Abstract
Chlorophyll is one of the key factors for photosynthesis and plays an important role in plant growth and development. We previously isolated an EMS mutagenized rapeseed chlorophyll-reduced mutant (crm1), which had yellow leaf, reduced chlorophyll content and fewer thylakoid stacks. Here, we found that crm1 showed attenuated utilization efficiency of both light energy and CO2 but enhanced heat dissipation efficiency and greater tolerance to high-light intensity. BSA-Seq analysis identified a single nucleotide change (C to T) and (G to A) in the third exon of the BnaA01G0094500ZS and BnaC01G0116100ZS, respectively. These two genes encode the magnesium chelatase subunit I 1 (CHLI1) that catalyzes the insertion of magnesium into protoporphyrin IX, a pivotal step in chlorophyll synthesis. The mutation sites resulted in an amino acid substitution P144S and G128E within the AAA+ domain of the CHLI1 protein. Two KASP markers were developed and co-segregated with the yellow leaf phenotype in segregating F2 population. Loss of BnaA01.CHLI1 and BnaC01.CHLI1 by CRISPR/Cas9 gene editing recapitulated the mutant phenotype. BnaA01.CHLI1 and BnaC01.CHLI1 were located in chloroplast and highly expressed in the leaves. Furthermore, RNA-seq analyses revealed the expression of chlorophyll synthesis–related genes were upregulated in the crm1 mutant. These findings provide a new insight into the regulatory mechanism of chlorophyll synthesis in rapeseed and suggest a novel target for improving the photosynthetic efficiency and tolerance to high-light intensity in crops.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01429-6.
Keywords: Photosynthetic characteristics, Chlorophyll synthesis, CHLI, Yellow leaf mutant, crm1, Brassica napus
Introduction
Leaf is the main organ for plants to perform photosynthesis and respiration, which directly affects the yield and quality of crops. Chlorophyll is one of the most abundant organic molecules in plant leaves and is an indispensable component of photosynthesis. The biosynthesis of chlorophyll in higher plants is a very complex enzymatic biochemical reaction process (Tanaka and Tanaka 2007; Ryouichi et al. 2011). Leaf color mutations are of great significance to the study on chlorophyll biosynthesis and metabolism (Colombo et al. 2008; Pogson and Albrecht 2011; Xu et al. 2013).
The most common type of leaf color mutants are the chlorophyll-deficient or reduced mutants, in which pigment content, proportion or component changed due to abnormal chlorophyll synthesis, consequently showing leaf color phenotype. For example, the rice leaf color mutant W1 was resulted from a possible inefficient synthesis from Pchlide to Chlide catalyzed by protochlorophyll ester oxygen reductase, thus attributing to the lowered chlorophylls (Xu et al. 2006). Barley light-green leaf mutant fch2 was caused by mutation of chlorophyll a oxygenase, which converts chlorophyllide a to chlorophyllide b, thus chlorophyll b reduction (Mueller et al. 2012). Premature termination of translation of OsPORB transcript, which encodes a protochlorophyllide oxidoreductase, reduced the chlorophyll content in rice faded green leaf (fgl) mutant, thus leading to severe degreening in leaves (Sakuraba et al. 2013). Additionally, the blocking of magnesium protoporphyrin IX synthesis, in which magnesium chelatase catalyzes the insertion of magnesium into protoporphyrin IX, a pivotal step in chlorophyll synthesis, results in lowered chlorophyll. Those leaf color mutants with impaired magnesium protoporphyrin IX synthesis have been found in barley (Hansson et al. 1999, 2002), Arabidopsis (Rissler et al. 2002; Huang and Li 2009), rice (Zhang et al. 2006;Tian et al. 2013; Jiang et al. 2015), cucumber (Gao et al. 2016), soybean (Du et al. 2018), wheat (Wang et al. 2020a), and strawberry (Ma et al. 2023).
Rapeseed (Brassica napus. L) is one of the important oil crops. The genetic basis of chlorophyll synthesis in rapeseed is not well understood. To date, a few leaf color mutants have been reported in rapeseed, such as Cr3529 (Zhao et al. 2001), chlorophyll-reduced mutant NY (Xiao et al. 2013), chlorophyll-deficient mutant (BnaC.ygl) (Zhu et al. 2014), chlorophyll-deficient mutant cde1 (Wang et al. 2016), yellow to green mutant ytg (Zhang et al. 2022), and white leaf mutant (WL) (Ye et al. 2022). However, only three of these leaf color mutations were identified. The BnaC.ygl mutant was controlled by BnaC07.HO1, which encodes heme oxygenase 1 and catalyzes heme conversion to form biliverdin IXα (Zhu et al. 2014; Zhu et al. 2017). The ytg mutant was controlled by BnaA02.YTG1, which encodes a tetratricopeptide repeat protein and is required for early chloroplast biogenesis in rapeseed (Zhang et al. 2022). The cde1 mutant was controlled by the cytochrome P450-like gene BnaC08g34840D, which is involved in the regulation of heme biosynthesis (Yang et al. 2023). Rapeseed mutant Cr3529 was resulted from the decrease of chlorophyll b, and the biosynthesis of chlorophyll was blocked at the step of converting porphobilinogen to uroporphyrinogen III (Zhao et al. 2001; Sun et al. 2007); however, its related gene(s) have not been identified.
We previously isolated an ethyl methanesulfonate (EMS) mutagenized rapeseed chlorophyll-reduced mutant (crm1), which showed yellow leaf during the whole growth period and reduced chlorophyll content (Yang et al. 2020). In this study, we further analyzed the photosynthetic characteristics and chlorophyll fluorescence kinetic parameters, and found that crm1 had enhanced heat dissipation efficiency and greater tolerance to high-light intensity, although utilization efficiency of both light energy and CO2 attenuated under natural light conditions. Genetic analysis indicated that the crm1 was controlled by double recessive nuclear genes. BnaA01.CHLI1 and BnaC01.CHLI1 were identified for the candidate genes by bulked segregant analysis coupled with whole-genome sequencing (BSA-Seq) and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene editing. According to these mutations, two functional kompetitive allele-specific polymerase chain reaction (KASP) markers were developed. The findings of this study revealed a new insight into the regulatory mechanism of chlorophyll synthesis in rapeseed, and also provided a novel target for molecular design breeding to improve the photosynthetic efficiency and tolerance to high-light intensity in crops.
Materials and methods
Plant materials and growth conditions
The crm1 was isolated following the ethyl methane sulfonate (EMS) mutagenesis of the seeds of an maintainer line of polima cytoplasmic male, 2B, as described previously (Liao et al. 2016; Yang et al. 2020). crm1 was crossed with L329 (Xiangyou 15), an commercial cultivar (Zhang et al. 2014). The resulting progeny was self-pollinated to produce the F2 population and backcrossed with crm1 to produce a backcross population (BC1F1). All plants were grown in open fields at experimental locations in Changsha (28.12° N, 112.59° E) in Hunan Province, China. Twelve plants were planted in each row, with a row spacing of 30 cm and a plant spacing of 20 cm. The initial flowering period, full flowering period, final flowering period, and growth period were counted as described (Wang et al. 2020b). Plant height was measured as described in our previous study (Li et al. 2021).
Leaf anatomy observation
Flat leaves in the same position in plants at five-leaf stage were sampled. The leaves were then sliced into 5 mm × 5 mm in size, and fixed in FAA solution (10% formaldehyde, 5% acetic acid, 50% ethanol) at 4°C for more than 24 h. The samples were then dehydrated in a graded series of ethanol and embedded in paraffin. Eight to ten micrometers of ultrathin sections of specimens were made with an ultramicrotome and a diamond knife. After staining with acid Schiff’s reagent and mounting with neutral gum, the specimens were observed and photographed using a light microscope (Nikon).
Determination of photosynthetic characteristic parameters
At five-leaf stage, by using LI-6400 portable photosynthesis instrument (LI-COR, USA), the instantaneous photosynthetic rate, stomatal conductance, transpiration rate, and intercellular CO2 concentration of the third flat leaf were measured every 2 h from 6:30 to 17:30 as described (Xiao et al. 2013). The LI6400-02B LED red and blue light sources were used for the measurement. The photosynthetically active radiation (PAR) was set to 1000 μmol m−2 s−1. The CO2 injection system was set to 400 μmol mol−1. The gas flow was 500 μmol s−1. Five individual samples were measured for each time point.
