Keywords: colonic uptake, human differentiated colonoid monolayers, LPS, SLC44A4, thiamin pyrophosphate
Abstract
This study investigated the effect of the bacterial endotoxin lipopolysaccharide (LPS) on colonic uptake of thiamin pyrophosphate (TPP), the biologically active form of vitamin B1 that is generated by gut microbiota. We used three complementary models in our study: in vitro (human-derived colonic epithelial NCM460), ex vivo (human differentiated colonoid monolayers), and in vivo (mouse colonic tissue). The results showed that exposure of NCM460 cells to LPS leads to a significant inhibition of carrier-mediated TPP uptake as well as in decreased expression of the colonic TPP transporter (cTPPT) protein, mRNA, and heterologous nuclear RNA (hnRNA) compared with untreated controls. Similarly, exposure of human differentiated colonoid monolayers and mice to LPS caused significant inhibition in colonic carrier-mediated TPP uptake and in cTPPT protein, mRNA, and hnRNA expression. The effect of LPS on colonic TPP uptake and cTTPT expression was also found to be associated with a significant reduction in activity of the SLC44A4 promoter as well as in decreased expression of the nuclear factor Elf-3 (E74-like ETS transcription factor 3), which is needed for promoter activity. Finally, we found that knocking down the Toll-like receptor 4 (TLR4) and blocking the nuclear factor kappa B (NF-κB), JNK, and p38 signaling pathways with the use of pharmacological inhibitors lead to significant abrogation in the degree of LPS-mediated inhibition in TPP uptake and cTPPT expression. These results demonstrated that exposure of colonic epithelia to LPS inhibits colonic TPP uptake via transcriptional mechanism(s) and that the effect is mediated via TLR4 receptor and NF-κB/p38/JNK signaling pathways.
NEW & NOTEWORTHY This study examined the effect of the bacterial lipopolysaccharide (LPS) on the colonic uptake of thiamin pyrophosphate (TPP), the biologically active form of vitamin B1. Three complementary models were used: in vitro (human NCM460 cells), ex vivo (human colonoids), and in vivo (mice). The results showed LPS to significantly suppress TPP uptake and the expression of its transporter, and that these effects are mediated via the membrane TLR4 receptor, and involve the NF-κB/p38/JNK signaling pathways.
INTRODUCTION
A member of the water-soluble family of vitamins, thiamin (vitamin B1), plays vital roles in a variety of metabolic reactions in all mammalian cells. In its principle metabolically active form, i.e., thiamin pyrophosphate (TPP), this essential micronutrient serves as a cofactor in pathways involved in oxidative energy metabolism and reduction of cellular oxidative stress; it also possesses anti-inflammatory properties (1, 2). Cellular deficiency/suboptimal levels of thiamin lead to impairment in energy metabolism and to oxidative stress; it also negatively impacts the normal physiology of mitochondria (3–5). Deficiency of vitamin B1 in humans occurs in a variety of pathophysiological conditions including sepsis, inflammatory bowel disease, chronic alcoholism, and diabetes mellitus (6–9).
Being unable to synthesize thiamin endogenously, humans and other mammals obtain the vitamin from exogenous sources via intestinal absorption. Two sources of vitamin B1 are available to the host: diet and the gut microbiota (10–14). Dietary vitamin B1 exists in the forms of free and phosphorylated thiamin, with the latter form being hydrolyzed (by the action of small intestinal surface phosphatases) to free thiamin before absorption. Absorption of free thiamin then proceeds via a specific carrier-mediated process that involves thiamin transporter-1 and -2 (THTR-1 and -2; products of the SLC19A2 and SLC19A3 genes, respectively) (14). With regards to the microbiota-generated vitamin B1, this source provides the vitamin in the free thiamin and TPP forms (10–12). Colonocytes, which lack the ability to hydrolyze TPP to free thiamin (they lack surface phosphatase), are capable of absorbing both forms of vitamin B1 (15); absorption, however, occurs via two distinct carrier-mediated processes. For free thiamin, colonic absorption is as in the small intestine being mediated via THTR-1 and -2 (14, 16). For TPP, colonic absorption occurs via a high-affinity and specific (does not transport free thiamin) process that involves the colonic TPP transporter (cTPPT; the product of the SLC44A4 gene) (17, 18). Studies from our laboratory have characterized different physiological/biological aspects of the colonic TPP uptake process and the system involved (17–19). These studies have shown the exclusive expression of the cTPPT system along the intestinal tract to be in the large intestine, that the expression is restricted to the apical membrane domain of the polarized colonocytes (17–20), and that a variety of internal/external factors affect the colonic TPP uptake process (21–23). To date, however, nothing has been known regarding the possible effect of bacterial lipopolysaccharide (LPS) on colonic TPP uptake. This endotoxin (which is a complex polymer that forms a major component of the outer membrane of Gram-negative bacteria) causes considerable clinical morbidity and mortality (24–26). The human/mammalian gut is exposed to significant levels of LPS in conditions like sepsis, necrotizing enterocolitis, and infections with enteric pathogens (e.g., Escherichia coli pathovars and Salmonella) (27–29). LPS affects a variety of cellular events, including transport processes at the cell membranes, with both inhibition and stimulation being observed (31, 32, 55). The effects of LPS on cell physiology are mediated via Toll-like receptors (mainly TLR4) that lead to the activation of intracellular signaling pathways like nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs); i.e., p38, ERK1/2, and JNK1/2 (26, 27, 33, 35–37, 56).
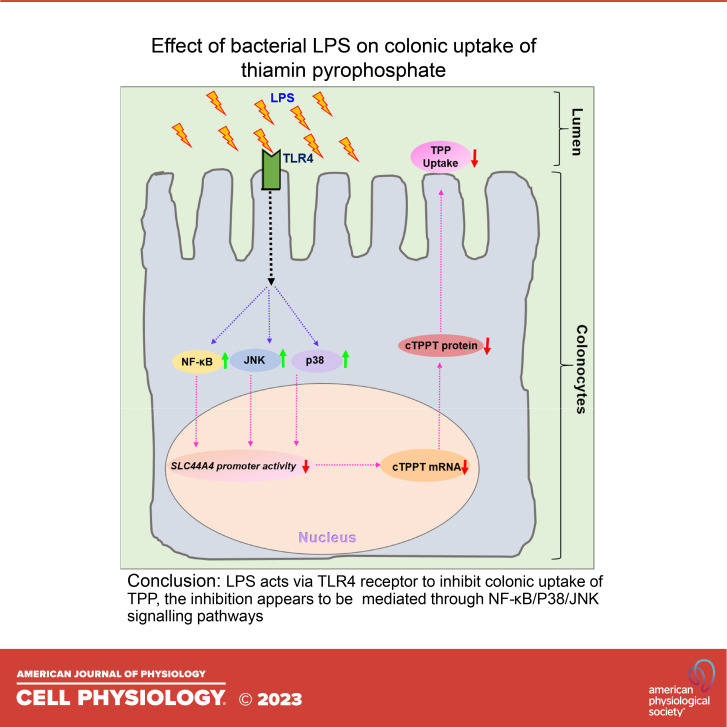
Our aim in this study was to examine the effect of LPS on colonic carrier-mediated uptake of TPP. Addressing this issue is of nutritional importance since the cTPPT contributes to host vitamin B1 nutrition/status; it is also important from the standpoint of colonocyte physiology since these cells (unlike their small intestinal counterparts) have limited capacity to synthesize the biologically active form of vitamin B1 (i.e., TPP) from free thiamin as they express a very low level of the critical enzyme, i.e., thiamin pyrophosphokinase, TPKase (38). We used complementary in vitro (human-derived colonic epithelial NCM460), ex vivo (human differentiated colonoid monolayers), and in vivo (mouse colonic tissue) models in our investigations. The results showed that exposure of colonic epithelial cells to LPS leads to inhibition in TPP uptake. This inhibition is associated with a significant reduction in the level of expression of cTPPT and transcription of the SLC44A4 gene. Further, the inhibition appears to be mediated via TLR4 receptor and the NF-κB/p38/JNK signaling pathways.
MATERIALS AND METHODS
Materials
[3H]-TPP (specific activity: >0.7 Ci/mmol; and purity: >98%) was procured from Moravek Biochemicals Inc. (Brea, CA). Purified lipopolysaccharide (LPS; Escherichia coli 0111: B4; Cat. No.: L5293) was obtained from Sigma Aldrich (St. Louis, MO). TLR4 receptor-specific siRNA (Cat. Nos.: 10620318 and 10620319) and its negative control (Cat. No.: 452002) were procured from Thermo-Fisher (Frederick, MD). Lipofectamine RNAiMAX transfection reagent (Cat. No.: 13778-150) was from Invitrogen, Carlsbad, CA. The pharmacological inhibitors MG-132 (Cat. No.: BML-PI102-0005), SB203580 (Cat. No.: 5633), SP600125 (Cat. No.: 8177), and PD98059 (Cat. No.: 1672186) were obtained from Enzo (Farmingdale, NY), Cell Signaling Technology (Danvers, MA) and BioGems (Westlake Village, CA), respectively. Affinity-purified human-specific anti-cTPPT rabbit polyclonal antibodies were custom-generated by Thermo-Fisher Scientific (Waltham, MA). The anti-β-actin mouse monoclonal primary antibody (Cat. No.: SC47778) was purchased from Santa Cruz Biotechnology (Dallas, TX); the anti-rabbit IRDye-800 (Cat. No.: 926–32211) and anti-mouse IRDye-680 (Cat. No.: 926–68020) were obtained from LI-COR Bioscience (Lincoln, NE) and were used as secondary antibodies. Other chemicals and reagents used in the study (all of the analytical/molecular biology grade) were purchased from authenticated sources.
