Abstract
A phage-inducible middle promoter (P15A10) from the lytic, lactococcal bacteriophage φ31, a member of the P335 species, is located in an 888-base pair fragment near the right cohesive end. Sequence analysis revealed extensive homology (>95%) to the right cohesive ends of two temperate phages of the P335 species, φr1t and φLC3. Sequencing upstream and downstream of P15A10 showed that the high degree of homology between φ31 and φr1t continued beyond the phage promoter. With the exception of one extra open reading frame in φ31, the sequences were highly homologous (95 to 98%) between nucleotides 13448 and 16320 of the published φr1t sequence. By use of a β-galactosidase (β-Gal) gene under the control of a smaller, more tightly regulated region within the P15A10 promoter, P566–888, it was established that mitomycin C induction of a lactococcal strain harboring the prophage φr1t induced the P566–888 promoter, as determined from an increase in β-Gal activity. Hybridization of nine other lactococcal strains with 32P-labeled P566–888 showed that the Lactococcus lactis strains C10, ML8, and NCK203 harbored sequences homologous to that of the phage-inducible promoter. Mitomycin C induced the resident prophages in all these strains and concurrently induced the P566–888 promoter, as determined from an increase in β-Gal activity. DNA restriction analysis revealed that the prophages in C10, ML8, and NCK203 had identical restriction patterns which were different from that of φr1t. In addition, DNA sequencing showed that the promoter elements in the three phages were identical to each other and to P566–888 from the lytic phage φ31. These results point to a conserved mechanism in the regulation of gene expression between the lytic phage φ31 and at least two temperate bacteriophages and provide further evidence for a link in the evolution of certain temperate phages and lytic phages.
Lactococcus lactis is an industrially important member of the lactic acid bacteria. It is used in the fermentation of many dairy products, including sour cream, buttermilk, and various cheeses such as cheddar. Lytic bacteriophages routinely disrupt these industrial milk fermentations. Despite the substantial research conducted to protect L. lactis, the appearance of new lytic phages continues to plague the dairy industries (16, 27). In previous years, it was observed that lytic and temperate phages showed little significant homology at the DNA level (13). Therefore, it was not considered likely that temperate phages contributed to the development of lytic phages for L. lactis (5, 15). However, the fairly recent emergence of the problematic P335 species (1, 19), composed of both lytic and temperate bacteriophages, has at least partly refuted this position. The lytic and temperate phages of the P335 species exhibit some DNA homology, suggesting that some temperate and lytic phages may have common ancestors (4, 5, 15, 17, 19, 25). In addition, recent evidence suggests that prophages may be an important source of DNA that contributes to the appearance of new lytic phages (7, 20).
The lactococcal bacteriophage φ31 is a small-isometric, cohesive-ended, lytic lactococcal bacteriophage of the P335 species (1) with a double-stranded DNA genome of 31.9 kb. Recently, the first phage-inducible middle promoter from a lytic, lactococcal bacteriophage was isolated from φ31 (22). The 888-base pair promoter fragment (P15A10) was cloned upstream of the β-galactosidase (β-Gal) gene (lacZ.st) from Streptococcus thermophilus (28). The promoter yielded a low level of β-Gal activity before phage infection and was induced three- to fourfold upon infection with phage φ31. P15A10 was subcloned to obtain a smaller, more tightly regulated phage promoter, encompassed within nucleotides 566 to 888 (P566–888 [32]). This promoter fragment yielded β-Gal activity only after phage infection of the lactococcal host, NCK203 (10).
The phage-inducible promoter (P15A10), located near the right cohesive end of the lytic bacteriophage φ31 (22), showed extensive DNA sequence homology (>96%) to two temperate bacteriophages of the P335 species, φr1t (31) and φLC3 (3). This high level of homology led to an interest in the distribution of this promoter in other temperate bacteriophages. The goals of the present study were twofold: (i) to determine whether or not other prophages harbored by lactococcal strains carried this promoter element, and (ii) to evaluate if these prophages could induce the P566–888 promoter, as determined from expression of β-Gal from a P566–888::lacZ.st fusion in pTRK477 (Fig. 1) (32).
