Abstract
Objective:
To evaluate if children and adolescents with a diagnosis of ASD or ADHD have distinct executive function (EF) profiles.
Methods:
Peer-reviewed articles comparing ASD, ADHD, and typically developing individuals under 19 years of age were identified. The domains evaluated were: working memory, response inhibition, planning, cognitive flexibility, attention, processing speed, and visuospatial abilities.
Results:
Fifty-eight articles met inclusion criteria. Analyses were performed on 45 performance metrics from 24 individual tasks. No differences in EF were found between individuals diagnosed with ASD and ADHD. Individuals diagnosed with ASD and ADHD exhibited worse performance in attention, flexibility, visuospatial abilities, working memory, processing speed, and response inhibition than typically developing individuals. Groups did not differ in planning abilities.
Conclusion:
Children and adolescents with ASD and ADHD have similar EF profiles. Further research is needed to determine if comorbidity accounts for the commonality in executive dysfunction between each disorder.
Keywords: autism spectrum disorders (ASD), children, adolescence, ADHD, executive function
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are impairing, childhood-onset, neurodevelopmental disorders. ADHD is characterized by developmentally inappropriate inattention, hyperactivity, and impulsivity (American Psychiatric Association [APA], 2013) and affects 5% to 7% of school-aged children (Polanczyk et al., 2014). ASD is characterized by restricted and repetitive behaviors, interests, and/or activities in conjunction with impairments in social interactions and communication (APA, 2013). About 2% of school-aged children are diagnosed with ASD (Maenner et al., 2020). A comorbid diagnosis of ASD and ADHD could not be made prior to the release of the DSM-V. Since then, it has become clear that approximately 40% to 70% of individuals with ASD exhibit comorbid ADHD or marked subthreshold symptoms (Hours et al., 2022). The overlap of symptoms in individuals with ASD and ADHD peaks during adolescence, where the majority of research has been focused (Hartman et al., 2016). ADHD and ASD share many behavioral, genetic, neural, and cognitive features suggesting some overlapping mechanisms (Antshel & Russo, 2019; Craig et al., 2016; Hartman et al., 2016).
Executive Functions (EFs) are higher-order neurocognitive processes that are critical for guiding and regulating attention, affect, and action (Hosenbocus & Chahal, 2012). Most models of EF include working memory, response inhibition, planning, cognitive flexibility, and attention (Diamond, 2013; Miyake et al., 2000; Stuss & Benson, 1987). Processing speed is often considered to be a distinct EF or is included in EF studies as a control condition (Cepeda et al., 2013) as is reaction time variability (Buczylowska & Petermann, 2018). Each EF domain can be evaluated using a variety of qualitative rating scales and quantitative neuropsychological tasks. Quantitative and qualitative EF data have little correlation and the former are more empirical (Toplak et al., 2014). Therefore, only quantitative neuropsychological tests were included in this meta-analysis.
EF deficits are present during childhood in various neurodevelopmental disorders including both ASD and ADHD (Barkley, 1997; Ozonoff & Jensen, 1999; Russell, 1997). There is considerable interest in the EF profiles of each psychopathology because EF are thought to link underlying genetic, neural, or environmental mechanisms to the behavioral manifestations of psychopathology and to predict current and future functional impairment such as quality of life and educational attainment (Mannuzza & Klein, 2000; Yang et al., 2022). Identifying distinct EF deficits in either group could clarify etiology, advance early detection, and improve screening and diagnosis (Uddin et al., 2017). However, it is unclear whether ASD and ADHD have distinct or common EF profiles. Shared EF deficits would support the hypothesis that ASD and ADHD have overlapping mechanisms. Current conclusions about EF profiles in ASD and ADHD are based on the results of comparisons of ASD and typically developing controls and studies of ADHD and typically developing controls (Antshel & Russo, 2019; Hosenbocus & Chahal, 2012; Schachar et al., 2007). A comparison of the results of these studies suggests that a diagnosis of ASD is associated with deficient cognitive flexibility and a diagnosis of ADHD is associated with deficient response inhibition, though the results are mixed (Antshel & Russo, 2019; Hosenbocus & Chahal, 2012; Schachar et al., 2007). More persuasive evidence about shared or distinct EF profiles requires direct comparison of the two diagnoses to ensure control over extraneous factors such as task variation, age, or diagnostic criteria that might influence results (Haidich, 2010; Craig et al., 2016). There has not been a meta-analysis that directly compares EF in children and adolescents with ASD and ADHD. Meta-analysis enables the pooling of results from similar primary research articles to derive a more precise estimate of the effect (Haidich, 2010).
Therefore, the primary aim of this systematic review and meta-analysis is to evaluate the differences in EF deficits in ADHD and ASD in studies where the two disorders have been compared directly. Based on the results of indirect comparisons, we hypothesized that ASD and ADHD would have distinct EF profiles-inhibition deficit in ADHD and cognitive flexibility deficit in ASD. The secondary aim of this review is to evaluate the differences in EF between ASD and ADHD with typically developing children and adolescents.
Methods
This systematic review and meta-analysis was pre-registered on PROSPERO (RecordID = 262654) prior to the commencement of the study. This review was completed following Cochrane guidelines for conducting reviews (Higgins et al., 2022), and the preferred reporting for systematic reviews and meta-analyses guidelines (PRISMA) for reporting the review (Liberati et al., 2009; see Supplemental PRISMA Checklist).
