Abstract
Chimeric antigen receptor T cells (CAR-T) therapy has shown great potential in tumor treatment. However, many factors impair the efficacy of CAR-T therapy, such as antigenic heterogeneity and loss, limited potency and persistence, poor infiltration capacity, and a suppressive tumor microenvironment. To overcome these obstacles, recent studies have reported a new generation of CAR-T cells expressing cytokines called armored CAR-T, TRUCK-T, or the fourth-generation CAR-T. Here we summarize the strategies of arming CAR-T cells with natural or synthetic cytokine signals to enhance their anti-tumor capacity. Moreover, we summarize the advances in CAR-T cells expressing non-cytokine proteins, such as membrane receptors, antibodies, enzymes, co-stimulatory molecules, and transcriptional factors. Furthermore, we discuss several prospective strategies for armored CAR-T therapy development. Altogether, these ideas may provide new insights for the innovations of the next-generation CAR-T therapy.
Keywords: chimeric antigen receptor, CAR, cytokines, cancer immunotherapy, biomedical engineering, synthetic biology
Graphical abstract
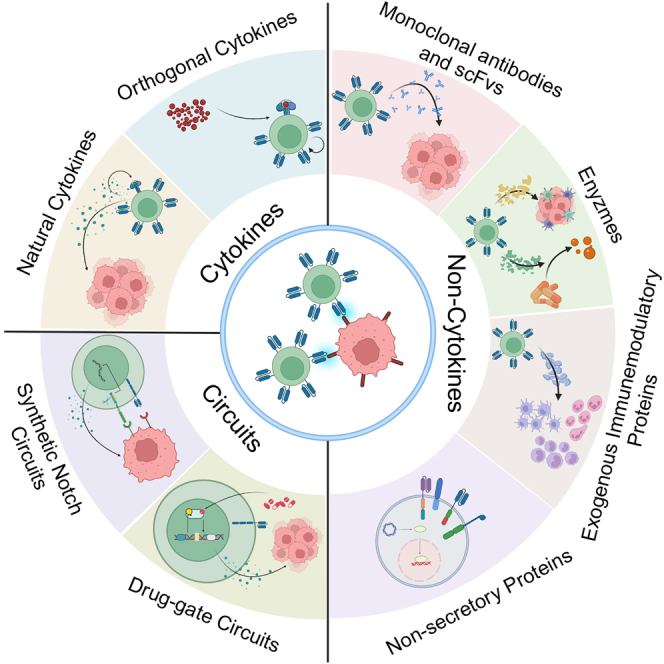
Qiang and colleagues review the advances in fourth-generation CAR-T therapy, which improve the functions of CAR-T itself, enhance supportive immunity, or directly arm CAR-T with tumor-killing payloads. These strategies could be summarized as “CAR+X”—arming CAR-T cells with certain biological payloads and carrying out corresponding functions.
Introduction
Chimeric antigen receptor T (CAR-T) cell therapy has succeeded dramatically in treating hematological malignancies. Adoptive transfer of autologous CD19-targeted CAR-T cells has become the first genetically modified cell-based therapy approved by the U.S. Food and Drug Administration.1 The first-generation CAR molecule consists of a single-chain variable fragment (scFv) derived from monoclonal antibodies that can specifically recognize antigens, a transmembrane domain, and an intracellular signaling domain of CD3.2 However, these CAR-T cells show low proliferative capacity and anti-tumor activity.3 Next, the second-generation CAR integrates a co-stimulating domain, CD28 or 4-1BB, to augment and prolong the activation of CAR-T cells.4 Moreover, the third-generation CARs simultaneously integrate two of the CD28, the 4-1BB, and OX40 domains, which further enhances the anti-tumor capacity of CAR-T cell therapy.5
In contrast with CAR-T cell therapy for hematological malignancies, the efficacy of CAR-T therapy for solid tumors remains limited, which is due to multiple factors, including tumor antigen heterogeneity, limited potency and persistence of T cells, poor infiltration capacity, and a suppressive tumor microenvironment (TME).6,7 To get around these challenges, researchers have tried to improve CAR-T cell function, enhance their infiltration, modify the TME to activate bystander immune cells, and use combined therapy. Recently, approaches that arm CAR-T cells with cytokine expression to boost their anti-tumor capacity have shown significant promise in overcoming the challenges, which are called armored CAR-T, T cells redirected for universal cytokine-mediated killing (TRUCK-T), or the fourth-generation CAR-T therapy.8,9 Moreover, the force-expressed cytokines in armored CAR-T cells can be replaced by a series of secretory or non-secretory functional proteins to further improve the efficacy of CAR-T therapy. In this review, we summarize the strategies for designing armored CAR-T cell therapies, mainly focusing on CAR-T cells expressing natural or synthetic cytokines, antibodies, enzymes, and other secretory proteins. Additionally, we discuss the advances in CAR-T cells expressing non-secretory proteins, including co-stimulatory molecules and transcriptional factors, which mainly enhance the capacity of CAR-T cells through signaling transduction and transcriptional regulation. Furthermore, potential strategies that may be engaged in the future development of next-generation CAR-T therapy are discussed.
TRUCK-T: Redirected for natural cytokine-mediated killing
CAR-T cells remain ineffective in most solid tumors, mainly because of poor infiltration, expansion and persistence of CAR-T cells in the TME.10 These results could be explained by the timely limited activation of the first and the second T cell signals (i.e., TCR and co-stimulatory signal).11 Therefore, cytokines are supplemented to CAR-T cells to maintain a sustained T cell response by producing a third signal.12 The fourth-generation CAR-T therapy makes CAR-T cells as biofactories or vehicles to produce transgenic cargo, like cytokines, to generate a third signal or other additional signals. TRUCK-T cells often produce one or two types of cytokines that improve the CAR-T functions or modify the TME to combat malignancies.8,13 In the following part, we discuss the advances in TRUCK-T therapeutics, mainly based on different structural and functional families of cytokines used in CAR-T therapies (Table 1).
Table 1.
Summary of CAR-T cells co-expressing cytokines in the treatment of tumors
| Cytokine-based cargos | Tumor | Targeted antigen | Model | Reference |
|---|---|---|---|---|
| IL-15 | Burkitt lymphoma | CD19 | immune-deficient tumor model | Hoyos et al.15 |
| IL-15 | lung cancer, neuroblastoma, and glioblastoma | GD2 | immune-deficient tumor model | Reppel et al., Chen et al. and Gargett et al.16,17,18 |
| IL-2 | pancreatic cancer and melanoma | CD19 | immune-deficient tumor model | Allen et al.20 |
| IL-2 | B cell malignancies | HER2 | immune-deficient tumor model | Li et al.21 |
| IL-2, IL-15, IL-7, IL-21 | hematological malignancies | CD19 | immune-deficient tumor model | Markley and Sadelain24 |
| IL-21 | chronic lymphocytic leukemia | CD19 | immune-deficient tumor model | Štach et al.22 |
| IL-15 and IL-21 | HCC | GPC3 | immune-deficient tumor model | Batra et al.26 |
| IL-23 | neuroblastoma | GD2 | immune-deficient/competent tumor model | Ma et al.27 |
| IL-23 | prostate cancer | PSMA | immune-deficient tumor model | Wang et al.28 |
| IL-7 and CCL19 | lung cancer, pancreatic ductal adenocarcinoma | CD20, MSLN | immune-competent tumor model | Adachi et al.31 |
| IL-12 | B cell malignancies, non-Hodgkin lymphomas | CD19 | immune-competent tumor model | Pegram et al.40 |
| IL-12 | colorectal tumors | CEA | immune-deficient tumor model | Chmielewski et al.13 |
| IL-18 | leukemia | CD19 | immune-deficient tumor model | Hu et al.44 |
| IL-18 | hematological malignancies and ovarian carcinoma | CD19, MUC | immune-deficient/competent tumor model | Avanzi et al.45 |
| IL-18 | pancreatic and lung tumors | CEA | immune-competent tumor model | Chmielewski and Abken46 |
| IL-36 | lymphoma | CD19 | immune-competent tumor model | Li et al.50 |
| Flt3L | mouse colon adenocarcinoma and sarcoma | HER2 | immune-competent tumor model | Lai et al.54 |
Enhance the potency and persistence of CAR-T cells
IL-2 and IL-15
Improving T cell activity with proliferative cytokines, such as IL-2 and IL-15, has been thought to drive powerful anti-tumor functions.14 IL-15 is crucial for T cell homeostasis and survival.15 A study reported that CD19 CAR-T cells with antigen-induced IL-15 expression showed superior survival, expansion, and anti-tumor activity compared with controlled CAR-T cells in xenogeneic models of B cell malignancies.15 Similarly, GD2 CAR-T cells expressing IL-15 show extraordinary abilities of infiltration and cytotoxicity in lung cancer, neuroblastoma, and glioblastoma in xenograft models.16,17,18 A study conducted a comparative analysis of the activity and toxicity in two phase 1 studies.19 One evaluated glypican-3 (GPC3) CAR-T therapy (NCT02932956, NCT02905188), and the other examined the same CAR combined with IL-15 expression (NCT04377932, NCT05103631). Six patients were infused with 3 × 107 GPC3 CAR-T cells and six with 3 × 107 IL-15 GPC3 CAR-T cells. The IL-15 GPC3 CAR-T cells showed higher peak expansion and comparable effective tumor trafficking. The increased expansion was associated with a higher response rate. Three of the patients (3/6 [50%]) treated with IL-15 GPC3 CAR-T cells showed partial responses (PR), including resolution of lung metastases, and the other three (3/6 [50%]) had progressive disease (PD). In the comparative GPC3 CAR-T trials, two patients (2/6 [33.3%]) exhibited PD, and four (4/6 [66.7%]) exhibited stable disease (SD). The responsive rate was 50% versus 0. However, increased expansion was also associated with a higher incidence of adverse events. The patients in the IL-15 GPC3 CAR-T group had higher levels of cytokine release syndrome (CRS): one patient with grade 2 and two with grade 4, versus one grade 2 event in the comparative GPC3 CAR-T group. Another trial of IL-15 GD2 CAR-T therapy for neuroblastoma and osteosarcoma (NCT03721068) is underway.