For light-response curve parameters measurement, LED red and blue light sources were used, and 14 photosynthetically effective radiation gradients (0, 25, 50, 100, 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800, and 2000 μmol m−2 s−1) were set. The CO2 injection system was set to 400 μmol mol−1. The right angle hyperbolic modified model was used to simulate and calculate the maximum net photosynthetic rate (Pn, max), light compensation point (LCP), light saturation point (LSP), dark respiration rate (Rd), and the apparent photosynthetic quantum efficiency (AQY).
For CO2-response curve parameters measurement, LED red and blue light sources were used, and ten CO2 concentration gradients (50, 100, 150, 200, 300, 400, 600, 800, 1000, and 1200 μmol mol−1) were set for the CO2 injection system. The photosynthetically effective radiation was set to 1000 μ mol m−2 s−1. The maximum net photosynthetic rate (Pn, max), CO2 compensation point (CCP), CO2 saturation point (CSP), photorespiration rate (Rp), and the carboxylation efficiency (CE) were simulated and calculated using the right angle hyperbolic modified model.
Determination of chlorophyll fluorescence kinetic parameters
By using LI-6400 portable photosynthetic apparatus fluorescence leaf chamber, the chlorophyll fluorescence kinetic parameters of the third flat leaf were measured at five-leaf stage according to the method described (Genty et al. 1989; Li et al. 2007). Plant leaves were dark adapted for about 16 h, and then the parameters were measured at 9:00–11:00 am. The parameters include minimum fluorescence under dark adaptation (Fo), maximum fluorescence under dark adaptation (Fm), minimum fluorescence under light adaptation (Fo'), and maximum fluorescence under light adaptation (Fm'). Subsequently, the maximum photochemical efficiency under dark adaptation (Fv/Fm), maximum photochemical efficiency under light adaptation (Fv'/Fm'), the quantum yield of PSII (ΦPSII), electron transfer rate (ETR), photochemical quenching (qP), and non-photochemical quenching (NPQ) were calculated as described by Genty et al.(Genty et al. 1989).
Construction of bulks
For bulked pools construction, 50 plants with normal green leaf and 50 plants with yellow leaf were selected from the F2 population generated from the cross between L329 and crm1. Green bulk (GB) and yellow bulk (YB) were pooled with same amount of DNA from the 50 selected plants with normal green leaf and 50 selected plants with yellow leaf, respectively.
BSA-seq analysis
Raw reads were processed with Fastp to remove low-quality reads and any adapter contamination (Bolger et al. 2014). Clean reads containing 150 bp paired-end reads were aligned to the ZhongShuang 11 reference genome (http://cbi.hzau.edu.cn/bnapus/) using Burrows–Wheeler Aligner (BWA, version 0.7.15). All potential polymorphic SNP sites in the whole genome are detected by the HaplotypeCaller tool of Genome Analysis Toolkit (GATK, version 3.8), CombineGVCFs tool was used to combine all GVCFs, and then further filtered according to quality value, depth, repeatability, etc., and finally a high-confidence SNP data set is obtained. BSA analysis was performed with QTLseqr (Mansfeld and Grumet 2018), the parameters window size = 1 Mb, popStruct = F2 bulkSize = c (50,50); analyze the SNP site frequency of each sample, and draw the SNP index ratio diagram. The Δ(SNP index) was calculated by subtracting the SNP index of the yellow leaf bulk (YB) from that of the green leaf bulk (GB), and the candidate region was determined based on G’ value and Δ(SNP index).
Candidate gene analysis and KASP markers analysis
The categories used for filtering SNPs linked to the yellow leaf phenotype were as follows: SNPs were (I) polymorphic between the wide type 2B and crm1; (II) C/G to T/A transitions (Tang et al. 2020); (III) nonsense mutations, frameshift mutations or missense mutations; (IV) SNPs located in A01 and C01 can cause mutations in homologous genes. Polymorphic SNPs linked to the yellow leaf phenotype were used for KASP marker development. KASP primers were developed following standard KASP guidelines (LGC Genomics, Hoddesdon, UK). Allele-specific primers were designed using the standard FAM (5′GAAGGTGACCAAGTTCATGCT 3′) and HEX (5′ GAAGGTCGGAG TCAACGGATT 3′) tails and with the targeted SNP at the 3′ end. A common primer was designed such that total amplicon length was less than 80 bp. The primer mixture comprised 46 μl ddH2O, 30 μl common primer (100 μM), and 12 μl of each tailed primer (100 μM). Assays were tested in 96-well formats and set up as ~0.8 μl reactions (20–50 ng dry DNA, 0.4 μl of 1× KASP master mixture, 0.4 μl ddH2O, and 0.056 μl of primer mixture). PCR cycling was performed using the following protocol: hot start at 95°C for 15 min, followed by 10 touchdown cycles (95°C for 20 s; touchdown at 65°C initially and decreasing by −1°C per cycle for 25 s), followed by 30 additional cycles of annealing (95°C for 10 s; 57°C for 60 s). The corresponding primer sequences used for KASP analysis are listed in Supplementary Table S1.
CRISPR/Cas9 construction and rapeseed transformation
To generate the CRISPR/Cas9 system targeting both BnaA01.CHLI1 and BnaC01.CHLI1, the target sites of BnaA01.CHLI1 and BnaC01.CHLI1 were screened and the primers containing guide RNA sequences targeting both BnaA01.CHLI1 and BnaC01.CHLI1 were designed using the online tool CRISPR-P2.0 (http://crispr.hzau.edu.cn/CRISPR2/) (Supplementary Table S2). The target site sgRNA were inserted into the pKSE401 vector (Xing et al. 2014). The construct was then introduced into rapeseed by Agrobacterium-mediated transformation as described (Dai et al. 2020).
Subcellular localization analysis
The full-length coding sequences of BnaA01.CHLI1 and BnaC01.CHLI1 were cloned into pCambia-1300-GFP vector under control of 35S promoter to generate BnaA01.CHLI1-GFP and BnaC01.CHLI1-GFP constructs, respectively. The constructs were then infiltrated into 3-week-old N. benthamiana (tobacco) leaves as described (Sparkes et al. 2006). The tobacco leaves were kept in the dark for 48 h, and then the BnaA01.CHLI1-GFP and BnaC01.CHLI1-GFP signal was observed using a confocal microscope (Nikon).
RNA-Seq data analysis
The transcriptome sequencing and analysis were conducted by OE Biotech Co., Ltd. (Shanghai, China). The libraries were sequenced on an illumina Novaseq 6000 platform and 150 bp paired-end reads were generated. About 48.94M raw reads for each sample were generated. Raw reads of fastq format were firstly processed using fastp and the low-quality reads were removed to obtain the clean reads.
Then, about 47.44M clean reads for each sample were retained for subsequent analyses. The clean reads were mapped to the reference genome (http://cbi.hzau.edu.cn/rape/download_ext/zs11. genome.fa) using HISAT2. FPKM of each gene was calculated and the read counts of each gene were obtained by HTSeq-count. PCA analysis was performed using R (v3.2.0) to evaluate the biological duplication of samples. Differential expression analysis was performed using the DESeq2. Q value < 0.05 and foldchange > 2 or foldchange < 0.5 was set as the threshold for significantly differential expression gene (DEGs). Hierarchical cluster analysis of DEGs was performed using R (v3.2.0) to demonstrate the expression pattern of genes in different groups and samples. Based on the hypergeometric distribution, GO, KEGG pathway, Reactome, and WikiPathways enrichment analysis of DEGs were performed to screen the significant enriched term using R (v3.2.0), respectively. Gene set enrichment analysis (GSEA) was performed using GSEA software. The analysis was used a predefined gene set, and the genes were ranked according to the degree of differential expression in the two types of samples. Then, it is tested whether the predefined gene set was enriched at the top or bottom of the ranking list.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using RNAiso Plus (TakaRa). The extracted RNA was then reverse transcribed using PrimeScript RT regent Kit With gDNA Eraser (TakaRa) to obtain cDNA. qRT-PCR was carried out using SYBR Green Premix pro Taq HS qPCR Kit (Accurate Biology) on BioRad CFX96 Touch Real-Time PCR instrument according to the instructions. BnActin7 was used as an internal reference. The primers were listed in Supplementary Table S3. Each experiment was biologically repeated three times.