Methods
Culturing of the human-derived colonic epithelial NCM460 cells and LPS treatment.
NCM460 cells (INCELL, San Antonio, TX) were routinely maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 20% of fetal bovine serum (FBS), 100 μg/mL of streptomycin, and 100 U/mL of penicillin, and then cells were incubated in 5% CO2 incubator at 37°C. To examine the effect of LPS on TPP uptake, confluent monolayers of NCM460 cells were grown in 24-well plates, and cells were starved overnight with the 0.5% FBS-containing culture medium. The serum-starved cells were then treated with 50 µg/mL of LPS (a concentration that has been previously shown to have no effect on cell viability) (39–41) in each well and incubation continued for 48 h. To determine the involvement of signaling pathways in mediating the inhibitory effects of LPS on TPP uptake physiology and molecular biology, specific pharmacological inhibitors (10 μM of MG-132, 1 μM of SB203580, 5 μM of SP600125, and 50 μM of PD98059) of the different signaling pathways (NF-κB, p38, JNK, and ERK1/2 pathways, respectively) were introduced 1 h before the treatment with LPS, and the incubation continued for an additional 48 h at 37°C.
Culturing of human primary differentiated colonoid monolayers and LPS treatment.
The human colonoid line (Hu235A) provided by the Fleckenstein laboratory for these studies was originally obtained from the Digestive Diseases Research Core Center (DDRCC) at Washington University in Saint Louis, School of Medicine and was routinely maintained and cultured in Matrigel droplets (BD Biosciences) as described previously (21, 22, 42). Briefly, isolated human colonoids were cultured in 24-well plates containing conditioned media (IntestiCult Organoid Growth Medium, Cat. No.: 06010, STEMCELL Technologies Inc., Canada) supplemented with 10 μM Y27632 (Cat. No.: 04-0012-02, Reprocell) and 10 μM SB431542 (Cat. No.: 04–0010-05, Reprocell). To generate differentiated colonoid monolayers, Transwell plates (Corning; Kennebunk, ME) were coated with type IV human collagen (Cat. No.: C6745, Sigma, St. Louis, MO) and then seeded with 5 × 104 cells per well. The cells were then allowed to grow for 4 days, and differentiation was induced by adding the differentiation media (5% conditioned media in 95% of Advanced DMEM/F12 supplemented with 20% FBS, 2 mM l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and only 10 μM Y-27632 inhibitor) followed by incubation for 2 days. The differentiated colonoid monolayers were then treated with LPS (50 µg/mL for 48 h) (30) and then used for physiological and molecular investigations.
In vivo treatment of mice with LPS.
Male C57BL/6J mice (10–12 wk old; Jackson Laboratory; Bar Harbor, ME) were used and were housed in a well-ventilated sterile cages, and had free (ad libitum) access to water and a standard diet. One group of the animals was administered a single dose of LPS (5 mg/kg body wt) by intraperitoneal (ip) injection as described previously (30, 31, 43, 44); control mice were injected (ip) with an equal volume of PBS. Forty-eight hours later, the animals were euthanized, and colonic sheets were prepared and utilized for [3H]-TPP uptake as well as for RT-qPCR and Western blot analysis as described before (21, 23). Use of animals as well as the in vivo experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine, CA.
Carrier-mediated [3H]-TPP uptake.
Carrier-mediated uptake of TPP by NCM460 cells and by human differentiated colonoid monolayers was evaluated as described by us previously (21, 22). Briefly, the control and LPS-treated samples were incubated (for 30 min at 37°C) in Krebs–Ringer (KR) buffer [123 mM NaCl, 4.93 mM KCl, 1.23 mM MgSO4, 0.85 mM CaCl2, 5 mM glucose, 5 mM glutamine, 10 mM HEPES, and 10 mM MES (pH 7.4)] containing [3H]-TPP (with and without 1 mM of unlabeled TPP) at 37°C. To assess TPP uptake by mouse colonic sheets, ∼1 cm sheets collected from the same colonic regions of control and LPS-treated mice were incubated in KR buffer containing 0.46 µM of [3H]-TPP (with and without 1 mM unlabeled TPP) (21, 23). All incubations (10 min for NCM460 [in 0.23 μM of [3H]-TPP] and colonic sheet preparation; 30 min for human differentiated colonoid monolayers [in 0.38 μM of [3H]-TPP]) were performed at 37°C. At the end of incubation of the cells/colonic sheets, ice-cold KR buffer (1 mL) was added to stop the reaction, followed by washing with the same buffer and digestion with NaOH as described before (21–23). Finally, levels of [3H] in the cells/sheets were determined using a liquid scintillation counter (LS6500; Beckman Coulter, Brea, CA). The protein quantity of each sample was estimated using Bio-Rad DC protein assay kit (Bio-Rad, Carlsbad, CA).
Quantitative real-time PCR analysis.
Total RNAs from NCM460 cells, human differentiated colonoid monolayers, and mouse colonic tissues were isolated using RNeasy Kit (QIAGEN, Hilden, Germany). Verso-cDNA Synthesis Kit (Thermo-Fisher Scientific, Waltham, MA) was utilized for cDNA synthesis from isolated RNA samples as per the manufacturer’s instruction. iQSYBER Green Supermix (Bio-Rad, Carlsbad, CA) was then used to quantify the level of mRNA expression in the CFX96 RT-qPCR system. Sequences of the gene-specific primers used in this study are shown in Table 1; relative gene expression was calculated using the method (45). The cycle threshold (Ct) of the β-actin gene (the internal control) was used to normalize the Ct values of the respective samples.
Table 1.
List of primer sequences used for RT-qPCR analysis
| Gene Name | Forward Primers (5′–3′) | Reverse Primers (5′–3′) |
|---|---|---|
| Human cTPPT | TGCTGATGCTCATCTTCCTGCG | GGACAAAGGTGACCAGTGGGTA |
| Human CREB-1 | TTAACCATGACCAATGCAGCA | TGGTATGTTTGTACGTCTCCAGA |
| Human Elf-3 | TCTTCCCCAGCGATGGTTTTC | TCCCGGATGAACTCCCACA |
| Human TLR4 | AGACCTGTCCCTGAACCCTAT | CGATGGTCTAAACCAGCCA |
| Human β-Actin | CATCCTGCGTCTGGACCT | TAATGTCACGCACGATTTCC |
| Mouse cTPPT | TGCCTACCAGAGTGTGAAGGAG | TGGCTTCCTTCAGCAGAGCGAT |
| Mouse Elf-3 | TCCTCCGACTACCTTTGGCACT | ACTCCAGAACCTGGGTCTTCGA |
| Mouse β-Actin | ATCCTCTTCCTCCCTGGA | TTCATGGATGCCACAGGA |
CREB-1, cAMP responsive element-binding protein 1; cTPPT, colonic thiamin pyrophosphate transporter; Elf-3, E74-like ETS transcription factor 3; TLR4, Toll-like receptor 4.
Western blotting.
Total protein from each colonic cells/tissue sample was isolated using radio-immunoprecipitation assay (RIPA; Sigma, St. Louis, MO) buffer containing 1% of protease inhibitor cocktail (Cat. No.: 78410, Thermo-Fisher Scientific, Rockford, IL); protein concentrations were estimated using Bio-Rad DC protein assay kit. Twenty-five micrograms of the total proteins were then loaded and resolved on 4%–12% Bris–Tris gels (NuPAGE, Invitrogen). The proteins on gels were blotted onto polyvinylidene difluoride membranes and probed with previously validated primary antibodies [for anti-cTPPT (1:500); for anti-β-actin (1:2,000); 23]. The anti-rabbit IR-800 dye (1: 30,000) and anti-mouse IR-680 dye (1: 30,000) were used as secondary antibodies to detect the primary antibody-bounded protein bands in the blots. The Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE) was utilized to quantify the relative expression of target protein (cTPPT) by normalizing the fluorescence intensity of target protein band using their respective internal control protein bands (β-actin) fluorescence intensity. Western blot membranes were subjected to two different scans of 680 nm (for control protein) and 800 nm (for target protein).
Transfection of SLC44A4 promoter constructs and luciferase assay.
Using Lipofectamine 2000 reagent (Life Technologies, Carlsbad, CA), both the full-length and minimal promoters (3 μg/well) of the human SLC44A4 genes (previously generated and characterized in our laboratory) were transiently transfected (together with 100 ng of Renilla luciferase-thymidine kinase plasmid construct) into NCM460 cells as described previously (46). Cells were then exposed to LPS (50 µg/mL; 48 h) followed by quantifying firefly luciferase reporter activity with the help of dual-luciferase assay system (Promega, Madison, WI). The data were normalized relative to Renilla-luciferase activity of each samples using a Glomax 20/20 Luminometer (Promega, Madison, WI).