IL-2 is commonly used in the in vivo culture of T cells,19 since it has a canonical function on T cell activation and expansion. However, systemic IL-2 treatment is extremely toxic and can lead to serious adverse effects.20 Constructing CAR-T cells with conditionally expressed IL-2 using synthetic gene circuits is a strategy reported in recent studies to overcome these obstacles.20,21 These cells showed a better ability to proliferate and infiltrate, less expansive effect of neighboring regulatory T cells (Tregs), and less toxicity compared with CAR-T cells with IL-2 expression dependent on CAR or T cell receptor (TCR) signals and systemic IL-2 treatment.20
IL-7 and IL-21
Enhancing the CAR-T cells’ potency and persistence in vivo is a vital issue.22 IL-7 can promote the homeostatic expansion of native and memory T cells, which also has been proven in early clinical trials.23 Moreover, compared with CD19-CAR-T cells expressing IL-2 or IL-15, the constitutive expression of IL-7 in CD19-CAR-T cells demonstrated enhanced anti-tumor activity, potentially attributed to the sustained killing of target cells and T cell expansion in immunodeficient mice.24 However, it should be noted that these IL-7-expressing CD19-CAR-T cells did not exhibit the long-term persistence observed in IL-15-expressing CD19-CAR-T cells.24
Like IL-15, IL-21 overexpression supported long-term T cell persistence in vivo.24 IL-21 is a pleiotropic cytokine that acts on a variety of lymphocyte subsets, such as B cells, T cells, and natural killer (NK) cells, which promotes the expansion and maintains the early memory phenotype of T cells.25 Štach et al. designed anti-CD19 CAR-T cells expressing IL-21 after antigen stimulation, demonstrating enhanced potential to expand and sustain the early memory phenotype in an immune-deficient chronic lymphocytic leukemia model.22 Furthermore, to investigate whether the combinational expression of IL-21 and IL-15 enhances the anti-tumor effect of CAR-T cells against hepatocellular carcinoma (HCC), Batra et al.26 designed GPC3 CAR-T cells co-expressing IL-15 and IL-21 upon the activation of CAR, which exhibited robust expansion and sustained persistence in HCC xenograft models, resulting in superior tumor control and survival compared with non-armored CAR-T or equipped with either cytokine alone. These results provided a strong rationale for evaluating GPC3 CAR-T cells co-expressing IL-15 and IL-21 in patients with liver cancers (NCT04715191, NCT02932956).26
IL-23
IL-23 is a heterodimer composed of IL-23α p19 and IL-12β p40 subunit, which supports the proliferation and survival of T cells.27 Ma et al.27 found that the activation of T cells induces the expression of both IL-23α p19 subunit and IL-23R. Based on this finding, they designed a type of T cell that produces IL-23 upon activation by forced expression of IL-12β p40 submitted in T cells (named p40-Td cells). These p40-expressing T cells couple the release and the activity of IL-23 with T cell activation, which drives T cell proliferation and survival. Incorporating p40 in CAR- or TCR-engineered T cells further enhanced their anti-tumor activity in xenograft and syngeneic mouse models.27 However, IL-23 produced by p40-Td cells functions predominantly in an autocrine way, with limited effects on bystander cells. Therefore, an IL-23-expressing PSMA CAR-T was designed, which showed greater tumor clearance and faster weight recovery in an immune-deficient model of prostate cancer.28
Improve CAR-T trafficking: IL-7 × CCL19
Inefficient homing and penetration of solid tumors impair the efficacy of CAR-T therapy.7 Studies showed that IL-7 and CCL19 produced by T zone fibroblastic reticular cells are essential for forming and maintaining the T cell zone in lymphoid organs, where both T cells and dendritic cells (DCs) are recruited from the periphery.29,30 To recruit T cells and DCs into solid tumors, Adachi et al.31 designed a mesothelin (MSLN)-targeting CAR-T expressing IL-7 and CCL19 under antigen stimulating (7 × 19 CAR-T), imitating the characteristics of T zone fibroblastic reticular cells. Histopathological analyses showed increased infiltration of DCs and T cells into tumor tissues following 7 × 19 CAR-T treatment in an immune-competent model.31 Moreover, BCMA-targeting 7 × 19 CAR-T cells preliminarily showed promising safety and efficacy in a clinical trial treating refractory or relapsed (R/R) multiple myeloma (NCT03778346). The first two enrolled patients achieved a complete response (CR) and very good PR with grade 1 CRS only 1 month after one dose of CAR-T cell infusion, and the responses lasted more than 12 months.32 Another multicenter phase 1b trial of 7 × 19 CAR-T in patients with R/R large B cell lymphoma (NCT03929107, NCT04833504) showed that 7 × 19 CD19 CAR-T cells can induce durable responses with a median overall survival of greater than 2 years and has a manageable safety profile in patients with R/R B cell lymphoma.33 Additionally, one trial of CD19 7 × 19 CAR-T therapy in B cell lymphoma (NCT05659628) is under way to evaluate the efficacy. Furthermore, a phase 1 trial (NCT03198546) was conducted for GPC3- or MSLN-targeted 7 × 19 CAR-T therapy in advanced HCC/pancreatic carcinoma (PC)/ovarian carcinoma patients. In the six-case preliminary cohort, one PC patient (1/6 [16.7%]) achieved CR; one HCC patient (1/6 [16.7%]) achieved PR, and two (2/6 [33.3%]) achieved steady disease. This result showed that 7 × 19 CAR-T cells had a great therapeutic potential.34 These studies all demonstrated that incorporating IL-7 and CCL19 into CAR-T cells significantly enhanced their anti-tumor capacity against hematological malignancies and solid tumors.
Reprogram T cell metabolism: IL-10
T cell exhaustion is one of the major hurdles in cancer immunotherapy. Studies showed that exhausted T cells exhibit suppressed mitochondrial respiration and/or glycolysis.35,36 Such poor metabolic fitness may further reinforce T cell exhaustion, while maintaining mitochondrial fitness can restore the proliferation and effector function of exhausted T cells, leading to enhanced anti-tumor immunity.35,36 IL-10 is known to enhance the mitochondrial oxidative phosphorylation of macrophages.37 Interestingly, Guo et al.38 found that IL-10 can also reprogram T cell metabolism and restore the functions of exhausted T cells. They produced a recombinant half-life-extended fusion protein of human IL-10 and IgG1 Fc (IL-10-Fc). IL-10-Fc fusion protein directly and potently enhanced the expansion and effector function of terminally exhausted CD8+ tumor-infiltrating lymphocytes by promoting oxidative phosphorylation. Moreover, CAR-T cells co-treated with IL-10-Fc fusion protein showed enhanced oxygen consumption rate, proliferation, and killing efficiency. Although IL-10 is considered an immunosuppressive factor, these findings showed that IL-10 can also potentially revitalize terminally exhausted T cells, which enhanced the efficacy of adoptive T cell therapy unexpectedly.38 However, the above study only examined the function of IL-10 on CAR-T therapy in a trans-delivered way. Whether cis-expressed IL-10 can enhance the efficacy of CAR-T remains unknown.
Modify the immunosuppressive TME
IL-12
IL-12 is a pivotal proinflammatory cytokine derived from phagocytes and DCs in response to pathogens, which has a strong antitumor effect on modulating the TME. IL-12 functions by improving activation of cytotoxic T cells and NK cells, promoting the response of Th1 cells to produce interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α), and polarizing macrophages to M1 phenotype.13,39 Based on these privileges, considerable efforts have been made to establish IL-12 in tumor therapy. CD19 CAR-T cells constitutively secreting IL-12 can eradicate systemic B cell lymphoma and stimulate T cells to secrete IFN-γ, which can resist Treg-mediated suppression in an immune-competent model.40 However, the severe toxicity of IL-12 therapy limited its systemic administration in solid tumor.13,41 To solve this problem, GD2 CAR-T cells with intratumoral injection of IL-12 were proven to reshape TME in an immune-competent model of glioblastoma, driving increased infiltration of proinflammatory CD4+ T cells, decreased proportion of Treg cells, and activation of myeloid compartment.42 Furthermore, researchers designed CAR-T cells with induced IL-12 expression upon antigen stimulation of carcinoembryonic antigen (CEA) to enrich IL-12 in tumor lesions locally. Tumors treated with inducible IL-12 CAR-T cells were infiltrated with increased numbers of M1 polarized macrophages compared with tumors treated with CAR-T cells without IL-12 in NIH-III mice deficient in NK cells, B cells, and T cells. These M1 polarized macrophages can directly eliminate cancer cells, which can help the inducible IL-12-secreting CAR-T cells to eliminate the mixed tumor with a substantial number of cancer cells lacking the targeted antigen.13 Moreover, a study incorporated IL-12 into the exodomain of CAR, which reprogrammed CD8+ CAR-T cells to a novel NK cell-like signature and a CD94+CD56+ CD62Lhigh phenotype, like cytokine-induced killer cells.26 This IL-12-inserted CAR-T achieved antigen-independent, human leukocyte antigen E restricted cytotoxicity, and gained the ability to eliminate antigen-negative cancer cells in Rag−/−cg−/− immunodeficient mice.41 Because f the pleiotropic effects on autocrine activation, antigen-independent killing and TME modulation, IL-12 CAR-T therapy is expected to be successful in solid tumor treatment.
However, the efficacy and safety of these IL-12 CAR-T therapies need to be further evaluated. In a phase 1 clinical trial, the best response of constitutive IL-12 secreting CAR-T was only SD (NCT02498912, MUC6ecto CAR-T, ovarian, primary peritoneal, fallopian tube carcinoma). Although no significant toxicity was found in patients treated with this CAR-T alone without lymphodepletion, two-thirds of patients in the lymphodepleting cohorts showed dose-limited toxicity,43 revealing the safety issue of constitutive IL-12 expression. As for inducible IL-12 CAR-T therapy, a clinical trial is still underway to evaluate its efficacy and safety (NCT03542799) (epidermal growth factor receptor [EGFR] CAR-T, metastatic colorectal cancer).
IL-18
IL-18 was initially characterized as an inducer of IFN-γ expression in T cells and can activate lymphocytes and monocytes.44 A recent study reported that NSG mice bearing Nalm6 leukemia cells injected with CD19 CAR-T cells secreting constitutively IL-18 can remain viable for more than 185 days after T cell injection.44 Moreover, further analysis revealed that the blood concentration of CAR-T cells secreting constitutively IL-18 was nearly 35 times that of traditional CAR-T cells during treatment.44 These results show that CAR-T cells secreting constitutive IL-18 have a stronger proliferative ability than traditional CAR-T cells.44 Another study reported constitutive IL-18-secreting CD19 CAR-T cells were capable of modulating and activating endogenous immune cells residing in the bone marrow of immunocompetent syngeneic mouse models of both hematologic and metastatic solid tumor malignancies.45 Moreover, to fight against solid tumors, Chmielewski et al.46 constructed CEA CAR-T cells with inducible IL-18, which exhibited superior activity against large pancreatic and lung tumors that are refractory to CAR-T cells without cytokines. They demonstrated that the anti-tumor process of inducible IL-18 CAR-T was accompanied by the overall change of TME.46 These CAR-T cells in immune-competent models can convert the tumor environment to acute inflammation and decrease the number of immunosuppressive cells, such as Treg, suppressive CD103+ DC, and M2 macrophage, whereas CD206+ M1 macrophages and NKG2D+ NK cells increase in number.46 Recently, a phase 1 clinical trial (NCT04684563) on IL-18 CD19 CAR-T therapy has been reported.47 In 11 patients, this CAR-T therapy-induced overall response rate was 82%, and CR and PR rate were 55% and 27%, respectively. Disease progression occurred in 18% of patients, and the median duration of response was not reached (NR) (range, 6.6 to NR). Additionally, the 12-month progression-free survival rate was 54% (95% confidence interval, 15%–81%). At a median follow-up of 12 months (range, 3–20 months), the 12-month overall survival rate was 100%. Furthermore, CRS was observed in 58% patients: grade 1, 33%; grade 2, 17%; and grade 3, 8%. Seventeen percent patients had immune effector cell-associated neurotoxicity syndrome: grade 1, 8%; and grade 2m 8%. These results show the IL-18 CD19 CAR-T therapy have a manageable safety profile and induces lasting remission.
IL-36
IL-36 is a newly discovered member of the IL-1 superfamily that has shown potent anti-tumor activity in preclinical models.48 IL-36 can transform the TME and promote type 1 lymphocyte-mediated anti-tumor immune responses.49 A study reported that CD19 CAR-T cells expressing constitutively IL-36 showed more extraordinary abilities of expansion and persistence than control CAR-T cells in lymphoblastic leukemia immune competent models.50 Moreover, CD19 CAR-T cells secreting IL-36 can enhance major histocompatibility complex class II and CD86 expression on splenic macrophages and DCs of tumor-bearing mice, suggesting the role of IL-36 in the maturation of antigen-presenting cells and stimulation of endogenous immunity, which can promote the antigen presentation to activate bystander T cells.50 Therefore, CAR-T cells expressing IL-36 might be a viable treatment option for solid tumors with antigen heterogeneity or those with antigen loss.