Results
crm1 mutant shows longer growth period and retarded growth
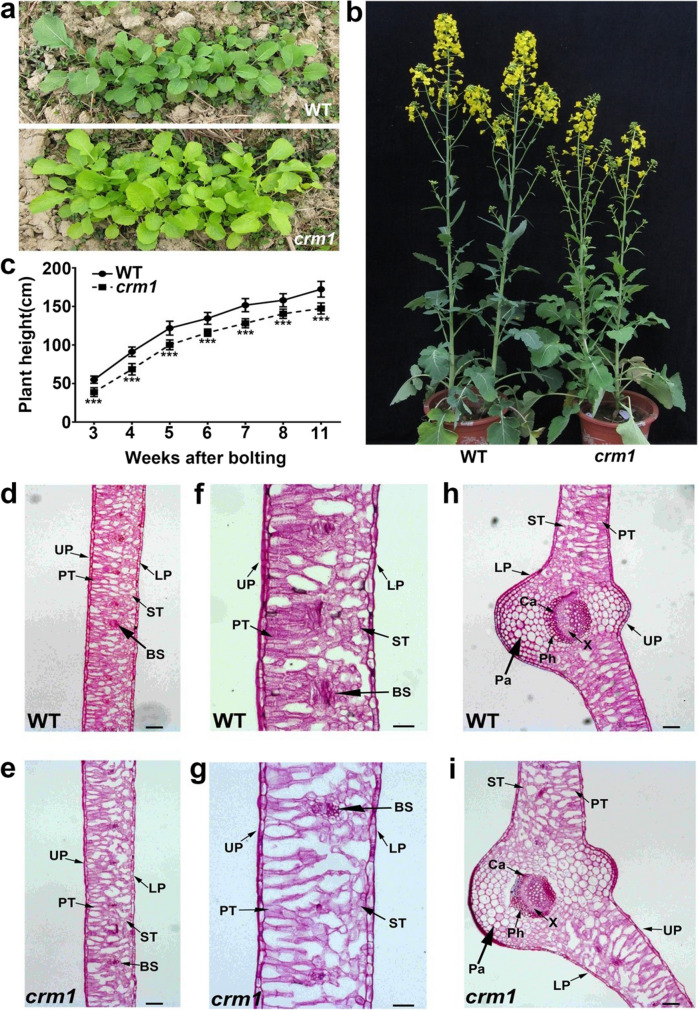
Our previous studies showed that the leaves of the crm1 mutant exhibits yellow phenotype throughout its entire developmental process (Yang et al. 2020). As shown in Fig. 1, crm1 mutant displayed yellow leaves both at the seedlings stage and flowering stage (Fig. 1a, b). The initial flowering stage, full flowering stage and final flowering stage in crm1mutant were delayed by 3–5 days compared with that in the wild-type (WT) (Fig. 1b; Supplementary Table S4). Thus, the life time was extended by approximately 4 days for the crm1mutant (Supplementary Table S4). However, the overall plant height of crm1 mutant was significantly shorter than that of the WT throughout the entire growth period (Fig. 1c). At the mature stage, the plant height in crm1 mutant was approximately 85.5% of that in the WT (Fig. 1c). These results indicated that the yellow leaf phenotype lead to the longer growth period and retarded growth in crm1 mutant.
Fig. 1.
Phenotype and leaf structure of crm1 and wild-type (WT) plants. a–b Plants phenotype at seedling stage and full flowering stage. c Plant height at indicated time after bolting. Standard deviations (n = 10) are shown. Statistically significant differences are indicated (*** p < 0.001; Student’s t-test). d, e, f, g Transverse section of leaf. h–i Transverse section of veins. WT, wild-type; UP, upper epidermis; LP, lower epidermis; PT, palisade tissue; BS, bundle sheath; ST, spongy tissue; Pa, parenchyma; X, xylem; Ph, phloem; Ca, cambium. Scale bars: 100 μm in d, e, h, and i; 500 μm in f and g
Leaf structure development is impaired in crm1
We observed the microstructure of leaves and veins in the crm1 mutant. The transverse section observation showed that the palisade tissue and spongy tissue of crm1 mutant leaves were loosely arranged compared with that of the WT (Fig. 1d–g). Additionally, the mesophyll cell arrangement and shape in crm1 mutant were irregular and had significant gaps (Fig. 1e, g). However, there was no notably difference of veins microstructure between crm1 mutant and WT (Fig. 1h, i). These results demonstrated that there were structural development defects in leaves but not in veins in crm1 mutant.
crm1 mutant shows attenuated utilization efficiency of both light energy and CO2 but greater tolerance to high-light intensity
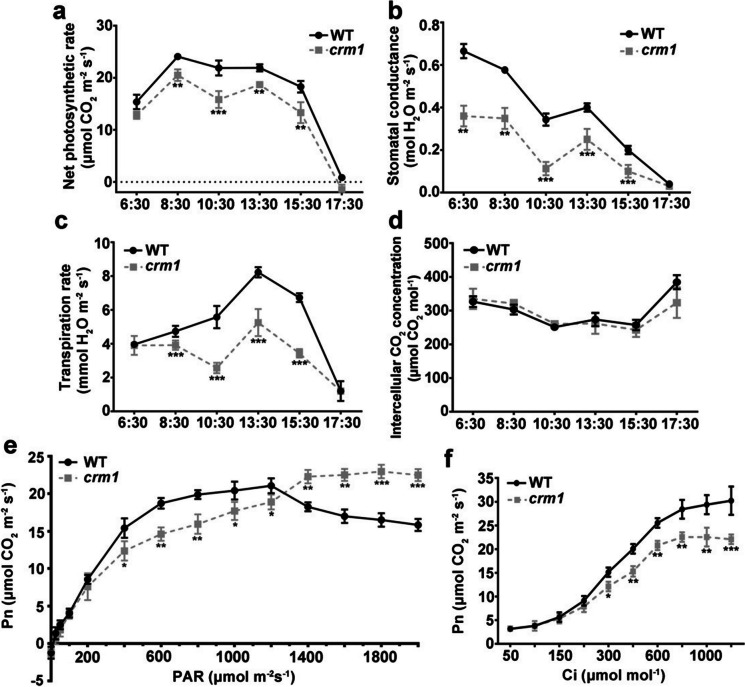
In comparison with WT, the crm1 mutant showed greatly decreased net photosynthetic efficiency, stomatal conductance and transpiration rate under natural light conditions (Fig. 2a–c). However, the intercellular CO2 concentration was similar to that of the WT (Fig. 2d). We previously showed that there were fewer thylakoid stacks in crm1 (Yang et al. 2020). These results suggested that the reduction in the photosynthetic efficiency in crm1 was not likely owing to a stomatal flaw, but was likely due to the chloroplasts development defects inside the mesophyll cells.
Fig. 2.
Photosynthetic characteristics of fully expanded leaf in crm1 and WT plants at five-leaf stage. a Daily change of photosynthetic rate. b Daily change of stomatal conductance. c Daily change of transpiration rate. d Daily change of intercellular carbon dioxide concentration. e Light-response curves (Pn-PAR). f CO2-response curves (Pn-Ci). WT, wild-type; Pn, net photosynthetic rate; PAR, photosynthetically active radiation; Ci, intercellular CO2 concentration. Standard deviations (n = 5) are shown. Statistically significant differences are shown (*p < 0.05; **p < 0.01; ***p < 0.001; Student’s t-test)
Concomitant with the increases of light intensity or carbon dioxide concentrations, the net photosynthetic rates both in the mutant and the WT increased. However, the net photosynthetic rate of crm1 was largely decreased in comparison to that in the WT under relatively low-light intensity (200–1000 μmol s−2 s−1) (Fig. 2e) or at different carbon dioxide concentrations (Fig. 2f). Interestingly, under high-light intensity (1200–2000 μmol s−2 s−1), the net photosynthetic rate was still increased in crm1 but declined in WT (Fig. 2e), indicating that crm1 mutant leaves had stronger tolerance under high-light intensity.