Search Strategy
A comprehensive search strategy created with the assistance and approval of a librarian at the University of Calgary was implemented from inception to May 2021 in CINAHL, EBM, Embase, Medline, and PsycINFO databases. The complete search strategy is available in the Supplemental Materials. The search strategy followed the framework of population age (e.g., “pediatric”, etc), intervention (e.g., “executive function” or “response inhibition,” etc), and diagnosis (e.g., “ADHD” and “ASD,” etc). An additional hand search of the reference lists of included articles and relevant reviews was also conducted to further exhaust the literature. No location or date limits were applied. A rigorous screening process was completed in Covidence (Release 17; StataCorp, 2021). Each article was screened at two levels: titles and abstracts, followed by full-text screening by two blinded reviewers (PT, SYL, PP, or CL). Discrepancies between reviewers were settled through consensus among the entire study team.
Inclusion Criteria
To be included, articles were required to be primary empirical research published in a peer-reviewed journal with an English full-text of the article available. Studies must have included one group with a primary diagnosis of ASD using DSM or ICD criteria, a group with a primary diagnosis of ADHD using DSM or ICD criteria, and a typically developing group. A control group was necessary to be included to evaluate if the measure was differential and sensitive to both ADHD and ASD conditions and if their performance was different from typically developing individuals in each study. Participants in all groups must have been under 19 years of age and matched in age and sex. Articles must have reported the mean and a measure of variation for each primary outcome of interest. Lastly, rating scales to measure EF, such as the Behavior Rating of Executive Function (BRIEF), were not included because of their low convergence with laboratory measures of EFs (McAuley et al., 2010).
Data Extraction
Charting of data for included articles was done in duplicate by two reviewers (PT, SYL, PP, or CL) with an online form that was calibrated a priori. Discrepancies between reviewers were resolved through consensus among the entire study team. The following information was recorded from each article: publication year, the country where the study was conducted, whether data was collected during an fMRI or EEG scan, sample size, mean age, number of females, IQ, stimulant medication use on the day of testing, the EF domain(s) reported to have been tested, and the task(s) used to evaluate EF.
Risk of Bias
An evaluation of the risk of bias for individual included articles was achieved using the Downs and Black risk of bias assessment (Downs & Black, 1998). Risk of bias assessments are imperative to evaluate the validity of results based on the quality of included literature and increase the transparency of secondary research (Higgins et al., 2011). The Downs and Black scale was chosen for its high internal consistency, test-retest reliability, inter-rater reliability, and high correlations with existing instruments (Downs & Black, 1998). Modifications to the assessment followed the recommended risk of bias assessment for cross-sectional studies. Question 13, “Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive?” was also removed because treatment studies were irrelevant to the current review.
Data Analysis
Processing speed, reaction time variability and visuospatial abilities were included in the review even though they are not always considered EFs (Dias et al., 2018). Literature was synthesized both by tasks employed and domains of EF measured. Most EF tasks generate multiple performance metrics. Performance metrics from each task were categorized under the domains that they were reported to have measured by included literature. A narrative synthesis of results was then performed for each EF domain.
Meta-analyses were conducted for task performance metrics that were reported by at least two separate studies using a weighted random-effects model with a DerSimonian-Laird model estimator (DerSimonian & Laird, 1986) and a standardized mean difference effect size (SMD). Effect sizes were reported as Hedge’s g. As a general rule of thumb, a small Hedges g represents (g = 0.2), medium (g = 0.5), and large (g = 0.8). A random effects model was used to cope with the heterogeneity in how tasks were administered by studies. Pooled effect sizes were not conducted within each EF domain due to the marked differences in what aspect of the respective EF domain each performance metric were reported to have measured. Pooling each performance metric would lack meaningful interpretations of results and undermine the underlying substantive and methodological issues present in the highly heterogeneous EF literature (Savitz & Forastiere, 2021).
Three separate meta-analyses were performed for each performance metric: ASD versus ADHD, ASD versus typically developing, and ADHD versus typically developing. The current study looked specifically at case-control differences between each disorder and did not evaluate dimensional measures within each participant or diagnosis. Meta-analyses were conducted using STATA (StataCorp, 2021). A significant positive effect size indicates a higher score on the respective metric in those with ASD or ADHD compared to typically developing individuals. In the case of the ASD versus ADHD analysis, a positive effect size indicates a higher score for those with ASD.
The Tau2 statistic was used as an estimate of the between-study variance in each meta-analysis, where the square root is the estimated standard deviation across studies (Higgins et al., 2022). The I2 statistic was also utilized for analyses of more than four articles as an evaluation of the proportion of total variance among studies due to heterogeneity (Higgins & Thompson, 2002). The I2 statistic is a transformation of the Q statistic and is reported as a percentage of the total variability and depends on the degrees of freedom for the sample. I2 resulting percentages of 75%, 50%, and 25% indicate a high, medium, and low heterogeneity, respectively (Huedo-Medina et al., 2006). Evidence for dysfunction in an EF domain is supported by many individual studies yielding a significant Hedge’s g (SMD) of medium or large effect size that is not heterogenous as indexed by I2 and Tau2.
