Abstract
A small-spored Alternaria was found from black spots of storaged Koerle pear (Pyrus sinkiangensis), one of the economically important fruit in Xinjiang province, China. The morphology is similar to A. limoniasperae but obviously different in secondary conidiophores and conidial septa. A phylogenetic analysis using sequence datasets of ITS, GAPDH, TEF1, RPB2, Alt a1, OPA10–2, and EndoPG genes revealed that it belonged to the Alternaria alternata complex group. Pathogenicity tests illustrated that the fungus was the causal pathogen of black spot on Koerle pear fruit.
Keywords: Alternaria, black spot disease, phylogenetic analysis, pathogenicity
Koerle pear (Pyrus sinkiangensis synonymy Pyrus sp. nr. communis) is mainly distributed in north-western China, especially in Xinjiang Autonomous Region [1]. It is one of the important agricultural fruit and primarily exported to the international market because the fruit has a distinctive nice flavor and scent, thin skin, crisper and succulence, fewer dregs, and high volume sugary [2]. In 2011, the incidence of calyx-end black spot disease of Koerle pear reached from 14.7% to 34.8% with high yield loss in some orchards of Shayidong horticultural field, Bazhou, Xinjiang, China, of which casual pathogen is identified as Alternaria alternata based on morphology and sequence analyses of ITS, GAPDH, and TEF1 [3].
Alternaria is initially described by Nees (1816), which can be found as saprophytic, endophytic, and pathogenic species not only in agricultural products but also in soil and organic matter [4–6]. Two taxonomic sections of Alternaria including large-spored taxa and small-spored taxa are described by Simmons [7] based on conidial morphology and sporulation patterns. Most of small-spored Alternaria species are challenging because some morphological characters are difficult to clearly characterize [8]. Phylogenetically, a total of 27 sections are proposed by Lawrence et al. [9] after a review of biodiversity and taxonomy on Altenraria. Among the phylogenetic sections, sect. Alternaria consists of 11 phylogenetic species and two species complexes, from which A. alternata species complex comprising 35 morphospecies [10]. Gannibal recommends that sec. Alternaria includes 59 species (1 type species, 21 phylogenetic species, and additional 37 morphospecies) [11].
Black spots of Koerle pear fruit were observed during storage in October 2017. An Alternaria alternata-like fungus was observed from the symptoms. The objectives of this study aim to test the pathogenicity of that fungus and clearly describe it based on morphology and sequence analyses of seven genes according to Simmons [7] and Woudenberg et al. [10], respectively. To get pure cultures, single spore was collected from infected fruit tissue segments and incubated on potato dextrose agar (PDA; Difco, Montreal, Canada) according to Luo et al. [12]. Five strains (YZU 171916, YZU 171918, YZU 171919, YZU 171920, and YZU 171921) were deposited in the Culture Collection at Yangtze University (YZU), Jingzhou, China (Table 1).
Table 1.
Strains and their accession numbers used in the study.
| Morphospecies | Strain | Host | Location | GenBank accession No. |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | GAPDH | TEF1 | RPB2 | Alt a1 | EndoPG | OPA10–2 | ||||
| A. alternata | CBS 916.96 T | Arachis hypogeae | India | AF347031 | AY278808 | KC584634 | KC584375 | AY563301 | JQ811978 | KP124632 |
| A. alternata | CBS 110977 T | Arachis hypogaea | India | AF347031 | AY278808 | KC584634 | KC584375 | AY563301 | JQ811978 | KP124647 |