To further analyze the photosynthetic light-response curve parameters, we calculated the maximum net photosynthetic rate (Pn, max), light compensation point (LCP), light saturation point (LSP), dark respiration rate (Rd), and apparent photosynthetic quantum efficiency (AQY) using a right angle hyperbolic modified model. The Pn, max, LSP, and AQY in crm1 mutant were lower than that in the WT, and decreased by 6.34%, 6.57%, and 25.40%, respectively. However, LCP was increased up to 40.00% compared to the WT (Supplementary table S5). Next, we analyzed the photosynthetic CO2-response curve parameters by calculating the Pn, max, CO2 compensation point (CCP), CO2 saturation point (CSP), photorespiration rate (Rp), and carboxylation efficiency (CE) using a modified right angle hyperbolic model simulation. The results showed that crm1 had a 28.81% reduction in Pn,max but a 133.96% increase in CCP when compared with WT (Supplementary table S6). Collectively, these results indicated that crm1 had attenuated utilization efficiency of both light energy and CO2 but greater tolerance to high-light intensity.
crm1 mutant shows alleviated photochemical conversion efficiency but enhanced heat dissipation efficiency
The changes of chlorophyll fluorescence in plants are closely related to the absorption, transmission, dissipation, and distribution of light energy by the photosystem during photosynthesis (Xiao et al. 2013). We therefore evaluated whether crm1 mutation affects the chlorophyll fluorescence parameters, including minimum fluorescence under dark adaptation (Fo), maximum fluorescence under dark adaptation (Fm), minimum fluorescence under light adaptation (Fo'), maximum fluorescence under light adaptation (Fm'), maximum photochemical efficiency under light adaptation (Fv'/Fm'), quantum yield of PSII (ΦPSII), and electron transfer rate (ETR). Chlorophyll fluorescence kinetic tests indicated that Fo, Fm, Fo', Fm', Fv'/Fm', ΦPSII, and ETR in the crm1 were significantly reduced compared to that in the WT. On average, 24.98%, 8.68%, 4.71%, 14.89%, 11.54%, 27.22%, and 27.23% reduction was observed in the crm1 mutant, respectively (Table 1). The chlorophyll fluorescence parameters were reported to be positively correlated with the chlorophyll content (Li et al. 2022). We previously showed that the crm1 mutant had reduced chlorophyll content (Yang et al. 2020). Thus, the reduction of the chlorophyll fluorescence parameters might be due to the reduced chlorophyll content in crm1 mutant. In addition, the crm1 mutant had a 18.06% less qP (the photochemical quenching) than WT. However, the non-photochemical quenching (NPQ) was higher than that of the WT. Indeed, 29.86% increase was observed in the crm1 mutant (Table 1). Together, these results demonstrate that the mutant crm1 had alleviated photochemical conversion efficiency but enhanced heat dissipation efficiency in its PSII reaction center, which might explain why crm1 mutant had stronger tolerance under high-light intensity.
Table 1.
The chlorophyll fluorescence parameters of wild-type (WT) and crm1 mutant plants at five-leaf stage
| Parameters | WT | crm1 |
|---|---|---|
| Fo | 554.91±6.02 | 416.32±6.79*** |
| Fm | 2490.73±138.24 | 2274.46±79.08** |
| Fo' | 429.93±10.59 | 409.69±15.56** |
| Fm' | 891.70±14.14 | 758.94±18.97*** |
| Fv/Fm | 0.78±0.01 | 0.81±0.01 |
| Fv'/Fm' | 0.52±0.01 | 0.46±0.03*** |
| ΦPSII | 0.38±0.01 | 0.27±0.07* |
| ETR | 164.51±6.98 | 119.72±30.08* |
| qP | 0.72±0.02 | 0.59±0.11* |
| NPQ | 1.44±0.13 | 1.87±0.14*** |
Values are means ± SD (n = 5). Statistically significant differences are shown (*p < 0.05; ***p < 0.001; Student’s t-test). Fo minimum fluorescence under dark adaptation, Fm maximum fluorescence under dark adaptation, Fo' minimum fluorescence under light adaptation, Fm' maximum fluorescence under light adaptation, Fv/Fm maximum photochemical efficiency under dark adaptation, Fv'/Fm' maximum photochemical efficiency under light adaptation; ΦPSII the quantum yield of PSII, ETR electron transfer rate, qP photochemical quenching, NPQ non-photochemical quenching
BnaA01.CHLI1 and BnaC01.CHLI1 are candidate genes for yellow leaf phenotype in crm1
To analyze the inheritance of the yellow leaf phenotype, crm1 was reciprocally crossed with its wild-type parent 2B, and the commercial cultivar L329 which possessed a normal green leaf color. The resulting heterozygous F1 plants crm1/2B, 2B/crm1, crm1/L329, and L329/crm1 displayed green leaf (Supplementary Fig. S1; Supplementary table S7), indicating that the yellow leaf phenotype is controlled by recessive nuclear gene(s). The F2 population derived from the crossing of crm1 with L329 included 2203 plants showing a green leaf phenotype and 145 plants showing a yellow leaf phenotype. This segregation ratio in the F2 generation was in line with the expected Mendelian inheritance ratio of 15:1 (green leaf: yellow leaf, χ2 = 2.04 < χ20.05, 1 = 3.84) (Supplementary table S7). A BC1F1 population generated from a cross between crm1/L329 F1 plant and crm1 showed a segregation ratio of 3:1 (green leaf: yellow leaf, χ2 = 1.46 < χ20.05, 1 = 3.84) (Supplementary table S7). Additionally, we constructed an F2 population between the wild-type parent 2B and crm1, which exhibited a segregation ratio of 15:1 (green leaf: yellow leaf, χ2 = 0.28 < χ20.05, 1 = 3.84) (Supplementary table S7). These results indicated that the yellow leaf phenotype of crm1 was controlled by two recessive loci, and both of which were likely formed through EMS mutagenesis.
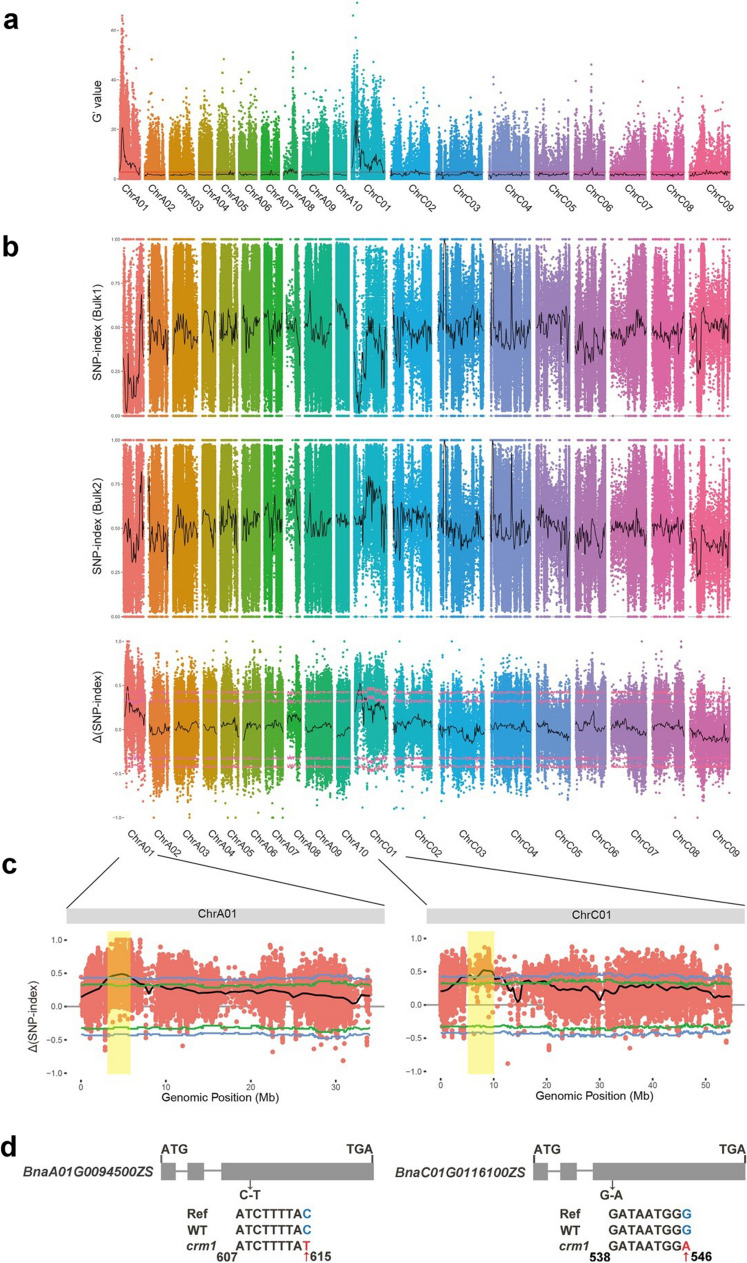
To map the gene underlying the crm1 phenotype, we used F2 population obtained from the crm1 and L329 crosses to perform bulked segregant analysis coupled with whole-genome sequencing (BSA-Seq). In the F2 population, we selected 50 plants with yellow leaves and 50 plants with green leaves to create separate bulks. Subsequently, the bulks, along with the parental plants, were subjected to sequencing. After applying quality control filtering, the total amount of data obtained was 116.8 G, of which 8.93, 13.62, 16.68, 38.16 and 39.52 Gb corresponded to the 2B, crm1, L329, the yellow bulk, and the green bulk, respectively, with a coverage depth of 48.21X, 19.19X, 25.42X, 13.68X and 48.3X, respectively (Supplementary Table S8). A total of 88,088,306, 134,229,902, 164,090,846, 376,009, and 387,975,976 clean reads were harvested for the 2B, crm1, L329, the yellow bulk, and the green bulk, respectively (Supplementary Table S8). The sequencing data revealed that each pool had a percentage of bases with a quality score of more than 30 (Q30) exceeding 92.84%, and a percentage of bases with a quality score of more than 20 (Q20) exceeding 97.53% (Supplementary Table S8). Furthermore, the average GC content was determined to be 37.18%, and the average genome coverage reached 93.17% (Supplementary Table S8). Therefore, we confirm that the sequencing data quality aligns with our expectations and is suitable for subsequent analysis. Based on the alignment with the ZhongShuang 11 reference genome, 4,679,083 single nucleotide polymorphisms (SNPs) were identified in the yellow bulk (Supplementary Table S9). In the green bulk, given the genotype ratios of A_B_: A_bb: aaB_ = 9:3:3, the expected allele frequency of the causative mutation would be 7/15. Conversely, in the yellow bulk (aabb), the expected allele frequency would be 1. Hence, SNPs that are closely linked to the causative mutation are likely to reflect a similar expected allele frequency as the causative mutation, resulting in a ∆(SNP index) value that approaches 8/15. Two notable peaks in the ∆(SNP-index) were identified, specifically in the region spanning from 3.24 to 7.16 Mb on chromosome A01 and from 2.67 to 12.72 Mb on chromosome C01 (Fig. 3a–c; Supplementary Table S10). Importantly, the Gprime method, which calculates a modified G statistic for each SNP based on observed and expected allele depths, also revealed these same peaks. These findings strongly suggested that these regions are promising candidate loci housing the crm1 genes.
