Abstract
Background
Although numerous modalities are currently in use for the treatment and prophylaxis of COVID-19, probiotics are a cost-effective alternative that could be used in diverse clinical settings. Hence, we conducted a meta-analysis to investigate the role of probiotics in preventing and treating COVID-19 infection.
Methods
We searched several databases from inception to 30 May 2023 for all randomized controlled trials (RCTs) and comparative observational studies that evaluated probiotics (irrespective of the regimen) for the treatment or prevention of COVID-19. We conducted our meta-analysis using RevMan 5.4 with risk ratio (RR) and mean difference (MD) as the effect measures.
Results
A total of 18 studies (11 RCTs and 7 observational studies) were included in our review. Probiotics reduced the risk of mortality (RR 0.40; 95% CI: 0.25–0.65, I2 = 0%). Probiotics also decreased the length of hospital stay, rate of no recovery, and time to recovery. However, probiotics had no effect on the rates of ICU admission. When used prophylactically, probiotics did not decrease the incidence of COVID-19 cases (RR 0.65; 95% CI: 0.37–1.12; I2 = 66%). The results for all outcomes were consistent across the subgroups of RCTs and observational studies (P for interaction >0.05).
Conclusion
The results of this meta-analysis support the use of probiotics as an adjunct treatment for reducing the risk of mortality or improving other clinical outcomes in patients with COVID-19. However, probiotics are not useful as a prophylactic measure against COVID-19. Large-scale RCTs are still warranted for determining the most efficacious and safe probiotic strains.
Systematic Review Registration
PROSPERO (CRD42023390275: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=390275).
Keywords: probiotics, COVID-19, SARS-CoV-2, synbiotics, meta-analysis
1. Introduction
New variants of COVID-19 continue to be reported worldwide significantly impacting morbidity and mortality. Thus, research to investigate novel treatment modalities still holds significance in the clinical setting. Numerous therapies have been investigated for treating COVID-19 (1, 2); however, questionable efficacy or safety, high costs, and the need for parenteral administration are among the issues that limit the widespread use of many of these agents (3–6). Probiotics can be a cost-effective alternative that could be used in diverse clinical settings.
Probiotics are defined as “live microorganisms that when administered in adequate amounts, confer a health benefit on the host (7).” Clinical trials have shown the efficacy of single or multiple strains of probiotics in the management of respiratory tract infections (8, 9). Recently, the use of probiotics for COVID-19 has also been proposed (10); however, the efficacy of probiotics in the treatment of COVID-19 remains inconclusive. Previous meta-analyses either did not include several key studies or only assessed the effect of probiotics on a limited number of clinical outcomes which limits the applicability of their findings (11–13). In this meta-analysis, we have thus included all the studies available in the literature to investigate the role of probiotics in preventing and treating COVID-19 infection.
2. Methods
Our meta-analysis was registered with PROSPERO (CRD42023390275) and conducted by following the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14).
2.1. Search strategy
We searched MEDLINE (via PubMed), Embase, the Cochrane Library, and ClinicalTrials.gov from inception to 30 May 2023, without using any filters or restrictions. The search strategy consisted of the following terms: (“COVID-19′′ or “coronavirus disease 2019′′ or “novel coronavirus” or “SARS-CoV-2′′) AND (“probiotics” or “S. thermophilus” or “L.acidophilus” or “synbiotics” or “Lactobacillus rhamnosus GG” or “SIM01” or “Bifidobacterium”). Reference lists of relevant articles were also manually screened to retrieve additional relevant studies. A partial search of Google Scholar was conducted to find any relevant grey literature.
2.2. Study selection and eligibility criteria
All the literature obtained from our searches was imported into Mendeley Desktop 1.19.8 and duplicates were removed. The remaining articles were subjected to a rigorous screening process by two independent reviewers. The inclusion criteria were: (1) study design: randomized controlled trials (RCTs) and comparative observational studies; (2) population: patients with COVID-19 irrespective of age or disease severity; (3) intervention: probiotics (irrespective of the regimen) used to treat or prevent COVID-19; and (4) comparator: placebo or standard care.
2.3. Data extraction and outcomes
We extracted all information relating to the study characteristics such as author names, location, study population, details of intervention and comparator groups, and our outcomes of interest. The primary outcome was the risk of all-cause mortality, while the secondary outcomes included the rates of ICU admission, length of hospital stay, time to recovery, and the rate of no recovery. For studies that assessed the use of probiotics as a prophylactic measure against COVID-19, our outcome was the incidence of COVID-19 cases.