Promote antigen epitope spreading: Flt3L
Enhancing endogenous supportive immunity to counteract the heterogeneity of tumor antigens is an important way to improve CAR-T efficacy against solid tumors.10,51,52 Strategies to boost endogenous immunity include stimulating bystander immune cells, reprogramming tumor-educated immunosuppressive cells, and, of utmost importance, promoting APCs to spread epitopes.10,12,51,53 Flt3L can promote hematopoietic progenitor commitment to the DC lineage as well as DC survival and proliferation in tissues.54,55 Lai et al.54 designed a novel CAR-T cell constitutively secreting Flt3L, which was able to expand intratumoral conventional type 1 DCs. This Flt3L CAR-T substantially activates DCs and T cells when combined with immune agonists poly (I:C) and anti-4-1BB antibody in immune-competent models. Importantly, this led to epitope spreading of antigens beyond those recognized by adoptively transferred T cells in solid tumor models of TCR- and CAR-T treatment, which suggests that enhancing the capabilities of endogenous DCs is a promising strategy to improve the anti-tumor ability of CAR-T cells.54 Other approaches for improving DC-based treatments include DC vaccination, expression of chemokine CXCL4 or type I IFNs (IFN-α/β), targeting the negative regulators of DC activation, and delivering DNA/RNA-containing nanoparticles.56,57 In the future, these approaches could be combined with CAR-T therapy, further enhancing the capacity of both exogenous and endogenous immunity.
From natural to orthogonal: Synthetic cytokines and receptors for CAR-T therapy
Although CAR-T cells co-expressing cytokines have great efficacy in many murine models, the risk of severe adverse events including neurotoxicity and CRS from the systemic accumulation of constitutively secreted cytokine cannot be ignored.20 This strategy limits the application of TRUCK-T cells and systemic cytokine accumulation is not seen so far in inducible CAR-T therapy.13 To avoid the potential risk of cytokine overproduction, engineering the downstream cytokine receptors or signaling transduction proteins instead of the cytokine itself might be an alternative option, although systemic cytokine accumulation has not been seen in the ongoing trials of CAR-T therapies with inducible cytokine expression yet. Moreover, a further strategy to minimize the potential risk of transgenic cytokine is to develop an orthogonal system that a certain engineered cytokine is only active on its modified receptor, but not on the natural one. Together, we discuss the recent advances in synthetic cytokine signals that have already been or might have the potential to be used in CAR-T therapy in the next part.
Constitutively active cytokine receptors
In an early attempt to decrease the systemic use of IL-2, researchers developed adoptive T cells overexpressing cytokine receptors such as IL-7R, which are responsive to IL-7 and might have fewer side effects.58 However, this design of IL-7R-overexpressed T cells did not eliminate the need for an exogenous cytokine supply. Based on it, Shum et al.59 constructed CAR-T cells expressing a constitutively active IL-7 cytokine receptor (C7R). This C7R harbors activating mutations derived from B cell precursor acute lymphoblastic leukemia patients, with its native extracellular domain replaced by ectodomains of CD34 to avoid additional activation by external ligands. The engineered C7R can activate CAR-T cells without the requirement of an exogenous supply or transgenic expression of cytokines, which avoids the overactivation of bystander immune cells and decreases side effects. Meanwhile, the growth and survival of C7R-expressing CAR-T cells remained antigen dependent, which demonstrated superior anti-tumor activity in metastatic neuroblastoma and orthotopic glioblastoma xenograft models.59 Two clinical trials (NCT04099797, NCT03635632) of C7R-expressing GD2 CAR-T cells against brain cancer and other cancers that express GD2 are underway to evaluate the safety and feasibility.
Chimeric cytokine receptors
Cytokine receptors are generally composed of several subunits, which makes it possible to be engineered modularly. Chimeric cytokine receptors have been invented to treat inflammatory diseases or expand hematopoietic progenitors in the last century.60,61 Recently, studies proved that chimeric cytokine receptors could be effective on CAR-T cells to convert inhibitory or neutral cytokine signals into activating ones.62,63 For example, IL-4 is a type 2 cytokine that inhibits Th1 polarization and promotes tumor growth.64 Based on this, chimeric IL-4 receptors were designed to combine an extracellular domain from IL-4R with an intracellular domain from the receptors of γc cytokines, such as IL-2R, IL-15R, and IL-7R, which invert the immunosuppressive effect of IL-4 in tumor into an activating signal.65,66 Unlike IL-4, which is secreted by tumors, granulocyte macrophage colony stimulating factor (GM-CSF) is a cytokine secreted by CAR-T cells upon activation.67 With a similar strategy, chimeric GM-CSF/IL-18 receptor (GM18) was reported to combine the extracellular domains of the GM-CSF receptor α/β chains with the transmembrane domain and the intracellular signaling domain of the IL-18 receptor α/β subunits. GM18 recognizes GM-CSF secreted by CAR-T cells and activates MyD88 downstream signaling, which sustains the effector function of CAR-T cells under chronic antigen exposure in solid tumor xenograft models.63 GM18 proved that an autocrine loop of cytokines secreted by CAR-T cells with their corresponding chimeric cytokine receptors can keep CAR-T self-activating. Another classic example is the hybrid IL-7Rα/IL-2Rβ receptor.68 IL-2 can counteract the transforming growth factor β (TGF-β) suppression on CD28-z CAR-T cells while activating Treg cells that infiltrate tumors.23 Unlike IL-2, IL-7 can counteract TGF-β suppression without stimulating Treg cells. However, the IL-7 receptor is rapidly downregulated by effector and central memory T cells upon ligand binding, which would desensitize CD28-z CAR-T cells to transgenic IL-7 in the long term and would lose TGF-β resistance. To prolong the protective effect of IL-7 and avoid IL-2 stimulation of Treg cells, Golumba-Nagy et al.68 engineered the hybrid IL-7Rα/IL-2Rβ receptor consisting of IL-7 receptor α chain in the extracellular moiety and the IL-2 receptor β chain in the intracellular moiety. The CAR-T cells with IL-7Rα/IL-2Rβ receptor and transgenic IL-7 can tolerate inhibitory signals from TGF-β in the TME of Rag2−/−γc−/− mice.68 Chimeric cytokine receptors can integrate the advantages of different cytokines/cytokine receptors and avoid the disadvantages, such as pluripotency toxicity, and immunosuppression. The development of chimeric cytokine receptors has greatly expanded the application scenarios of cytokines.
Equipping CAR-T with JAK-STAT signaling domains
The γc family cytokines, such as IL-2, IL-7, IL-15, IL-21, and IL-23, transmit signals via the JAK-STAT pathway. As summarized previously, these cytokines play an important role in activating and proliferating T cells and NK cells.69 To enhance the downstream signal of these cytokines, Kagoya et al.70 designed a novel CAR molecule containing JAK-STAT signaling domains. This CD19-targeted CAR encodes a truncated cytoplasmic domain from the IL-2 receptor β-chain (IL-2Rβ) and a STAT3-binding tyrosine-X-X-glutamine (YXXQ) motif, together with the CD28 co-stimulatory domain and the CD3ζ TCR signaling domain (referred to as 28-ΔIL2RB-z(YXXQ)). The 28-ΔIL2RBz(YXXQ) CAR-T cells showed antigen-dependent activation of the JAK kinase and the STAT3 and STAT5 transcription factors signaling pathways, promoting their proliferation and preventing terminal differentiation in vitro.70 Similarly, Zhang et al.71 designed a MUC1-targeted CAR with JAK-STAT signaling domains and verified its enhanced efficacy in esophageal cancers in NSG mice. These CAR-T cells transmit JAK-STAT signals in an antigen-dependent way, which demonstrated superior anti-tumor effects with minimal toxicity. This novel strategy suggests that more intracellular signaling domains could be incorporated into a CAR molecule to promote its function, and recent work on decoding the CAR-T cell phenotype with machine learning has proved this concept systemically.72
Pursuing orthogonal cytokine signaling
In addition to modifying cytokine receptors or CAR molecules, engineering cytokine to narrow down its bioactive spectrums provides another way to reduce its dose-related toxicities. A recent study reported a strategy designing a two-component synthetic cytokine that could independently target two surface markers but require colocalization for activity. Typically, they constructed a neoleukin-2/15 (Neo-2/15) that mimics a combination of IL-2 and IL-15, which can trans-activate immune cells surrounding targeted tumor cells or directly cis-activate targeted immune cells. Neo-2/15 could selectively expand CD8T cells or promote CAR-T cell activation in a CD25-independent way, resulting in enhanced anti-tumor efficacy and decreased toxicity in immunocompetent melanoma and lymphoma models.73 Therefore, constructing CAR-T cells expressing a couple of half cytokines might be a novel way to limit the universal adverse effects of cytokines.
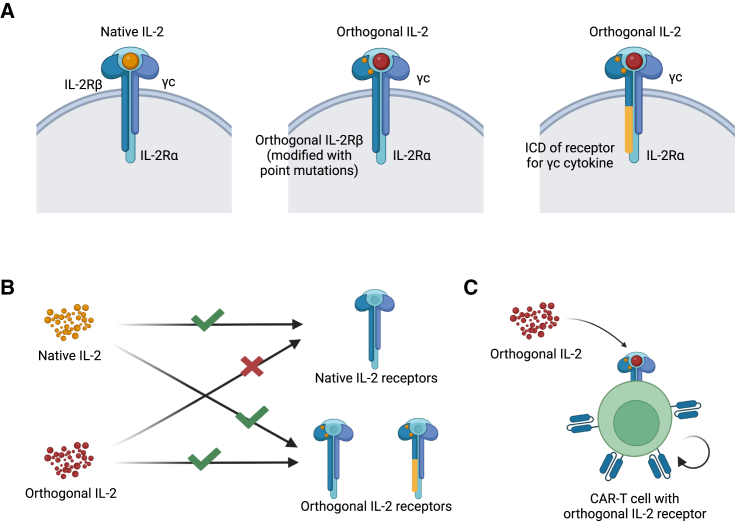
Further studies proceeded to limit the effect of cytokines on orthogonal functions. In this way, a certain engineered cytokine could only act on its modified receptor in correspondence (Figure 1). Sockolosky et al.74 designed a pair of orthogonal IL-2/IL-2Rβ molecules based on structural modifications. The modified ligand and receptor bind to each other specifically and transmit IL-2 signals independent from native cytokines and receptors. Next, they introduced the orthogonal IL-2Rβ into T cells and proved the orthogonal IL-2 acts specifically on engineered T cells in vitro and in vivo, avoiding the effect of IL-2 on other immune cells.74 Based on this study, researchers completed the humanization of orthogonal IL-2/IL-2Rβ and combined them with CAR-T therapy to limit the side effects of armored CAR-T cells.75,76 A clinical trial (NCT05665062) of IL-2Rβ CD19 CAR-T therapy for hematologic malignancies is underway. Furthermore, Kalbasi et al.77 designed a series of chimeric orthogonal receptors that fuse the extracellular domain (ECD) of the orthogonal IL-2 receptor with the intracellular domain (ICD) of the common γ chain (γc) cytokine (IL-4, IL-7, IL-9, and IL-21) receptors, which allows orthogonal IL-2/IL-2R interaction to initiate the downstream signaling of different γc cytokines.77 Among them, the activation of chimeric orthogonal IL-2Rβ-ECD-IL-9R-ICD could significantly promote the activation of stem cell-like memory T cells and consequently improve the efficacy of adoptive cell therapy in immune-competent models.77 So far, the main idea of orthogonal cytokine design has gone from initially limiting the side effect of IL-2 to now broadening the range of cytokine signals orthogonally, which makes cytokine cargos not only safer, but also more flexible to be facilitated in armored CAR-T therapy.