| A. alstroemeriae | CBS 118808 R | Alstroemeria sp. | United States | KP124296 | KP124153 | KP125071 | KP124764 | KP123845 | KP123993 | KP124601 |
| A. angustiovoidea | CBS 195.86 T | Euphorbia esula | Canada | KP124317 | KP124173 | KP125093 | KP124785 | JQ646398 | KP124017 | KP124624 |
| A. arborescens | CBS 115189 | Citrus clementina | South Africa | KP124402 | KP124254 | KP125180 | KP124872 | KP123949 | KP124106 | KP124716 |
| A. arborescens | CPC 25266 | Pyrus sp. | Austria | KP124418 | KP124269 | KP125196 | KP124887 | KP123965 | KP124122 | KP124732 |
| A. arborescens | CBS 124283 | Oryza sp. | Russia | KP124416 | KP124267 | KP125194 | KP124885 | KP123963 | KP124120 | KP124730 |
| A. arborescens | CBS 102605 T | Solanum lycopersicum | United States | AF347033 | AY278810 | KC584636 | KC584377 | AY563303 | AY295028 | KP124712 |
| A. astragali | CBS 127672 T | Astragalus bisulcatus | United States | KP124382 | KP124234 | KP125160 | KP124852 | KP123930 | KP124086 | KP124695 |
| A. betae–kenyensis | CBS 118810 T | Beta vulgaris var. cicla | Kenya | KP124419 | KP124270 | KP125197 | KP124888 | KP123966 | KP124123 | KP124733 |
| A. broussonetiae | CBS 121455 T | Broussonetia papyrifera | China | KP124368 | KP124220 | KP125146 | KP124838 | KP123916 | KP124072 | KP124681 |
| A. brassicinae | CBS 118811 T | Brassica oleracea | United States | KP124356 | KP124210 | KP125132 | KP124824 | KP123904 | KP124057 | KP124667 |
| A. burnsii | CBS 107.38 T | Cuminum cyminum | India | KP124420 | JQ646305 | KP125198 | KP124889 | KP123967 | KP124124 | KP124734 |
| A. caudata | CBS 121544 R | Cucumis sativus | United States | KP124371 | KP124223 | KP125149 | KP124841 | KP123919 | KP124075 | KP124684 |
| A. cerealis | CBS 119544 T | Avena sativa | New Zealand | KP124408 | JQ646321 | KP125186 | KP124878 | KP123955 | KP124112 | KP124722 |
| A. cirtri | CBS 102.47 R | Citrus sinensis | United States | KP124304 | KP124161 | KP125080 | KP124773 | KP123855 | KP124002 | KP124610 |
| A. citricancri | CBS 119543 T | Citrus paradisi | United States | KP124363 | KP124215 | KP125139 | KP124831 | KP123911 | KP124065 | KP124674 |
| A. citriarbusti | CBS 102598 T | Minneola tangelo | United States, | KP124329 | KP124184 | KP125105 | KP124797 | KP123878 | KP124031 | KP124638 |
| A. citrimacularis | CBS 102596 T | Citrus jambhiri | United States | KP124328 | KP124183 | KP125104 | KP124796 | KP123877 | KP124030 | KP124637 |
| A. daucifolii | CBS 118812 T | Daucus carota | United States | KC584193 | KC584112 | KC584652 | KC584393 | KP123905 | KP124058 | KP124668 |
| A. destruens | CBS 121454 T | Cuscuta gronovii | United States | MH863109 | AY278812 | KP125145 | KP124837 | JQ646402 | KP124071 | KP124680 |
| A. dumosa | CBS 102604 T | Minneola tangelo | Israel | KP124334 | AY562410 | KP125110 | KP124802 | AY563305 | KP124035 | KP124643 |
| A. eichhorniae | CBS 489.92 T | Eichhornia crassipes | India | KC146356 | KP124276 | KP125204 | KP124895 | KP123973 | KP124130 | KP124740 |
| A. gaisen | CBS 118488 R | Pyrus pyrifolia | Japan | KP124427 | KP124278 | KP125206 | KP124897 | KP123975 | KP124132 | KP124743 |
| A. gaisen | CBS 118389 R | Pyrus pyrifolia | Japan | KP124407 | KP124259 | KP125185 | KP124877 | KP123954 | KP124111 | KP124721 |
| A. geophila | CBS 101.13 T | Peat soil | Switzerland | KP124392 | KP124244 | KP125170 | KP124862 | KP123940 | KP124096 | KP124705 |
| A. gossypina | CBS 104.32 T | Gossypium sp. | Zimbabwe | KP124430 | JQ646312 | KP125209 | KP124900 | JQ646395 | KP124135 | KP124746 |
