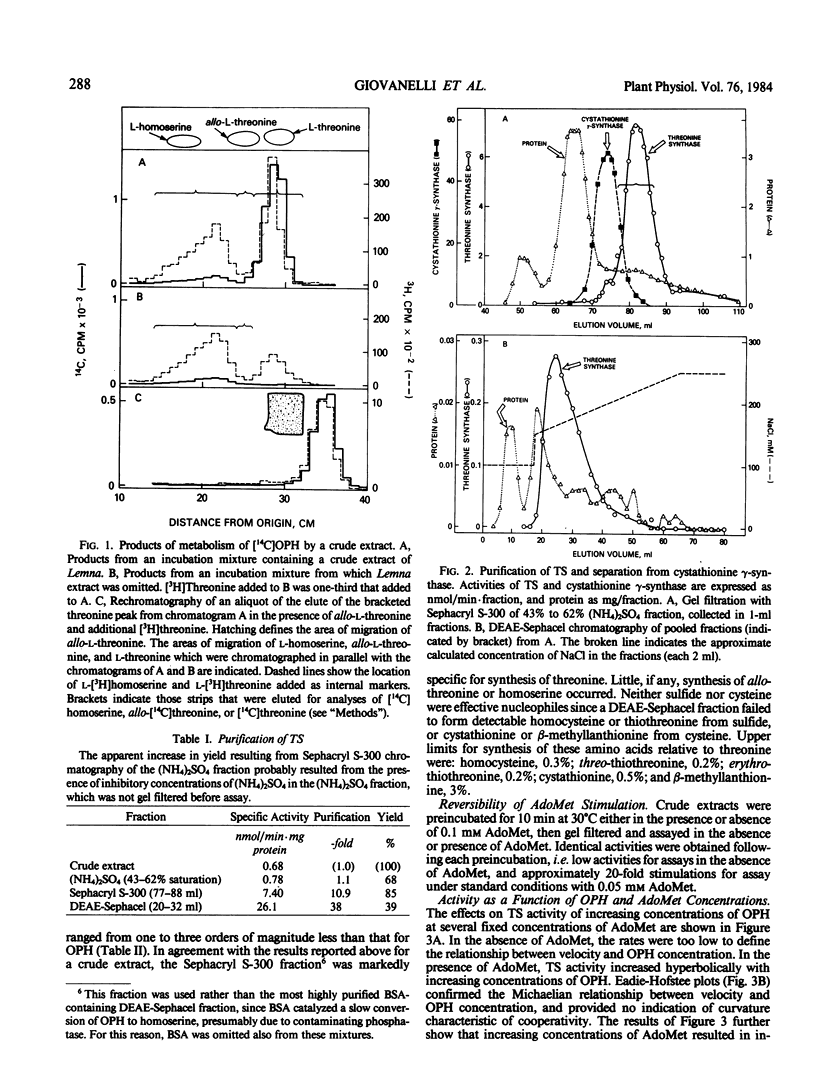
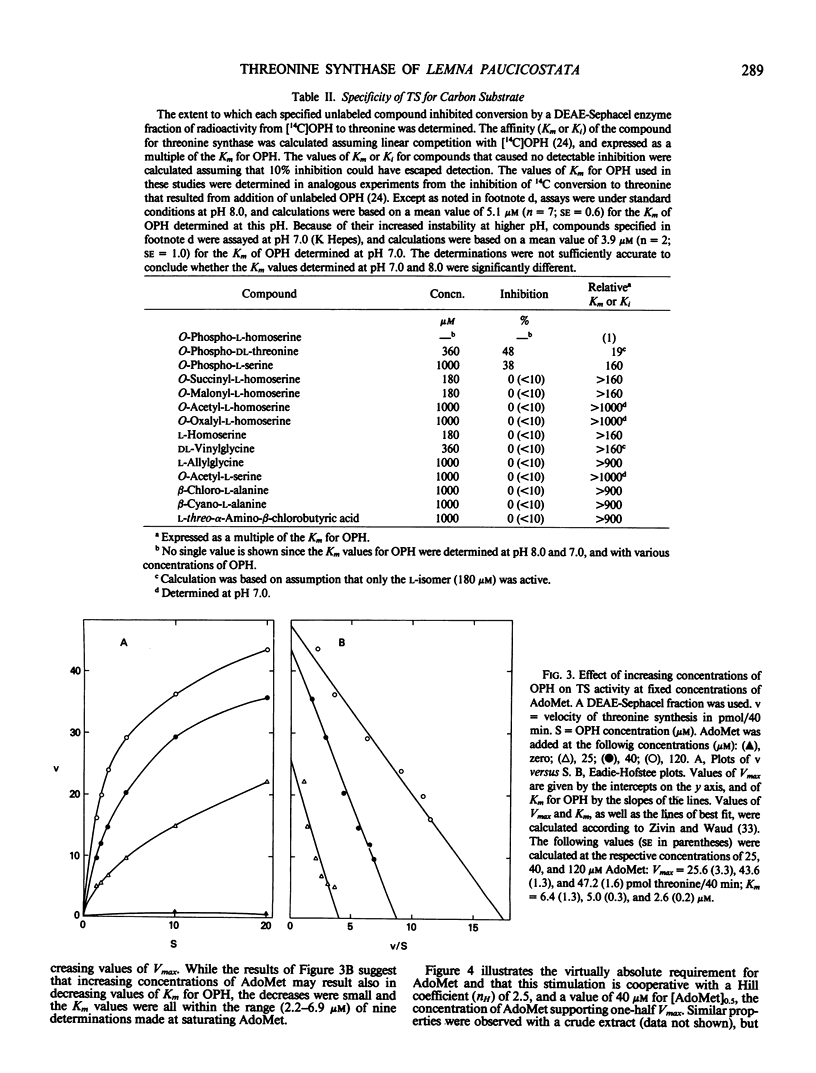
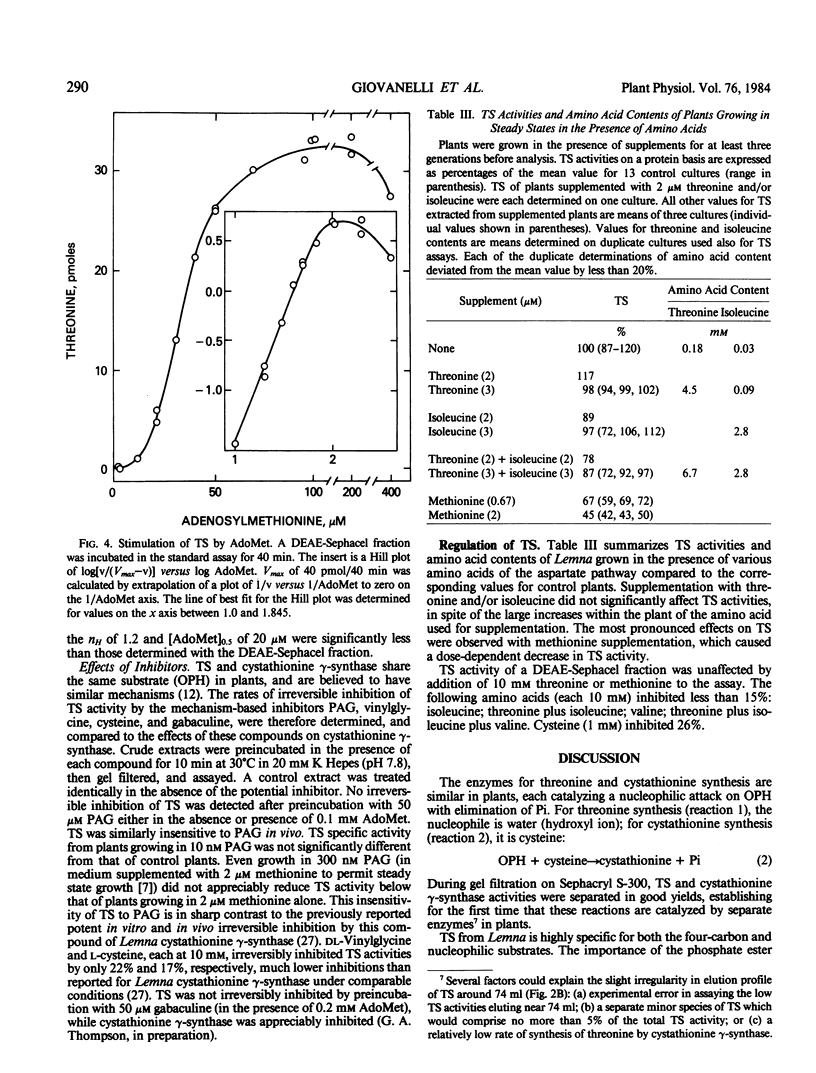
Abstract
Threonine synthase (TS) was purified approximately 40-fold from Lemna paucicostata, and some of its properties determined by use of a sensitive and specific assay. During the course of its purification, TS was separated from cystathionine γ-synthase, establishing the separate identity of these enzymes. Compared to cystathionine γ-synthase, TS is relatively insensitive to irreversible inhibition by propargylglycine (both in vitro and in vivo) and to gabaculine, vinylglycine, or cysteine in vitro. TS is highly specific for O-phospho-l-homoserine (OPH) and water (hydroxyl ion). Nucleophilic attack by hydroxyl ion is restricted to carbon-3 of OPH and proceeds sterospecifically to form threonine rather than allo-threonine. The Km for OPH, determined at saturating S-adenosylmethionine (AdoMet), is 2.2 to 6.9 micromolar, two orders of magnitude less than values reported for TS from other plant tissues. AdoMet markedly stimulates the enzyme in a reversible and cooperative manner, consistent with its proposed role in regulation of methionine biosynthesis. Cysteine (1 millimolar) caused a slight (26%) reversible inhibition of the enzyme. Activities of TS isolated from Lemna were inversely related to the methionine nutrition of the plants. Down-regulation of TS by methionine may help to limit the overproduction of threonine that could result from allosteric stimulation of the enzyme by AdoMet.