Fig. 3.
Gene mapping of crm1 by bulked segregant analysis using resequencing. a The G’-value is represented as the y-axis. The physical position (unit: Mbp) of each chromosome in Brassica napus is represented as the x-axis. b The SNP index of 1-Mb interval with 10-kb sliding window each time is represented by the y-axis. The Δ(SNP index) is calculated by subtracting the SNP index of the yellow leaf bulk (bulk1) from that of the green leaf bulk (bulk2). The red line in outside and inter is indicate 95% and 99% confidence intervals, respectively. c ΔSNP index plot of chromosome A01 and C01, green lines and blue lines indicate 95% and 99% confidence intervals, respectively. The significant genomic region is shaded (3.24–7.16 Mb on A01 and 2.67–12.72 Mb on C01). d Structure of the BnaA01G0094500ZS and BnaC01G0116100ZS, a single nucleotide substitution (C-T) and (G-A) between crm1 and its wild-type parent 2B is identified in the third exon, respectively. Exons and introns are represented as gray boxes or grey lines, respectively
To identify the candidate gene, the SNPs located in the candidate region were further analyzed. Of the 25, 279 SNPs in chromosome A01 candidate region, 213 SNPs were polymorphic between crm1 and 2B and considered as true EMS induced mutations. Of the 25, 945 SNPs in chromosome C01 candidate region, 323 SNPs were polymorphic between crm1 and 2B. Among these SNPs, 119 (55.87%) and 253 (78.33%) were C/G to T/A transitions, of which 26 and 29 were nonsense mutations, frameshift mutations, or missense mutations in chromosome A01 and C01 candidate region, respectively. We further searched for functional mutations by annotation information and identified BnaA01G0094500ZS and BnaC01G0116100ZS as the candidate genes for A01 and C01 loci, respectively. Both genes encode the magnesium chelatase subunit CHLI1, which catalyzes the insertion of Mg2+ into the protoporphyrin IX in the chlorophyll biosynthesis pathway (Luo et al. 2013), hereafter named BnaA01.CHLI1 and BnaC01.CHLI1, respectively. A single nucleotide change from C to T occurred in the third exon region at position 615 bp of BnaA01.CHLI1 (Fig. 3d; Supplementary Fig. S2). A single nucleotide change from G to A occurred in the third exon region at position 546 bp of BnaC01.CHLI1 (Fig. 3d; Supplementary Fig. S3). The C to T transition on chromosome A01 of BnaA01.CHLI1 caused a substitution of proline (Pro) to serine (Ser) at the 144st amino acid (P144S) (Supplementary Fig. S4a). The G to A transition on chromosome C01 of BnaC01.CHLI1 caused a substitution of glycine (Gly) to glutamate (Glu) at the 128st amino acid (G128E) (Supplementary Fig. S4b). The P144S and G128E were located within the ATPases associated with various cellular activities (AAA+) domain of the CHLI1 protein. Noticeably, the proline and glycine residues in the AAA+ domain are absolutely conserved in almost all the known CHLI1 proteins from different plant species (Supplementary Fig. S4).
To further determine whether these SNPs were associated with the yellow leaf phenotype of crm1, two KASP markers, KASP-A01 and KASP-C01, were designed based on the candidate SNPs (Supplementary Table S1). We used KASP-A01 and KASP-C01 to genotype 1254 plants from the original F2 population derived from a crm1/L329 cross, revealing a 1:2:1:2:4:2:1:2:1 segregation ratio across the 9 marker genotypes. Only plants with homozygous mutations at both loci exhibited a yellowing phenotype (Table 2). Moreover, we used these two markers to genotype an additional 428 yellow leaf plants from a larger F2 population, which also derived from the crossing of crm1 with L329. The genotypes determined by KASP-A01 and KASP-C01 in these 428 yellow leaf plants were the same as those in the crm1 parent (Supplementary Fig. S5). Together, these results suggested that BnaA01.CHLI1 and BnaC01.CHLI1 are the possible candidate genes for yellow leaf phenotype in crm1.
Table 2.
Summary of the genotype and phenotype segregation ratios of F2 population derived from the crossing of crm1 with L329
| Genotypea | Genotypic segregationb | Phenotypic segregationc | ||
|---|---|---|---|---|
| Observed | Expected | Green leaf plants | Yellow leaf plants | |
| AACC | 65 | 78.375 | 65 | 0 |
| AACc | 165 | 156.75 | 165 | 0 |
| AAcc | 79 | 78.375 | 79 | 0 |
| AaCC | 173 | 156.75 | 173 | 0 |
| AaCc | 291 | 313.5 | 291 | 0 |
| Aacc | 158 | 156.75 | 158 | 0 |
| aaCC | 90 | 78.375 | 90 | 0 |
| aaCc | 161 | 156.75 | 161 | 0 |
| aacc | 72 | 78.375 | 0 | 72 |
| Total | 1254 | 1254 | 1182 | 72 |
| Chi-squared test | χ2 (1:2:1:2:4:2:1:2:1) | 8.34 | χ2 (15:1) | 0.553 |
| χ2 P value | 0.396 | χ2 P value | 0.457 | |
aThe genotypes of the A01 and C01 loci are determined by KASP-A01 and KASP-C01, respectively. At the A01 locus, uppercase “A” represents the wild-type genotype, while lowercase “a” denotes the mutant genotype. Similarly, for the C01 locus, uppercase “C” signifies the wild-type genotype, and lowercase “c” indicates the mutant genotype. bχ2(1:2:1:2:4:2:1:2:1) > χ20.05 =15.507 was considered significant. cχ2(15:1) > χ20.05 =3.84 was considered significant
To verify whether the BnaA01.CHLI1 and BnaC01.CHLI1 genes are responsible for the yellow phenotype of crm1, we generated the double mutant of BnaA01.CHLI1 and BnaC01.CHLI1 using the CRISPR/Cas9 (Fig. 4a). Among 53 T0 transgenic positive plants derived from 2B, two plants, which had yellow leaf phenotype (Fig. 4b), were edited in BnaC01.CHLI1 and BnaC01.CHLI1 (Fig. 4c), hereafter named bnachli1-1 and bnachli1-19. Consistent with the yellow leaf phenotype, the bnachli1-1 and bnachli1-19 had largely reduced levels of Chl a and Chl b. On average, the Chl a was 21.63% and 22.06 % lower, and the Chl b was 33.51% and 38.87% lower than that in the WT, respectively (Fig. 4d). The Chl a to Chl b ratio in bnachli1-1 and bnachli1-19 was increased 17.87% and 27.5%, respectively, but the ratio of chlorophyll to carotenoid appeared unchanged (Fig. 4e). These results reconfirmed that BnaA01.CHLI1 and BnaC01.CHLI1 are the possible candidate genes for yellow leaf phenotype in crm1.