Figure 1.
The system of orthogonal IL-2
(A) Schematic of native IL-2R, orthogonal IL-2R, or γc family chimeric orthogonal receptor. (B) Native IL-2 and orthogonal IL-2 can only match their corresponding receptors. (C) Orthogonal IL-2 acts on CAR-T cells armed with orthogonal IL-2R, which promotes the activation and proliferation of the CAR-T cells.
Systematic discovery on synthetic cytokines and receptors
Recently, synthetic cytokines and receptors have become emerging tools to improve the design of CAR-T therapies.78 Therefore, the systematic discovery of synthetic cytokines and receptors is an area that urgently needs further research. Usually, cytokine signals are induced by natural biological stimuli, but recently designed synthetic cytokine receptors have shown the ability to be responsive to non-physiological ligands, even if they are not structurally cytokine-like.79 Engelowski et al.79 engineered a series of synthetic cytokine receptors (SyCyRs) sensitive to fluorescent fusion proteins like GFP and mCherry, with their specific nanobodies as ECDs. The transmembrane and ICDs of these SyCyRs mimic the IL-23R, which phosphorylates STAT3 upon ligand stimulation.79 This work proved the concept that SyCyRs can be designed to sense almost any kind of protein signal, which provides an opportunity to program the third signal of CAR-T cells at will. To further explore the relationship between synthetic cytokines and downstream signals, Yen et al.80 developed a high-throughput platform for the discovery of surrogate cytokine agonists. They generated combinatorial matrices of single-chain bispecific ligands using nanobodies and scFvs of natural cytokine receptors, exhibiting diverse spectrums of functional activities.80 This work provided a new framework to study the cellular effects of synthetic cytokines, which may accelerate the systemic exploration of orthogonal cytokines or other therapeutic cytokine-related signals. With these systemic advances in synthetic immunology, armored CAR-T cell therapy will embrace many opportunities in the next era.
Broadening the field of fourth-generation CAR-T therapy: Non-cytokine proteins
Classically, the fourth-generation CAR-T cells are defined as CAR-T cells engineered with enhanced cytokine signals. However, similar strategies to arm CAR-T cells with gene-edited non-cytokine proteins could also be an emerging field of fourth-generation CAR-T therapeutics. In the next part, we discuss the functional proteins that CAR-T cells were engineered to express to enhance their anti-tumor efficacy. These proteins are secretory and non-secretory based on their functional spaces (Table 2). The former usually acts on the immunosuppressive TME, while the latter mainly focuses on improving CAR-T. Encouragingly, both of them have shown great potential to treat hematological malignancies and solid tumors in pre-clinical studies.
Table 2.
Summary of CAR-T cells co-expressing non-cytokines in the treatment of tumors
| Non-cytokine based cargos | Tumor | Targeted antigen | Model | Reference |
|---|---|---|---|---|
| PD-1-blocking scFv | ovarian carcinoma | CD19, MUC16 | immune-deficient/competent tumor model | Rafiq et al.85 |
| Anti-PD-L1 antibody | renal cell carcinoma | CAIX | immune-deficient tumor model | Suarez et al.86 |
| HPSE | neuroblastoma | GD2 | immune-deficient tumor model | Caruana et al.89 |
| Drug activating enzymes | leukemia | CD19 | immune-deficient/competent tumor model | Gardner et al.92 |
| Neutrophil-activating protein from Hp. | lymphoma and neuroblastoma | CD19, GD2 | immune-competent tumor model | Jin et al.51 |
| 12 co-stimulatory receptors | B cell lymphomas | CD20 | immune-deficient tumor model | Zhang et al.99 |
| Anti-CD38 CCR | multiple myeloma | BCMA, CD19 | immune-deficient/competent tumor model | Katsarou et al.100 |
| Anti-B7-H3 CCR | neuroblastoma | GD2 | immune-deficient tumor model | Hirabayashi et al.101 |
| PD-L1-specific CSR | chronic myeloid leukemia and lung cancer | CD19, MSLN | immune-deficient tumor model | Qin et al.102 |
| TBBR | prostate cancer | PSCA | immune-deficient tumor model | Sukumaran et al.103 |
| Anti-PSMA CCR | prostate cancer | PSCA | immune-deficient tumor model | Kloss et al.105 |
| c-Jun | neuroblastoma | GPC-2 | immune-deficient tumor model | Heitzeneder et al.111 |
Secretory proteins: Enabling CAR-T cells with further functions
CAR-T cells have been engineered as vehicles to generate and secrete antibodies, enzymes, or pathogen-derived immunomodulatory proteins in situ. Based on the proliferation of CAR-T cells upon activation, these cargos can be enriched in the targeting microenvironment, allowing CAR-T cells to have further therapeutic functions. Here, we summarize the payloads that armored CAR-T cells could be programmed to secrete in addition to cytokines (Figure 2).
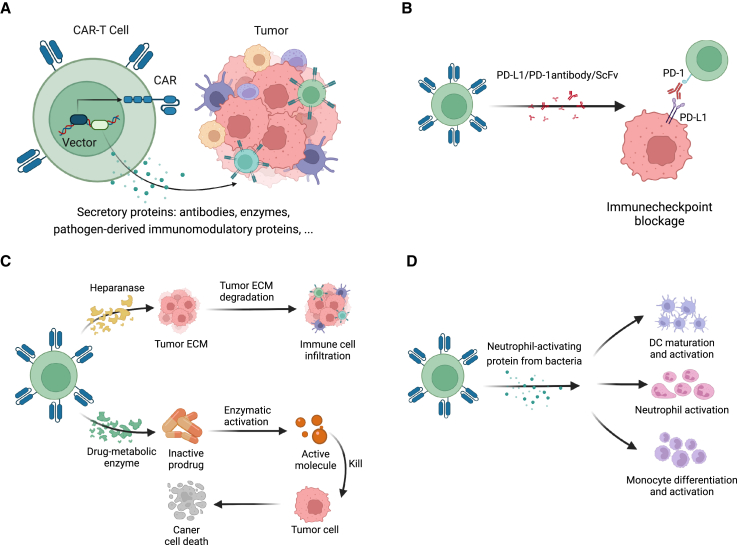
Figure 2.
Engineering CAR-T cells as vehicles to secrete antitumor proteins into TME
(A) Armored CAR-T cells engineered to release a variety of secretory proteins to enhance their antitumor capacity. (B) Representatives of antibodies and ScFvs that armored CAR-T cells can generate, the functions of which include immunocheckpoint blockage (PD-1/PD-L1 antibody/ScFv). (C) Representative enzymes that armored CAR-T cells secrete, which can degrade ECM to improve infiltration (HPSE) or convert prodrugs to cytotoxic agents in situ (drug-metabolic enzyme). (D) Representative pathogen-derived immunomodulatory protein that CAR-T cells release (neutrophil-activating protein from H. pylori), which activates various innate immune cells in TME.
Monoclonal antibodies and ScFvs
The immunosuppressive TME essentially limits the effect of CAR-T cell therapy. Antibody-based therapy is now one of the most successful strategies in tumor patients to improve the TME.81 Combining with antibody-based therapy might overcome the challenges that CAR-T therapy is facing in solid tumors.
Immune checkpoints are one of the main components of immunosuppressive TME. Engagement of programmed cell death 1 (PD-1) with PD ligand 1 (PD-L1) leads to suppression of T cell immune responses.82 Some studies have shown that combining anti-PD-1 antibody and CAR-T can significantly improve CAR-T cells’ ability in syngeneic and xenogeneic mouse models.83,84 Based on these studies, Rafiq et al.85 designed CAR-T cells secreting PD-1-blocking ScFv. These CAR-T cells demonstrated enhanced survival of PD-L1+ tumor-bearing mice in syngeneic and xenogeneic models.85 Similarly, Suarez et al.86 designed CAR-T cells secreting anti-PD-L1 antibodies and proved their effect on suppressing T cell exhaustion in xenogeneic mouse models, which provided the renewed potential for CAR-T cells.85,86 Moreover, four clinical trials of PD-1 antibodies secreting CAR-T therapy and a clinical trial of anti-PD-L1 scFv CAR-T therapy are underway (NCT03615313 mesothelin CAR-T, mesothelin-positive advanced solid tumors; NCT03030001 mesothelin CAR-T, mesothelin-positive advanced solid tumors; NCT02873390 EGFR CAR-T, EGFR family member-positive advanced solid tumor; NCT02862028 EGFR CAR-T, Lung, Liver and Stomach) (NCT04556669 CD22 CAR-T cervical cancer, sarcoma, non-small cell lung cancer). Meanwhile, several clinical trials (NCT03182816, NCT03182803, NCT03179007) (EGFR CAR-T, EGFR-positive advanced solid tumors; mesothelin CAR-T, mesothelin-positive advanced solid tumors; MUC1 CAR-T, MUC1-positive advanced solid tumors) of CTLA-4, and PD-1 antibody CAR-T therapy are underway to evaluate safety and efficacy. Furthermore, CAR-T cells secreting antibodies targeting other immune checkpoints are also worth studying, such as LAG3, TIGIT, TIM3, B7H3, CD39, CD73, CD47, and adenosine A2A receptor. Besides, other functional antibodies can also be taken into the design of the fourth-generation CAR-T.
Enzymes
Dysregulation of the extracellular matrix (ECM) composition, structure, stiffness, and abundance contributes to tumor progression,87 while the remodeling of ECM can modulate TME.88 To regulate the ECM of tumors, one strategy is designing CAR-T cells targeting cancer-associated fibroblasts,88 and another strategy is breaking down ECM by proteases. For example, heparanase (HPSE) is a protease degrading heparan sulfate proteoglycans, the main components of ECM.89 The CAR-T cells secreting HPSE can promote immune cell infiltration and enhance the anti-tumor efficacy in an immune-deficient model.89 Similar to HPSE, sulphatase was reported to alter the properties of ECM.90 Designing CAR-T cells secreting sulphatase might also achieve a great anti-tumor effect. Furthermore, other enzymes that break down ECM can also be used to design armored CAR-T cells, such as metalloproteinases, adamalysins, and meprins.91
Researchers have been looking for other enzymes to provide CAR-T cells access to new functions in light of the ECM enzyme-armored CAR-T experiments discussed above. One such is drug-metabolizing enzymes that in situ activate small-molecule prodrugs given orally. Gardner et al.92 designed CAR-T cells expressing bacterium-derived enzymes that convert inactive prodrugs into cytotoxic agents at the tumor site. This live cell-directed enzyme-prodrug therapy exhibited enhanced anti-tumor activity both in vitro and in vivo in xenogeneic and syngeneic mouse tumor models, which provide synergy with the immune functions of CAR-T cells and enable far higher levels of drug amplification, compared with antibody-drug conjugates and antibody-directed enzyme-prodrug therapy.92
Exogenous immunomodulatory proteins
Many pathogen-derived molecules have strong immunomodulatory properties. For example, neutrophil-activating protein of Helicobacter pylori (HP-NAP) can promote the adhesion of neutrophils to endothelial cells.93 Moreover, some studies reported that HP-NAP could induce the secretion of the proinflammatory cytokines TNF-α, IL-8, IL-6, IL-12, and IL-23.94 Furthermore, HP-NAP has the potential to shift antigen-specific T cell responses from a predominant Th2 to a polarized Th1 cytotoxic phenotype and promote the maturation of DC with Th1 polarization.95 Based on these results, Jin et al.51 designed CAR-T cells secreting HP-NAP to treat solid tumors. They demonstrated that, in tumors with heterogeneous antigen expression, NAP secreted by CAR-T cells induced DC maturation and bystander T cell responses, as indicated by epitope spreading and infiltration of cytotoxic CD8+ T cells targeting tumor-associated antigens other than the CAR-targeted antigen in syngeneic mouse models of lymphoma and neuroblastoma.51 This strategy effectively stimulates endogenous immunity against tumors using exogenous proteins given by CAR-T cells, motivating us to seek more immunostimulatory secretory proteins to develop armored CAR-T therapy. However, further research into the safety of these exogenous proteins is required to prevent severe CRS and other pathogen-related illnesses.