| A. godetiae | CBS 117.44 T | Godetia sp. | Denmark | KP124303 | KP124160 | KP125079 | KP124772 | KP123854 | KP124001 | KP124609 |
| A. herbiphorbicola | CBS 119408 T | Euphorbia esula | United States | KP124362 | JQ646326 | KP125138 | KP124830 | JQ646410 | KP124064 | KP124673 |
| A. iridiaustralis | CBS 118404 R | Iris sp. | New Zealand | KP124434 | KP124283 | KP125213 | KP124904 | KP123980 | KP124139 | KP124750 |
| A. interrupta | CBS 102603 T | Minneola tangelo | Israel | KP124333 | KP124188 | KP125109 | KP124801 | KP123882 | KP124034 | KP124642 |
| A. jacinthicola | CBS 133751 T | Eichhomrnia crassipes | Mali | KP124438 | KP124287 | KP125217 | KP124908 | KP123984 | KP124143 | KP124754 |
| A. kikuchiana | CBS 107.53 | Pyrus pyrifolia | Japan | KP124305 | KP124162 | KP125081 | KP124774 | KP123858 | KP124005 | KP124613 |
| A. lini | CBS 106.34 T | Linum usitatissimum | unknown | Y17071 | JQ646308 | KP125078 | KP124771 | KP123853 | KP124000 | KP124608 |
| A. limoniasperae | CBS 102595 T | Citrus jambhiri | United States | FJ266476 | AY562411 | KC584666 | KC584408 | AY563306 | KP124029 | KP124636 |
| The present fungus | YZU 171916 | Pyrus sinkiangensis | China | MK391581 | MK391582 | MK415954 | MK391583 | MK391585 | MK415955 | MK391584 |
| YZU 171918 | Pyrus sinkiangensis | China | MK391594 | MK415938 | MK415946 | MK391586 | MK415950 | MK415942 | MK391590 | |
| YZU 171919 | Pyrus sinkiangensis | China | MK391596 | MK415940 | MK415948 | MK391588 | MK415952 | MK415944 | MK391592 | |
| YZU 171920 | Pyrus sinkiangensis | China | MK391595 | MK415939 | MK415947 | MK391587 | MK415951 | MK415943 | MK391591 | |
| YZU 171921 T | Pyrus sinkiangensis | China | MK391597 | MK415941 | MK415949 | MK391589 | MK415953 | MK415945 | MK391593 | |
| A. longipes | CBS 540.94 R | Nicotiana tabacum | United States | AY278835 | AY278811 | KC584667 | KC584409 | AY563304 | KP124147 | KP124758 |
| A. mali | CBS 106.24 T | Malus sylvestris | United States | KP124298 | KP124155 | KP125073 | KP124766 | KP123847 | AY295020 | JQ800620 |
| A. palandui | CBS 121336 T | Allium sp. | United States | KJ862254 | KJ862255 | KP125141 | KP124833 | KJ862259 | KP124067 | KP124676 |
| A. pellucida | CBS 479.90 T | Citrus unshiu | Japan | KP124319 | KP124174 | KP125095 | KP124787 | KP123870 | KP124019 | KP124626 |
| A. perangusta | CBS 102602 T | Minneola tangelo | Turkey | KP124332 | KP124187 | KP125108 | KP124800 | KP123881 | AY295023 | KP124641 |
| A. platycodonis | CBS 121348 T | Platycodon grandiflorus | China | KP124367 | KP124219 | KP125144 | KP124836 | KP123915 | KP124070 | KP124679 |
| A. postmessia | CBS 119399 T | Minneola tangelo | United States | KP124361 | JQ646328 | KP125137 | KP124829 | KP123910 | KP124063 | KP124672 |
| A. pulvinifungicola | CBS 194.86 T | Quercus sp. | United States | KP124316 | KP124172 | KP125092 | KP124784 | KP123869 | KP124016 | KP124623 |
| A. rhadina | CBS 595.93 T | Pyrus pyrifolia | Japan | KP124320 | KP124175 | KP125096 | KP124788 | JQ646399 | KP124020 | KP124627 |
| A. sanguisorbae | CBS 121456 T | Sanguisorba officinalis | China | KP124369 | KP124221 | KP125147 | KP124839 | KP123917 | KP124073 | KP124682 |
| A. seleniiphila | CBS 127671 T | Stanleya pinnata | United States | KP124381 | KP124233 | KP125159 | KP124851 | KP123929 | KP124085 | KP124694 |
| A. senecionicola | CBS 119545 T | Senecio skirrhodon | New Zealand | KP124409 | KP124260 | KP125187 | KP124879 | KP123956 | KP124113 | KP124723 |
| A. soliaegyptiaca | CBS 103.33 T | Soil | Egypt | KP124302 | KP124159 | KP125077 | KP124770 | KP123852 | KP123999 | KP124607 |