Fig. 4.
Double mutants with BnaA01.CHLI1 and BnaC01.CHLI1 generated by targeted gene editing showing yellow leaf phenotype. a The BnaA01.CHLI1 and BnaC01.CHLI1 gene structure includes exons (box) and introns (line). The vertical line in the gene structure indicates the target site. The target sequences are shown with the PAM in red color. b Transgenic plants of bnachli1-1 and bnachli1-9. c Sequences at the sgRNA target sites identified from bnachli1-1 and bnachli1-9. The wild-type sequence is shown at the top with the PAM in red color. The mutation sites are highlighted in red. +, insertion. −, deletion. d Chlorophyll (Chl) and carotenoid content in leaves. e Ratio of the Chl a to Chl b and the Chl to carotenoids in leaves. Bars represent the standard deviations of three independent experiments. Statistically significant differences are shown (***p < 0.001; Student’s t-test)
Expression profiling of BnaA01.CHLI1 and BnaC01.CHLI1 in rapeseed
To investigate the potential role of BnaA01.CHLI1 and BnaC01.CHLI1 in regulation of plant growth and development, we examined their expression in different tissues and organs, including cotyledons, hypocotyls, and root of seedlings, and flowers, stems, buds, siliques, rosette leaves, and caulin leaves of adult plant. The qRT-PCR analysis results showed that the expression of BnaA01.CHLI1 and BnaC01.CHLI1 were detected in all tissues. They highly expressed in cotyledon and caulin leaves and relatively low in root tissues and flower (Fig. 5a, b). These expression patterns were consistent with their role in chlorophyll biosynthesis.
Fig. 5.
Expression profiling and subcellular localization of BnaA01.CHLI1 and BnaC01.CHLI1. a Expression levels of BnaA01.CHLI1 and BnaC01.CHLI1 in root, hypocotyl, and cotyledon of seedlings. b Expression levels of BnaA01.CHLI1 and BnaC01.CHLI1 in different organs of adult plants. Bars represent the standard deviations of three independent experiments. c Subcellular localization of BnaA01.CHLI1 and BnaC01.CHLI1 in tobacco leaves. Bright, bright field; Chloroplast, chloroplast fluorescence; GFP, GFP fluorescence; Merge, overlay of the chloroplast fluorescence; GFP, fluorescence and bright field images. Scale bar = 100μm
BnaA01.CHLI1 and BnaC01.CHLI1 locate in the chloroplast
CHLI was identified as a stromal protein of chloroplasts in soybean (Nakayama et al. 1995). It was reported to be a target protein of the chloroplast thioredoxin in Arabidopsis (Ikegami et al. 2007). To investigate whether BnaA01.CHLI1 and BnaC01.CHLI1 were localized in chloroplasts, we generated BnaA01.CHLI1-GFP and BnaC01.CHLI1-GFP constructs and introduced them into tobacco leaves, respectively. Strong fluorescence signal was observed in the chloroplast transformed with BnaA01.CHLI1-GFP or BnaC01.CHLI1-GFP. However, no signal was observed in chloroplasts transformed with the control GFP, although the GFP fluorescence signal was present in the nucleus and the cytoplasm (Fig. 5c). Together, these results indicated that BnaA01.CHLI1 and BnaC01.CHLI1 locate in the chloroplast of plant cells.
Chlorophyll biosynthesis–related genes are upregulated in crm1
To evaluate the effect of the crm1 mutation on the gene expression, we performed RNA-seq analysis by using wild-type 2B and crm1 leaves. A total of 1028 differentially expressed genes (DEGs) were identified, of which 620 were upregulated and 408 were downregulated in crm1 mutant (Fig. 6a, b; Supplementary Tables S11 and S12). To clarify the functional significance of DEGs, we further performed GO enrichment analysis. The DEGs were assigned to three main GO categories (Supplementary Fig. S6). Leaf color variation is closely related to chlorophyll synthesis. Therefore, we performed a detailed analysis of the DEGs related to chlorophyll biosynthesis. Importantly, the DEGs involved in the process of chlorophyll biosynthesis were almost upregulated in crm1 (Fig. 6c). To further verify the reliability of RNA-seq data, we randomly selected five upregulated genes for qRT-PCR analysis. As expected, qRT-PCR results were generally consistent with those of RNA-seq analysis (Fig. 6d). Collectively, these results suggested that the crm1 mutation resulted in feedback regulation of chlorophyll biosynthesis–related gene expression.
Fig. 6.
Differential expressed gene analysis in crm1 mutant. a Volcano plot of all differential expressed genes in crm1 mutant compared with WT, and the criteria for significant differentially expressed gene screening were as follows: q ≤ 0.05 and log2FC ≥ 1 for upregulated genes, and q ≤ 0.05 and log2FC ≤ −1 for downregulated genes. b Quantitative analysis of the differential expressed genes between WT and crm1. c Cluster analysis of differentially expressed chlorophyll synthesis related genes in WT and crm1 mutant, and three biological replicates are shown. d qRT-PCR analysis of randomly selected chlorophyll synthesis related genes expression in WT and crm1 plant leaves. The gene expression was normalized to ACTIN7 (ACT7) expression. Bars represent the standard deviations of three independent experiments. Statistically significant differences are shown (***p < 0.001; Student’s t-test)
Discussion
Magnesium chelatase is a heterotrimeric enzyme complex composed of three subunits CHLH, CHLD, and CHLI. The enzyme catalyzes the insertion of Mg2+ into protoporphyrin IX, a key regulatory and enzymatic reaction in chlorophyll biosynthesis (Walker and Willows 1997; Al-Karadaghi et al. 2006). CHLI belongs to the AAA+ family, has an active ATPases activity, and is responsible for ATP hydrolysis (Gibson et al. 1995; Jensen et al. 1999; Lundqvist et al. 2010). A few chli mutants with leaf phenotypes from pale green, to light green, to yellow, and to albino have been isolated from various species. The Arabidopsis T-DNA insertion mutant ch42-3 in AtCHLI1 was pale green in appearance and had reduced chlorophyll accumulation (Rissler et al. 2002). The cs215 mutant had a C to T mutation at nucleotide 584 that converted a Thr at residue 195 to an Ile (T195I). The homozygous cs215 was albino (Huang and Li 2009). Rice Chlorina-9 (chl9) mutant was due to a missense mutation at the third exon (R307C), consequently showing reduced chlorophyll levels and a yellowish-green leaf phenotype (Zhang et al. 2006). Rice etiolated leaf and lethal (ell) mutant, resulting from an amino acid substitution (G177R) in a highly conserved region, showed yellow leaf in young seedlings and became lethal after three-leaf stage (Jiang et al. 2015). The cucumber plant C582 had an amino acid substitution (G269R), thus leading to reduced chlorophyll synthesis and the golden leaf color (Gao et al. 2016). The barley semidominant mutants Chlorina-125, -157, and -161, which had the G559A (D187N), G806A (R269K), and C271T (L91F) mutations, respectively, developed light-green phenotype in heterozygotes and yellow phenotype in homozygotes. They showed deficiency in ATP hydrolysis and magnesium chelatase activity (Hansson et al. 1999, 2002). The wheat chli mutant, which exhibited decreased chlorophyll accumulation and pale-green leaf phenotype at the seedling stage, resulted from an amino acid substitution (D221N) in the highly conserved domain of CHLI (Wang et al. 2020a, 2020b). Recently, it was reported that a mutation E186K of the CHLI in strawberry p240 mutant resulted in a yellow-green leaf and a low chlorophyll level in heterozygosis mutation (Ma et al. 2023). However, there has been no identification of a CHLI mutant in rapeseed. We previously reported an EMS mutagenized crm1 mutant in rapeseed which showed reduced chlorophyll levels and a yellow leaf phenotype (Yang et al. 2020). In the present study, we identified a single nucleotide change (C to T) and (G to A) in the third exon of the BnaA01.CHLI1 and BnaC01.CHLI1 (Fig. 3). These two mutations resulted in an amino acid substitution P144S and G128E in the AAA+ domain of the CHLI1 protein in the crm1, respectively (Supplementary Fig. S4). The double mutation of BnaA01.CHLI1 and BnaC01.CHLI1 generated by CRISPR/Cas9 gene editing recapitulated the reduced chlorophyll content and yellow leaf phenotype (Fig. 4). We therefore concluded that BnaA01.CHLI1 and BnaC01.CHLI1 functioned redundantly, and both the P144 and G128 played a key role in CHLI function to affect leaf coloration via regulating chlorophyll synthesis in rapeseed.