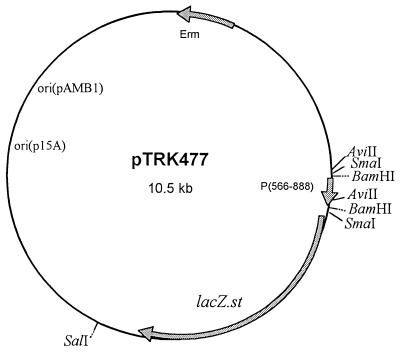
FIG. 1.
Representation of pTRK477. The vector encodes the β-Gal gene (lacZ.st) from S. thermophilus (28) under control of the tightly regulated middle promoter (P566–888) from the lytic bacteriophage φ31. P566–888 was cloned as a BamHI fragment (32) in the promoter screening vector pTRK390 (22). ori, origin of replication; Erm, erythromycin resistance.
Activation of P566–888 by φr1t.
Phages φ31 and φr1t show extensive homology near the right cohesive (cos) ends (22, 31). Therefore, L. lactis R1, the lactococcal strain harboring φr1t in its chromosome, was initially examined for activation of P566–888 concurrent with prophage induction. R1Cs/r1t (prophage positive) and R1Cs, a prophage-cured derivative, were available from previous experiments (14). Both strains were transformed with pTRK477 (P566–888::lacZ.st [Fig. 1]) by the procedure of Holo and Nes (12) as modified by Walker and Klaenhammer (32). One transformed colony was obtained for R1Cs/r1t. Small-scale plasmid isolation (21) and restriction analysis confirmed the presence of pTRK477. No transformants were obtained for the cured derivative, R1Cs, even upon repeated transformation attempts with increasing concentrations of pTRK477. The vector pTRK477 was also isolated from the R1Cs/r1t transformant and electroporated to R1Cs. No transformants were obtained, indicating that a restriction/modification barrier was not responsible. The reason for the difficulty in transforming R1 is not known.
The unsuccessful attempts to transform R1Cs forced us to create a prophage-negative control strain from R1Cs/r1t containing pTRK477. To cure φr1t, R1Cs/r1t (pTRK477) was propagated in GM17 (30) at 30°C to an optical density at 600 nm (OD600) of ∼0.2. Erythromycin (EM) (2 μg/ml) was added to maintain pTRK477. Mitomycin C (2 μg/ml) was added to induce φr1t, and induction was allowed to proceed for 45 min. The cells were diluted in sterile phosphate-buffered saline buffer and plated onto GM17 containing 2 μg of EM per ml [GM17 (EM)] and 1.5% agar so that individual colonies could be isolated. Individual colonies were then patched onto fresh GM17 (EM) plates containing 10 mM CaCl2 and spotted with 10 μl of a φr1t lysate. The cured derivatives were identified by their sensitivity to φr1t. The cure rate was greater than 15% for the survivors. Plasmid mini-preps and restriction analysis confirmed the presence of pTRK477 in the cured derivatives. The plasmid isolated from R1Cs (pTRK477) was then transformed into the original host, L. lactis NCK203. β-Gal assays were performed during a phage φ31 infection (18, 22) to ensure that pTRK477 had not suffered mutations during mitomycin C treatment. No change in the level of β-Gal induced from the P566–888 promoter was observed (data not shown).
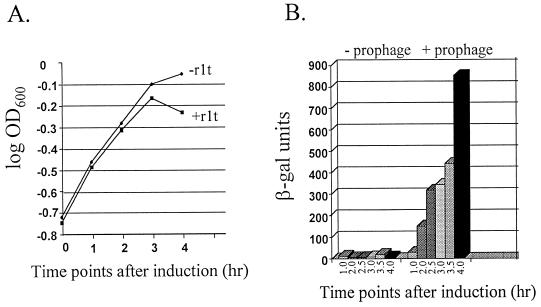
To evaluate whether or not φr1t could activate P566–888, R1Cs/r1t (pTRK477) and R1Cs (pTRK477) were propagated in GM17 (EM) at 30°C to an initial OD600 of ∼0.2. Mitomycin C was then added to a concentration of 2 μg/ml, and growth continued for 4 h, with OD600 readings and β-Gal activity assays performed every 30 min. The results are shown in Fig. 2. R1Cs/r1t (pTRK477) showed evidence of lysis within 3 h (lysis was complete upon overnight incubation), while the cured derivative did not (Fig. 2A). β-Gal activity was detected in R1Cs/r1t (pTRK477) upon induction with mitomycin C and continued to increase during the lytic cycle (Fig. 2B). No β-Gal activity was observed in the cured derivative. Therefore, induction of phage φr1t activated the P566–888 promoter.