| Alternaria sp. | CBS 632.93 R | Pyrus pyrifolia | Japan | KC584197 | KC584116 | KC584658 | KC584399 | KP123974 | AY295033 | KP124742 |
| A. tenuissima | CBS 918.96 R | Dianthus chinensis | UK | AF347032 | AY278809 | KC584693 | KC584435 | AY563302 | KP124026 | KP124633 |
| A. tomato | CBS 114.35 | Solanum lycopersium | Unknown | KP124446 | KP124295 | KP125225 | KP124916 | KP123992 | KP124152 | KP124763 |
| A. tomaticola | CBS 118815 R | Solanum lycopersicum | United States | KP124358 | KP124212 | KP125134 | KP124826 | KP123907 | KP124060 | KP124670 |
| A. tomaticola | CBS 118814 T | Solanum lycopersicum | United States | KP124357 | KP124211 | KP125133 | KP124825 | KP123906 | KP124059 | KP124669 |
| A. turkisafria | CBS 102599 T | Minneola tangelo | Turkey | KP124330 | KP124185 | KP125106 | KP124798 | KP123879 | KP124032 | KP124639 |
| A. turkisafria | CBS 121344 R | Minneola tangelo | Israel | KP124365 | KP124217 | KP125142 | KP124834 | KP123913 | KP124068 | KP124677 |
| A. turkisafria | CBS 121346 R | Minneola tangelo | South Africa | KP124366 | KP124218 | KP125143 | KP124835 | KP123914 | KP124069 | KP124678 |
| A. toxicogenica | CBS 102600 T | Citrus reticulat | United States | KP124331 | KP124186 | KP125107 | KP124799 | KP123880 | KP124033 | KP124640 |
| A. vaccinii | CBS 118818 T | Vaccinium sp. | United States | KP124359 | KP124213 | KP125135 | KP124827 | KP123908 | KP124061 | KP124671 |
| A. yali–inficiens | CBS 121547T | Pyrus bretschneideri | China | KP124372 | KP124224 | KP125150 | KP124842 | KP123920 | KP124076 | KP124685 |
T: ex-type strain; R: representative strain.
Bold contents are related to the present fungus generated in this study.
To determine the morphological characteristics, mycelia disks (6 mm diam.) were cut from 3-day-old colony and transferred on PDA kept at 25 °C for 7 days in darkness. The colony features were recorded and the colors were determined using the color chart of Rayner [13]. The fungus was incubated on potato carrot agar (PCA) for 7 days at 22 °C under the daily fluorescent light/dark cycle of 8/16 h to describe the conidial morphology [7]. To describe the morphology from host, diseased tissue was incubated for 4 days under the same condition as conidial description on PCA. The sporulation patterns and conidia were photographed using a compound light microscope (Nikon DS-Ri2, Tokyo, Japan). The conidia were mounted in lactophenol solution. Fifty conidia were investigated for the description of each of characteristics.
After 7 days, the colonies reached to 65–66 mm in diam. on PDA with umber to olivaceous color surrounding with white margin (Figure 1(A,B)). On PCA (Figure 1(C–F)), primary conidiophores were 15–146 × 3–5 µm producing 4–10 units catenulate conidia and the secondary conidiophores to develop lateral intra-conidia were 3–20 × 3–4 µm forming branched chains of 1–4 units. Conidia comprising 1–7 transverse septa were narrow-ellipsoid (13–50 × 6–11 µm) or ovoid (6–23 × 4–13 µm) in the initial lower part of the chains, gradually becoming ovoid (7–22 × 5–9 µm) and considerably smaller in the distal part, with apical conidia (2–12 × 2–4 µm). On the host (Figure 1(G–I)), the primary conidiophores reached 3–107 × 2–4 µm producing catenulate conidia (3–10 units) and the secondary ones to produce lateral intra-conidia (catenulate with 1–4 units) were 3–23 × 2–4 µm in size. Normally, conidia were 13–44 × 2–28 µm, with 1–6 transverse septa and false beaks 2–32 × 2–7 µm in size. The present fungus was morphologically similar to the species Alternaria limoniasperae, A. perangusta, A. interrupta, and A. turkisafria (Table 2).