TLR4 receptor-specific siRNA transfection.
Thirty picomoles of TLR4-specific siRNA were transfected into 60%–80% confluence NCM460 cells using Lipofectamine RNAiMAX transfection reagent and followed the manufacturer’s protocol. Twenty-four hours after transfection, cells were exposed to LPS for 48 h followed by measuring TPP uptake, and cTPPT as well as Elf-3 (E74-like ETS transcription factor 3) mRNA expression levels.
Statistical analysis.
Generated data are expressed as means ± SE of three to six separate determinations and presented in the figures as a percentage relative to simultaneously performed controls using GraphPad Prism 9 software (GraphPad Software, Inc.). Unpaired Student’s t test (Welch’s t test) and ANOVA (followed by Dunnett’s post hoc test) were used for statistical analyses, and a P value of <0.05 was considered as statistically significant.
RESULTS
Effect of Bacterial LPS on Colonic Carrier-Mediated Uptake of TPP
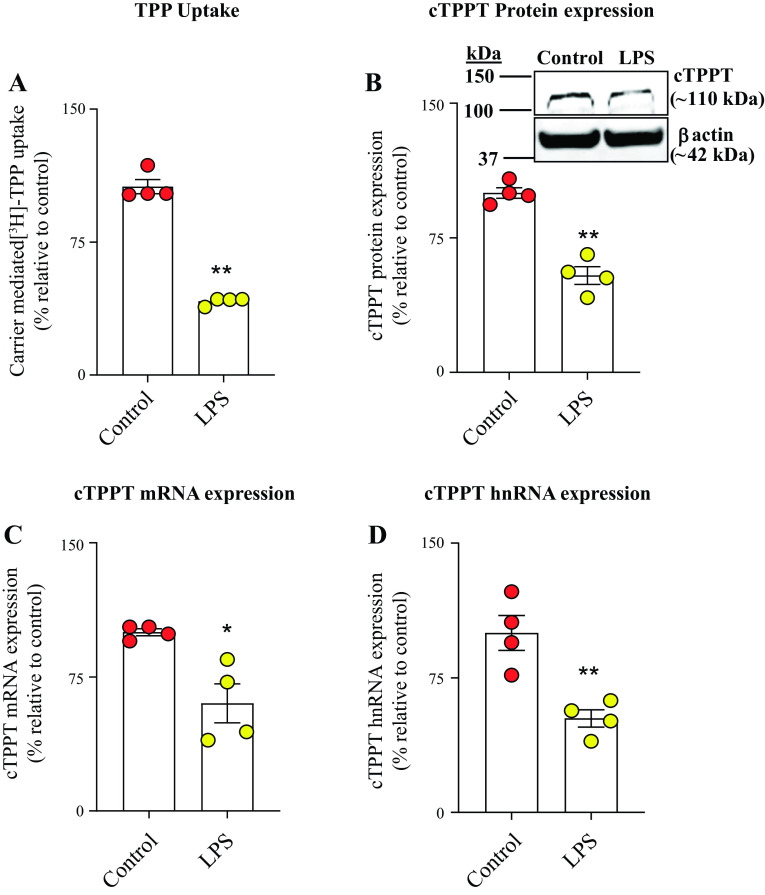
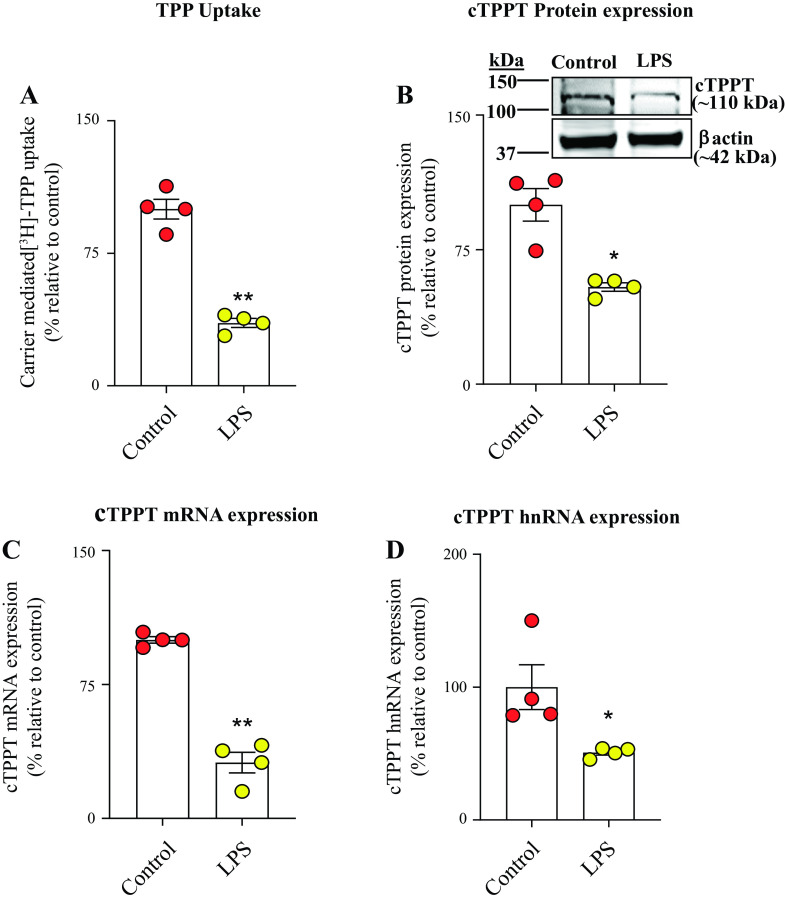
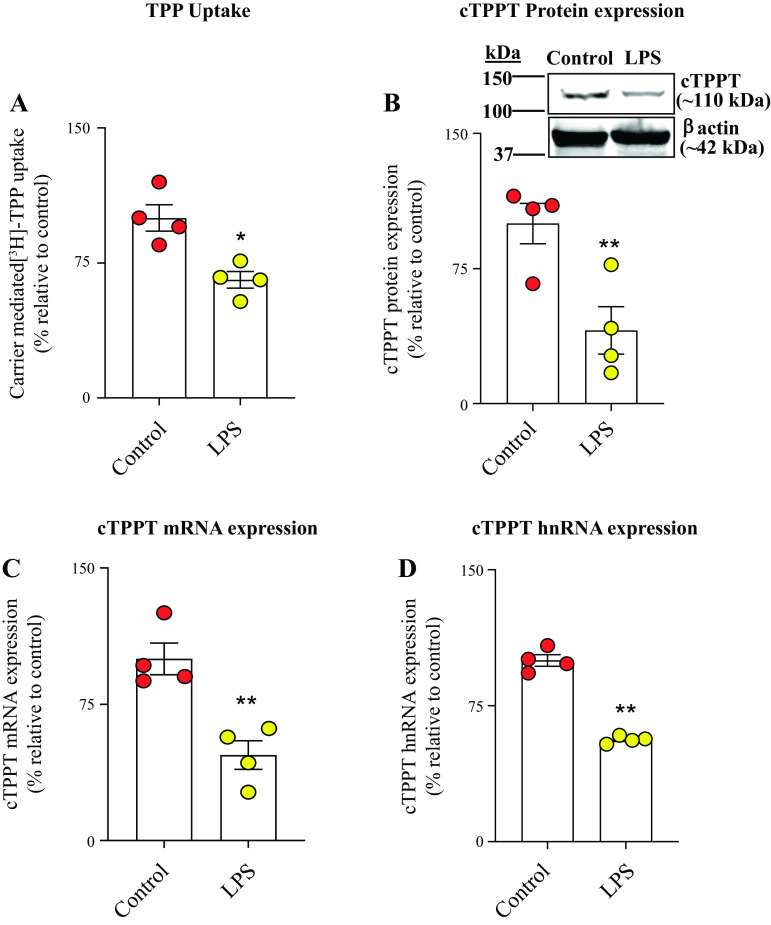
In this study, we used in vitro (human-derived colonic epithelial NCM460), ex vivo (human differentiated colonoid monolayers), and in vivo (mouse colonic tissue) models to assess the influence of bacterial LPS on colonic TPP uptake. In the in vitro model, we examined the effect of exposure of human-derived colonic epithelial NCM460 cells to LPS (50 μg/mL, 48 h) (30, 31 on carrier-mediated TPP uptake (0.23 μM; 7 min). The results showed a significant (P < 0.01) inhibition in TPP uptake in LPS-treated cells compared with simultaneously performed untreated controls (Fig. 1A). This inhibition was found to be associated with a significant reduction in the level of expression of the cTPPT protein (P < 0.01), mRNA (P < 0.05), and hnRNA (P < 0.01) levels in LPS-treated cells compared with untreated controls (Fig. 1, B–D). To assess the translational relevance of these in vitro findings, we tested the effect of LPS (50 μg/mL; 48 h) on carrier-mediated uptake of TPP using the ex vivo model, human differentiated colonoid monolayers. The results again showed a significant inhibition in carrier-mediated TPP uptake (P < 0.01) that is associated with the suppression in the level of expression of the cTPPT protein (P < 0.05), mRNA (P < 0.01), and hnRNA (P < 0.05) in the LPS-treated colonoid monolayers compared with the untreated controls (Fig. 2, A–D). We also sought in vivo confirmation of the above-described in vitro and ex vivo findings, and for this, we examined the effect of treating mice with LPS (5 mg/kg body wt, ip) (30, 31) on colonic TPP (0.46 μM, 7 min) uptake. The results again showed a significant (P < 0.05) inhibition in colonic carrier-mediated TPP uptake by colonic sheets from LPS-treated mice compared with control (vehicle-treated) mice (Fig. 3A). Again, the inhibition was found to be associated with a significant (P < 0.01 for all) reduction in the level of expression of the cTPPT protein, mRNA, and hnRNA (Fig. 3, B–D).