2.4. Quality assessment
For the quality assessment, the revised Cochrane Risk of Bias Tool (RoB 2.0) (15) was used for RCTs, while the Newcastle Ottawa Scale (NOS) was used for observational studies (16).
2.5. Data analysis
We conducted our meta-analysis using RevMan 5.4 with risk ratio (RR) and mean difference (MD) as the effect measures for categorical and continuous variables, respectively. We utilized a random-effects model as we anticipated our included studies to be substantially heterogeneous (17). We evaluated heterogeneity using the Chi2 test and the I2 statistic. We conducted a subgroup analysis for all of our outcomes on the basis of the type of study (RCT vs. observational study). In addition, we conducted a sensitivity analysis on our primary outcome by excluding Shah et al. (18) which combined probiotics with a systemic enzyme complex. We could not assess publication bias as no outcome included 10 studies or more.
3. Results
3.1. Search results and study characteristics
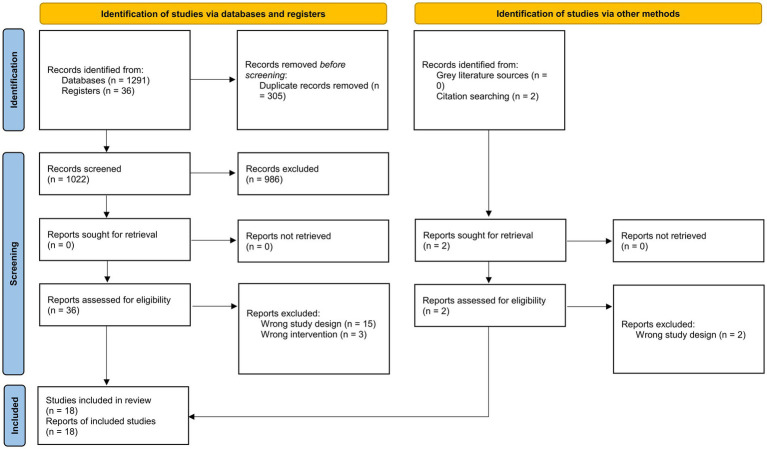
We included 18 studies (11 RCTs and 7 observational studies) in our review (18–35). The details of the screening process are presented in Figure 1.
Figure 1.
PRISMA 2020 flowchart.
Most of the studies had small sample sizes while the retrospective cohort study by Louca et al. was the largest with 445,850 subjects (33). The studies employed a variety of probiotic regimens and most used standard of care as the comparator. The detailed characteristics of each study are presented in Supplementary Table S1.
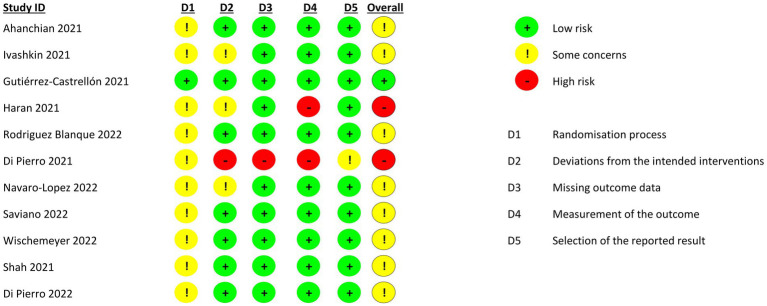
3.2. Risk of bias assessment
Two trials were at a high risk of bias, only one was at a low risk of bias, and the rest had some concerns of bias (Figure 2). The most frequent bias was in the randomization process. Of the observational studies, only two were deemed to be of high quality (Table 1).
Figure 2.
Quality assessment of included trials.
Table 1.
Quality assessment of observational studies.