FIG. 2.
Induction of P566–888 by phage r1t. (A) Growth of R1Cs/r1t and R1Cs, the prophage-cured derivative, in the presence of mitomycin C. The strains were grown to an initial OD600 of 0.2 before the addition of 2.5 μg of mitomycin C per ml. Time zero represents the point of addition of mitomycin C. (B) β-Gal activity expression from R1Cs/r1t (pTRK477) and R1Cs (pTRK477) after induction of the resident prophage with mitomycin C. β-Gal activity was measured by the procedure of Miller (18) as modified by O’Sullivan et al. (22). Activity is expressed as units/OD600 of the cell culture (22). β-Gal results reported are the averages of two separate assays. In each assay, time points were performed (or assayed) in duplicate.
Different Lactococcus prophages activate P566–888.
To determine if other prophages were capable of activating P566–888, several lactococcal strains (Table 1) were tested for the presence of DNA homologous to the fragment from positions 566 to 888 by using slot blot Southern hybridization. As a negative control, the original φr1t-cured derivative R1Cs (14), which could not be transformed, was included. The probe was the 32P-labeled fragment from positions 566 to 888 (P566–888). Results are shown in Fig. 3. The DNA from three L. lactis subsp. lactis strains, NCK203, ML8, and C10, showed homology to P566–888. These results were confirmed by transferring HindIII digests of each DNA sample to a MagnaCharge membrane (MSI, Westborough, Mass.) and probing with the 32P-labeled fragment P566–888 by using standard procedures (26). Identical HindIII fragments showing homology to P566–888 were observed for all three strains (data not shown). DNA sequencing with the Thermosequenase kit (Amersham Life Sciences, Arlington Heights, Ill.) was then used to determine if the homologous DNA identified in each strain by hybridization with P566–888 was identical to that isolated from φ31. The regions of C10, ML8, and NCK203 showing homology to P566–888 were amplified by PCR from the genomes of C10, ML8, and NCK203 with one primer consisting of nucleotides 566 to 582 and a second primer complementary to nucleotides 865 to 888 of the published sequence from φ31 (22, 32). The PCR products were then cloned into the T vector, pGEM-T easy (Promega, Madison, Wis.) and transformed to JM110 (33) by the procedure of Hanahan (9) as modified by Dinsmore and Klaenhammer (6). Sequencing was performed with the T7 promoter primer, and the first 200 nucleotides of each region (corresponding to nucleotides 566 to 766 of P566–888) could be easily read. The sequences of the homologous regions in the three prophages were identical to the corresponding region of the P566–888 sequence of phage φ31. The sequenced regions showed identical putative transcriptional start sites, −10 consensus regions, and inverted or direct repeats positioned in the −35 regions (22, 32).
TABLE 1.
L. lactis strains used to determine homology with P566–888
| Strain | Source or descriptiona | Source or reference |
|---|---|---|
| L. lactis subsp. lactis | ||
| KP1 derivative | NCK203 | 10 |
| CNRZ 481 | NCK329 | 23 |
| ML8 | NCK341 | University of Lavalb |
| C6 | NCK394 | NZDRIc |
| C10 | NCK428 | 8 |
| ATCC 11454 | NCK654 | ATCCd |
| L. lactis subsp. cremoris | ||
| R1Cs | No NCK number | 14 |
| MM 210 | Industrial strain | Our laboratory |
| HP | NCK435 | 29 |
| KH | NCK436 | 29 |
NCK, NCK culture collection at North Carolina State University, Raleigh, N.C.
University of Laval, Quebec, Canada.
NZDRI, New Zealand Dairy Research Institute.
ATCC, American Type Culture Collection.
FIG. 3.
Slot blot analysis of genomic DNA from 10 lactococcal strains. Total DNA was isolated from each strain by the procedure of Hill et al. (11). Slot blot Southern hybridizations were performed with the Zeta probe membrane (BioRad, Richmond, Calif.) and a BioRad apparatus according to the manufacturer’s protocol. The probe (the fragment from positions 566 to 888) was 32P-labeled with the Multiprime DNA labeling system (Amersham Life Sciences).
