Abstract
The physical bases for enhancement of growth rates induced by auxin involve changes in cell wall structure. Changes in the chemical composition of the primary walls during maize (Zea mays L. cv WF9 × Bear 38) coleoptile development were examined to provide a framework to study the nature of auxin action. This report documents that the primary walls of maize cells vary markedly depending on developmental state; polymers synthesized and deposited in the primary wall during cell division are substantially different from those formed during cell elongation.
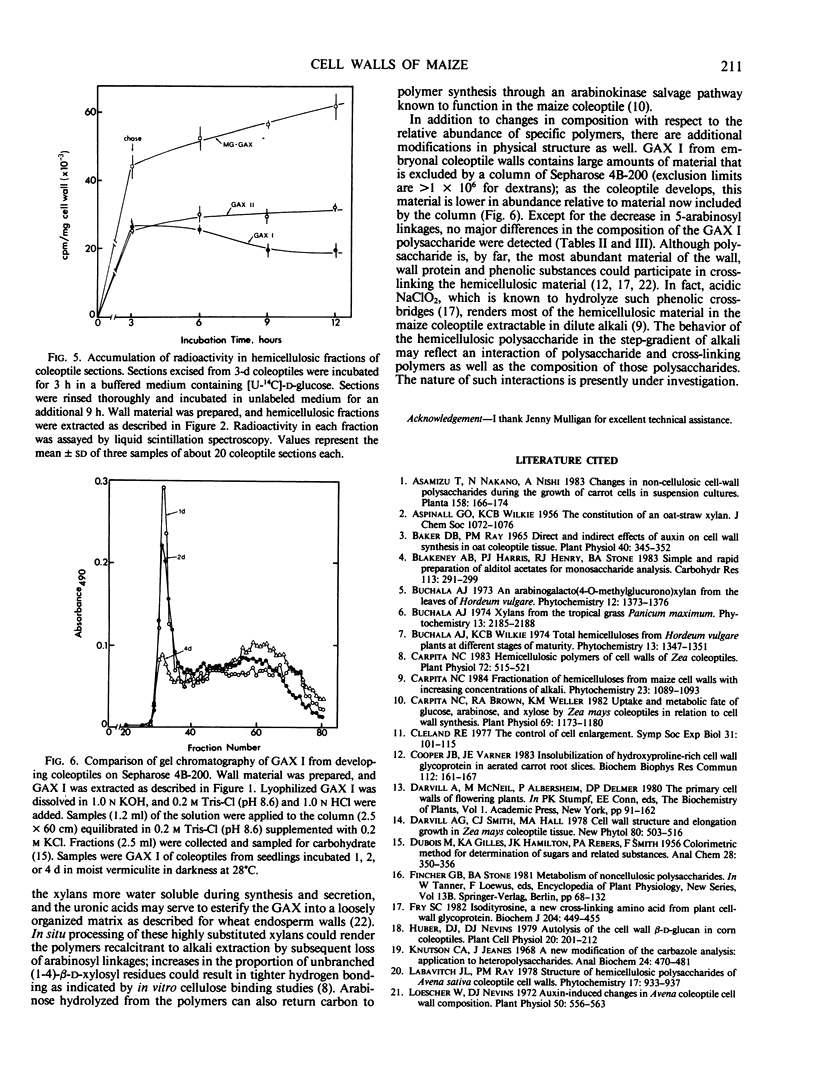
The embryonal coleoptile wall is comprised of mostly glucuronoarabinoxylan (GAX), xyloglucan, and polymers enriched in 5-arabinosyl linkages. During development, both GAX and xyloglucan are synthesized, but the 5-arabinosyls are not. Rapid coleoptile elongation is accompanied by synthesis of a mixed-linked glucan that is nearly absent from the embryonal wall. A GAX highly substituted with mostly terminal arabinofuranosyl units is also synthesized during elongation and, based on pulse-chase studies, exhibits turnover possibly to xylans with less substitution via loss of the arabinosyl and glucuronosyl linkages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. B., Ray P. M. Direct and Indirect Effects of Auxin on Cell Wall Synthesis in Oat Coleoptile Tissue. Plant Physiol. 1965 Mar;40(2):345–352. doi: 10.1104/pp.40.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Brown R. A., Weller K. M. Uptake and Metabolic Fate of Glucose, Arabinose, and Xylose by Zea mays Coleoptiles in Relation to Cell Wall Synthesis. Plant Physiol. 1982 May;69(5):1173–1180. doi: 10.1104/pp.69.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Hemicellulosic polymers of cell walls of zea coleoptiles. Plant Physiol. 1983 Jun;72(2):515–521. doi: 10.1104/pp.72.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. The control of cell enlargement. Symp Soc Exp Biol. 1977;31:101–115. [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Insolubilization of hydroxyproline-rich cell wall glycoprotein in aerated carrot root slices. Biochem Biophys Res Commun. 1983 Apr 15;112(1):161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]