Figure 1.
Morphological characteristics of Alternaria sp. YZU 171921 from Pyrus sinkiangensisi. Colony on PDA (A) and (B); sporulation patterns, conidiophores and conidia on the PCA (C)–(F); sporulation patterns, conidiophores and conidia on the host plant (G)–(I). Bars: (D)–(F) = 25 μm, (H) and (I) = 25 μm, (C) = 100 μm, (G) = 100 μm.
Table 2.
Morphological comparison of the present fungus and its closely related species described by Simmons [7].
| Species | Conidia |
Conidiophore (μm) | Secondary conidiophore (μm) | Conidia per primary chain (lateral branched chain) | |
|---|---|---|---|---|---|
| Shape and size (μm) | Septa | ||||
| A. limoniasperae | Narrow-ellipsoid 30 − 50 × 8 − 10 or ovoid 20 − 35 × 8 − 12 in the initial lower part of the chain, ovoid 8 − 12 × 4 − 8 in the distal part of the chain | 1–4 | 100 | 2 − 4 × 2 − 3 | up to 20 (4 − 10) |
| YZU 171921 | Narrow-ellipsoid 13 − 50 × 6 − 11 or ovoid 6 − 23 × 4 − 13 in the initial lower part of the chain, ovoid 7 − 22 × 5 − 9 in the distal part of the chain | 1–7 | 15 − 146 × 3–5 | 3 − 20 × 3–4 | 4 − 10 (1 − 4) |
| A. turkisfria | Narrow-ovoid to long-ovoid or long-ellipsoid 20 − 50 × 6− 8, conidia have small 1-cell secondary conidiophores | 3–8 | 30 − 60 × 4 | 3 − 5 × 2–3 | 8 − 20 (4 − 10 ) |
| A. perangusta | Long narrow-ellipsoid, rarely wide enough to be termed obclavate 15 − 40 × 3–7 | 3–7 | 100 − 200 × 3–4 | 3 − 5 × 2–3 | 10+ (unknown) |
| A. interrupta | Narrow-ellipsoid or narrow-obclavate 35 − 40 × 7–8 | 7–8 | 140 × 4 | 3 − 8 × 2–3 | 10 − 15 (unknown) |
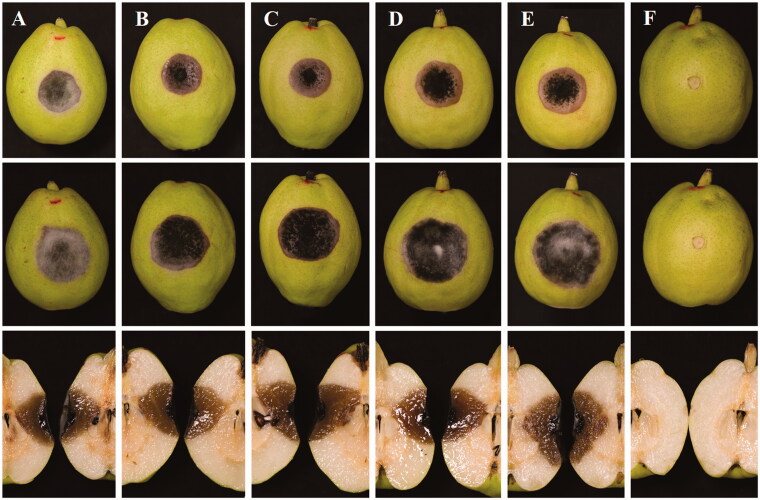
To test its pathogenicity, healthy Koerle pear fruits were obtained from the seller of Xinjiang market. Eighteen fruits were surface sterilized by dipping in 1% sodium hypochlorite (NaOCl) for 2 min, and then, washed with sterilized distilled water for 3 times. Each fruit was wounded two sites (one for mycelia plug and another for spore suspension) by a puncher (4 mm diam.) and placed into moist containers maintained at 25 °C. Mycelia plugs (4 mm diam.) of each strain were cut from the edge of 3-day-old colonies and placed on wounded sites. Sterile PDA plugs were used as controls. Conidia were harvested from PCA to obtain the spore suspension (106 conidia/mL). A volume of 20 µL spore suspension was inoculated and distilled water was used as controls. Each strain was conducted with three replications and the experiment was repeated for three times. The disease development was checked daily.