Adding non-secretory proteins to CAR-T: Augmenting the efficiency and persistence
Insufficient stimulatory signals may affect the full activation of CAR-T cells.96 However, continuous antigen stimulation will contribute to the exhaustion of CAR-T cells.96,97 Increasing the efficiency and persistence of CAR-T cells is thus an essential issue. In addition to engineering the cytokine signals discussed previously, modifying non-secretory proteins is also a promising strategy, such as expressing co-stimulatory molecules or transcriptional factors in CAR-T cells (Figure 3).
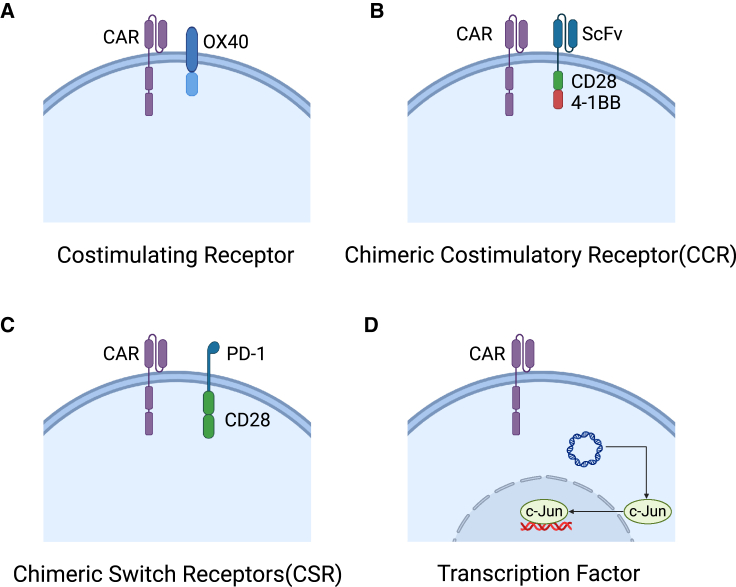
Figure 3.
Equipping CAR-T cells with non-secretory proteins
(A) CAR-T cells armed with an additional co-stimulatory receptor, such as OX-40L, to enhance the co-stimulatory signal. (B) CAR-T cells co-expressing CCR, which recognizes a second antigen with an extracellular ScFv and transmits co-stimulatory signals via intracellular CD28 and 4-1BB co-stimulatory domains. (C) CAR-T cells co-expressing CSR, which switches an inhibitory signal (e.g., PD-1/PD-L1 signal) outside into an activating signal (e.g., CD28 signal) inside. (D) CAR-T cells overexpressing transcription factors coming from vectors, such as c-Jun, to enhance the persistence of CAR-T cells against exhaustion upon activation.
Co-stimulatory molecules
Co-stimulatory molecules provide a second signal to T cells after TCR activation, which enhances the proliferation, cytokine secretion, cytotoxic function, memory formation, and survival of T cells.98 In general, co-stimulatory domains are incorporated into CAR molecules in the second or third generation of CAR-T therapy.4,5 An alternative strategy engineers CAR-T cells with an additional co-stimulatory receptor, which is activated independent of tumor antigens to recapitulate physiological stimulations. Zhang et al.99 screened 12 co-stimulatory receptors and identified OX40 as the most effective CAR-T function enhancer in immune-deficient models. The CAR-T cells with OX-40L showed a stronger capacity for proliferation, cytotoxicity, and anti-tumor efficacy compared with controlled CAR-T cells.99
Further studies engineered the ectodomain of co-stimulatory molecules with scFvs to form chimeric co-stimulatory receptors (CCRs). CCRs are able to deliver a co-stimulatory signal when recognizing a second antigen, complementary to the first signal caused by CAR activation. Cooperating with CARs, these CCRs could serve as signal amplifiers to boost the efficacy of CAR-T therapy under certain conditions,100 or form logic gates to achieve precise control of CAR-T activation. Katsarou et al.100 reported that the co-expression of CD38-CCR with first- or second-generation BCMA-CAR could significantly improve the cytotoxicity and the expansion of CAR-T cells when both the antigens were provided. They found that co-expression of CD38-CCR enhanced the efficacy of BCMA CAR-T when treating antigen-low variants of multiple myeloma under challenging conditions with low T cell dosing in a humanized mouse model. This work proved that the dual-target strategy of a CAR+CCR combination can enhance the sensitivity of CAR-T against low antigen-density tumors.100 Furthermore, Hirabayashi et al.101 revealed that dual-target CAR+CCR had a stronger capacity compared with a combination of dual CARs. They proved that dual targeting of GD2 and B7-H3, or MSLN and CSPG4, with split co-stimulation and shared CD3ζ promoted the activation, expansion, and persistence of CAR-T cells. Consequently, they had an enhanced efficacy in preventing tumor escape because of antigen loss in immunodeficient solid tumor models. The above studies showed that the co-expression of CCRs in CAR-T cells with a dual-target strategy could improve the efficacy of CAR-T therapy in both hematopoietic malignancies and solid tumors under the conditions of low antigen density or prevent antigen loss.101
Additionally, the ectodomain of CCR not only can be scFvs or nanobodies to recognize antigens, but also can be ligand binding motifs to recognize various extracellular signals. Chimeric switch receptors (CSRs) are designed to invert an inhibitory co-stimulating signal into an activating one. Qin et al.102 designed CAR-T cells co-expressing a CSR that combines the ECD of PD-1 with the transmembrane and cytoplasmic signaling domains of CD28. This PD-L1-specific CSR could not only block the intrinsic PD-1 signal competitively, but also convert the inhibitory signal from PD-L1 into an activating signal downstream, which seemed to have greater effects than co-expressing native co-stimulatory molecules simply in mouse xenograft models.102 Furthermore, Sukumaran et al.103 designed a novel CCR named TBBR (TGFβR 4-1BB hybrid receptor), with its ECD derived from TGFβRII and ICD from 4-1BB, which converts an inhibitory cytokine signal into a co-stimulating signal.
In addition, CAR+CCR could also serve as AND or NOT logic gates to avoid the on-target off-tumor toxicity (OTOT) caused by CAR-T cells recognizing targets on normal tissue.104 Kloss et al.105 modified T cells with both a CAR that provides suboptimal activation upon binding of one antigen and a CCR that recognizes a second antigen via ScFv and transmits co-stimulating signals via CD28 and 4-1BB. These CAR-T cells cannot activate without simultaneous CCR recognition of the second antigen.105 However, this CCR-based logic gate along with other AND-gate CAR-T strategies, such as synNotch ROR1 CAR-T,106 did not fully solve the problem of OTOT, since leakage of toxicity still exists in these designs.107 Interestingly, a recent study co-opted proximal signaling molecules LAT and SLP-76 to form a rapid and reversible AND-gated CAR-T platform, which outperforms CCR and other logic systems in terms of both efficacy and prevention of on-target off-tumor toxicity.107
Transcription factor
T cell exhaustion plays an important role in the resistance of CAR-T therapy in solid tumors, partially because of the transcriptional dysregulation of CAR-T cells during exhaustion.108 The transcription factor NFAT promotes the exhaustion of activated CD8+ T cells by binding at sites that do not require cooperation with a master transcription factor complex, activator protein-1 (AP-1).109 c-Jun is a major component of AP-1, which functions downstream of the TCR and CD8 co-stimulatory receptor.110 Constitutively overexpressing c-Jun on CAR-T cells rendered them resistant to exhaustion, thereby enhancing their capacity to control tumor growth in immune-deficient models.111 A further study demonstrated that overexpression of c-Jun decreased the antigen density threshold of GPC2-CAR, enabling potent and durable eradication of neuroblastoma expressing clinically relevant GPC2 antigen density without toxicity in immune-competent models.111 These results showed that overexpression of c-Jun in CAR-T cells is a promising way to enhance their efficiency and persistence. Further studies on transcriptional regulations of CAR-T cells in the progress of activation and exhaustion are under investigation.
Gene circuits: Improving the armored CAR-T cells by controlling the expression of payloads
Transgenic payloads provide CAR-T cells with greater anti-tumor capacity, but the lack of control for payload expression can impair the safety and efficacy of CAR-T therapy. For example, the constitutive production of IL-2 can inhibit CAR-T proliferation through activation-induced cell death,20 which demonstrated the significance of when and how cytokines were produced in CAR-T cells. Gene circuits typically refer to artificially designed, self-regulating genetic devices, which can turn on and off the expression of payloads under certain circumstances. With the aid of gene circuits, we are able to further improve the safety and the efficacy of armored CAR-T therapy.20,21
A gene circuit consists of three processes: input, biological circuits, and output.112 Synthetic Notch receptor (synNotch) is a representative, which is activated when its exodomain recognizes a specific antigen, then cuts itself to release an intracellular transcription factor, and finally initiates the transcription of downstream genetic payloads.113,114 A recent study reported a novel CAR-T armed with a synNotch receptor that drives IL-2 production when recognizing specific antigens.20 This armored CAR-T demonstrated great infiltration into immune-excluded tumors and strong efficacy compared with CAR-T cells with constitutive IL-2 expression or TCR/CAR-dependent IL-2 production.20 This circuit allows CAR-T cells to express cytokines timely. First, it enriches the expression of IL-2 around the tumor, which reduces the impact of IL-2 on systemic immune cells, including nearby immune-suppressive Tregs. Meanwhile, the conditional expression of IL-2 avoids its continuous stimulation on CAR-T itself, which decreases activation-induced cell death. Moreover, the synNotch circuit can be activated independently when CAR signaling is partially suppressed by the sinks from the TME. Therefore, this synNotch-based antigen-dependent cytokine release strategy allows for an efficient expansion of CAR-T cells in immune-desert tumors and greatly improves the efficacy of armored CAR-T in solid tumors.20 Another recent work created a set of drug-gated circuits to control the expression of different payloads independently.21 This study designed a bundle of synthetic zinc finger transcription regulators, which orthogonally transcript different downstream genes under the management of various small-molecule inducers. These gene circuits can instruct the T cells to sequentially activate multiple cellular programs, such as proliferation, CAR expression, and production of other payloads.21 This strategy enables flexible and multidimensional control of CAR-T activity and payload expression,21 which might prevent the early exhaustion of T cells caused by continuous activation and enhance the efficacy of CAR-T therapy. Additionally, the drug-gated circuit could be timely discontinued by the withdrawal of inducers when necessary, which might decrease the risk of severe adverse effects caused by the sustained existence or unexpected activation of CAR-T cells in vivo.