| Study | Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C | O1 | O2 | O3 | |||
| Li et al. (32) | * | * | * | * | * | * | * | 7* | ||
| Louca et al. (33) | * | * | * | * | * | * | * | 7* | ||
| Trinchieri et al. (24) | * | * | * | * | * | * | * | 7* | ||
| Zhang et al. (34) | * | * | * | * | * | * | * | * | * | 9* |
| Ceccarelli et al. (29) | * | * | * | * | * | * | * | * | * | 8* |
| Ceccarelli et al. (35) | * | * | * | * | * | * | * | 7* | ||
| d’Ettorre et al. (19) | * | * | * | * | * | * | * | 7* | ||
3.3. Results of the meta-analysis
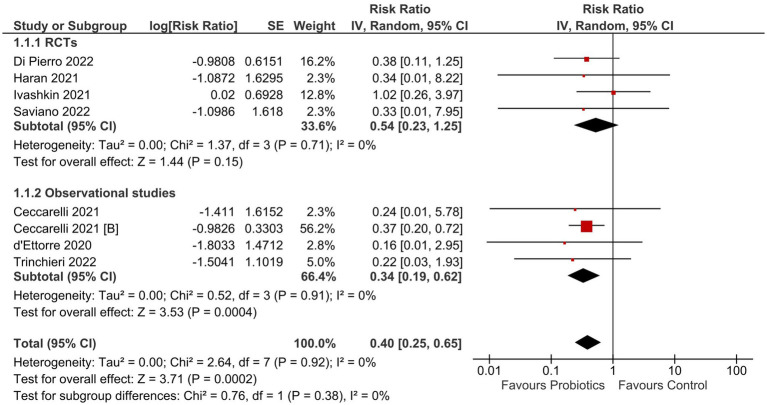
The results of our meta-analysis showed that probiotics reduced the risk of mortality (RR 0.40; 95% CI: 0.25–0.65, I2 = 0%; Figure 3).
Figure 3.
Effect of probiotics on all-cause mortality in COVID-19 patients.
Probiotics had no effect on the rates of ICU admission (RR 0.79; 95% CI: 0.52–1.20; I2 = 0%; Supplementary Figure S1). The length of hospital stay was significantly reduced with probiotics (MD -2.52 days; 95% CI: −4.66 to −0.38 days; I2 = 67%; Supplementary Figure S2). Probiotics reduced the rate of no recovery (RR 0.66; 95% CI: 0.55–0.78; I2 = 0%; Supplementary Figure S3) and decreased the time to recovery (MD -2.18 days; 95% CI: −3.87 to −0.48 days; I2 = 82%; Supplementary Figure S4). When taken prophylactically, probiotics did not decrease the incidence of COVID-19 cases (RR 0.65; 95% CI: 0.37–1.12; I2 = 66%; Supplementary Figure S5). The results for all outcomes were consistent across the subgroups of RCTs and observational studies (P for interaction >0.05).
Sensitivity analysis by excluding Shah et al. (18) which combined probiotics with a systemic enzyme complex produced no significant changes in the results of the length of hospital stay (MD -3.47 days; 95% CI: −5.22 to −1.72 days; I2 = 0%) and recovery time (MD -2.49 days; 95% CI: −4.51 to −0.48 days; I2 = 83%).
4. Discussion
To the best of our knowledge, this is the most comprehensive meta-analysis to date which evaluates the therapeutic efficacy of probiotics in treating and preventing COVID-19. A previous meta-analysis by Neris Almeida Viana et al. assessed only the symptomatic recovery of COVID-19 patients and did not include several large and important studies (11). Other meta-analyses also have an outdated search and did not include several recent studies (12, 13). Moreover, none of these meta-analyses evaluated the role of probiotics as a prophylactic therapy. In this study, we assessed several important clinical outcomes such as mortality and ICU admission, which increases the reliability of our conclusions regarding the efficacy of probiotics. The primary findings of our study indicate that probiotics are effective in reducing the risk of mortality in COVID-19 patients by 60%. Probiotics also decreased the duration of hospitalization and recovery time. However, no benefit was found when given as prophylaxis for COVID-19.
The effectiveness of probiotics in therapy can be explained through intricate pathways and potential anatomical connections, primarily involving the gut-lung axis (GLA) (36, 37). The mesenteric lymphatic system serves as the conduit between the intestines and the lungs, facilitating the passage of intact bacteria, their components, or metabolites across the intestinal barrier into the systemic circulation. This process can subsequently impact the immune response within the lungs (38, 39). Dysbiosis in the gut microbiota has been documented in individuals with COVID-19, and this dysbiosis may arise either as a result of the COVID-19 infection itself or due to the antiviral medications administered during treatment (40). Consequently, the use of probiotics in COVID-19 patients could potentially offer advantages in preserving the equilibrium of the gut microbiota (10). In addition to their effects within the gastrointestinal tract, probiotics have demonstrated the potential to confer health benefits through various mechanisms. These mechanisms encompass immunomodulation, the maintenance of epithelial barrier function, and the modulation of signal transduction pathways (41). Therefore, probiotics may improve the clinical outcomes in COVID-19 patients along with other COVID-19 symptoms like diarrhea.