Results
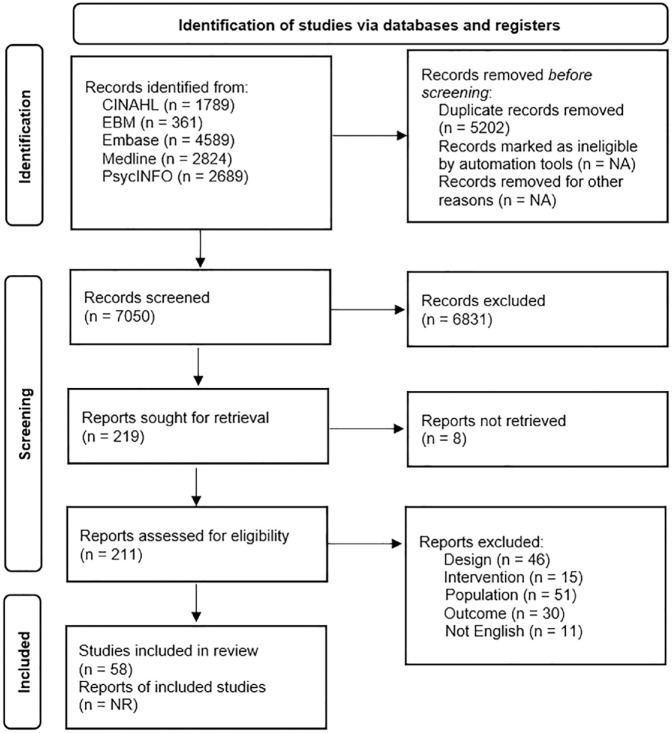
Fifty-eight articles met inclusion criteria and were included following the comprehensive screening process (see Figure 1). A full reference list of all the included articles is presented in the Supplemental Material. There was moderate inter-rater reliability during the screening process (Kappa = 0.53). There was high inter-rater reliability for data extraction. See Table 1 for aggregate data of the study and participant demographic characteristics for included studies. Ethnicity was only reported in 10 of the 58 articles. A comorbid group was included in 11 articles which precluded the ability to include the group in any analysis. Two articles stratified their outcomes into visual and auditory performance during the Continuous Performance Test (Kim et al., 2018) and the Go/No-Go task (Nydén et al., 1999). The results from the visual versions of these tasks were used because visual tasks are far more common than auditory ones.
Figure 1.
PRISMA flow diagram.
Note. Articles were reviewed in duplicate by two reviewers blind to each other’s decision following a two level screening process. Discrepancies were settled through consensus between the entire study team.
Table 1.
Aggregate Data of Study and Participant Characteristics for Included Studies.
| Evaluated characteristics | Units | ASD group | ADHD group | TD group |
|---|---|---|---|---|
| Articles reporting each group | K (%) | 58 (100.0) | 58 (100.0) | 58 (100.0) |
| Total sample size | N (%) | 2,092 (23.5) | 2,800 (31.5) | 3,367 (37.8) |
| Total female participants | N (%) | 217 (11.2) | 538 (27.9) | 1,035 (53.6) |
| Age | Mean (SD) | 10.3 (1.9) | 9.8 (1.8) | 10.1 (1.8) |
| Range | 3.5–18.9 | 3.3–18 | 3.5–18.33 | |
| Articles reporting stimulant medication use on the day of testing | ||||
| No | K (%) | 41 (70.7) | 41 (70.7) | — |
| Yes | K (%) | 1 (1.7) | 2 (3.4) | — |
| Not reported | K (%) | 16 (27.6) | 15 (25.9) | — |
Note. A total of 58 primary research articles, involving 8,259 participants with an average age of 9.5 (SD = 1.8) years old. Data collected for three groups including autism spectrum disorder (ASD), ADHD, and typically developing (TD). N = number of participants; K = number of articles.
Risk of Bias
The average score of articles on the Downs and Black risk of bias assessment was 11.22 out of 14 total points that could be awarded, revealing moderate-high validity. The minimum score for an article was 8 points (Unterrainer et al., 2020), and the maximum points awarded to an article was 13 (Christakou et al., 2013; Hutchison et al., 2016). The included articles were awarded 662 points (80.15%) out of the total 826 possible points that articles could collectively receive. There was a high overall agreement for each article rating between reviewers. See the Supplemental Material for a full breakdown.
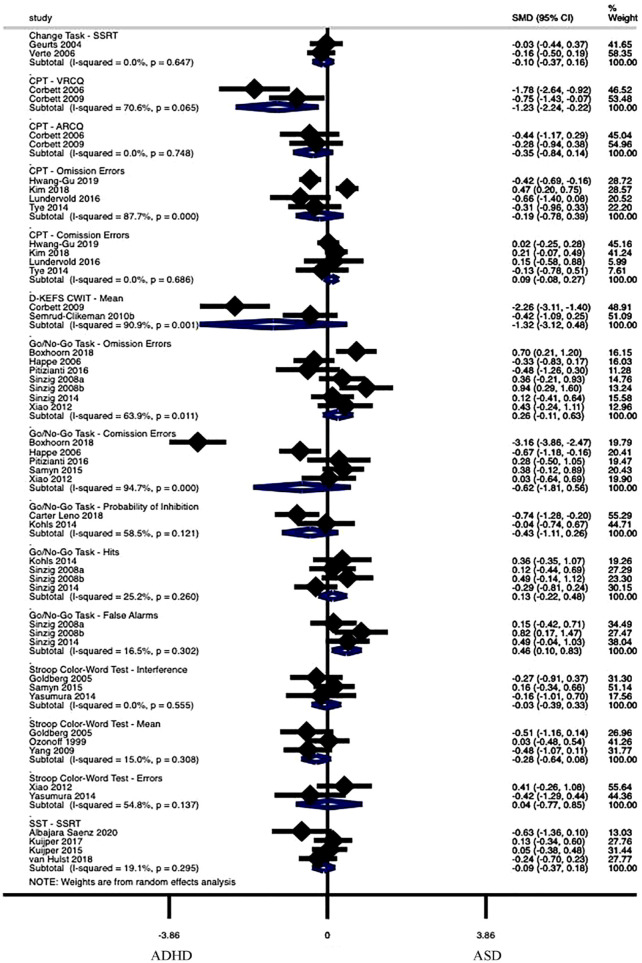
Response Inhibition
Fifteen performance metrics derived from six tasks evaluated response inhibition. The group with ASD performed significantly worse than the group with ADHD in two performance metrics. See Figure 2 and Supplemental Table 2a for the complete set of analyses. The group with ASD (n = 33) scored significantly worse than the group with ADHD (n = 33) in studies (k = 2) on the Visual Response Control Quotient from the Continuous Performance Test (CPT) (Hedge’s g = −1.23, p = .02, 95% CI [−2.24, −0.22], τ2 = .38). The group with ASD (n = 66) also scored significantly worse than the group with ADHD (n = 80) in studies (k = 3) on the number of False Alarms from the Go/No-Go Task (Hedge’s g = 0.46, p = .01, CI [0.10, 0.83], τ2 = .02). The remaining 13 nonsignificant performance metrics had effect sizes ranging from Hedge’s g = −1.32 to 0.26, p = .12 to .92, τ2 = .00 to 1.73.