Figure 1.
Effect of exposure of the human-derived colonic epithelial NCM460 cells to LPS (50 µg/mL; for 48 h) on: carrier-mediated [3H]-TPP uptake (A), and level of expression of cTPPT (B) protein (by Western blotting), mRNA (by RT-qPCR) (C), and hnRNA (by RT-qPCR) (D). All protein, mRNA, and hnRNA data were normalized relative to β-actin, and comparison was made relative to simultaneously performed untreated controls. Data are presented as means ± SE of 4 independent experiments. Statistical analysis of all the data was assessed by the Student’s t test (Welch’s t test). *P < 0.05; **P < 0.01. cTPPT, colonic thiamin pyrophosphate transporter; hnRNA, heterologous nuclear RNA; LPS, lipopolysaccharide; RT-qPCR, real-time quantitative PCR; TPP, thiamin pyrophosphate.
Figure 2.
Effect of exposure of human differentiated and polarized colonoid monolayers to LPS (50 µg/mL; for 48 h) on: carrier-mediated [3H]-TPP uptake (A), and on level of expression of cTPPT protein (B), mRNA (C), and hnRNA (D). Results for protein and mRNA were normalized relative to β-actin, and comparison was made relative to simultaneously performed untreated controls. Data are presented as means ± SE of 4 independent experiments. Statistical analysis was assessed by mean of the Student’s t test (Welch’s t test). *P < 0.05; **P < 0.01. cTPPT, colonic thiamin pyrophosphate transporter; hnRNA, heterologous nuclear RNA; LPS, lipopolysaccharide; TPP, thiamin pyrophosphate.
Figure 3.
Effect of treating mice with LPS (5 mg/kg body wt; ip) on colonic: carrier-mediated [3H]-TPP uptake (A), and level of expression of cTPPT protein (B), mRNA (C), and hnRNA (D). Uptake was performed using colonic sheets (1 cm in length) as described in methods. The cTPPT protein and mRNA data were normalized relative to β-actin expression, and comparison was made relative to control (vehicle treated) mice. Data are presented as means ± SE of 4 independent experiments; statistical significance was evaluated by the Student’s t test (Welch’s t test). *P < 0.05; **P < 0.01. cTPPT, colonic thiamin pyrophosphate transporter; hnRNA, heterologous nuclear RNA; LPS, lipopolysaccharide; TPP, thiamin pyrophosphate.
Effect of Bacterial LPS on Activity of the SLC44A4 Gene Promoter in Human Colonic Epithelial Cells: Role of Transcriptional Mechanism(s)
The inhibition caused by LPS in the level of expression of the cTPPT mRNA and hnRNA suggests a possible involvement of transcriptional mechanism(s) affecting the SLC44A4 gene. To assess this possibility, we exposed colonic epithelial NCM460 cells that have been transfected with full-length and minimal SLC44A4 promoters (fused to the luciferase reporter gene) to LPS (50 µg/mL for 48 h) followed by determination of promoter activity. The results showed that LPS treatment of cells caused significant (P < 0.01 for both) reduction in activity of both the full-length and the minimal SLC44A4 promoters (Fig. 4, A and B). These findings suggest that transcriptional mechanism(s) are involved in mediating the effect of LPS on cTPPT expression in human colonocytes. The data also suggest that the LPS-responsive region(s) is localized in the minimal promoter region of this gene.
Figure 4.
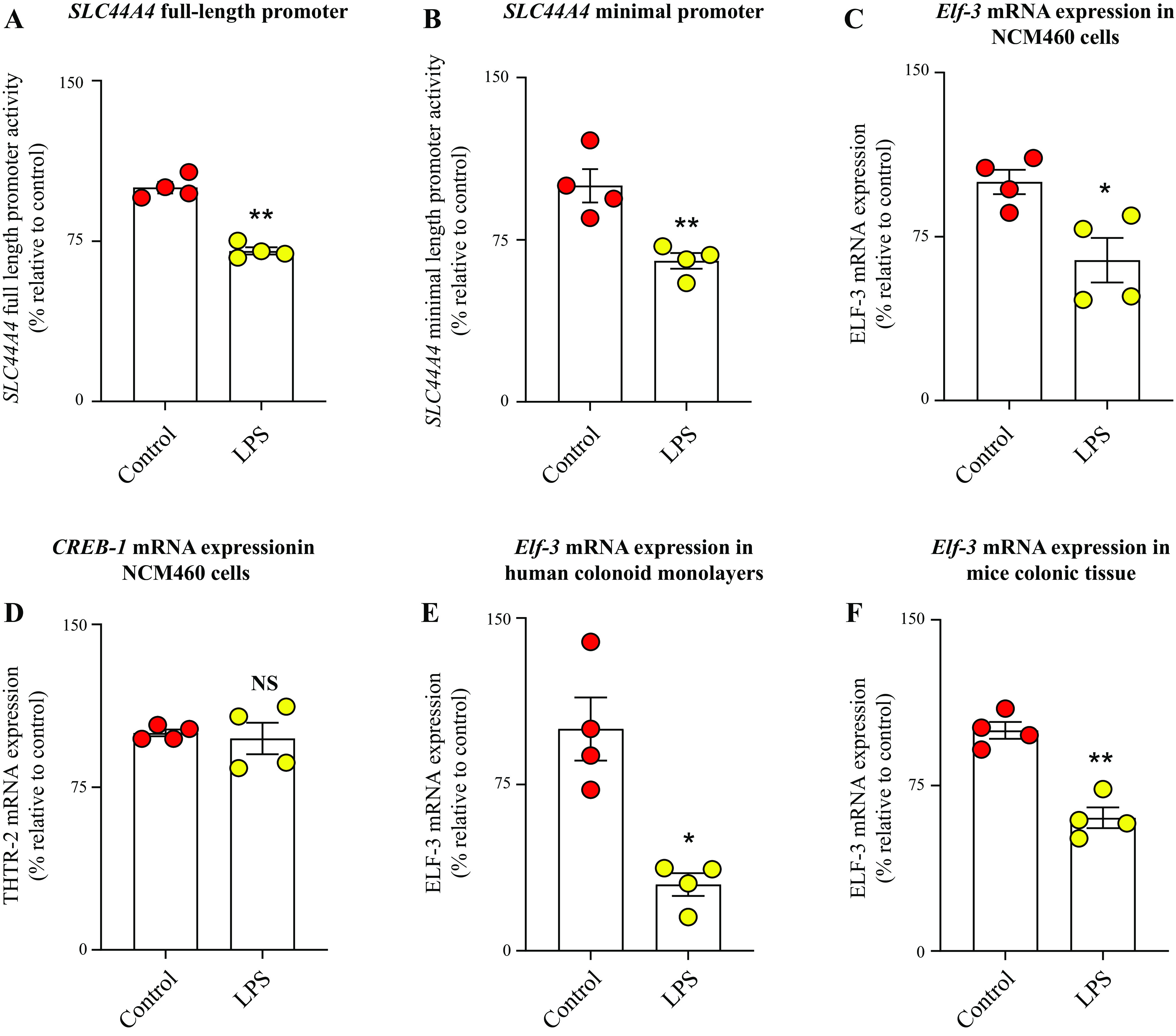
Effect of exposure of human colonic epithelial cells to LPS on activity of the SLC44A4 promoter and on level of expression of relevant transcription activators. Activity of the full-length (A) and minimal SLC44A4 (B) promoters, respectively; levels of mRNA expression of the transcription factors Elf-3 (C) and CREB-1 (D) in human colonic epithelial NCM460 cells; level of expression of Elf-3 mRNA in human differentiated colonoid monolayers (E); and level of expression of Elf-3 mRNA in colonic tissue of mice treated with LPS (F). Activity of the luciferase reporter was normalized relative to renilla luciferase activity, and mRNA levels were normalized relative to Ct value of β-actin gene of the respective samples. Data are presented as means ± SE of 4 independent experiments. Statistical analysis was assessed using the Student’s t test (Welch’s t test). *P < 0.05; **P < 0.01. CREB-1, cAMP responsive element-binding protein 1; Ct, cycle threshold; Elf-3, E74-like ETS transcription factor 3; LPS, lipopolysaccharide; NS, not significant; THTR-2, thiamin transporter-2.