Additionally, modular design is an important concept of gene circuits, which divides a circuit into modules with independent biological functions to reduce the complexity of synthetic biological systems. Zhu et al.115 optimized the design of synNotch receptors by dividing the synthetic intramembrane proteolysis receptors into four modules, including the extracellular regulatory element, transmembrane domain, intracellular juxtamembrane domain, and transcriptional regulator. Each module has different cores. The combination of cores from different modules provides synNotch receptors tunable sensing and transcriptional response abilities, which will help us filter out the optimal combination.115 The modular design also can promote CAR optimization. Daniels et al.72 constructed a library of CARs containing approximately 2,300 synthetic co-stimulatory domains, built from combinations of 13 signaling motifs. These CARs can promote different functions of cells due to different motif components and conformational differences, unlocking various functions corresponding to different CARs through machine learning and other methods.72 The modular design strategy allows for more precise tuning of CAR-T functions and gives CAR-T therapy greater potential in the future.
Conclusions
CAR-T therapies have made significant advances in the treatment of hematological malignancies. Nevertheless, achieving analogous responses in solid malignancies remains a formidable challenge.116 Antigenic heterogeneity, limited potency and persistence, poor infiltration capacity, and a suppressive TME are the primary obstacles. The fourth-generation CAR-T cells can overcome a variety of obstacles by both improving the functions of CAR-T itself and enhancing the endogenous supportive immunity, as well as directly arming CAR-T with tumor-killing payloads. We propose summarizing the above designs of next-generation CAR-T therapies as a CAR+X strategy, or Gen-X CAR-T—arming CAR-T cells with certain biological payloads and carrying out corresponding functions. The X was cytokines in the past decade, and more kinds of proteins have joined the list. Expectedly, we think the X will include a broader range of bioactive molecules in the future, such as small peptides, non-coding RNAs, functional DNAs, and metabolites. Additionally, the X can be a bundle of elements that cooperate together, forming biological circuits or microsystems that may fulfill the dreams of therapeutic intelligent cells.117
However, arming with bioactive payloads could also bring new issues to CAR-T therapeutics, such as systemic toxicity of constitutively secreted proteins.39 This toxicity issue, as well as the remodeling effect of additional payloads on the TME and the immune system, might not be presented in pre-clinical murine models, particularly in immune-deficient xenografts for most cases. These would be an important limitation of current pre-clinical studies and need further investigations. Several clinical trials have released early results of fourth-generation CAR-T therapy, while more high-grade evidence is needed to further evaluate the safety and efficacy of armored CAR-T in clinics (Table 3). Therefore, in the next era, the main focus of CAR+X development might move from what to express, to when to express, where to express, how to control the expression, and how much to express. Currently, advances in biomedical engineering and synthetic biology can further promote the improvement of next-generation CAR-T therapy.20,118 In addition, artificial intelligence has prompted us to understand the nature of CARs or other synthetic molecules,72 which might also change our minds about the principles of therapeutic cell design.
Table 3.
Clinical trial summary of CAR-T cells with additional function proteins
| NCT number | Target antigen | Gene-delivery proteins | Expression pattern of cargo | Tumor | Clinical stage | Status | Locations | Result |
|---|---|---|---|---|---|---|---|---|
| NCT04377932 | GPC3 | IL-15 | unknown | liver cancer | phase 1 | recruiting | Texas Children’s Hospital, Houston, Texas, USA | Steffin et al.19 |
| NCT05103631 | GPC3 | IL-15 | unknown | liver cancer | phase 1 | recruiting | Houston Methodist Hospital, Houston, Texas, United States | Steffin et al.19 |
| NCT03721068 | GD2 | IL-15 | unknown | neuroblastoma, osteosarcoma | phase 1 | recruiting | Emory Winship Cancer Institute, Atlanta, Georgia, USA Lineberger Comprehensive Cancer Center at University of North Carolina Chapel Hill, Chapel Hill, North Carolina, USA |
unknown |
| NCT04715191 | GPC3 | IL-15,IL-21 | unknown | liver cancer | phase 1 | not yet recruiting | Texas Children’s Hospital, Houston, Texas, USA | unknown |
| NCT02932956 | GPC3 | IL-15, IL-21 | unknown | liver cancer | phase 1 | active, not recruiting | Texas Children’s Hospital, Houston, Texas, USA | unknown |
| NCT03778346 | BCMA | IL-7 and CCL19 | unknown | relapsed/refractory multiple myeloma | phase 1 | unknown | The Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang, China | Duan et al.32 |
| NCT03929107 | CD19 | IL-7 and CCL19 | unknown | B cell lymphoma | phase 2 | unknown status | The first affiliated hospital of Zhejiang University, Hangzhou, Zhejiang, China | Lei et al.33 |
| NCT04833504 | CD19 | IL-7 and CCL19 | unknown | relapsed/refractory B cell lymphoma | completed | Second Affiliated Hospital, School of Medicine, Zhejiang University | Lei et al.33 | |
| NCT05659628 | CD19 | IL-7 and CCL19 | unknown | diffuse large B cell lymphoma | phase 1 | recruiting | The Second Affiliated Hospital of Zhejiang University, Ningbo First Hospital, Hangzhou, Zhejiang, China | unknown |
| NCT03198546 | GPC3 | IL-7 and CCL19 | unknown | liver cancer | phase 1 | recruiting | The First Affiliated Hospital of Sun Yat-sen University Guangzhou, Guangdong, China The Second Affiliated Hospital of Guangzhou Medical University Guangzhou, Guangdong, China |
Pang et al.34 |
| NCT02498912 | MUC6ecto | IL-12 | constitutive | ovarian, primary peritoneal, fallopian tube carcinoma | phase 1 | active, not recruiting | Memorial Sloan Kettering Cancer Center New York, New York, USA | O’Cearbhaill et al.43 |
| NCT03542799 | EGFR | IL-12 | inducible | metastatic colorectal cancer | phase 1 | unknown | Shenzhen Second People’s Hospital, Shenzhen, Guangdong, China | unknown |
| NCT04684563 | CD19 | IL-18 | constitutive | non-Hodgkin lymphoma, chronic lymphocytic leukemia, acute lymphoblastic leukemia | phase 1 | recruiting | University of Pennsylvania, Philadelphia, Pennsylvania, USA | Svoboda et al.47 |
| NCT04099797 | GD2 | C7R | unknown | high-grade glioma, diffuse intrinsic pontine glioma, medulloblastoma or other rare brain cancer that expresses GD2 | phase 1 | recruiting | Texas Children’s Hospital, Houston, Texas, USA | unknown |
| NCT03635632 | GD2 | C7R | unknown | neuroblastoma, sarcoma, uveal melanoma, breast cancer, or another cancer that expresses a substance on the cancer cells called GD2 | phase 1 | recruiting | Houston Methodist Hospital, Houston, Texas, USA Texas Children’s Hospital, Houston, Texas, USA |
unknown |
| NCT05665062 | CD19 | orthogonal IL-2Rβ | unknown | hematological malignancies | phase 1 | recruiting | City of Hope, Duarte, California, USA Roswell Park, Buffalo, New York, USA Columbia University Irving Medical Center, New York, New York, USA Memorial Sloan Kettering Cancer Center, New York, New York, USA Cleveland Clinic, Cleveland, Ohio, USA |
unknown |
| NCT04556669 | CD22 | anti-PD-L1 scFv | unknown | cervical cancer, sarcoma, non small cell lung cancer | phase 1 | recruiting | Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China | unknown |
| NCT03615313 | MSLN | PD-1 antibody | unknown | mesothelin-positive advanced solid tumors | phase 1/2 | unknown | Shanghai Cell Therapy Research Institute, Shanghai, China | unknown |
| NCT03030001 | MSLN | PD-1 antibody | unknown | mesothelin-positive advanced solid tumors | phase 1/2 | unknown | Ningbo No.5 Hospital (Ningbo Cancer Hospital), Ningbo, Zhejiang, China | unknown |
| NCT02873390 | EGFR | PD-1 antibody | unknown | EGFR family member positive advanced solid tumor | phase 1/2 | unknown | Ningbo No.5 Hospital (Ningbo Cancer Hospital), Ningbo, Zhejiang, China | unknown |
| NCT02862028 | EGFR | PD-1 antibody | unknown | lung, liver and stomach cancers | phase 1/2 | unknown | Shanghai International Medical Center, Shanghai, Shanghai, China | unknown |
| NCT03182816 | EGFR | CTLA-4 and PD-1 antibody | unknown | EGFR-positive advanced solid tumors | phase 1/2 | unknown | Ningbo No.5 Hospital (Ningbo Cancer Hospital), Ningbo, Zhejiang, China | unknown |
| NCT03182803 | MSLN | CTLA-4 and PD-1 antibody | unknown | mesothelin-positive advanced solid tumors | phase 1/2 | unknown | Ningbo No.5 Hospital (Ningbo Cancer Hospital), Ningbo, Zhejiang, China | unknown |
| NCT03179007 | MUC1 | CTLA-4 and PD-1 antibody | unknown | MUC1-positive advanced solid tumors | phase 1/2 | unknown | Ningbo No.5 Hospital (Ningbo Cancer Hospital), Ningbo, Zhejiang, China | unknown |
All clinical trials were downloaded at www.clinicaltrials.gov (access date: August 10, 2023).
Above all, the X is now moving from constitutive to regulatable, from monolithic to modular, from natural to orthogonal, from bulk to compact, and immunogenic to humanized. Generally, the X is becoming more and more programmable, bringing it closer to the clinic with stronger capability and more safety.115,119 In summary, armed with the CAR+X strategy, the fourth-generation CAR-T therapy shows a promising future in treating human diseases.
Acknowledgments
This work was supported by grants from Key Research and Development Plan of Zhejiang Province (No.2021C03118), National Key Research and Development Program of China (No. 2021YFA1100500), and the Major Research Plan of the National Natural Science Foundation of China (No.92159202). All figures are created with BioRender.com. Thanks to Prof. Songmin Ying, Miss Fang Xu, Mr. Chaojie Zhu, and Dr. Haijun Guo for their comments during the revision process.
Author contributions
L.T. and S.P. were the major contributors to reviewing the literature, writing the manuscript, and creating descriptive figures; X.W. was a major contributor to revising the manuscript and figures; Q.W. and X.X. designed the table and figures and supervised the completion of the manuscript. All authors read and approved the final manuscript.
Declaration of interests
None declared.
Contributor Information
Xiao Xu, Email: zjxu@zju.edu.cn.
Qiang Wei, Email: zjuwq@zju.edu.cn.