Figure 2.
Forest plot of response inhibition meta-analysis.
Note. Standardized Mean Difference (SMD) estimates are reported using Hedge’s g. I2 estimates are only relevant for analyses of five or more articles. Significance has been reached when the Subtotal does not cross 0. Positive values indicate greater scores in the ASD group. SSRT = stop signal response time; CPT = continuous performance test; VRCQ = visual response control quotient; ARCQ = auditory response control quotient; D-KEFS = Delis-Kaplan executive function system; CWIT = color-word interference test; SST = stop signal task.
Eight performance metrics demonstrated significantly worse performance in the group with ASD compared to the typically developing group (see Supplemental Table 2b). Six performance metrics demonstrated significantly worse performance in the group with ADHD compared to the typically developing group (see Supplemental Table 2c).
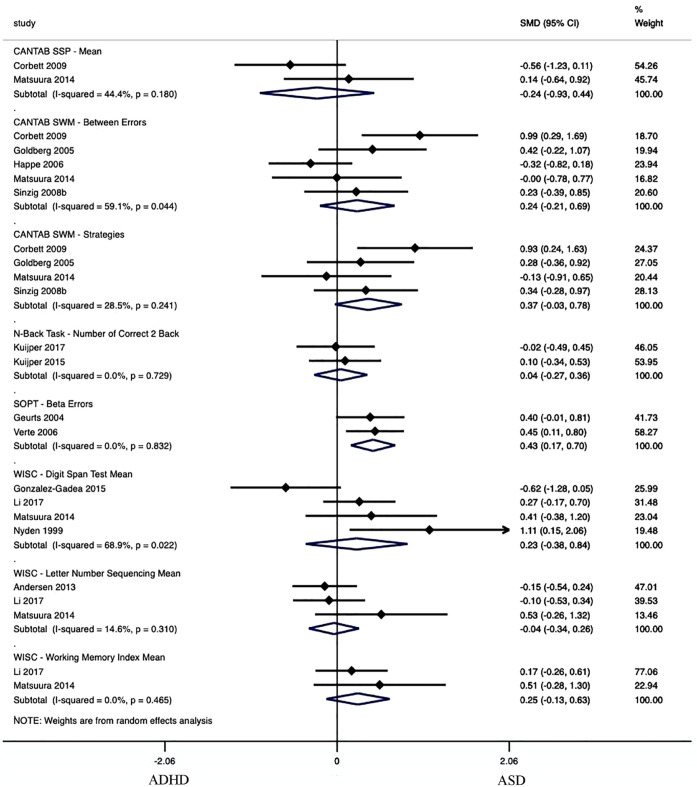
Working Memory
Working Memory was evaluated in eight performance metrics as part of seven tasks, where one performance metric showed significantly worse performance in ASD compared to ADHD (see Figure 3 and Supplemental Table 2a). The group with ASD (n = 107) had significantly more Beta Errors on the Self-Ordered Pointing Task than the group with ADHD (n = 119) in studies (k = 2; Hedge’s g = 0.43, p < .01, 95% CI 0.17, 0.70], τ2 = .00). The remaining seven performance metrics had effect sizes ranging from Hedge’s g = −0.24 to 0.37, p = .07 to .81, τ2 = .00 to .26.
Figure 3.
Forest plot of working memory meta-analysis.
Note. Standardized Mean Difference (SMD) estimates are reported using Hedge’s g. I2 estimates are only relevant for analyses of five or more articles. Significance has been reached when the Subtotal does not cross 0. Positive values indicate greater scores in the ASD group. CANTAB = cambridge neuropsychological test automated battery; SSP = spatial span; SWM = spatial working memory; SOPT = self-ordered pointing task; WISC = Wechsler intelligence scale for children.
Four performance metrics showed significantly worse performance in the group with ASD in comparison to the typically developing group (see Supplemental Table 2b). In ADHD versus controls, six performance metrics expressed significantly worse performance in the group with ADHD compared to the typically developing group (see Supplemental Table 2c).
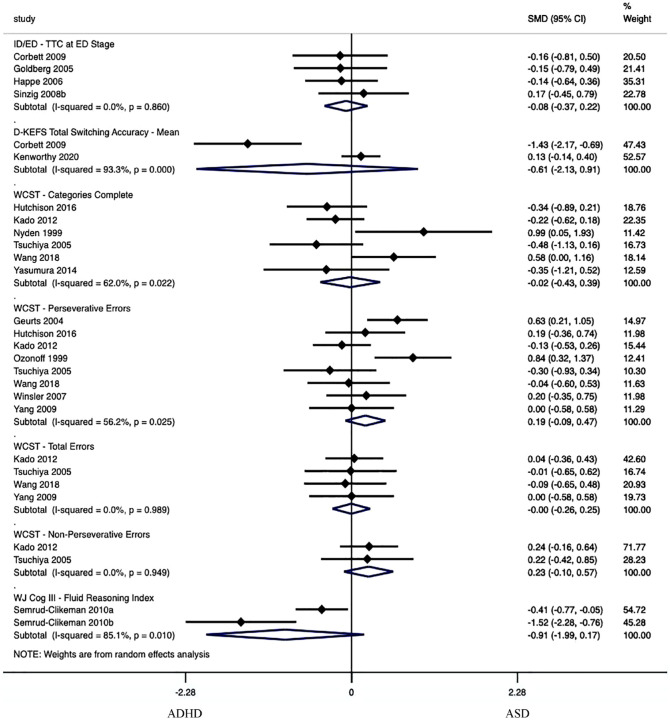
Cognitive Flexibility / Set-Shifting
Cognitive Flexibility/Set-Shifting was evaluated in seven performance metrics as part of four tasks, where no performance metrics showed significantly worse performance in either group (see Figure 4 and Supplemental Table 2a). The seven performance metrics had effect sizes ranging from Hedge’s g = −0.91 to 0.23, p = .10 to .97, τ2 = .00 to 1.13.