Previous studies from our laboratory have demonstrated an important role for the nuclear factors CREB-1 (cAMP responsive element-binding protein 1) and Elf-3 (E74-like ETS transcription factor 3) in driving basal activity of the SLC44A4 promoter (21, 22, 46). Thus, we also examined whether the effect of LPS on the activity of the SLC44A4 promoter is (at least in part) mediated via inhibition in the level of expression of these transcription factors. For this, we treated NCM460 cells with LPS (50 µg/mL), followed by determining (by mean of RT-qPCR) the levels of expression of CREB-1 and Elf-3. The results showed significant (P < 0.05) inhibition in the level of expression of Elf-3 (but not CREB-1) in cells treated with LPS compared with untreated controls (Fig. 4, C and D). Similarly, the level of expression of the Elf-3 mRNA in LPS-exposed human differentiated colonoid monolayers (P < 0.05) and colonic sheets of mice treated with the endotoxin in vivo (P < 0.01) were found to be significantly reduced compared with respective controls (Fig. 4, E and F).
Role of the TLR4 Receptor and the NF-κB/p38/JNK/ERK1/2 Signaling Pathways in Mediating the Inhibitory Effect of LPS on Colonic TPP Uptake and on Level of Expression of cTPPT and Elf-3 mRNAs
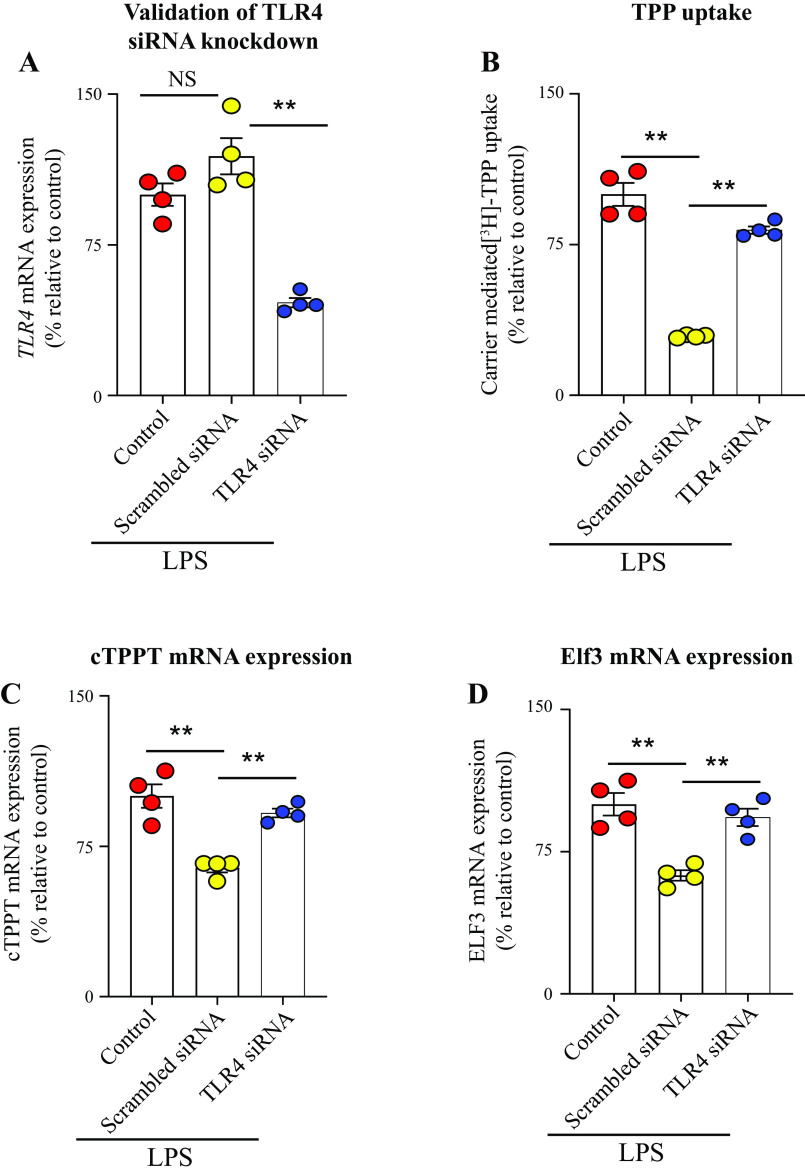
As mentioned earlier, the effects of LPS on cell physiology are mediated mainly via TLR4 receptor and lead to the activation of intracellular signaling pathways including NF-kB, p38, JNK, and ERK1/2 (27, 33–35). Thus, we studied the effect of knocking down the TLR4 receptor of the colonic epithelial NCM460 cells with gene-specific siRNA on the effect of LPS on TPP uptake. The results showed that knocking down the TLR4 receptor (verified by mean of RT-qPCR) led to a significant abrogation in the inhibitory effect of LPS on colonic TPP uptake (P < 0.01) as well as the level of expression of the cTPPT (P < 0.01) and Elf-3 (P < 0.01) mRNAs (Fig. 5, A–D). These results suggested that LPS effect on colonic uptake of TPP is mediated via the TLR4 receptor.
Figure 5.
Effect of TLR4 receptor knockdown on the inhibitory effect of LPS on TPP uptake and the level of expression of cTPPT and Elf-3 in human colonic epithelial NCM460 cells. qPCR data showing the effect of transfection with TLR4 siRNAs (see methods) on level of expression of the TLR4 receptor in NCM460 cells (A); carrier-mediated [3H]-TPP uptake (B); and levels of mRNA expression of the cTPPT (C) and Elf-3 (D) following TLR4 receptor knockdown. The mRNA expressions were normalized relative to level of expression of β-actin. Data are presented as means ± SE of 4 independent experiments. The Student’s t test (Welch’s t test) and ANOVA (Dunnett’s post hoc test) were used for statistical analysis. **P < 0.01. cTPPT, colonic thiamin pyrophosphate transporter; Elf-3, E74-like ETS transcription factor 3; LPS, lipopolysaccharide; NS, not significant. TLR4, Toll-like receptor 4; TPP, thiamin pyrophosphate.
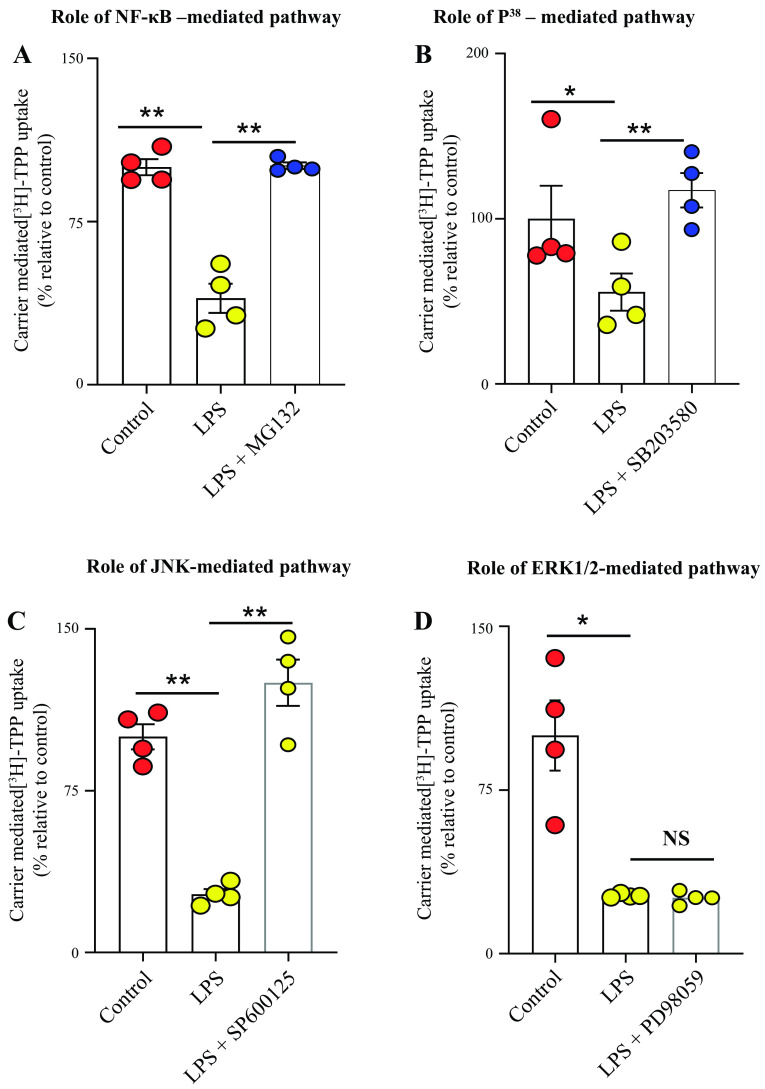
We also examined the role of the NF-κB, p38, JNK, and ERK1/2 signaling mechanisms in mediating the inhibitory effect of LPS on the physiological and molecular parameters of the colonic TPP uptake process. This was done by examining the effect of blocking these pathways with the use of specific pharmacological inhibitors (10 μM of MG-132, 1 μM of SB203580, 5 μM of SP600125, and 50 μM of PD98059) on the degree of inhibition of LPS in TPP uptake by colonic epithelial NCM460 cells. The results showed that blocking the NF-κB, JNK, and p38 pathways (but not the ERK1/2 pathway) led to significant (P < 0.01 for all) abrogation in the degree of LPS-mediated inhibition in TPP uptake (Fig. 6, A–D). We also tested the effect of treating the colonic epithelia NCM460 cells with the earlier described inhibitors on the level of expression of cTPPT mRNA and protein. The results showed that the treatment with inhibitors of the NF-κB, JNK, and p38 pathways led to significant abrogation (P < 0.01 for NF-κB; P < 0.05 for p38, and P < 0.01 for JNK) in the inhibitory effect of LPS on the level of expression of the cTPPT mRNA (Fig. 7, A–C).