Numerous treatment modalities such as antivirals, immunomodulatory agents, monoclonal antibodies, repurposed drugs, and herbal therapies have been investigated for COVID-19 since the beginning of the pandemic (42–46). However, many factors such as low availability, high costs, and questionable efficacy hinder their widespread use (2, 3, 47, 48). Thus, probiotics prove to be an efficacious, inexpensive, and readily available treatment alternative for COVID-19. However, the findings of our study do not support the use of probiotics for prophylaxis as the association with the incidence of COVID-19 cases was reported to be insignificant. Future research should evaluate the efficacy of probiotics against newer COVID-19 variants as well as comparative efficacy in relation to other treatment modalities.
Several limitations should be considered when interpreting the findings of our study. First, this is a pooled analysis of individual studies, and a patient-level analysis was not conducted as we did not have access to the individual patient data. Second, the inclusion of observational studies might have introduced confounding bias; however, this was mitigated by pooling RCTs separately from observational studies. Third, the considerable heterogeneity in the population and intervention across the included studies in terms of disease severity, and composition and dose of probiotics precluded any attempts to conduct subgroup analyses on these potential effect modifiers. Therefore, our results may not be generalizable to some probiotic strains or a spectrum of disease severity. Lastly, only a few included studies were of high quality as most demonstrated poor internal validity.
In conclusion, treatment with probiotics contributes to improved clinical outcomes including a decreased mortality rate and faster recovery. However, further large-scale trials are warranted for determining the most efficacious probiotic strains and regimens and evaluating the safety of these regimens.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ArS: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. HC: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MM: Formal analysis, Writing – original draft, Writing – review & editing. AbS: Data curation, Writing – original draft, Writing – review & editing. AhN: Formal analysis, Writing – original draft, Writing – review & editing. AH: Formal analysis, Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. AbN: Writing – original draft, Writing – review & editing. IC-O: Conceptualization, Writing – original draft, Writing – review & editing. RA: Conceptualization, Writing – original draft, Writing – review & editing. SA: Conceptualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
AN was employed by Hamad Medical Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1274122/full#supplementary-material
References
- 1.Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ. (2020) 370:m3379. doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 2.Rayner CR, Dron L, Park JJH, Decloedt EH, Cotton MF, Niranjan V, et al. Accelerating clinical evaluation of repurposed combination therapies for COVID-19. Am J Trop Med Hyg. (2020) 103:1364–6. doi: 10.4269/ajtmh.20-0995, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal-Ré R, Becker SL, Bottieau E, Holm S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect Dis. (2022) 22:e231–8. doi: 10.1016/s1473-3099(22)00119-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatima M, Azeem S, Saeed J, Shahid A, Cheema HA. Efficacy and safety of molnupiravir for COVID-19 patients. Eur J Intern Med. (2022) 102:118–21. doi: 10.1016/j.ejim.2022.05.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheema HA, Jafar U, Sohail A, Shahid A, Sahra S, Ehsan M, et al. Nirmatrelvir–ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. (2023) 95:e28471. doi: 10.1002/jmv.28471 [DOI] [PubMed] [Google Scholar]
- 6.Cheema HA, Ali A, Ali M, Shahid A, Ghafoor MS, Ur Rehman ME, et al. Efficacy and safety of Favipiravir for the treatment of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials. Am J Ther. (2023). doi: 10.1097/MJT.0000000000001649, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Martín R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol. (2019) 10:1047. doi: 10.3389/fmicb.2019.01047, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. (2022) 8:CD006895. doi: 10.1002/14651858.CD006895.pub4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheema HA, Shahid A, Ayyan M, Mustafa B, Zahid A, Fatima M, et al. Probiotics for the prevention of ventilator-associated pneumonia: an updated systematic review and Meta-analysis of randomised controlled trials. Nutrients. (2022) 14:1600. doi: 10.3390/nu14081600, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier-Santos D, Padilha M, Fabiano GA, Vinderola G, Gomes Cruz A, Sivieri K, et al. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: a bibliometric analysis and systematic review. Trends Food Sci Technol. (2022) 120:174–92. doi: 10.1016/j.tifs.2021.12.033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neris Almeida Viana S, do Reis Santos Pereira T, de Carvalho Alves J, Tianeze de Castro C, Santana C Da Silva L, Henrique Sousa Pinheiro L, et al. Benefits of probiotic use on COVID-19: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2022):1–13. doi: 10.1080/10408398.2022.2128713, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Wu J-Y, Huang P-Y, Liu T-H, Kuo C-Y, Tsai Y-W, Tang H-J, et al. Clinical efficacy of probiotics in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Anti-Infect Ther. (2023) 21:667–74. doi: 10.1080/14787210.2023.2189100, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Pitre T, Ching C, Zeraatkar D, Gruchy S. Safety and efficacy of probiotic supplements as adjunctive therapies in patients with COVID-19: a systematic review and meta-analysis. PLoS One. (2023) 18:e0278356. doi: 10.1371/journal.pone.0278356, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hosp Res Inst; (2000) Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Shah N, Parate R, Vispute A. Potential of the combination of a systemic enzyme complex and probiotics administration to combat COVID-19: a randomized open label prospective analysis. Adv Clin Toxicol. (2021) 6:205. doi: 10.23880/act-16000205 [DOI] [Google Scholar]
- 19.d'Ettorre G, Ceccarelli G, Marazzato M, Campagna G, Pinacchio C, Alessandri F, et al. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med. (2020) 7:389. doi: 10.3389/fmed.2020.00389, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez-Castrellón P, Gandara-Martí T, Abreu AT AY, Nieto-Rufino CD, López-Orduña E, Jiménez-Escobar I, et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. (2022) 14:2018899. doi: 10.1080/19490976.2021.2018899, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro-López V, Hernández-Belmonte A, Pérez Soto MI, Ayo-González M, Losa-Rodríguez G, Ros-Sánchez E, et al. Oral intake of Kluyveromyces marxianus B0399 plus Lactobacillus rhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: a randomized open label clinical trial. Med Microecol. (2022) 14:100061. doi: 10.1016/J.MEDMIC.2022.100061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Blanque R, Sánchez-García JC, Cobos-Vargas Á, Aguilar Quesada A, Maldonado-Lobón JA, Olivares M, et al. Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19. Front Nutr. (2022) 9:1771. doi: 10.3389/FNUT.2022.962566/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saviano A, Potenza A, Siciliano V, Petruzziello C, Tarli C, Migneco A, et al. COVID-19 pneumonia and gut inflammation: the role of a mix of three probiotic strains in reducing inflammatory markers and need for oxygen support. J Clin Med. (2022) 11:3758. doi: 10.3390/jcm11133758, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinchieri V, Marazzato M, Ceccarelli G, Lombardi F, Piccirilli A, Santinelli L, et al. Exploiting Bacteria for improving hypoxemia of COVID-19 patients. Biomedicine. (2022) 10:1851. doi: 10.3390/biomedicines10081851, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wischmeyer PE, Tang H, Ren Y, Bohannon L, Ramirez ZE, Andermann TM, et al. Daily Lactobacillus probiotic versus placebo in COVID-19-exposed household contacts (PROTECT-EHC): a randomized clinical trial. medRxiv. (2022). doi: 10.1101/2022.01.04.21268275 [DOI] [Google Scholar]
- 26.Di Pierro F, Iqtadar S, Mumtaz SU, Bertuccioli A, Recchia M, Zerbinati N, et al. Clinical effects of Streptococcus salivarius K12 in hospitalized COVID-19 patients: results of a preliminary study. Microorganisms. (2022) 10:1926. doi: 10.3390/MICROORGANISMS10101926, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivashkin V, Fomin V, Moiseev S, Brovko M, Maslennikov R, Ulyanin A, et al. Efficacy of a probiotic consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the treatment of hospitalized patients with COVID-19: a randomized controlled trial. Probiotics Antimicrob Proteins. (2023) 15:460–8. doi: 10.1007/s12602-021-09858-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahanchian H, Ranjbar A, Reihani H, Yazdi AP, Jafari SA, Kiani MA, et al. Synbiotic for prevention of SARS-Cov2 infection in high risk hospital staffs: a randomized controlled trial. Open J Nurs. (2021) 11:281–90. doi: 10.4236/OJN.2021.115025 [DOI] [Google Scholar]