Figure 4.
Forest plot of cognitive flexibility/set-shifting meta-analysis.
Note. Standardized Mean Difference (SMD) estimates are reported using Hedge’s g. I2 estimates are only relevant for analyses of five or more articles. Significance has been reached when the Subtotal does not cross 0. Positive values indicate greater scores in the ASD group. ID/ED = CANTAB intra-dimensional/extra-dimensional set shifting; TTC = number of trials needed to reach criterion; D-KEFS = Delis-Kaplan executive function system; WCST = Wisconsin card sorting task; WJ Cog = Woodcock-Johnson tests of cognitive abilities.
Six performance metrics had significantly worse performance in the group with ASD compared to the typically developing group (see Supplemental Table 2b). Three performance metrics had significantly worse performance in the group with ADHD compared to the typically developing group (see Supplemental Table 2c).
Planning
Planning was evaluated in five performance metrics as part of four tasks, where no performance metric showed significantly worse performance in either group (see Supplemental Figure and Supplemental Table 2a). The five performance metrics had effect sizes ranging from Hedge’s g = −0.61 to 0.31, p = .22 to .77, τ2 = .00 to 1.13.
The group with ASD performed significantly worse than the typically developing group on one performance metric (see Supplemental Table 2b). The group with ADHD did not perform significantly worse than the typically developing group on any performance metrics (see Supplemental Table 2c).
Processing Speed
Processing Speed was evaluated in 10 performance metrics as part of seven tasks, where one performance metric exemplified significantly worse performance in ASD and one performance metric exemplified significantly worse performance in ADHD (See Supplemental Figure and Supplemental Table 2a). The Initial Thinking Time—from the CANTAB—Stockings of Cambridge (SOC) task found a significant result in studies (k = 2) with the group with ADHD (n = 38) scoring higher than the group with ASD (n = 38) (Hedge’s g = −0.69, p < .01, 95% CI [−1.16, −0.23], τ2 = .00). The Mean—from the WISC—Processing Speed Index (PSI) found a significant result in studies (k = 3) with the group with ADHD (n = 308) scoring higher than ASD (n = 220; Hedge’s g = −0.43, p < .001, 95% CI [−0.60, −0.25], τ2 = .00). The remaining eight performance metrics had effect sizes ranging from Hedge’s g = −0.23 to 0.26, p = .17 to .95, τ2 = .00 to .25.
Five performance metrics identified significantly worse performance in the group with ASD compared to the typically developing group (see Supplemental Table 2b). Five performance metrics also identified significantly worse performance in the group with ADHD compared to the typically developing group (see Supplemental Table 2c).
Attention
Attention was evaluated in 10 performance metrics as part of four tasks, where one performance metric showed significantly worse performance in ASD compared to ADHD (see Supplemental Figure and Supplemental Table 2a). The Visual Response Control Quotient from the (CPT) found a significant result in studies (k = 2) where the group with ADHD (n = 33) scored higher than the group with ASD (n = 33; Hedge’s g = −1.23, p = .02, 95% CI [−2.24, −0.22], τ2 = .38). The remaining nine performance metrics had effect sizes ranging from Hedge’s g = −0.62 to 0.26, p = .16 to .75, τ2 = .00 to 1.73.
Eight performance metrics expressed significantly worse performance in the group with ASD compared to the typically developing group (see Supplemental Table 2b). Eight performance metrics also expressed significantly worse performance in the group with ADHD compared to the typically developing group (see Supplemental Table 2c).
Visuospatial Abilities
Visuospatial Abilities were evaluated in four performance metrics as part of four tasks, where one performance metric showed significantly worse performance in ADHD compared to ASD (see Supplemental Figure and Supplemental Table 2a). The Mean from the WISC Block Design found a significant result in studies (k = 4) where the group with ASD (n = 125) scored higher than the group with ADHD (n = 144; Hedge’s g = 0.34, p = 0.01, 95% CI [0.10, 0.59], τ2 = .00). The remaining nine performance metrics had effect sizes ranging from Hedge’s g = −0.26 to 0.08, p = .19 to .68, τ2 = .00 to .00.
Three performance metrics showed significantly worse performance in the ASD group compared with the typically developing group (see Supplemental Table 2b). Two performance metrics showed significantly worse performance in ADHD and one performance metric showed significantly better performance in the group with ADHD than the typically developing group (see Supplemental Table 2c).
Discussion
This is the first meta-analysis of EF studies focusing on direct comparisons of children and adolescents with ASD and those with ADHD. Limiting this review to direct comparisons allowed for tighter control over the tasks, task conditions, and factors such as age, sex, medication status at the time of testing, and testing environment (e.g., during an fMRI or EEG scan), which could confound the interpretation of results of indirect comparisons where ASD and ADHD are first compared to typically developing individuals and then the results are compared between groups.
The results of this meta-analysis found no evidence to support the hypothesis that children and adolescents with ASD and those with ADHD have distinct EF profiles. The current meta-analysis did confirm that both ADHD and ASD exhibited EF deficits compared to the typically developing peers. Both ASD and ADHD groups performed significantly worse than the typically developing group in response inhibition, cognitive flexibility, visuospatial abilities, and attention. Less consistent deficits were also found in processing speed and working memory. No deficits in planning were found in ASD or ADHD. The current results support the hypothesis that ASD and ADHD share some underlying mechanism even though they are distinct clinical disorders.