Recently, it was reported that the yellow-green leaf mutant p240 in strawberry exhibited a photosensitive phenotype under high light conditions. Heterozygous chli mutants, with the phenotype comparable to the heterozygosis p240 mutants, produced lower net photosynthetic rates under full light, but higher net photosynthetic rates under low-light conditions (Ma et al. 2023). Unlike strawberry chli mutants, our rapeseed crm1 mutant had higher net photosynthetic rate under high-light intensity (1200–2000 μmol s−2 s−1) (Fig. 2e). This result suggested that crm1 mutant leaves have stronger tolerance under high-light intensity and adapt better to reduce the stress from high light. The different phenotypes are possibly due to different mutations that differentially affect activity of CHLI or intermolecular interactions between subunits CHLI, CHLH, and CHLD, which in turn affecting magnesium chelatase function. However, the underlying molecular mechanism needs to be further studied. Additionally, chlorophyll fluorescence kinetic parameters are usually used to characterize the intrinsic role of PSII, which is associated with the photosynthetic capacity (Hao et al. 2012). The Fo, Fm, Fo', and Fm' in the crm1 mutant were significantly decreased in comparison to the WT. However, the NPQ was dramatically increased in the crm1 (Table 1). These results suggested that the crm1 mutant PSII reaction center had higher heat dissipation consumption, which might partially explain why crm1 mutant had stronger tolerance under high-light intensity. Therefore, CHLI may be a novel target for improving photosynthetic efficiency and tolerance of rapeseed under high-light intensity conditions.
Supplementary information
Acknowledgements
We thank Dr. Zhongsong Liu for providing L329 (xiangyou 15) seeds, Prof. Wusheng Peng for 2B seeds.
Author contribution
HZ, WZ, FX, XinmeiL, BL, and XZ designed the research; HZ, WZ, FX, ZZ, YG, and TC performed the experiments; FD, QZ, XinL, MF, and XinmeiL provided technical assistance to HZ, WZ, and FX; HZ, WZ, FX, XinmeiL, BL, and XZ analyzed the data; HZ, BL, and XZ supervised and completed the writing; HZ, WZ, and FX contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (U20A2029), Natural Science Foundation of Changsha city (kq2202150).
Data availability
The sequencing data have been deposited in the NCBI database under BioProject PRJNA912866, the SRA accession numbers are: SRR12968239, SRR12968240, SRR12968241, and SRR12968242. The data that support the findings of this study are available in the main text and supporting materials of this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work and share first authorship
Contributor Information
Bao Li, Email: Libao@hunaas.cn.
Xiaoying Zhao, Email: xiaoyzhao@hnu.edu.cn.
References
- Al-Karadaghi S, Franco R, Hansson M, Shelnutt JA, Isaya G, Ferreira GC. Chelatases: distort to select? Trends Biochem Sci. 2006;31(3):135–142. doi: 10.1016/j.tibs.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N, Emanuel C, Lainez V, Maldonado S, Prina AR, Borner T. The barley plastome mutant CL2 affects expression of nuclear and chloroplast housekeeping genes in a cell-age dependent manner. Mol Genet Genomics. 2008;279:403–414. doi: 10.1007/s00438-008-0321-x. [DOI] [PubMed] [Google Scholar]
- Dai C, Li Y, Li L, Du Z, Lin S, Tian X, Li S, Yang B, Yao W, Wang J, Guo L, Lu S. An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol Breeding. 2020;40(10):96. doi: 10.1007/s11032-020-01174-0. [DOI] [Google Scholar]
- Du H, Qi M, Cui X, Cui Y, Yang H, Zhang J, Ma Y, Zhang S, Zhang X, Yu D. Proteomic and functional analysis of soybean chlorophyll-deficient mutant cd1 and the underlying gene encoding the CHLI subunit of Mg-chelatase. Mol Breeding. 2018;38:71. doi: 10.1007/s11032-018-0819-9. [DOI] [Google Scholar]
- Gao M, Hu L, Li Y, Weng Y. The chlorophyll-deficient golden leaf mutation in cucumber is due to a single nucleotide substitution in CsChlI for magnesium chelatase I subunit. Theor Appl Genet. 2016;129:1961–1973. doi: 10.1007/s00122-016-2752-9. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990(1):87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- Gibson LC, Willows RD, Kannangara CG, Von WD, Hunter CN. Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli. Proc Natl Acad Sci. 1995;92(6):1941–1944. doi: 10.1073/pnas.92.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A, Kannangara CG, von Wettstein D, Hansson M. Molecular basis for semidominance of missense mutations in the XANTHA-H (42-kDa) subunit of magnesium chelatase. Proc Natl Acad Sci. 1999;96(4):1744–1749. doi: 10.1073/pnas.96.4.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A, Willows RD, Roberts TH, Hansson M. Three semidominant barley mutants with single amino acid substitutions in the smallest magnesium chelatase subunit form defective AAA+ hexamers. Proc Natl Acad Sci. 2002;99(21):13944–13949. doi: 10.1073/pnas.212504499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D, Chao M, Yin Z, Yu D. Genome-wide association analysis detecting significant single nucleotide polymorphisms for chlorophyll and chlorophyll fluorescence parameters in soybean (Glycine max) landraces. Euphytica. 2012;186:919–931. doi: 10.1007/s10681-012-0697-x. [DOI] [Google Scholar]
- Huang YS, Li HM. Arabidopsis CHLI2 can substitute for CHLI1. Plant Physiol. 2009;150:636–645. doi: 10.1104/pp.109.135368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Yoshimura N, Motohashi K, Takahashi S, Romano P, Hisabori T, Takamiya KI, Masuda T. The CHLI1 subunit of Arabidopsis thaliana magnesium chelatase is a target protein of the chloroplast thioredoxin. J Biol Chem. 2007;282:19282–19291. doi: 10.1074/jbc.M703324200. [DOI] [PubMed] [Google Scholar]
- Jensen P, Gibson L, Hunter C. ATPase activity associated with the magnesium-protoporphyrin IX chelatase enzyme of synechocystis PCC6803: evidence for ATP hydrolysis during Mg2+ insertion, and the MgATP-dependent interaction of the ChlI and ChlD subunits. Biochem J. 1999;339:127–134. doi: 10.1042/bj3390127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Chen S, Zheng T, Zeng Z, Liu L. A point mutation of magnesium chelatase OsCHLI gene dampens the interaction between CHLI and CHLD subunits in Rice. Plant Mol Biol Rep. 2015;33:1975–1987. doi: 10.1007/s11105-015-0889-3. [DOI] [Google Scholar]
- Li F, Zhang H, Yang T, Wang J, Gu X. Relationgship between fluorescence parameters ans chlorophyll content in soybean leaves at pod filling stage. J Nuclear Agric Sci. 2022;36:2519–2527. [Google Scholar]
- Li M, Yang D, Li W. Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding. Photosynthetica. 2007;45:222–228. doi: 10.1007/s11099-007-0036-y. [DOI] [Google Scholar]
- Li X, Xiang F, Zhang W, Yan J, Li X, Zhong M, Yang P, Chen C, Liu X, Mao D, Zhao X. Characterization and fine mapping of a new dwarf mutant in Brassica napus. BMC Plant Biol. 2021;21:117. doi: 10.1186/s12870-021-02885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Yan J, Zhong M, Zhuo Y, Wu D, He R, Zhao X, Liu X. EMS mutagenesis and analysis of multi-branched and long-silique mutants in Brassica napus L. Life Sci Res. 2016;20:435–441. [Google Scholar]