Necrotic symptoms were observed obviously at 3 days in both inoculations. After 7 days, the symptoms developed up to 23 mm (diam.) inoculated with mycelia plugs (Figure 2) and 14 mm with spore suspensions. After 14 days, the symptoms turned to be rotten reaching to 30 mm in mycelium block and 22 mm in spore suspension. Any control was symptomless during the experiment. By the way, unwounded fruits were symptomless either by mycelia plug or spore suspension (data not shown). The Koch’s postulates were fulfilled by a re-isolation from inoculated fruits. The results showed that the present fungus was the causal agent of black spot of Koerle pear fruit (Figure 2).
Figure 2.
Pathogenicity tests on Koerle pear fruit (Pyrus sinkiangensis) inoculated with mycelia plugs of five strains (A–F) of the present fungus for 7 days (upper) and for 14 days (middle and down) at 25 °C. (A) YZU 171916; (B) YZU 171918; (C) YZU 171919; (D) YZU 171920; (E) YZU 171921; (F) Control.
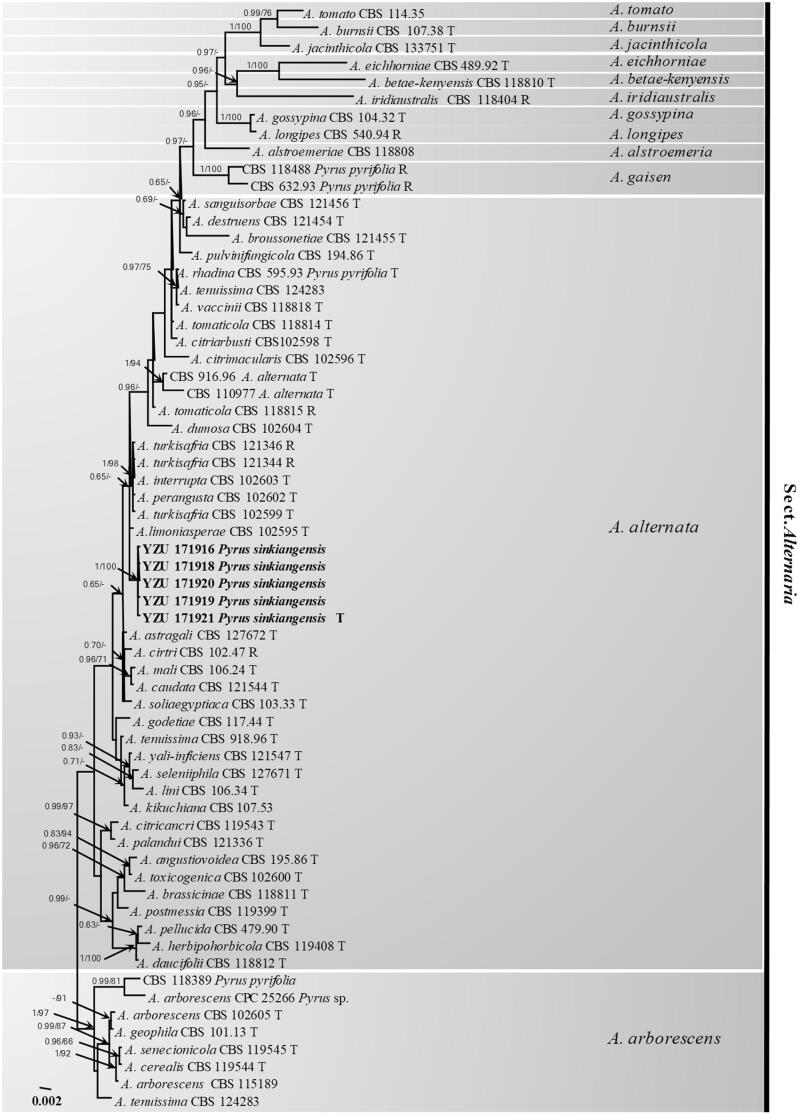
To confirm the phylogenetic position of the present fungus, the genomic DNA was extracted using mycelia grown on PDA according to the method of Cenis [14]. Seven genes including internal transcribed spacer rDNA regions (ITS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), partial translation elongation factor 1 alpha (TEF1), RNA polymerase second largest subunit (RPB2), Alternaria major allergen gene (Alt a1), an anonymous gene region (OPA10-2), and endopolygalacturonase gene (EndoPG) were amplified using the primer pairs ITS4/ITS5 [15], gpd1/gpd2 [16], EF1-728F/EF1-986R [17], RPB2-5F [18]/RPB2-7CR [19], Alt-for/Alt-rev [20], OPA 10-2 L/OPA 10-2 R [8], and PG3/PG2b [8], respectively. PCR amplification was performed in a 25 µL reaction volume containing 8 µL ddH2O, 2 µL DNA solution, 1.25 µL each primer, and 12.5 µL 2 × Taq PCR StarMix (Genstar, Beijing, China). The PCR products were checked in 1% agarose gel, run in 0.5 × TBE buffer and visualized under UV illumination. Successfully amplified PCR products were sequenced by Beijing Genomics Institute (BGI, Beijing, China) using both primers. The resulting sequences were compared with those of morphospecies described by Simmons (2007) derived from Woudenberg et al. [10]. Each of seven gene sequences was aligned and combined using the MEGA v. 6.0.0 software [21]. The best-fit model GTRGAMMAI was selected by MrModeltest v. 2.3.6. [22]. Bayesian analyses were performed with MrBayes v. 3.1.2 [23]. The parameters including 2,000,000 Markov chain Monte Carlo (MCMC) generations and a sampling frequency of every 100 generations were set for the combined analysis of seven