The results of this meta-analysis of direct comparisons using a meta-analytical approach of ASD and ADHD differ from the conclusions reached by previous reviews (Antshel & Russo, 2019; Banaschewski et al., 2005; Craig et al., 2016). Craig et al. (2016) compared children with ASD and/or ADHD and found both groups had similar executive function impairments in attention and working memory. Craig et al. (2016) further identified cognitive flexibility and planning deficits in those with ASD only. Lastly, Craig et al. (2016) found response inhibition impairments in those with ADHD only. Antshel and Russo (2019) also reviewed the literature on executive functioning in those with ASD and ADHD, though their review was not systematic and they did not look specifically at youth. Antshel and Russo (2019) concluded that only individuals with ADHD exhibited challenges in response inhibition and planning. They also found only individuals with ASD expressed challenges in cognitive flexibility (Antshel & Russo, 2019). Another review comparing those with ADHD to different disorders including those with ASD found mixed results in children, where individuals with ASD exemplified greater difficulties in cognitive flexibility and planning (Banaschewski et al., 2005).
This meta-analysis was not able to determine if the similarity in EF deficits in ASD and ADHD were due to the high rate of comorbidity of these disorders (Hours et al., 2022). Few studies were found that included a group with comorbid ASD and ADHD and fewer that controlled for comorbidity using quantitative trait measures. Some studies which included a comorbid group found greater deficits in the comorbid group compared to the ASD, ADHD and typically developing groups (Antshel & Russo, 2019; Craig et al., 2016; Hwang-Gu et al., 2019). These studies do not clarify whether EFs are associated with ASD or ADHD or whether the comorbid group represents a distinct diagnostic entity (Caron & Rutter, 1991; Krakowski et al., 2021). Studies in which ADHD and ASD traits were controlled indicate that, at least response inhibition and response variability, may be more strongly associated with ADHD than with ASD traits (Karalunas et al., 2018; Schachar et al., 2022). Taken together, these results are not inferring that all children with ASD should be suspected of having ADHD because each disorder present differently clinically in other symptom areas such as in social functioning (Hours et al., 2022). Rather, executive functioning deficits are apparent across both neurodevelopmental disorders.
Limitations
The literature that was available for meta-analysis covered a wide range of EF processes using many different tests. Theoretical limitations prevented a pooled estimated across all performance metrics evaluating each EF domain. Many individual analyses included a small number of articles and participants, precluding the ability to evaluate moderating variables, such as age, that may significantly influence results. Many analyses also had large between-study variations. While the risk of bias assessment found a medium-high quality of articles, external validity categories (extent findings can be generalized to the respective population) were low. Only English articles were included, which may have excluded some international publications. We found few studies that included a comorbid group, controlled for traits of ASD and ADHD, or distinguished between ADHD subtypes precluding adequate consideration of the effect of comorbidity and subtype on EF performance (Schachar et al., 2022; Semrud-Clikeman et al., 2010).
Future Directions
EFs were measured by a plethora of tasks with variation among studies in what EFs were attributed to each task, which has been a long-standing issue in the literature (Lezak, 1982). In the future, standardization of EF tests and performance metrics would greatly increase the consistency of reporting and reliability of results improving data integration across studies (Verbruggen et al., 2019). One method to accomplish this goal would be to adopt the NIMH Research Domain Criteria (RDoC) approach (Insel et al., 2010) and standardized measures of each EF such as in the NIH ToolKit (Hodes et al., 2013). Future evaluations of the effect of comorbidity, dimensional ADHD traits, age, and sex on executive functioning are necessary.
Conclusion
This meta-analysis provides the most systematic and up-to-date summary of the executive function profiles in children and adolescents with ASD and those with ADHD. No differences were found between children and adolescents with ASD and ADHD in any executive function domain. However, individuals with ASD and individuals with ADHD exhibited significant deficits compared to controls.
Supplemental Material
Supplemental material, sj-docx-1-jad-10.1177_10870547231190494 for Do ASD and ADHD Have Distinct Executive Function Deficits? A Systematic Review and Meta-Analysis of Direct Comparison Studies by Parker Townes, Chunlin Liu, Prabdeep Panesar, Daniel Devoe, Soo Youn Lee, Gracie Taylor, Paul D. Arnold, Jennifer Crosbie and Russell Schachar in Journal of Attention Disorders
Acknowledgments
The authors would like to thank Nicole Dunnewold, University of Calgary Health Sciences Library.
Author Biographies
Parker Townes is a research coordinator in the Department of Psychiatry at The University of Calgary, Canada.
Chunlin Liu is a summer research student at SickKids Hospital, University of Toronto, Canada.
Prabdeep Panesar is a summer research student at SickKids Hospital, University of Toronto, Canada.
Daniel Devoe is a contract faculty member in the Psychology Department at Mount Royal University, Canada
Soo Youn Lee is a summer research student at SickKids Hospital, University of Toronto, Canada.
Gracie Taylor is a summer research student in the Psychology Department at Mount Royal University, Canada.
Paul D. Arnold is the Academic Director at The Hotchkiss Brain Institute, Mathison Centre for Mental Health Research and Education at The University of Calgary, Canada.
Jennifer Crosbie is a Psychologist and an Associate Scientist in the Department of Psychiatry at The University of Toronto SickKids Hospital, Canada.
Russell Schachar is a Psychiatrist and a Senior Scientist in the Department of Psychiatry at The University of Toronto SickKids Hospital, Canada.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Canadian Institutes of Health Research (R.J.S., MOP-93696 and P.D.A., MOP-106573), the TD Bank Financial Group Chair in Child and Adolescent Psychiatry (R.J.S.), and the Alberta Innovates Translational Health Chair in Child and Youth Mental Health (P.D.A.).