References
- 1.O’Leary M.C., Lu X., Huang Y., Lin X., Mahmood I., Przepiorka D., Gavin D., Lee S., Liu K., George B., et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2019;25:1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 2.Panagopoulou T.I., Rafiq Q.A. CAR-T immunotherapies: Biotechnological strategies to improve safety, efficacy and clinical outcome through CAR engineering. Biotechnol. Adv. 2019;37 doi: 10.1016/j.biotechadv.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmielewski M., Abken H. TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 5.Hombach A.A., Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int. J. Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 6.Majzner R.G., Mackall C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019;25:1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 7.Wagner J., Wickman E., DeRenzo C., Gottschalk S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020;28:2320–2339. doi: 10.1016/j.ymthe.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chmielewski M., Hombach A.A., Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 2014;257:83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 9.Hong M., Clubb J.D., Chen Y.Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell. 2020;38:473–488. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Johnson L.R., Lee D.Y., Eacret J.S., Ye D., June C.H., Minn A.J. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184:4981–4995.e14. doi: 10.1016/j.cell.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A., Gill S. CAR-T cell persistence in the treatment of leukemia and lymphoma. Leuk. Lymphoma. 2021;62:2587–2599. doi: 10.1080/10428194.2021.1913146. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S., Abken H. CAR T cell therapy becomes CHIC: “cytokine help intensified CAR” T cells. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielewski M., Kopecky C., Hombach A.A., Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Lundqvist A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers (Basel) 2020;12:E3586. doi: 10.3390/cancers12123586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reppel L., Tsahouridis O., Akulian J., Davis I.J., Lee H., Fucà G., Weiss J., Dotti G., Pecot C.V., Savoldo B. Targeting disialoganglioside GD2 with chimeric antigen receptor-redirected T cells in lung cancer. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Sun C., Landoni E., Metelitsa L., Dotti G., Savoldo B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clin. Cancer Res. 2019;25:2915–2924. doi: 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 18.Gargett T., Ebert L.M., Truong N.T.H., Kollis P.M., Sedivakova K., Yu W., Yeo E.C.F., Wittwer N.L., Gliddon B.L., Tea M.N., et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2022-005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffin D., Ghatwai N., Zhang C., Rathi P., Courtney A., Ramos C., Lulla P., Armaghanny T., Grilley B., Metelitsa L., et al. ARMORED GLYPICAN 3-CAR T CELLS CO-EXPRESSING INTERLEUKIN-15 FOR PATIENTS WITH SOLID TUMORS (ASPHO 2023) http://aspho.org/uploads/2023_ASPHO_Conference_Paper_and_Poster_Index.pdf
- 20.Allen G.M., Frankel N.W., Reddy N.R., Bhargava H.K., Yoshida M.A., Stark S.R., Purl M., Lee J., Yee J.L., Yu W., et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science. 2022;378 doi: 10.1126/science.aba1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H.-S., Israni D.V., Gagnon K.A., Gan K.A., Raymond M.H., Sander J.D., Roybal K.T., Joung J.K., Wong W.W., Khalil A.S. Multidimensional control of therapeutic human cell function with synthetic gene circuits. Science. 2022;378:1227–1234. doi: 10.1126/science.ade0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Štach M., Ptáčková P., Mucha M., Musil J., Klener P., Otáhal P. Inducible secretion of IL-21 augments anti-tumor activity of piggyBac-manufactured chimeric antigen receptor T cells. Cytotherapy. 2020;22:744–754. doi: 10.1016/j.jcyt.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Perna S.K., Pagliara D., Mahendravada A., Liu H., Brenner M.K., Savoldo B., Dotti G. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clin. Cancer Res. 2014;20:131–139. doi: 10.1158/1078-0432.CCR-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markley J.C., Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis M.R., Zhu Z., Hansen D.M., Bai Q., Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358:107–114. doi: 10.1016/j.canlet.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 26.Batra S.A., Rathi P., Guo L., Courtney A.N., Fleurence J., Balzeau J., Shaik R.S., Nguyen T.P., Wu M.-F., Bulsara S., et al. Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma. Cancer Immunol. Res. 2020;8:309–320. doi: 10.1158/2326-6066.CIR-19-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X., Shou P., Smith C., Chen Y., Du H., Sun C., Porterfield Kren N., Michaud D., Ahn S., Vincent B., et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Shao Y., Zhang X., Lu G., Liu B. IL-23 and PSMA-targeted duo-CAR T cells in Prostate Cancer Eradication in a preclinical model. J. Transl. Med. 2020;18:23. doi: 10.1186/s12967-019-02206-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry T.J., Mackall C.L. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida R., Nagira M., Imai T., Baba M., Takagi S., Tabira Y., Akagi J., Nomiyama H., Yoshie O. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 1998;10:901–910. doi: 10.1093/intimm/10.7.901. [DOI] [PubMed] [Google Scholar]
- 31.Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36:346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 32.Duan D., Wang K., Wei C., Feng D., Liu Y., He Q., Xu X., Wang C., Zhao S., Lv L., et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei W., Ai Z., Liu H., Yang C., Wei C., Guo S., Chen Z., Guo Q., Li L., Zhao M., et al. Safety and Feasibility of Anti-CD19 CAR-T Expressing IL-7 and CCL19 in Patients with Relapsed or Refractory Large B-Cell Lymphoma. Blood. 2022;140:12722. doi: 10.1182/blood-2022-156635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang N., Shi J., Qin L., Chen A., Tang Y., Yang H., Huang Y., Wu Q., Li X., He B., et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol. 2021;14:118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharping N.E., Rivadeneira D.B., Menk A.V., Vignali P.D.A., Ford B.R., Rittenhouse N.L., Peralta R., Wang Y., Wang Y., DePeaux K., et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021;22:205–215. doi: 10.1038/s41590-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vardhana S.A., Hwee M.A., Berisa M., Wells D.K., Yost K.E., King B., Smith M., Herrera P.S., Chang H.Y., Satpathy A.T., et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 2020;21:1022–1033. doi: 10.1038/s41590-020-0725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ip W.K.E., Hoshi N., Shouval D.S., Snapper S., Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y., Xie Y.-Q., Gao M., Zhao Y., Franco F., Wenes M., Siddiqui I., Bevilacqua A., Wang H., Yang H., et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 2021;22:746–756. doi: 10.1038/s41590-021-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Morgan R.A., Beane J.D., Zheng Z., Dudley M.E., Kassim S.H., Nahvi A.V., Ngo L.T., Sherry R.M., Phan G.Q., et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pegram H.J., Lee J.C., Hayman E.G., Imperato G.H., Tedder T.F., Sadelain M., Brentjens R.J. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacy M.Q., Jacobus S., Blood E.A., Kay N.E., Rajkumar S.V., Greipp P.R. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): a trial of the Eastern Cooperative Oncology Group. Leuk. Res. 2009;33:1485–1489. doi: 10.1016/j.leukres.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agliardi G., Liuzzi A.R., Hotblack A., De Feo D., Núñez N., Stowe C.L., Friebel E., Nannini F., Rindlisbacher L., Roberts T.A., et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun. 2021;12:444. doi: 10.1038/s41467-020-20599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Cearbhaill R.E., Park J.H., Halton E.F., Diamonte C.R., Mead E., Lakhman Y., Kane P., Riviere I.C., Brentjens R.J. SGO; 2020. A phase I clinical trial of autologous chimeric antigen receptor (CAR) T cells genetically engineered to secrete IL-12 and to target the MUC16ecto antigen in patients (pts) with MUC16ecto+ recurrent high-grade serous ovarian cancer (HGSOC) [Google Scholar]
- 44.Hu B., Ren J., Luo Y., Keith B., Young R.M., Scholler J., Zhao Y., June C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avanzi M.P., Yeku O., Li X., Wijewarnasuriya D.P., van Leeuwen D.G., Cheung K., Park H., Purdon T.J., Daniyan A.F., Spitzer M.H., Brentjens R.J. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018;23:2130–2141. doi: 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chmielewski M., Abken H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017;21:3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 47.Svoboda J., Landsburg D.L., Chong E.A., Barta S.K., Nasta S.D., Ruella M., Hexner E.O., Marshall A., Leskowitz R., Four M., et al. Fourth Generation Hucart19-Il18 Produces Durable Responses in Lymphoma Patients Previously Relapsed/Refractory to Anti-Cd19 Car T-Cell Therapy. Hematol. Oncol. 2023;41:35–37. doi: 10.1002/hon.3163_6. [DOI] [Google Scholar]
- 48.Gabay C., Towne J.E. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J. Leukoc. Biol. 2015;97:645–652. doi: 10.1189/jlb.3RI1014-495R. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Zhao X., Feng C., Weinstein A., Xia R., Wen W., Lv Q., Zuo S., Tang P., Yang X., et al. IL-36γ Transforms the Tumor Microenvironment and Promotes Type 1 Lymphocyte-Mediated Antitumor Immune Responses. Cancer Cell. 2015;28:296–306. doi: 10.1016/j.ccell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., Daniyan A.F., Lopez A.V., Purdon T.J., Brentjens R.J. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia. 2021;35:506–521. doi: 10.1038/s41375-020-0874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin C., Ma J., Ramachandran M., Yu D., Essand M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers. Nat. Biomed. Eng. 2022;6:830–841. doi: 10.1038/s41551-022-00875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma L., Hostetler A., Morgan D.M., Maiorino L., Sulkaj I., Whittaker C.A., Neeser A., Pires I.S., Yousefpour P., Gregory J., et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell. 2023;186:3148–3165.e20. doi: 10.1016/j.cell.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi B.D., Yu X., Castano A.P., Bouffard A.A., Schmidts A., Larson R.C., Bailey S.R., Boroughs A.C., Frigault M.J., Leick M.B., et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 54.Lai J., Mardiana S., House I.G., Sek K., Henderson M.A., Giuffrida L., Chen A.X.Y., Todd K.L., Petley E.V., Chan J.D., et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 2020;21:914–926. doi: 10.1038/s41590-020-0676-7. [DOI] [PubMed] [Google Scholar]
- 55.Salmon H., Idoyaga J., Rahman A., Leboeuf M., Remark R., Jordan S., Casanova-Acebes M., Khudoynazarova M., Agudo J., Tung N., et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burdin N., Moingeon P. Cancer vaccines based on dendritic cells loaded with tumor-associated antigens. Cell Biol. Toxicol. 2001;17:67–75. doi: 10.1023/a:1010944003649. [DOI] [PubMed] [Google Scholar]