- Lundqvist J, Elmlund H, Wulff RP, Berglund L, Elmlund D, Emanuelsson C, Hebert H, Willows RD, Hansson M, Lindahl M, Al-Karadaghi S. ATP-induced conformational dynamics in the AAA+ motor unit of magnesium chelatase. Structure. 2010;18(3):354–365. doi: 10.1016/j.str.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Luo T, Luo S, Araujo WL, Schlicke H, Rothbart M, Yu J, Fan T, Fernie AR, Grimm B, Luo M. Virus-induced gene silencing of pea CHLI and CHLD affects tetrapyrrole biosynthesis, chloroplast development and the primary metabolic network. Plant Physiol Biochem. 2013;65:17–26. doi: 10.1016/j.plaphy.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shi J, Wang D, Liang X, Wei F, Gong C, Qiu L, Zhou H, Folta K, Wen Y, Feng J (2023) A point mutation in the gene encoding Mg-chelatase subunit I influences strawberry leaf color and metabolism. Plant Physiol. 10.1093/plphys/kiad247 [DOI] [PubMed]
- Mansfeld BN, Grumet R. QTLseqr: an R package for bulk segregant analysis with next-generation sequencing. Plant Genome. 2018;11:180006. doi: 10.3835/plantgenome2018.01.0006. [DOI] [PubMed] [Google Scholar]
- Mueller AH, Dockter C, Gough SP, Lundqvist U, von Wettstein D, Hansson M. Characterization of mutations in barley fch2 encoding chlorophyllide a oxygenase. Plant Cell Physiol. 2012;53:1232–1246. doi: 10.1093/pcp/pcs062. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Masuda T, Sato N, Yamagata H, Bowler C, Ohta H, Shioi Y, Takamiya K. Cloning, subcellular localization and expression of CHL1, a subunit of magnesium-chelatase in soybean. Biochem Bioph Res Co. 1995;215:422–428. doi: 10.1006/bbrc.1995.2481. [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Albrecht V. Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol. 2011;155:1545–1551. doi: 10.1104/pp.110.170365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissler HM, Collakova E, DellaPenna D, Whelan J, Pogson BJ. Chlorophyll biosynthesis. Expression of a second chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002;128:770–779. doi: 10.1104/pp.010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryouichi T, Koichi K, Tatsuru M. Tetrapyrrole metabolism in Arabidopsis thaliana. Arabidopsis Book. 2011;9:e0145. doi: 10.1199/tab.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Rahman ML, Cho SH, Kim YS, Koh HJ, Yoo SC, Paek NC. The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J. 2013;74:122–133. doi: 10.1111/tpj.12110. [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc. 2006;1:2019–2025. doi: 10.1038/nprot.2006.286. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang N, Du L. Chlorophyll biosynthesis in a chlorophyll b-deficient oilseed rape mutant Cr3529. Acta Bot Boreal-Occident Sin. 2007;27(10):1962–1966. [Google Scholar]
- Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol. 2007;58:321. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- Tang S, Liu D, Lu S, Yu L, Li Y, Lin S, Li L, Du Z, Liu X, Li X, Ma W, Yang Q, Guo L. Development and screening of EMS mutants with altered seed oil content or fatty acid composition in Brassica napus. Plant J. 2020;104:1410–1422. doi: 10.1111/tpj.15003. [DOI] [PubMed] [Google Scholar]
- Tian X, Ling Y, Fang L, Peng D, He G. Gene cloning and functional analysis of yellow green leaf3 (ygl3) gene during the whole-plant growth stage in rice. Genes genom. 2013;35:87–93. doi: 10.1007/s13258-013-0069-5. [DOI] [Google Scholar]
- Walker CJ, Willows RD. Mechanism and regulation of Mg-chelatase. Biochem J. 1997;327(2):321–333. doi: 10.1042/bj3270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang L, Li Y, Ali Buttar Z, Wang N, Xie Y, Wang C. Single nucleotide mutagenesis of the TaCHLI gene suppressed chlorophyll and fatty acid biosynthesis in common wheat seedlings. Front Plant Sci. 2020;11:97. doi: 10.3389/fpls.2020.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei L, Wang J, Xie L, Li Y, Ran S, Ren L, Lu K, Li J, Timko MP, Liu L. Integrating GWAS, linkage mapping and gene expression analyses reveals the genetic control of growth period traits in rapeseed (Brassica napus L.) Biotechnol Biofuels. 2020;13:134. doi: 10.1186/s13068-020-01774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He Y, Yang M, He J, Guan R. Fine mapping of a dominant gene conferring chlorophyll-deficiency in Brassica napus. Sci Rep. 2016;6:31419. doi: 10.1038/srep31419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HG, Yang HW, Rao Y, Yang B, Zhu Y. Photosynthetic characteristics and chlorophyll fluorescence kinetic parameters analyses of chlorophyll-reduced mutant in Brassica napus L. Acta Agronomica Sinica. 2013;39(3):520–529. doi: 10.3724/SP.J.1006.2013.00520. [DOI] [Google Scholar]
- Xing H, Dong L, Wang Z, Zhang H, Han C, Liu B, Wang X, Chen Q. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang J, Wu Z, Liu H, Huang F, Wu Y, Carrie C, Narsai R, Murcha M, Whelan J, Wu P. Identification of a dual-targeted protein belonging to the mitochondrial carrier family that is required for early leaf development in rice. Plant Physiol. 2013;161:2036–2048. doi: 10.1104/pp.112.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Li Y, Yuan C, Zhang H, Peng H, Lin H, Wang X, Wu X. Studies of photosystem complexes and chlorophyll synthesis in chlorophyll-deficient rice mutant W1. Sci Agric Sin. 2006;39(7):1299–1305. [Google Scholar]
- Yang M, Wan S, Chen J, Chen W, Wang Y, Li W, Wang M, Guan R (2023) Mutation to a cytochrome P450-like gene alters the leaf color by affecting the heme and chlorophyll biosynthesis pathways in Brassica napus. Plant J. 10.1111/tpj.16382 [DOI] [PubMed]
- Yang P, Li Y, He C, Yan J, Zhang W, Li X, Xiang F, Zuo Z, Li X, Zhu Y, Liu X, Zhao X. Phenotype and TMT-based quantitative proteomics analysis of Brassica napus reveals new insight into chlorophyll synthesis and chloroplast structure. J Proteomics. 2020;214:103621. doi: 10.1016/j.jprot.2019.103621. [DOI] [PubMed] [Google Scholar]
- Ye S, Yang J, Huang Y, Liu J, Ma X, Zhao L, Ma C, Tu J, Shen J, Fu T, Wen J. Bulk segregant analysis-sequencing and RNA-Seq analyses reveal candidate genes associated with albino phenotype in Brassica napus. Front Plant Sci. 2022;13:994616. doi: 10.3389/fpls.2022.994616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li J, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC. Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol. 2006;62:325–337. doi: 10.1007/s11103-006-9024-z. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li X, Yang Y, Hu K, Zhou X, Wen J, Yi B, Shen J, Ma C, Fu T. Bna A02.YTG1,encoding a tetratricopeptide repeat protein,is required for early chloroplast biogenesis in Brassica napus. Crop J. 2022;10:597–610. doi: 10.1016/j.cj.2021.06.010. [DOI] [Google Scholar]
- Zhang Z, Xiao G, Liu R, Tan T, Guan C, Wang G, Chen S. Proteomic analysis of differentially expressed proteins between Xiangyou 15 variety and the mutant M15. Front Biol. 2014;9:234–243. doi: 10.1007/s11515-014-1311-5. [DOI] [Google Scholar]
- Zhao Y, Du L, Yang S, Li S, Zhang Y. Chloroplast composition and structural differences in a chlorophyll-reduced mutant of oilseed rape of seedlings. Acta Botanica Sinica. 2001;43:877–880. [Google Scholar]
- Zhu L, Yang Z, Zeng X, Gao J, Liu J, Yi B, Ma C, Shen J, Tu J, Fu T, Wen J. Heme oxygenase 1 defects lead to reduced chlorophyll in Brassica napus. Plant Mol Biol. 2017;93:579–592. doi: 10.1007/s11103-017-0583-y. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zeng X, Chen Y, Yang Z, Qi L, Pu Y, Yi B, Wen J, Ma C, Shen J, Tu J, Fu T. Genetic characterisation and fine mapping of a chlorophyll-deficient mutant (BnaC.ygl) in Brassica napus. Mol Breeding. 2014;34:603–614. doi: 10.1007/s11032-014-0060-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data have been deposited in the NCBI database under BioProject PRJNA912866, the SRA accession numbers are: SRR12968239, SRR12968240, SRR12968241, and SRR12968242. The data that support the findings of this study are available in the main text and supporting materials of this article.