ORCID iD: Parker Townes  https://orcid.org/0000-0002-9314-1616
https://orcid.org/0000-0002-9314-1616
Data Availability: The data that support the findings of this study are available from the corresponding author under reasonable request.
Supplemental Material: Supplemental material for this article is available online.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI]
- Antshel K. M., Russo N. (2019). Autism spectrum disorders and ADHD: Overlapping phenomenology, diagnostic issues, and treatment considerations. Current Psychiatry Reports, 21(5), 34. 10.1007/s11920-019-1020-5 [DOI] [PubMed] [Google Scholar]
- Banaschewski T., Hollis C., Oosterlaan J., Roeyers H., Rubia K., Willcutt E., Taylor E. (2005). Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Developmental Science, 8(2), 132–140. 10.1111/j.1467-7687.2005.00400.x [DOI] [PubMed] [Google Scholar]
- Barkley R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Buczylowska D., Petermann F. (2018). Intraindividual variability in executive function performance in healthy adults: Cross-sectional analysis of the NAB executive functions module. Frontiers in Psychology, 9, 329. 10.3389/fpsyg.2018.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron C., Rutter M. (1991). Comorbidity in child psychopathology: Concepts, issues and research strategies. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 32(7), 1063–1080. 10.1111/j.1469-7610.1991.tb00350.x [DOI] [PubMed] [Google Scholar]
- Cepeda N. J., Blackwell K. A., Munakata Y. (2013). Speed isn’t everything: Complex processing speed measures mask individual differences and developmental changes in executive control. Developmental Science, 16(2), 269–286. 10.1111/desc.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Murphy C. M., Chantiluke K., Cubillo A. I., Smith A. B., Giampietro V., Daly E., Ecker C., Robertson D., Murphy D. G., Rubia K. (2013). Disorder-specific functional abnormalities during sustained attention in youth with Acttention Deficit Hyperactivity Disorder (ADHD) and with Autism. Molecular Psyhiatry, 18(2), 236–244. 10.1038/mp.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig F., Margari F., Legrottaglie A. R., Palumbi R., de Giambattista C., Margari L. (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment, 12, 1191–1202. 10.2147/NDT.S104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. (1986) Meta-analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias B. F., Rezende L. O., Malloy-Diniz L. F., Paula J. J. D. (2018). Relationship between visuospatial episodic memory, processing speed and executive function: Are they stable over a lifespan? Arquivos De Neuro-psiquiatria, 76(2), 89–92. 10.1590/0004-282x20170186 [DOI] [PubMed] [Google Scholar]
- Downs S. H., Black N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health, 52(6), 377–384. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidich A. B. (2010). Meta-analysis in medical research. Hippokratia, 14(1), 29–37. [PMC free article] [PubMed] [Google Scholar]
- Hartman C. A., Geurts H. M., Franke B., Buitelaar J. K., Rommelse N. N. J. (2016). Changing ASD-ADHD symptom co-occurrence across the lifespan with adolescence as crucial time window: Illustrating the need to go beyond childhood. Neuroscience and Biobehavioral Reviews, 71, 529–541. 10.1016/j.neubiorev.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Sterne J. A. C. (2011). Chapter 8: Assessing risk of bias in a randomized trial. In Higgins J. P. T., Green S. (Eds.), Cochrane handbook for systematic reviews of interventions (version 5.1.0). The Cochrane Collaboration. https://www.training.cochrane.org/handbook [Google Scholar]
- Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., Welch V. A. (Eds.). (2022). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. https://www.training.cochrane.org/handbook [Google Scholar]
- Higgins J. P. T., Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hodes R. J., Insel T. R., Landis S. C., & NIH Blueprint for Neuroscience Research. (2013). The NIH toolbox: Setting a standard for biomedical research. Neurology, 80(11 Suppl 3), S1. 10.1212/WNL.0b013e3182872e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosenbocus S., Chahal R. (2012). A review of executive function deficits and pharmacological management in children and adolescents. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 21(3), 223–229. [PMC free article] [PubMed] [Google Scholar]
- Hours C., Recasens C., Baleyte J. M. (2022). ASD and ADHD comorbidity: What are we talking about? Frontiers in Psychiatry, 13, 837424. 10.3389/fpsyt.2022.837424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina T. B., Sánchez-Meca J., Marín-Martínez F., Botella J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- Hutchison L., Feder M., Abar B., Winsler A. (2016). Relations between parenting stress, parenting style, and child executive functioning for children with ADHD or autism. Journal of Child and Family Studies, 25(12), 3644–3656. 10.1007/s10826-016-0518-2 [DOI] [Google Scholar]
- Hwang-Gu S. L., Lin H. Y., Chen Y. C., Tseng Y. H., Hsu W. Y., Chou M. C., Chou W. J., Wu Y. Y., Gau S. S. (2019). Symptoms of ADHD affect intrasubject variability in youths with autism spectrum disorder: An ex-Gaussian analysis. Journal of Clinical Child and Adolescent Psychology, 48(3), 455–468. 10.1080/15374416.2018.1452151 [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D. S., Quinn K., Sanislow C., Wang P. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Karalunas S. L., Hawkey E., Gustafsson H., Miller M., Langhorst M., Cordova M., Fair D., Nigg J. T. (2018). Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. Journal of Abnormal Child Psychology, 46(8), 1705–1716. 10.1007/s10802-017-0394-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. M., Lim M. H., Kwon H.-J., Yoo S.-J., Kim E., Kim J. W., Ha M., Paik K. C. (2018). Associations between urinary cotinine and symptoms of attention deficit/hyperactivity disorder and autism spectrum disorder. Environmental Research, 166, 481–486. 10.1016/j.envres.2018.06.018 [DOI] [PubMed] [Google Scholar]