- 58.Vera J.F., Hoyos V., Savoldo B., Quintarelli C., Giordano Attianese G.M.P., Leen A.M., Liu H., Foster A.E., Heslop H.E., Rooney C.M., et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol. Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shum T., Omer B., Tashiro H., Kruse R.L., Wagner D.L., Parikh K., Yi Z., Sauer T., Liu D., Parihar R., et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn J., Chen J., Goenka S., Aronica M.A., Mora A.L., Correa V., Sheller J.R., Boothby M. In vivo function of an interleukin 2 receptor beta chain (IL-2Rbeta)/IL-4Ralpha cytokine receptor chimera potentiates allergic airway disease. J. Exp. Med. 1998;188:1803–1816. doi: 10.1084/jem.188.10.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takagi M., Nakamura T., Sawada T., Kaneko A., Nozaki-Ukai M., Nakahata T., Yokota T., Heike T. Chimeric cytokine receptor can transduce expansion signals in interleukin 6 receptor alpha (IL-6Ralpha)-IL-11Ralpha-and gp130-low to -negative primitive hematopoietic progenitors. Mol. Biol. Cell. 1999;10:3633–3642. doi: 10.1091/mbc.10.11.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roca H., Craig M.J., Ying C., Varsos Z.S., Czarnieski P., Alva A.S., Hernandez J., Fuller D., Daignault S., Healy P.N., Pienta K.J. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin up-regulation. J. Cell. Biochem. 2012;113:1569–1580. doi: 10.1002/jcb.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lange S., Sand L.G.L., Bell M., Patil S.L., Langfitt D., Gottschalk S. A Chimeric GM-CSF/IL18 Receptor to Sustain CAR T-cell Function. Cancer Discov. 2021;11:1661–1671. doi: 10.1158/2159-8290.CD-20-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Todaro M., Lombardo Y., Francipane M.G., Alea M.P., Cammareri P., Iovino F., Di Stefano A.B., Di Bernardo C., Agrusa A., Condorelli G., et al. Correction to: Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2019;26:2808–2809. doi: 10.1038/s41418-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilkie S., Burbridge S.E., Chiapero-Stanke L., Pereira A.C.P., Cleary S., van der Stegen S.J.C., Spicer J.F., Davies D.M., Maher J. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leen A.M., Sukumaran S., Watanabe N., Mohammed S., Keirnan J., Yanagisawa R., Anurathapan U., Rendon D., Heslop H.E., Rooney C.M., et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mata M., Gerken C., Nguyen P., Krenciute G., Spencer D.M., Gottschalk S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017;7:1306–1319. doi: 10.1158/2159-8290.CD-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golumba-Nagy V., Kuehle J., Hombach A.A., Abken H. CD28-ζ CAR T Cells Resist TGF-β Repression through IL-2 Signaling, Which Can Be Mimicked by an Engineered IL-7 Autocrine Loop. Mol. Ther. 2018;26:2218–2230. doi: 10.1016/j.ymthe.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z., Miao L., Ren Z., Tang F., Li Y. Gene-Edited Interleukin CAR-T Cells Therapy in the Treatment of Malignancies: Present and Future. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.718686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagoya Y., Tanaka S., Guo T., Anczurowski M., Wang C.-H., Saso K., Butler M.O., Minden M.D., Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H., Zhao H., He X., Xi F., Liu J. JAK-STAT Domain Enhanced MUC1-CAR-T Cells Induced Esophageal Cancer Elimination. Cancer Manag. Res. 2020;12:9813–9824. doi: 10.2147/CMAR.S264358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Daniels K.G., Wang S., Simic M.S., Bhargava H.K., Capponi S., Tonai Y., Yu W., Bianco S., Lim W.A. Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning. Science. 2022;378:1194–1200. doi: 10.1126/science.abq0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quijano-Rubio A., Bhuiyan A.M., Yang H., Leung I., Bello E., Ali L.R., Zhangxu K., Perkins J., Chun J.-H., Wang W., et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat. Biotechnol. 2023;41:532–540. doi: 10.1038/s41587-022-01510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sockolosky J.T., Trotta E., Parisi G., Picton L., Su L.L., Le A.C., Chhabra A., Silveria S.L., George B.M., King I.C., et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science. 2018;359:1037–1042. doi: 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q., Hresko M.E., Picton L.K., Su L., Hollander M.J., Nunez-Cruz S., Zhang Z., Assenmacher C.-A., Sockolosky J.T., Garcia K.C., Milone M.C. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abg6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aspuria P.-J., Vivona S., Bauer M., Semana M., Ratti N., McCauley S., Riener R., de Waal Malefyt R., Rokkam D., Emmerich J., et al. An orthogonal IL-2 and IL-2Rβ system drives persistence and activation of CAR T cells and clearance of bulky lymphoma. Sci. Transl. Med. 2021;13:eabg7565. doi: 10.1126/scitranslmed.abg7565. [DOI] [PubMed] [Google Scholar]
- 77.Kalbasi A., Siurala M., Su L.L., Tariveranmoshabad M., Picton L.K., Ravikumar P., Li P., Lin J.-X., Escuin-Ordinas H., Da T., et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature. 2022;607:360–365. doi: 10.1038/s41586-022-04801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheller J., Engelowski E., Moll J.M., Floss D.M. Immunoreceptor Engineering and Synthetic Cytokine Signaling for Therapeutics. Trends Immunol. 2019;40:258–272. doi: 10.1016/j.it.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Engelowski E., Schneider A., Franke M., Xu H., Clemen R., Lang A., Baran P., Binsch C., Knebel B., Al-Hasani H., et al. Synthetic cytokine receptors transmit biological signals using artificial ligands. Nat. Commun. 2018;9:2034. doi: 10.1038/s41467-018-04454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yen M., Ren J., Liu Q., Glassman C.R., Sheahan T.P., Picton L.K., Moreira F.R., Rustagi A., Jude K.M., Zhao X., et al. Facile discovery of surrogate cytokine agonists. Cell. 2022;185:1414–1430.e19. doi: 10.1016/j.cell.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 82.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.John L.B., Devaud C., Duong C.P.M., Yong C.S., Beavis P.A., Haynes N.M., Chow M.T., Smyth M.J., Kershaw M.H., Darcy P.K. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 84.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J., Song M., Miele M.M., Li Z., Wang P., et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suarez E.R., Chang D.K., Sun J., Sui J., Freeman G.J., Signoretti S., Zhu Q., Marasco W.A. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piersma B., Hayward M.-K., Weaver V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer. 2020;1873 doi: 10.1016/j.bbcan.2020.188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bughda R., Dimou P., D’Souza R.R., Klampatsa A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: Launching an Attack on Tumor Stroma. Immunotargets Ther. 2021;10:313–323. doi: 10.2147/ITT.S291767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caruana I., Savoldo B., Hoyos V., Weber G., Liu H., Kim E.S., Ittmann M.M., Marchetti D., Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardner T.J., Lee J.P., Bourne C.M., Wijewarnasuriya D., Kinarivala N., Kurtz K.G., Corless B.C., Dacek M.M., Chang A.Y., Mo G., et al. Engineering CAR-T cells to activate small-molecule drugs in situ. Nat. Chem. Biol. 2022;18:216–225. doi: 10.1038/s41589-021-00932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark-Lewis I., Moser B., Walz A., Baggiolini M., Scott G.J., Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide. Biochemistry. 1991;30:3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- 94.de Bernard M., D’Elios M.M. The immune modulating activity of the Helicobacter pylori HP-NAP: Friend or foe? Toxicon. 2010;56:1186–1192. doi: 10.1016/j.toxicon.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 95.Ramachandran M., Jin C., Yu D., Eriksson F., Essand M. Vector-encoded Helicobacter pylori neutrophil-activating protein promotes maturation of dendritic cells with Th1 polarization and improved migration. J. Immunol. 2014;193:2287–2296. doi: 10.4049/jimmunol.1400339. [DOI] [PubMed] [Google Scholar]
- 96.Chen J., Qiu S., Li W., Wang K., Zhang Y., Yang H., Liu B., Li G., Li L., Chen M., et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 2023;33:341–354. doi: 10.1038/s41422-023-00789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber E.W., Parker K.R., Sotillo E., Lynn R.C., Anbunathan H., Lattin J., Good Z., Belk J.A., Daniel B., Klysz D., et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372 doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinkove R., George P., Dasyam N., McLellan A.D. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin. Transl. Immunol. 2019;8:e1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H., Li F., Cao J., Wang X., Cheng H., Qi K., Wang G., Xu K., Zheng J., Fu Y.-X., Yang X. A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.aba7308. [DOI] [PubMed] [Google Scholar]
- 100.Katsarou A., Sjöstrand M., Naik J., Mansilla-Soto J., Kefala D., Kladis G., Nianias A., Ruiter R., Poels R., Sarkar I., et al. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abh1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirabayashi K., Du H., Xu Y., Shou P., Zhou X., Fucá G., Landoni E., Sun C., Chen Y., Savoldo B., Dotti G. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer. 2021;2:904–918. doi: 10.1038/s43018-021-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qin L., Cui Y., Yuan T., Chen D., Zhao R., Li S., Jiang Z., Wu Q., Long Y., Wang S., et al. Co-expression of a PD-L1-specific chimeric switch receptor augments the efficacy and persistence of CAR T cells via the CD70-CD27 axis. Nat. Commun. 2022;13:6051. doi: 10.1038/s41467-022-33793-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sukumaran S., Watanabe N., Bajgain P., Raja K., Mohammed S., Fisher W.E., Brenner M.K., Leen A.M., Vera J.F. Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment. Cancer Discov. 2018;8:972–987. doi: 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ebert L.M., Yu W., Gargett T., Brown M.P. Logic-gated approaches to extend the utility of chimeric antigen receptor T-cell technology. Biochem. Soc. Trans. 2018;46:391–401. doi: 10.1042/BST20170178. [DOI] [PubMed] [Google Scholar]
- 105.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srivastava S., Salter A.I., Liggitt D., Yechan-Gunja S., Sarvothama M., Cooper K., Smythe K.S., Dudakov J.A., Pierce R.H., Rader C., Riddell S.R. Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell. 2019;35:489–503.e8. doi: 10.1016/j.ccell.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tousley A.M., Rotiroti M.C., Labanieh L., Rysavy L.W., Kim W.-J., Lareau C., Sotillo E., Weber E.W., Rietberg S.P., Dalton G.N., et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature. 2023;615:507–516. doi: 10.1038/s41586-023-05778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delgoffe G.M., Xu C., Mackall C.L., Green M.R., Gottschalk S., Speiser D.E., Zehn D., Beavis P.A. The role of exhaustion in CAR T cell therapy. Cancer Cell. 2021;39:885–888. doi: 10.1016/j.ccell.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 109.Martinez G.J., Pereira R.M., Äijö T., Kim E.Y., Marangoni F., Pipkin M.E., Togher S., Heissmeyer V., Zhang Y.C., Crotty S., et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lynn R.C., Weber E.W., Sotillo E., Gennert D., Xu P., Good Z., Anbunathan H., Lattin J., Jones R., Tieu V., et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heitzeneder S., Bosse K.R., Zhu Z., Zhelev D., Majzner R.G., Radosevich M.T., Dhingra S., Sotillo E., Buongervino S., Pascual-Pasto G., et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40:53–69.e9. doi: 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee S., Khalil A.S., Wong W.W. Recent progress of gene circuit designs in immune cell therapies. Cell Syst. 2022;13:864–873. doi: 10.1016/j.cels.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choe J.H., Watchmaker P.B., Simic M.S., Gilbert R.D., Li A.W., Krasnow N.A., Downey K.M., Yu W., Carrera D.A., Celli A., et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abe7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morsut L., Roybal K.T., Xiong X., Gordley R.M., Coyle S.M., Thomson M., Lim W.A. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu I., Liu R., Garcia J.M., Hyrenius-Wittsten A., Piraner D.I., Alavi J., Israni D.V., Liu B., Khalil A.S., Roybal K.T. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell. 2022;185:1431–1443.e16. doi: 10.1016/j.cell.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milone M.C., Xu J., Chen S.-J., Collins M.A., Zhou J., Powell D.J., Melenhorst J.J. Engineering-enhanced CAR T cells for improved cancer therapy. Nat. Cancer. 2021;2:780–793. doi: 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allen G.M., Lim W.A. Rethinking cancer targeting strategies in the era of smart cell therapeutics. Nat. Rev. Cancer. 2022;22:693–702. doi: 10.1038/s41568-022-00505-x. [DOI] [PubMed] [Google Scholar]
- 118.Labanieh L., Mackall C.L. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–648. doi: 10.1038/s41586-023-05707-3. [DOI] [PubMed] [Google Scholar]
- 119.Lim W.A. The emerging era of cell engineering: Harnessing the modularity of cells to program complex biological function. Science. 2022;378:848–852. doi: 10.1126/science.add9665. [DOI] [PubMed] [Google Scholar]