To determine whether or not prophages were present which could activate P566–888, each of the three strains was transformed with pTRK477 as described above for R1Cs/r1t. The strains containing pTRK477 were propagated in GM17 (EM) to an OD600 of ∼0.2. Mitomycin C was added to 10 μg/ml to attempt induction of the resident prophage. Since repeated attempts to cure these strains were unsuccessful, cultures to which no mitomycin C was added were used as controls in this experiment. OD600-monitored lysis and β-Gal activity assays were used to monitor the subsequent induction of P566–888. Lysis of all three strains began 2 h after the addition of mitomycin C and was virtually complete within 4 h. In addition, although no β-Gal activity was detected in the uninduced cultures, β-Gal activity was induced to approximately the same level (400 to 500 β-Gal units) for all three strains when mitomycin C was present. These data provided strong evidence that P566–888 was activated by induction of the resident prophages.
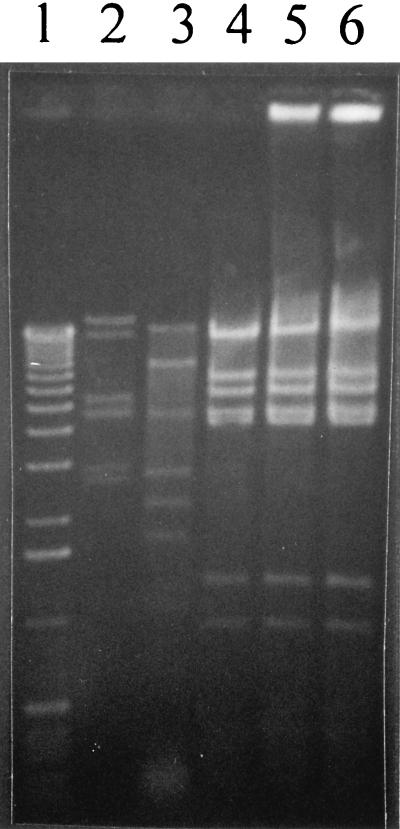
DNA restriction analysis was used to determine if the prophages from the lactococcal strains NCK203, C10, and ML8 were similar to each other or to φr1t. Prophage DNA was isolated as described by Raya et al. (24). Briefly, each strain was propagated in GM17 at 30°C to an OD600 of ∼0.25 to 0.3. Mitomycin C was then added to a level of 10 μg/ml for NCK203 and ML8 and to a level of 7.5 μg/ml for C10 and R1 (r1t) to induce the resident prophages. After lysis, the sample was centrifuged, filter sterilized through a 0.45-μm-pore-size filter to remove cell debris, and treated with DNaseI and RNaseA (1 μg of each per ml) for 30 min at 37°C. The phages were then precipitated with NaCl (3%) and PEG 8000 (10%) overnight, centrifuged at 8,000 rpm for 20 min, and resuspended in 2 ml of sterile distilled water. Phage DNA was isolated by successive phenol-chloroform-isoamyl alcohol extractions and precipitation with ethanol. DNA restriction analysis with EcoRI clearly showed that the three additional prophages identified by DNA hybridization were similar to each other (identical restriction patterns) but not to φr1t (Fig. 4). The similarity between the temperate phages isolated from C10, ML8, and NCK203 was also confirmed with HindIII and EcoRV (data not shown).
FIG. 4.
EcoRI digest patterns of DNA isolated from the temperate phages of L. lactis C10, ML8, and NCK203. Lane 1, 1-kb ladder (Gibco-BRL, Gaithersburg, Md.); lane 2, lytic bacteriophage φ31; lane 3, φr1t; lane 4, temperate phage isolated from NCK203; lane 5, temperate phage from ML8; lane 6, temperate phage from C10.
Sequencing upstream and downstream of the phage-inducible promoter.