- Krakowski A. D., Szatmari P., Crosbie J., Schachar R., Duku E., Georgiades S., Anagnostou E. (2021). Latent structure of combined autistic and ADHD symptoms in clinical and general population samples: A scoping review. Frontiers in Psychiatry, 12, 654120. 10.3389/fpsyt.2021.654120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M. D. (1982). The problem of assessing executive functions. International Journal of Psychology, 17, 281–297. 10.1080/00207598208247445 [DOI] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gotzsche P. C., Ioannidis J. P. A., Clarke M., Devereaux P. J., Kleijnen J., Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339(1), b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner M. J., Shaw K. A., Baio J., Washington A., Patrick M., DiRienzo M., Christensen D. L., Wiggins L. D., Pettygrove S., Andrews J. G., Lopez M., Hudson A., Baroud T., Schwenk Y., White T., Rosenberg C. R., Lee L.-C., Harrington R. A., Huston M., . . . Dietz P. M. (2020). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR. Surveillance Summaries, 69(4), 1–12. 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S., Klein R. G. (2000). Long-term prognosis in attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America, 9(3), 711–726. [PubMed] [Google Scholar]
- McAuley T., Chen S., Goos L., Schachar R., Crosbie J. J. (2010). Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? International Neuropsychological Society, 16(3), 495–505. 10.1017/S1355617710000093 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Rettinger D. A., Shah P., Hegarty M. (2000). How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. Journal of Experimental Psychology: General, 129(4), 611–628. 10.1037/0096-3445.129.4.611 [DOI] [PubMed] [Google Scholar]
- Nydén A., Gillberg C., Hjelmquist E., Heiman M. (1999). Executive function/attention deficits in boys with asperger syndrome, attention disorder and reading/writing disorder. Autism, 3(3), 213–228. 10.1177/1362361399003003002 [DOI] [Google Scholar]
- Ozonoff S., Jensen J. (1999). Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 29(2), 171–177. 10.1023/a:1023052913110 [DOI] [PubMed] [Google Scholar]
- Polanczyk G. V., Willcutt E. G., Salum G. A., Kieling C., Rohde L. A. (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. 10.1093/ije/dyt261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. (1997). Autism as an executive disorder. Oxford University Press. [Google Scholar]
- Savitz D. A., Forastiere F. (2021). Do pooled estimates from meta-analyses of observational epidemiology studies contribute to causal inference? Occupational and Environmental Medicine, 78(9), 621–622. 10.1136/oemed-2021-107702 [DOI] [PubMed] [Google Scholar]
- Schachar R. J., Dupuis A., Arnold P. D., Anagnostou E., Kelley E., Georgiades S., Nicolson R., Townes P., Burton C. L., Crosbie J. (2022). Autism spectrum disorder and attention-deficit/hyperactivity disorder: Shared or unique neurocognitive profiles? Research on Child and Adolescent Psychopathology, 51, 17–31. 10.1007/s10802-022-00958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R. J., Logan G. D., Robaey P., Chen S., Ickowicz A., Barr C. (2007). Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 35(2), 229–238. 10.1007/s10802-006-9075-2 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M., Walkowiak J., Wilkinson A., Butcher B. (2010). Executive functioning in children with asperger syndrome, ADHD-combined type, ADHD-predominately inattentive type, and controls. Journal of Autism and Developmental Disorders, 40(8), 1017–1027. 10.1007/s10803-010-0951-9 [DOI] [PubMed] [Google Scholar]
- StataCorp. (2021) Stata statistical software (Release 17). StataCorp LLC. Covidence systematic review software, Veritas Health Innovation, Melbourne. https://www.covidence.org [Google Scholar]
- Stuss D. T., Benson F. (1987). The frontal lobes and control of cognition and memory. In Perecman E. (Ed.), The frontal lobes revisited (1st ed., pp. 141–158). Psychology Press. 10.4324/9781315788975 [DOI] [Google Scholar]
- Toplak M. E., West R. F., Stanovich K. E. (2014). Rational thinking and cognitive sophistication: Development, cognitive abilities, and thinking dispositions. Developmental Psychology, 50(4), 1037–1048. 10.1037/a0034910 [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Dajani D. R., Voorhies W., Bednarz H., Kana R. K. (2017). Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Translational Psychiatry, 7(8), e1218. 10.1038/tp.2017.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterrainer J. M., Rahm B., Loosli S. V., Rauh R., Schumacher L. V., Biscaldi M., Kaller C. P. (2020). Psychometric analyses of the Tower of London planning task reveal high reliability and feasibility in typically developing children and child patients with ASD and ADHD. Child Neuropsychology, 26(2), 257–273. 10.1080/09297049.2019.1642317 [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Aron A. R., Band G. P., Beste C., Bissett P. G., Brockett A. T., Brown J. W., Chamberlain S. R., Chambers C. D., Colonius H., Colzato L. S., Corneil B. D., Coxon J. P., Dupuis A., Eagle D. M., Garavan H., Greenhouse I., Heathcote A., Huster R. J., Jahfari S., . . . Boehler C. N. (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife, 8, e46323. 10.7554/eLife.46323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Shields G. S., Zhang Y., Wu H., Chen H., Romer A. L. (2022). Child executive function and future externalizing and internalizing problems: A meta-analysis of prospective longitudinal studies. Clinical Psychology Review, 97, 102194. 10.1016/j.cpr.2022.102194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jad-10.1177_10870547231190494 for Do ASD and ADHD Have Distinct Executive Function Deficits? A Systematic Review and Meta-Analysis of Direct Comparison Studies by Parker Townes, Chunlin Liu, Prabdeep Panesar, Daniel Devoe, Soo Youn Lee, Gracie Taylor, Paul D. Arnold, Jennifer Crosbie and Russell Schachar in Journal of Attention Disorders