loci. And the run was stopped when the average standard deviation of split frequencies fell under 0.01. Burn–in was set to 25% after which the likelihood values were constant. A maximum-likelihood analysis was additionally run using RAxML v. 7.2.8 [24]. Bootstrap analysis was performed with 1000 replications for the combined analysis of seven loci. The Alternaria arborescens species complex (AASC) was used as root branch. The resulting tree was plotted and edited by FigTree v. 1.3.1 [25]. A total of 64 Alternaria isolates were included in the aligned sequence matrix. In the multigene phylogeny, 3445 characters were calculated including 502 of ITS, 446 of GAPDH, 240 of TEF1, 710 of RPB2, 472 of Alt a1, 633 of OPA10–2, and 442 of EndoPG. The Bayesian posterior probabilities (PP) >0.65 and RAxML bootstrap support values (BP) >65% were plotted in the phylogeny (Figure 3). Based on the seven genes, the fives strains used in this study were identical to each other. The phylogenetic results showed that the present fungus was belonging to Alternaria alternata species complex (AALSC) group of Alternaria and fell into a mono-clade highly supported by PP (1.00) and BP (100%) values. They were closely related to Alternaria limoniasperae, A. perangusta, A. interrupta, and A. turkisafria. However, the present fungus was the closest to A. limoniasperae with seven nucleotide position differences: Alt a 1 position 350 (C), RPB2 position 546 (G), and OPA10–2 position 369 (T), 618 (C), 624 (G), 639 (C), 648 (G). Morphologically, the present fungus was obviously different from A. limoniasperae by producing more septa in conidia with shorter chains. All the previous results indicated that the species might be a new morphospecies in AALSC. All thirty-five morphospecies under one species Alternaria alternata [10] is not a great way for the taxonomy based on phylogeny. More works should be done to better understand the taxonomy of Alternaria alternata species complex based on morphology and molecular at the same times. In the present study, the fungus collected from black spot of Koerle pear fruit (Pyrus sinkiangensis) was found as a causal agent and illustrated clearly in morphology generated from authentic culture and host. Phylogenetically, it should be considered as a new member of AALSC.
Figure 3.
The phylogentic tree using 64 strains of sect. Alternaria based on ITS, GAPDH, TEF1, RPB2, Alt a1, OPA10-2, and EndoPG gene sequences. The Bayesian posterior probabilities (PP) > 0.65 and RAxML bootstrap support values (BP) > 65% are given at the nodes (PP/BP). The type strain or ex-type strain is indicated with T and representative strain is R.