- 29.Ceccarelli G, Marazzato M, Celani L, Lombardi F, Piccirilli A, Mancone M, et al. Oxygen sparing effect of bacteriotherapy in COVID-19. Nutrients. (2021) 13:2898. doi: 10.3390/nu13082898, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Pierro F, Colombo M. The administration of S. salivarius K12 to children may reduce the rate of SARS-CoV-2 infection. Minerva Med. (2021) 112:514–6. doi: 10.23736/S0026-4806.21.07487-5, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Haran JP, Zheng Y, Knobil K, Palma NA, Lawrence JF, Wingertzahn MA. Targeting the microbiome with KB109 in outpatients with mild to moderate COVID-19 reduced medically attended acute care visits and improved symptom duration in patients with comorbidities. medRxiv. (2021). doi: 10.1101/2021.03.26.21254422 [DOI] [Google Scholar]
- 32.Li Q, Cheng F, Xu Q, Su Y, Cai X, Zeng F, et al. The role of probiotics in coronavirus disease-19 infection in Wuhan: a retrospective study of 311 severe patients. Int Immunopharmacol. (2021) 95:107531. doi: 10.1016/J.INTIMP.2021.107531, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louca P, Murray B, Klaser K, Graham MS, Mazidi M, Leeming ER, et al. Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 symptom study app. BMJ Nutr Prev Health. (2021) 4:149–57. doi: 10.1136/bmjnph-2021-000250, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Han H, Li X, Chen C, Xie X, Su G, et al. Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19. Ther Adv Gastroenterol. (2021) 14:175628482110356. doi: 10.1177/17562848211035670, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceccarelli G, Borrazzo C, Pinacchio C, Santinelli L, Innocenti GP, Cavallari EN, et al. Oral bacteriotherapy in patients with COVID-19: a retrospective cohort study. Front Nutr. (2021) 7:341. doi: 10.3389/fnut.2020.613928, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Ma W-T, Pang M, Fan Q-L, Hua J-L. The commensal microbiota and viral infection: a comprehensive review. Front Immunol. (2019) 10:10. doi: 10.3389/fimmu.2019.01551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. (2020) 10:9. doi: 10.3389/fcimb.2020.00009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon J-Y, Bernalier-Donadille A, et al. Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol. (2017) 2017:5035371–15. doi: 10.1155/2017/5035371, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. (2018) 48:39–49. doi: 10.1002/eji.201646721, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguila EJT, Lontok MADC, Aguila EJT. Letter: role of probiotics in the COVID-19 pandemic. Aliment Pharmacol Ther. (2020) 52:931–2. doi: 10.1111/apt.15898, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan LYM, Chen ZJ, Shah NP, El-Nezami H. Modulation of intestinal epithelial defense responses by probiotic Bacteria. Crit Rev Food Sci Nutr. (2016) 56:2628–41. doi: 10.1080/10408398.2014.905450, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Forrest JI, Rayner CR, Park JJH, Mills EJ. Early treatment of COVID-19 disease: a missed opportunity. Infect Dis Ther. (2020) 9:715–20. doi: 10.1007/s40121-020-00349-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheema HA, Shafiee A, Athar MMT, Shahid A, Awan RU, Afifi AM, et al. No evidence of clinical efficacy of famotidine for the treatment of COVID-19: a systematic review and meta-analysis. J Infect. (2023) 86:154–225. doi: 10.1016/j.jinf.2022.11.022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheema HA, Jafar U, Elrashedy AA, Shahid A, Awan RU, Ehsan M, et al. Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Infect. (2022) 85:702–69. doi: 10.1016/j.jinf.2022.10.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Levi J, Ellis L, Hill A. Minimum manufacturing costs, national prices, and estimated global availability of new repurposed therapies for coronavirus disease 2019. Open Forum Infect Dis. (2022) 9:ofab581. doi: 10.1093/ofid/ofab581, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheema HA, Sohail A, Fatima A, Shahid A, Shahzil M, Ur Rehman ME, et al. Quercetin for the treatment of COVID-19 patients: a systematic review and meta-analysis. Rev Med Virol. (2023) 33:e2427. doi: 10.1002/rmv.2427, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Fatima U, Rizvi SSA, Raina N, Fatima S, Rahman S, Kamal MA, et al. Therapeutic management of COVID-19 patients: clinical manifestation and limitations. Curr Pharm Des. (2021) 27:4223–31. doi: 10.2174/1381612826666201125112719 [DOI] [PubMed] [Google Scholar]
- 48.Sharun K, Tiwari R, Yatoo MI, Natesan S, Megawati D, Singh KP, et al. A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19. Narra J. (2022) 2:e92–2. doi: 10.52225/NARRA.V2I3.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.