Noting the extent of sequence homology of the phage-inducible promoter, P15A10, to the temperate bacteriophage φr1t (22, 31), we also performed sequencing upstream and downstream of the 15A10 fragment on phage φ31 to determine if this high level of homology continued. Phage φ31 genomic DNA was isolated as described earlier (24). Initially, sequencing was performed directly on the phage φ31 genome with the fmol Cycle Sequencing Kit (Promega, Madison, Wis.) and a 32P-end-labeled primer (Table 2) according to manufacturer’s instructions. Most recently, cycle sequencing was performed with the Thermosequenase kit (Amersham). This method utilizes 33P-labeled dideoxynucleotides to terminate sequencing reactions on the 3′ end, resulting in cleaner sequencing runs. To sequence across the cos site, the phage φ31 genome was annealed and ligated by standard protocols (26). Approximately 2,300 base pairs were sequenced downstream of P15A10, and 360 base pairs were sequenced upstream. Homology searches with the BLAST algorithm (2) revealed that the entire region, with the exception of one open reading frame (ORF), continued to show extensive DNA homology to φr1t (95 to 98% identity; nucleotides 13448 to 16320 of the published r1t sequence [31]). At a site adjacent to the right cohesive end (cos), which is identical to the r1t cos site, the sequences diverge. In this region, φ31 contains an additional ORF not found in φr1t (ORF4, 246 amino acids) (Fig. 5). The function of this protein is not known. At the end of ORF4, the sequences of φ31 and φr1t converge again (Fig. 5). A representation of the homology between φ31 and φr1t in this region is shown in Fig. 6. The functions of only two of the depicted putative ORFs have been published. ORF2 (φ31) is the transcriptional activator for the P566–888 promoter (32). ORF27 from φr1t has been identified as a putative structural protein (31).
TABLE 2.
Primers used in sequencing upstream and downstream of P15A10
| Primer | Sequence (5′–3′) | Location on submitted sequence (nucleotides) |
|---|---|---|
| L1153 | TGGTGCGCATCGTGTAG | 1153–1169 |
| L1436 | CGTGATTGGTCTTCTTATG | 1436–1454 |
| L1727 | GATAAAGCAAGATATGAG | 1727–1744 |
| L2091 | CATATTCTTATGGAAATGTAGTTC | 2091–2114 |
| L2339 | ACATGTTGATAGATTCAAAGG | 2339–2359 |
| L2636 | CATTGACCCAATTACTGGATTACT GACAGAG | 2636–2666 |
| L3070 | AACCAAGCATGTCGCCATTTACT GAAC | 3070–3096 |
| R455 | CTGGCATTAATACATCTTTAGAT TCATCA | 455–483 (complement) |
| R1429 | GACCAATCACGGCTCTGT | 1429–1446 (complement) |
| R1727 | CTCATATCTTGCTTTATC | 1727–1744 (complement) |
| R1884 | GTCATTCGTCGTCTTGTAGGAT | 1884–1905 (complement) |
| R2339 | CCTTTGAATCTATCAACATGT | 2339–2359 (complement) |
| R2636 | CTCTGTCAGTAATCCAGTAATTG GGTCAATG | 2636–2666 (complement) |
| R3070 | GTTCAGTAAATGGCGACATGCT TGGTT | 3070–3096 (complement) |
FIG. 5.
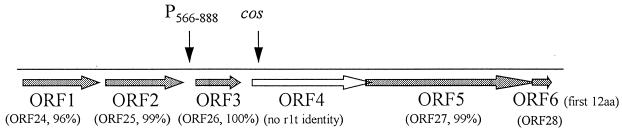
Representation of the DNA sequence of ORF4. The sequences in gray represent areas with extensive homology to φr1t. The homology extends both upstream and downstream of this depicted sequence, as described in the text. The solid underline denotes the cohesive site of φr1t. This exact sequence is present on φ31. The beginning of ORF4 is denoted by arrows. Two possible start codons, each with a potential Shine-Dalgarno sequence upstream (broken line), exist for ORF4. The first start codon is actually in the region of homology to φr1t. On φr1t, a one-nucleotide difference in the region results in a stop codon after the first three amino acids (codon TAG, resulting in MGA−). The second start codon is after the region of homology (denoted by ORF4alt). The end of ORF4 overlaps the beginning of ORF5, which shares extensive homology with φr1t’s ORF27, a putative structural protein (31).
FIG. 6.