Figure 6.
Role of the NF-κB, p38, JNK, and ERK1/2 signaling pathways in mediating the inhibitory effect of LPS on colonic carrier-mediated [3H]-TPP uptake by human colonic epithelial NCM460 cells. NCM460 cells were pretreated with the specific inhibitor of the different pathways (see methods) followed by the determination of [3H]-TPP uptake. The following inhibitors were used: MG-132 (inhibitor of the NF-κB pathway; A); SB203580 (inhibitor of the p38 pathway; B); SP600125 (inhibitor of the JNK pathway; C); and PD98059 (inhibitor of the ERK1/2 pathway; D). Uptake data are presented as means ± SE of multiple (n = 4) independent experiments. The Student’s t test (Welch’s t test) and ANOVA (Dunnett’s post hoc test) were used for statistical analysis. *P < 0.05; **P < 0.01. LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B; NS, not significant; TPP, thiamin pyrophosphate.
Figure 7.
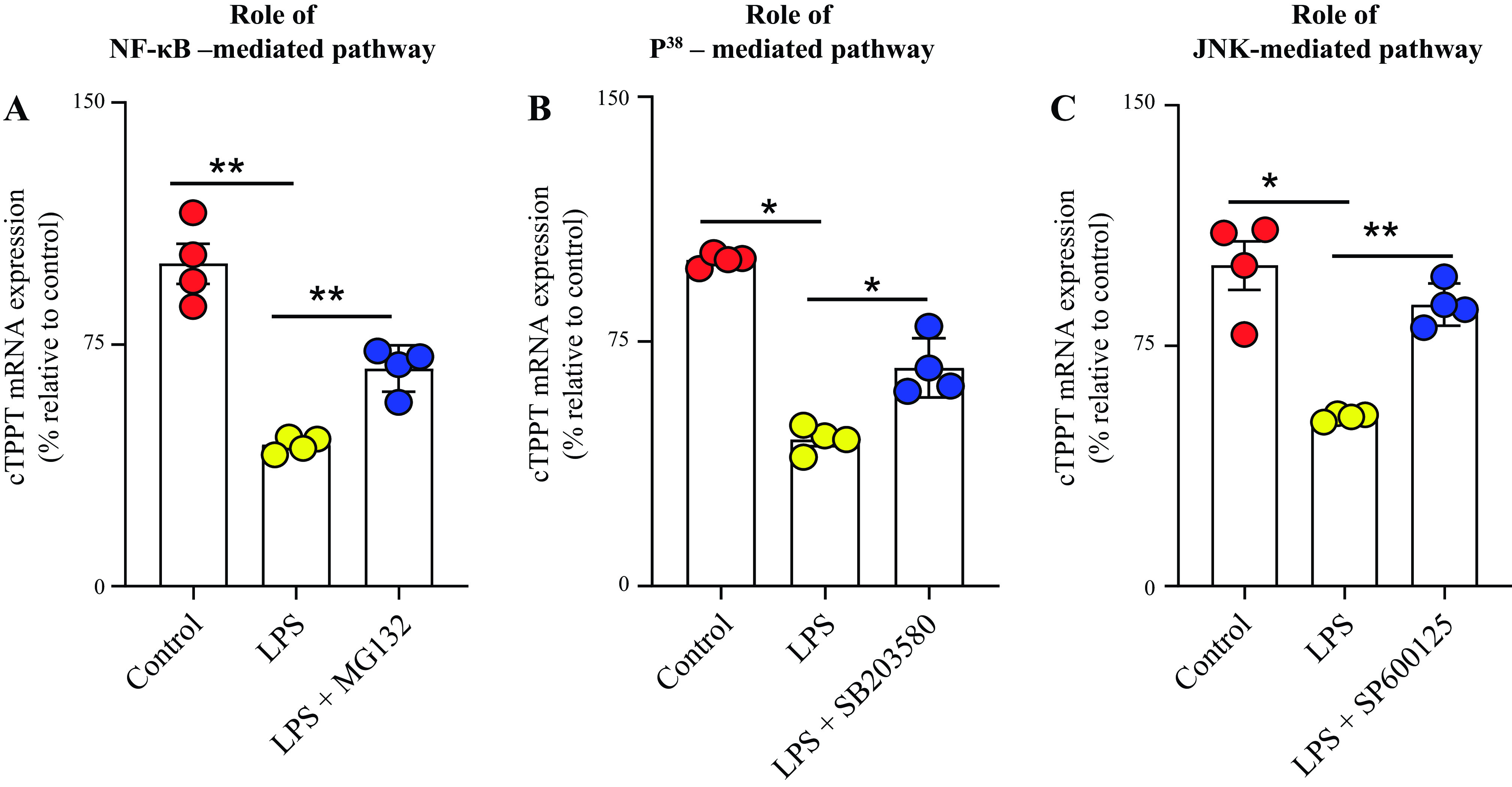
The role of NF-κB (A), p38 (B), and JNK (C) signaling pathways in mediating the inhibitory effect of LPS on level of expression of cTPPT mRNA in human colonic epithelial NCM460 cells. Levels of cTPPT mRNA were normalized relative to level of expression of β-actin. Data are presented as means ± SE of 4 independent experiments. The Student’s t test (Welch’s t test) and ANOVA (Dunnett’s post hoc test) were used for statistical analysis. *P< 0.05; **P < 0.01. cTPPT, colonic thiamin pyrophosphate transporter; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B.
In other studies, we examined the effect of blocking the NF-κB, JNK, and p38 signaling pathways on the level of inhibition caused by LPS in mRNA expression of the transcription factor Elf-3. The results showed a significant (P < 0.05 for NF-κB; P < 0.01 for JNK; and P < 0.01 for p38) reduction in the degree of inhibition caused by LPS in the level of expression of Elf-3 mRNA (Fig. 8, A–C). The above findings suggest that the NF-κB, JNK, and p38 signaling pathways are involved in mediating the inhibitory effects of LPS on the physiology and molecular biology of the colonic TPP uptake process.
Figure 8.
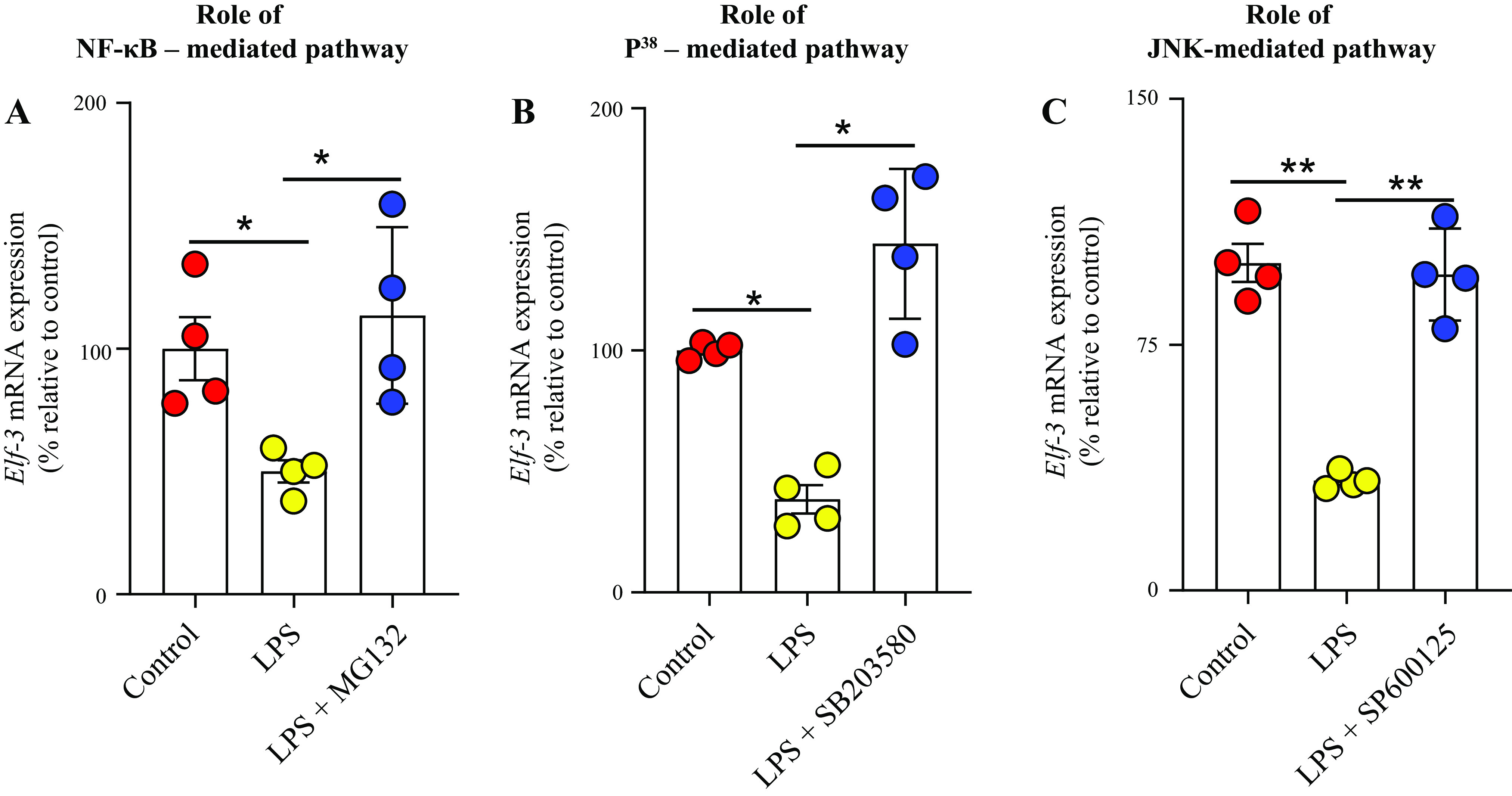
Role of NF-κB (A), p38 (B), and JNK (C) signaling pathways in mediating the inhibitory effect of LPS on the level of mRNA expression of Elf-3 in human-derived colonic epithelial NCM460 cells. Levels of Elf-3 mRNA were normalized relative to β-actin gene, and comparison was made relative to simultaneously performed (untreated) controls. Data are presented as means ± SE of 4 independent experiments. The Student’s t test (Welch’s t test) and ANOVA (Dunnett’s post hoc test) were used for statistical analysis. *P < 0.05; **P < 0.01. Elf-3, E74-like ETS transcription factor 3; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B.
