for gastroprotection-a cross-sectional population-based study. Pharmacoepidemiol Drug Saf. 2017;26(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang ST, Tseng LY, Chen LK, Peng LN, Hsiao FY. Does long-term proton pump inhibitor use increase risk of dementia? Not really! Results of the group-based trajectory analysis. Clin Pharmacol Ther. 2019;106(3):616-622. [DOI] [PubMed] [Google Scholar]
- 10.Gray SL, Walker RL, Dublin S, et al. . Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc. 2018;66(2):247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang IC, Chang J, Park SM. A nationwide population-based cohort study of dementia risk among acid suppressant users. Am J Geriatr Psychiatry. 2018;26(11):1175-1183. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65(9):1969-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomm W, von Holt K, Thomé F, et al. . Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416. [DOI] [PubMed] [Google Scholar]
- 14.Haenisch B, von Holt K, Wiese B, et al. . Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428. [DOI] [PubMed] [Google Scholar]
- 15.Tai SY, Chien CY, Wu DC, et al. . Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 2017;12(2):e0171006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Yuan Y, Iqbal U, et al. . No association linking short-term proton pump inhibitor use to dementia: systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2020;115(5):671-678. [DOI] [PubMed] [Google Scholar]
- 17.Hussain S, Singh A, Zameer S, et al. . No association between proton pump inhibitor use and risk of dementia: evidence from a meta-analysis. J Gastroenterol Hepatol. 2020;35(1):19-28. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis risk in communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 19.Wright JD, Folsom AR, Coresh J, et al. . The ARIC (Atherosclerosis risk in communities) study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, Gottesman RF, Sharrett AR, et al. . Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volcik KA, Barkley RA, Hutchinson RG, et al. . Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol. 2006;164(4):342-348. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing, R version 3.6.1. R Foundation for Statistical Computing; 2019. [computer program]. [Google Scholar]
- 23.Taipale H, Tolppanen AM, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer's disease. Am J Gastroenterol. 2017;112(12):1802-1808. [DOI] [PubMed] [Google Scholar]
- 24.Badiola N, Alcalde V, Pujol A, et al. . The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8(3):e58837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435-2442. [DOI] [PubMed] [Google Scholar]
- 26.Vogiatzoglou A, Smith AD, Nurk E, et al. . Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med. 2013;75(1):20-29. [DOI] [PubMed] [Google Scholar]
- 27.Fallahzadeh MK, Borhani Haghighi A, Namazi MR. Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther. 2010;35(2):125-126. [DOI] [PubMed] [Google Scholar]
- 28.Sehested TSG, Gerds TA, Fosbøl EL, et al. . Long-term use of proton pump inhibitors, dose-response relationship and associated risk of ischemic stroke and myocardial infarction. J Intern Med. 2018;283(3):268-281. [DOI] [PubMed] [Google Scholar]
- 29.Wang YF, Chen YT, Luo JC, Chen TJ, Wu JC, Wang SJ. Proton-pump inhibitor use and the risk of first-time ischemic stroke in the general population: a nationwide population-based study. Am J Gastroenterol. 2017;112(7):1084-1093. [DOI] [PubMed] [Google Scholar]
- 30.Lazarus B, Chen Y, Wilson FP, et al. . Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klatte DCF, Gasparini A, Xu H, et al. . Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153(3):702-710. [DOI] [PubMed] [Google Scholar]
- 32.Corraini P, Henderson VW, Ording AG, Pedersen L, Horváth-Puhó E, Sørensen HT. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke. 2017;48(1):180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006-1018. [DOI] [PubMed] [Google Scholar]
- 34.Savva GM, Stephan BC, Alzheimer's Society Vascular Dementia Systematic Review Group. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41(1):e41-e46. [DOI] [PubMed] [Google Scholar]
- 35.Zammit AR, Katz MJ, Bitzer M, Lipton RB. Cognitive impairment and dementia in older adults with chronic kidney disease: a review. Alzheimer Dis Assoc Disord. 2016;30(4):357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmer C, Stengel B, Metzger M, et al. . Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77(23):2043-2051. [DOI] [PubMed] [Google Scholar]
- 37.Koton S, Pike JR, Johansen M, et al. . Association of ischemic stroke incidence, severity, and recurrence with dementia in the Atherosclerosis risk in communities cohort study. JAMA Neurol. 2022;79(3):271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruno G, Zaccari P, Rocco G, et al. . Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25(22):2706-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Gao J, Liu K, Zhang HL. Microbiota-gut-brain axis and Alzheimer's disease: implications of the blood-brain barrier as an intervention target. Mech Ageing Dev. 2021;199:111560. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Gao J, Zhu M, Liu K, Zhang HL. Gut microbiota and dysbiosis in Alzheimer's disease: implications for pathogenesis and treatment. Mol Neurobiol. 2020;57(12):5026-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Yu NW, Wang DZ, et al. . Helicobacter pylori infection is associated with long-term cognitive decline in older adults: a two-year follow-up study. J Alzheimers Dis. 2023;91(4):1351-1358. [DOI] [PubMed] [Google Scholar]
- 42.Xie J, Cools L, Van Imschoot G, et al. . Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer's disease pathogenesis via C3-C3aR signalling. J Extracell Vesicles. 2023;12(2):e12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yadav P, Lee YH, Panday H, et al. . Implications of microorganisms in Alzheimer's disease. Curr Issues Mol Biol. 2022;44(10):4584-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boustani M, Hall KS, Lane KA, et al. . The association between cognition and histamine-2 receptor antagonists in African Americans. J Am Geriatr Soc. 2007;55(8):1248-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coupland CAC, Moore M, Hippisley-Cox J. Association of anticholinergic drug exposure with increased occurrence of dementia-reply. JAMA Intern Med. 2019;179(12):1730-1731. [DOI] [PubMed] [Google Scholar]
- 47.Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med. 2019;179(8):1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson K, Fox C, Maidment I, et al. . Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may obtain access to ARIC study data through the NIH National Heart, Lung, and Blood Institute (NHLBI) BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/).