Funding Statement
The work was supported by the National Natural Science Foundation of China [31400014] and by the Young Scientist Foundation of Yangtze University [2016cqr08].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.ISBN, AQSIQ . Import risk analysis: Pears (Pyrus bretschneideri, Pyrus pyrifolia, and Pyrus sp. nr. communis) fresh fruit from China (Final version), Policy and Risk, MAF Biosecurity New Zealand; 2009. [Google Scholar]
- 2.Qi X, Wu J, Wang L, et al. . Identifying the candidate genes involved in the calyx abscission process of ‘Kuerlexiangli’ (Pyrus sinkiangensis Yu) by digital transcript abundance measurements. BMC Genomics. 2013;14(1):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song B, Zhu XF, Xu BQ, et al. . Identification of Koerle pear calyx-end black spot pathogen and its sequence analysis of ITS, GPD and EF-1α. Acta Hortic Sin. 2016;43(2):329–336. [Google Scholar]
- 4.Pavón MM, González AI, Martín de Santos R, et al. . The importance of genus Alternaria in mycotoxins production and human diseases. Nutr Hosp. 2012;27:1772–1981. [DOI] [PubMed] [Google Scholar]
- 5.Polizzotto R, Andersen B, Martini M, et al. . A polyphasic approach for the characterization of endophytic Alternaria strains isolated from grapevines. J Microbiol Methods. 2012;88(1):162–171. [DOI] [PubMed] [Google Scholar]
- 6.Neergaard P. Danish species of Alternaria and Stemphylium: taxonomy, parasitism, economical significance. Copenhagen: Einar Munksgard; 1945. [Google Scholar]
- 7.Simmons EG. Alternaria: an identification manual, CBS biodiversity series 6. Utrecht: Centraalbureau voor Schimmelcultures; 2007. [Google Scholar]
- 8.Andrew M, Peever TL, Pryor BM. An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia. 2009;101(1):95–109. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence DP, Rotondo F, Gannibal PB. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol Progress. 2016;15(1):3. [Google Scholar]
- 10.Woudenberg JHC, Seidl MF, Groenewald JZ, et al. . Alternaria section Alternaria: species, formae speciales or pathotypes? Stud Mycol. 2015;82:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gannibal PB. Distribution of Alternaria species among sections.2. Section Alternaria. Mycotaxon. 2016;130(4):941–949. [Google Scholar]
- 12.Luo H, Xia ZZ, Chen YY, et al. . Morphology and molecular characterization of Alternaria argyranthemi on Chrysanthemum coronarium in China. Mycobiology. 2018;46(3):278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayner RW. A mycological colour chart. London: Commonwealth Mycological Institute (Kew; ); 1970. [Google Scholar]
- 14.Cenis JL. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992;20(9):2380–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TJ, Bruns T, Lee S, et al. . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. p. 315–322. [Google Scholar]
- 16.Berbee ML, Pirseyedi M, Hubbard S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia. 1999;91(6):964–977. [Google Scholar]
- 17.Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–556. [Google Scholar]
- 18.Sung G-H, Sung J-M, Hywel-Jones NL, et al. . A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Mol Phylogen Evol. 2007;44(3):1204–1223. [DOI] [PubMed] [Google Scholar]
- 19.Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA Polymerse II Subunit. Mol Biol Evol. 1999;16(12):1799–1808. [DOI] [PubMed] [Google Scholar]
- 20.Hong SG, Cramer RA, Lawrence CB, et al. . Alt a 1 allergen homologs from Alternaria and related taxa: analysis of phylogenetic content and secondary structure. Fungal Genet Biol. 2005;42(2):119–129. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K, Stecher G, Peterson D, et al. . MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nylander J. MrModeltest v2. Program distributed by the author. Uppsala, Sweden: Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]
- 23.Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. [DOI] [PubMed] [Google Scholar]
- 24.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. [DOI] [PubMed] [Google Scholar]
- 25.Rambaut A, Drummond A. FigTree v.1.3.1. Institute of evolutionary biology. Edinburgh, UK: University of Edinburgh; 2010. [Google Scholar]