DISCUSSION
The purpose of this study was to investigate the effect(s) of exposure of colonic epithelial cells to the bacterial endotoxin LPS on physiological and molecular parameters of the colonic carrier-mediated uptake of the microbiota-generated TPP, and to uncover the mechanism(s) involved. Addressing this issue is of both nutritional and physiological importance. Nutritionally, the cTPPT system is the only uptake system that is capable of absorbing the microbiota-generated form of vitamin B1, i.e., TPP. Physiologically, colonocytes have limited capability to generate the active form of vitamin B1 (TPP) from free thiamin (they express low level of the required enzyme, thiamin pyrophosphokinase; TPKase) (38), and thus, this source of TPP is important for their normal energy metabolism. We have previously delineated different physiological and molecular biology aspects of the colonic TPP uptake process; we have also investigated how certain external and internal factors and conditions affect this process and the transport system involved (17–23). Nothing, however, is known about the possible effect of the bacterial endotoxin LPS on colonic TPP uptake process and the transport system involved. We addressed these issues in the current study using three complementary models of colonocytes that includes cultured human-derived colonic epithelial NCM460 cells, human differentiated colonoid monolayers, and intact mouse colonic tissue.
Results of the current investigations showed that exposing the human-derived colonic epithelial NCM460 cells exposed to LPS caused a significant inhibition in carrier-mediated TPP uptake. This inhibition was associated with a notable suppression in the level of expression of the cTPPT protein, mRNA, and hnRNA levels. Similar results were obtained when human differentiated colonoid monolayers as well as mouse colonic tissue were exposed to LPS. Findings with the latter approaches also demonstrated the translational as well as the in vivo relevance of our findings. This effect of LPS appears to be mediated (at least in part) at the level of transcription of the SLC44A4 gene as suggested by the findings of inhibition in activity of the SLC44A4 promoters (both full-length and minimal promoters) in NCM460 cells expressing these constructs. Knowing that the nuclear factors Elf-3 and CREB-1 play important roles in driving the basal activity of the SLC44A4 promoter in colonic epithelial cells (21, 22, 46), we also tested whether the effect of LPS on SLC44A4 transcription is mediated via an effect of the endotoxin on the expression of these nuclear factors. Indeed, the results showed significant suppression in the level of expression of Elf-3 (but not CREB-1) in colonic epithelial cells treated with LPS.
As mentioned earlier, LPS effects on cell physiology are mediated via Toll-like receptors (mainly TLR4, which in gut epithelial cells is mostly expressed at the apical membrane domain) (47–49) and lead to the activation of intracellular signaling pathways like NF-κB, p38, JNK1/2, and ERK1/2 (27, 33–37, 47–54). Thus, we confirmed the involvement of TLR4 receptor in mediating the effect of LPS on colonic carrier-mediated uptake of TPP by demonstrating a significant abrogation in the inhibitory effect of the endotoxin on TPP uptake and on cTPPT expression upon knocking down the TLR4 receptor. Furthermore, we obtained evidence that shows the involvement of the NF-κB, p38, and JNK1/2 signaling pathways in mediating the effect of LPS on the physiology and molecular biology of colonic TPP uptake process (no role for ERK1/2 was found). The latter is in reference to the findings that specific pharmacological inhibitors of these pathways led to a significant attenuation of the inhibitory effects of LPS on colonic TPP uptake process.
In summary, results of these investigations show that exposure of colonic epithelial cells to bacterial LPS negatively impact TPP uptake and expression of the involved transporter (cTPPT), and that this is occurring (at least in part) via interference with SLC44A4 transcription. The results also show that the TLR4 receptor and the NF-κB/p38/JNK signaling pathways are involved in mediating these effects.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by grants from the Department of Veterans Affairs (I01BX001142 to HMS; 5I01BX001469-05 to JMF), and the National Institutes of Health (DK56061, AA018071, and DK056061-23S1 to HMS; AI089894 and AI170949 to JMF; T32AI007172 to AS; and P30 DK052574 (NIH) to the Washington University Digestive Diseases Research Center grant). HMS is a recipient of a Senior Research Career Scientist award (# IK6BX006189) from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A., S.S., and H.M.S. conceived and designed research; S.A., S.S., K.L., A.S., and H.M.S. performed experiments; S.A., S.S., K.L., A.S., J.M.F., and H.M.S. analyzed data; S.A., S.S., A.S., J.M.F., and H.M.S. interpreted results of experiments; S.A., S.S., and H.M.S. prepared figures; S.A., S.S., and H.M.S. drafted manuscript; S.A., S.S., A.S., J.M.F., and H.M.S. edited and revised manuscript; S.A., S.S., K.L., A.S., J.M.F., and H.M.S. approved final version of manuscript.