Putative ORFs identified near the right cohesive end of phage φ31. The overlapping arrows of ORFs 4, 5, and 6 indicate that the stop and start codons of these ORFs overlap each other. The extent of homology to putative ORFs of φr1t is shown in parentheses below each phage φ31 ORF. The positions of the cos site and the phage-inducible promoter, P566–888, relative to the identified ORFs are indicated by vertical arrows. The sizes of the ORFs are as follows: ORF1, incomplete (161 amino acids [aa] sequenced); ORF2, 143 amino acids; ORF3, 110 amino acids; ORF4, 216 or 246 amino acids (two possible start sites); ORF5, 409 amino acids; and ORF6, the nucleotides encoding the first 12 amino acids have been sequenced. The accession number for this sequence is AF022773.
Conclusions.
Due to the extensive homology of the phage φ31 middle promoter to several temperate phages, this study investigated whether or not other lactococcal strains harbored sequences homologous to that of P566–888. Of the nine strains tested, three lysogenic strains were found to have homologous sequences. Mitocycin C induction of a residing prophage in these strains concurrently activated the P566–888 promoter, generating β-Gal activity. These results clearly point to a conserved mechanism in the regulation of gene expression between the lytic phage φ31 and at least two temperate bacteriophages (φr1t and the residing prophage in C10, ML8, and NCK203). Therefore, lytic and temperate phages of the P335 species appear to share a common ancestor, strengthening the evidence for recent proposals (7, 20) that resident prophage sequences contribute to the evolution of new lytic phages in the dairy lactococci.
Nucleotide sequence accession number.
The nucleotide sequence of the region of φ31 depicted in Fig. 6 has been submitted to the EMBL databases under accession number AF022773.
Acknowledgments
This research was supported, in part, by USDA/NRIGCP project number 97-35503-4368 and by Rhone Poulenc, Inc., Madison, Wis. S.W. was supported by a USDA-GAANN Biotechnology Fellowship.
We thank John McCormick, Evelyn Durmaz, and David Mills for helpful discussions and for critical review of the manuscript.
Footnotes
Paper no. FSR 97-34 of the Department of Food Science, North Carolina State University, Raleigh.
REFERENCES
- 1.Alatossava T, Klaenhammer T R. Molecular characterization of three small isometric-headed bacteriophages which vary in their sensitivity to the lactococcal phage resistance plasmid pTR2030. Appl Environ Microbiol. 1991;57:1346–1353. doi: 10.1128/aem.57.5.1346-1353.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Birkeland N-K, Lonneborg A-M. The cos region of lactococcal bacteriophage φLC3. DNA Sequence. 1993;4:211–214. doi: 10.3109/10425179309015634. [DOI] [PubMed] [Google Scholar]
- 4.Braun V, Hertwig S, Neve G, Geis A, Teuber M. Taxonomic differentiation of bacteriophages of Lactococcus lactis by electron microscopy, DNA-DNA hybridization, and protein profiles. J Gen Microbiol. 1989;135:2551–2560. [Google Scholar]
- 5.Davidson B E, Powell I B, Hillier A J. Temperate bacteriophages and lysogeny in lactic acid bacteria. FEMS Microbiol Rev. 1990;87:79–90. doi: 10.1111/j.1574-6968.1990.tb04880.x. [DOI] [PubMed] [Google Scholar]
- 6.Dinsmore P K, Klaenhammer T R. Molecular characterization of a genomic region in a Lactococcus bacteriophage that is involved in its sensitivity to the phage defense mechanism AbiA. J Bacteriol. 1997;179:2949–2957. doi: 10.1128/jb.179.9.2949-2957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durmaz E, Klaenhammer T R. Abstracts of the 5th Symposium on Lactic Acid Bacteria: Genetics, Metabolism and Applications. Veldhoven, The Netherlands. 1996. Characterization and sequencing of recombinant lactococcal phage φ31.1, abstr. F27. [Google Scholar]