REFERENCES
- 1. Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med 1: 197–207, 2001. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- 2. Sambon M, Wins P, Bettendorff L. Neuroprotective effects of thiamine and precursors with higher bioavailability: focus on benfotiamine and dibenzoylthiamine. Int J Mol Sci 22: 5418, 2021. doi: 10.3390/ijms22115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol 44: 141–147, 2009. doi: 10.1093/alcalc/agn120. [DOI] [PubMed] [Google Scholar]
- 4. Hernandez-Vazquez AJ, Garcia-Sanchez JA, Moreno-Arriola E, Salvador-Adriano A, Ortega-Cuellar D, Velazquez-Arellano A. Thiamine deprivation produces a liver ATP deficit and metabolic and genomic effects in mice: findings are parallel to those of biotin deficiency and have implications for energy disorders. J Nutrigenet Nutrigenomics 9: 287–299, 2016. doi: 10.1159/000456663. [DOI] [PubMed] [Google Scholar]
- 5. Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 6. Woolum JA, Abner EL, Kelly A, Bastin MLT, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med 46: 1747–1752, 2018. doi: 10.1097/CCM.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 7. Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care 18: 576–581, 2015. doi: 10.1097/MCO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 8. Gomes F, Bergeron G, Bourassa MW, Fischer PR. Thiamine deficiency unrelated to alcohol consumption in high‐income countries: a literature review. Ann N Y Acad Sci 1498: 46–56, 2021. doi: 10.1111/nyas.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 50: 2164–2170, 2007. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet 6: 148, 2015. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR et al. Enterotypes of the human gut microbiome. Nature 473: 174–180, 2011. [Erratum in Nature 474: 666, 2011, and in Nature 506: 516, 2014]. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wrong OM, Edmonds CJ, Chadwick VS. The Large Intestine. Its Role in Mammalian Nutrition and Homeostasis. New York, NY: Wiley and Sons, 1981, p. 157–166. doi: 10.1136/gut.22.9.780. [DOI] [Google Scholar]
- 13. Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med 224: 246–255, 2000. doi: 10.1046/j.1525-1373.2000.22428.x. [DOI] [PubMed] [Google Scholar]
- 14. Said HM, Nexo E. Gastrointestinal handling of water-soluble vitamins. Compr Physiol 8: 1291–1311, 2018. doi: 10.1002/cphy.c170054. [DOI] [PubMed] [Google Scholar]
- 15. Dawson I, Pryse-Davies J. The distribution of certain enzyme systems in the normal human gastrointestinal tract. Gastroenterology 44: 745–760, 1963. doi: 10.1016/S0016-5085(63)80084-0. [DOI] [PubMed] [Google Scholar]
- 16. Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am J Physiol Gastrointest Liver Physiol 281: G144–G150, 2001. doi: 10.1152/ajpgi.2001.281.1.G144. [DOI] [PubMed] [Google Scholar]
- 17. Nabokina SM, Said HM. A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 303: G389–G395, 2012. doi: 10.1152/ajpgi.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nabokina SM, Inoue K, Subramanian VS, Valle JE, Yuasa H, Said HM. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. J Biol Chem 289: 4405–4416, 2014. [Erratum in J Biol Chem 292: 16526, 2017]. doi: 10.1074/jbc.M113.528257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabokina SM, Ramos MB, Said HM. Mechanism(s) involved in the colon-specific expression of the thiamine pyrophosphate (TPP) transporter. PLoS One 11: e0149255, 2016. [Erratum in PLoS One 12: e0186550, 2017]. doi: 10.1371/journal.pone.0149255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anandam KY, Srinivasan P, Subramanian VS, Said HM. Molecular mechanisms involved in the adaptive regulation of the colonic thiamin pyrophosphate uptake process. Am J Physiol Cell Physiol 313: C655–C663, 2017. doi: 10.1152/ajpcell.00169.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anthonymuthu S, Sabui S, Sheikh A, Fleckenstein JM, Said HM. Tumor necrosis factor α impedes colonic thiamin pyrophosphate and free thiamin uptake: involvement of JNK/ERK1/2-mediated pathways. Am J Physiol Cell Physiol 323: C1664–C1680, 2022. doi: 10.1152/ajpcell.00458.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabui S, Ramamoorthy K, Romero JM, Simoes RD, Fleckenstein JM, Said HM. Hypoxia inhibits colonic uptake of the microbiota-generated forms of vitamin B1 via HIF-1α-mediated transcriptional regulation of their transporters. J Biol Chem 298: 101562, 2022. doi: 10.1016/j.jbc.2021.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anandam KY, Sabui S, Thompson MM, Subramanian S, Said HM. Enterohemorrhagic Escherichia coli infection inhibits colonic thiamin pyrophosphate uptake via transcriptional mechanism. PLoS One 14: e0224234, 2019. doi: 10.1371/journal.pone.0224234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Page MJ, Kell DB, Pretorius E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress (Thousand Oaks) 6: 24705470221076390, 2022. doi: 10.1177/24705470221076390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev 40: 480–493, 2016. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 182: 375–387, 2013. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rhee SH. Lipopolysaccharide: basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest Res 12: 90–95, 2014. doi: 10.5217/ir.2014.12.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohr AE, Crawford M, Jasbi P, Fessler S, Sweazea KL. Lipopolysaccharide and the gut microbiota: considering structural variation. FEBS Lett 596: 849–875, 2022. doi: 10.1002/1873-3468.14328. [DOI] [PubMed] [Google Scholar]
- 29. Guerville M, Boudry G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am J Physiol Gastrointest Liver Physiol 311: G1–G15, 2016. doi: 10.1152/ajpgi.00098.2016. [DOI] [PubMed] [Google Scholar]
- 30. Subramanian VS, Sabui S, Moradi H, Marchant JS, Said HM. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim Biophys Acta Biomembr 1860: 556–565, 2018. doi: 10.1016/j.bbamem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakhan R, Said HM. Lipopolysaccharide inhibits colonic biotin uptake via interference with membrane expression of its transporter: a role for a casein kinase 2-mediated pathway. Am J Physiol Cell Physiol 312: C376–C384, 2017. doi: 10.1152/ajpcell.00300.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun 15: 243–250, 2009. doi: 10.1177/1753425909104781. [DOI] [PubMed] [Google Scholar]
- 33. Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Laboratory Investigation 86: 9–22, 2005. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 34. Lannoy V, Côté-Biron A, Asselin C, Rivard N. Phosphatases in toll-like receptors signaling: the unfairly-forgotten. Cell Commun Signal 19: 10, 2021. doi: 10.1186/s12964-020-00693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 72: 557–581, 2015. doi: 10.1007/s00018-014-1762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng Y-L, Zhang M-Q, Zhao Y, Chen J, Li B, Cai W. JNK inhibitor SP600125 protects against lipopolysaccharide-induced acute lung injury via upregulation of claudin-4. Exp Ther Med 8: 153–158, 2014. doi: 10.3892/etm.2014.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kong Q, Hua H, Cui A, Shao T, Song P, Jiang Y. SP600125 induces Src and type I IGF receptor phosphorylation independent of JNK. Int J Mol Sci 15: 16246–16256, 2014. doi: 10.3390/ijms150916246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao R, Gao F, Goldman ID. Molecular cloning of human thiamin pyrophosphokinase. Biochim Biophys Acta 1517: 320–322, 2001. doi: 10.1016/s0167-4781(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 39. Santoru ML, Piras C, Murgia F, Spada M, Tronci L, Leoni VP, Serreli G, Deiana M, Atzori L. Modulatory effect of nicotinic acid on the metabolism of Caco-2 cells exposed to IL-1β and LPS. Metabolites 10: 204, 2020. doi: 10.3390/metabo10050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang W, Zhao P, Shen A, Liu L, Chen H, Chen Y, Peng J, Sferra T, Sankararaman S, Luo Y, Ke X. Effects of Qing Hua Chang Yin on lipopolysaccharide-induced intestinal epithelial tight junction injury in Caco-2 cells. Mol Med Rep 23: 205, 2021. doi: 10.3892/mmr.2021.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirotani Y, Ikeda K, Kato R, Myotoku M, Umeda T, Ijiri Y, Tanaka K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 128: 1363–1368, 2008. doi: 10.1248/yakushi.128.1363. [DOI] [PubMed] [Google Scholar]
- 42. Sheikh A, Tumala B, Vickers TJ, Alvarado D, Ciorba MA, Bhuiyan TR, Qadri F, Singer BB, Fleckenstein JM. CEACAMs serve as toxin-stimulated receptors for enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA 117: 29055–29062, 2020. doi: 10.1073/pnas.2012480117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soromou LW, Jiang L, Wei M, Chen N, Huo M, Chu X, Zhong W, Wu Q, Baldé A, Deng X, Feng H. Protection of mice against lipopolysaccharide-induced endotoxic shock by pinocembrin is correlated with regulation of cytokine secretion. J Immunotoxicol 11: 56–61, 2014. doi: 10.3109/1547691x.2013.792886. [DOI] [PubMed] [Google Scholar]
- 44. An N, Song Y, Zhang X, Ci X, Li H, Cao Y, Zhang M, Cui J, Deng X. Pretreatment of mice with rifampicin prolongs survival of endotoxic shock by modulating the levels of inflammatory cytokines. Immunopharmacol Immunotoxicol 30: 437–446, 2008. doi: 10.1080/08923970802135146. [DOI] [PubMed] [Google Scholar]
- 45. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46. Nabokina SM, Ramos MB, Valle JE, Said HM. Regulation of basal promoter activity of the human thiamine pyrophosphate transporter SLC44A4 in human intestinal epithelial cells. Am J Physiol Cell Physiol 308: C750–C757, 2015. [Erratum in Am J Physiol Cell Physiol 313: C473, 2017]. doi: 10.1152/ajpcell.00381.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 160: 165–173, 2002. doi: 10.1016/S0002-9440(10)64360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68: 7010–7017, 2000. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu S, Gao N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci 72: 3343–3353, 2015. doi: 10.1007/s00018-015-1931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, Barton GM. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity 49: 560–575.E6, 2018. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu C, Chen Y, Zhang L, Liu M, Yang J, Huang F, Wang Y, Huang L. Curcumin analog C66 alleviates inflammatory colitis by inhibiting the activation of NF-κB. Inflammopharmacology 30: 2167–2179, 2022. doi: 10.1007/s10787-022-01085-w. [DOI] [PubMed] [Google Scholar]
- 52. Yuan Y, Sun M, Li K-S. Astragalus mongholicus polysaccharide inhibits lipopolysaccharide-induced production of TNF-α and interleukin-8. World J Gastroenterol 15: 3676, 2009. doi: 10.3748/wjg.15.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Drosatos K, Drosatos-Tampakaki Z, Khan R, Homma S, Schulze PC, Zannis VI, Goldberg IJ. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor α expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. J Biol Chem 286: 36331–36339, 2011. doi: 10.1074/jbc.m111.272146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan MA, Farahvash A, Douda DN, Licht J-C, Grasemann H, Sweezey N, Palaniyar N. JNK activation turns on LPS- and gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci Rep 7: 3409, 2017. doi: 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gabarin RS, Li M, Zimmel PA, Marshall JC, Li Y, Zhang H. Intracellular and extracellular lipopolysaccharide signaling in sepsis: avenues for novel therapeutic strategies. J Innate Immun 13: 323–332, 2021. doi: 10.1159/000512713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Akiba Y, Maruta K, Takajo T, Narimatsu K, Said H, Kato I, Kuwahara A, Kaunitz JD. Lipopolysaccharides transport during fat absorption in rodent small intestine. Am J Physiol Gastrointest Liver Physiol 318: G1070–G1087, 2020. doi: 10.1152/ajpgi.00079.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.