- 8.Efstathiou J D, Mckay L L. Plasmids in Streptococcus lactis: evidence that lactose metabolism and proteinase activity are plasmid-linked. Appl Environ Microbiol. 1976;32:38–44. doi: 10.1128/aem.32.1.38-44.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Pierce K, Klaenhammer T R. The conjugative plasmid pTR2030 encodes two bacteriophage defense mechanisms in lactococci, restriction/modification (R+/M+) and abortive infection (HSP+) Appl Environ Microbiol. 1989;55:2416–2419. doi: 10.1128/aem.55.9.2416-2419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill C, Massey I J, Klaenhammer T R. Rapid method to characterize lactococcal bacteriophage genomes. Appl Environ Microbiol. 1991;57:283–288. doi: 10.1128/aem.57.1.283-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis A W. DNA-DNA homology between lactic streptococci and their temperate and lytic phages. Appl Environ Microbiol. 1984;47:1031–1038. doi: 10.1128/aem.47.5.1031-1038.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis A W, Klaenhammer T R. Bacteriophage resistance plasmid pTR2030 inhibits lytic infection of r1t temperate bacteriophage but not induction of r1t prophage in Streptococcus cremoris R1. Appl Environ Microbiol. 1987;53:385–389. doi: 10.1128/aem.53.2.385-389.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvis A W. Bacteriophages of lactic acid bacteria. J Dairy Sci. 1989;72:3406–3428. [Google Scholar]
- 16.Klaenhammer T R, Fitzgerald G F. Bacteriophages and bacteriophage resistance. In: Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. Glasgow, United Kingdom: Blackie Academic and Professional; 1994. pp. 106–108. [Google Scholar]
- 17.Lautier M, Novel G. DNA-DNA hybridization in lactic streptococcal temperate and virulent phages belonging to distinct lytic groups. J Ind Microbiol. 1987;2:151–158. [Google Scholar]
- 18.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 19.Moineau S, Pandian S, Klaenhammer T R. Restriction/modification systems and restriction endonucleases are more effective on lactococcal bacteriophages that have emerged recently in the industry. Appl Environ Microbiol. 1993;59:197–202. doi: 10.1128/aem.59.1.197-202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moineau S, Pandian S, Klaenhammer T R. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl Environ Microbiol. 1994;60:1832–1841. doi: 10.1128/aem.60.6.1832-1841.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Sullivan D J, Klaenhammer T R. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59:2730–2733. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Sullivan D J, Walker S A, West S G, Klaenhammer T R. Development of an expression strategy using a lytic phage to trigger explosive plasmid amplification and gene expression. Bio/Technology. 1996;14:82–87. doi: 10.1038/nbt0196-82. [DOI] [PubMed] [Google Scholar]
- 23.Piard J-C, Delorme F, Giraffa G, Commissaire J, Desmazeaud M. Evidence for a bacteriocin produced by Lactococcus lactis CNRZ 481. Neth Milk Dairy J. 1990;44:143–158. [Google Scholar]
- 24.Raya R R, Kleeman E G, Luchansky J B, Klaenhammer T R. Characterization of the temperate bacteriophage φadh and plasmid transduction in Lactobacillus acidophilus ADH. Appl Environ Microbiol. 1989;55:2206–2213. doi: 10.1128/aem.55.9.2206-2213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Relano P, Mata M, Bonneau M, Ritzenthalen P. Molecular characterization and comparison of 38 virulent and temperate bacteriophages of Streptococcus lactis. J Gen Microbiol. 1987;133:3053–3063. doi: 10.1099/00221287-133-11-3053. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sanders M E. Bacteriophages of industrial importance. In: Goyal S M, Gerba C P, Bitton G, editors. Phage ecology. New York, N.Y: Wiley Interscience; 1987. pp. 211–244. [Google Scholar]
- 28.Schroeder C J, Robert C, Lenzen G, McKay L L, Mercenier A. Analysis of the lacZ sequences from two Streptococcus thermophilus strains: comparison with the Escherichia coli and Lactobacillus bulgaricus β-galactosidase sequences. J Gen Microbiol. 1991;137:369–380. doi: 10.1099/00221287-137-2-369. [DOI] [PubMed] [Google Scholar]
- 29.Sing W D, Klaenhammer T R. Conjugal transfer of bacteriophage resistance determinants on pTR2030 into Streptococcus cremoris strains. Appl Environ Microbiol. 1986;51:1264–1271. doi: 10.1128/aem.51.6.1264-1271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terzaghi B, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Sinderon D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage r1t. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 32.Walker S A, Klaenhammer T R. Molecular characterization of a phage-inducible middle promoter and its transcriptional activator from the lactococcal bacteriophage φ31. J Bacteriol. 1998;180:921–931. doi: 10.1128/jb.180.4.921-931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]