No evidence was obtained for feedback inhibition, repression, or covalent modification of TS by threonine and/or isoleucine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cafferata R. L., Freundlich M. Evidence for channeling of homoserine in Salmonella typhimurium. Biochim Biophys Acta. 1970 Dec 29;222(3):671–674. doi: 10.1016/0304-4165(70)90196-0. [DOI] [PubMed] [Google Scholar]
- Datka A. H., Mudd S. H., Giovanelli J. Homocysteine biosynthesis in green plants: studies of the homocysteine-forming sulfhydrylase. J Biol Chem. 1977 May 25;252(10):3436–3445. [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Responses of Sulfur-Containing Compounds in Lemna paucicostata Hegelm. 6746 to Changes in Availability of Sulfur Sources. Plant Physiol. 1984 Jun;75(2):474–479. doi: 10.1104/pp.75.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki N., Suzuki T., Tanaka H., Soda K., Rando R. R. Deamination and gamma-addition reactions of vinylglycine by L-methionine gamma-lyase. FEBS Lett. 1977 Dec 15;84(2):309–312. doi: 10.1016/0014-5793(77)80713-8. [DOI] [PubMed] [Google Scholar]
- FLAVIN M., SLAUGHTER C. Threonine synthetase mechanism: studies with isotopic hydrogen. J Biol Chem. 1960 Apr;235:1112–1118. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Johnston M., Marcotte P., Donovan J., Walsh C. Mechanistic studies with vinylglycine and beta-haloaminobutyrates as substrates for cystathionine gamma-synthetase from Salmonella typhimurium. Biochemistry. 1979 May 1;18(9):1729–1738. doi: 10.1021/bi00576a015. [DOI] [PubMed] [Google Scholar]
- Madison J. T., Thompson J. F. Threonine synthetase from higher plants: stimulation by S-adenosylmethionine and inhibition by cysteine. Biochem Biophys Res Commun. 1976 Jul 26;71(2):684–691. doi: 10.1016/0006-291x(76)90842-1. [DOI] [PubMed] [Google Scholar]
- Matthews B. F., Widholm J. M. Expression of aspartokinase, dihydrodipicolinic acid synthase and homoserine dehydrogenase during growth of carrot cell suspension cultures on lysine- and threonine-supplemented media. Z Naturforsch C. 1979 Dec;34(12):1177–1185. doi: 10.1515/znc-1979-1216. [DOI] [PubMed] [Google Scholar]
- Soper T. S., Manning J. M. Inactivation of pyridoxal phosphate enzymes by gabaculine. Correlation with enzymic exchange of beta-protons. J Biol Chem. 1982 Dec 10;257(23):13930–13936. [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H. Methionine Synthesis in Lemna: Inhibition of Cystathionine gamma-Synthase by Propargylglycine. Plant Physiol. 1982 Nov;70(5):1347–1352. doi: 10.1104/pp.70.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Intracellular localization of aspartate kinase and the enzymes of threonine and methionine biosynthesis in green leaves. Plant Physiol. 1983 Apr;71(4):780–784. doi: 10.1104/pp.71.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin J. A., Waud D. R. How to analyze binding, enzyme and uptake data: the simplest case, a single phase. Life Sci. 1982 Apr 26;30(17):1407–1422. doi: 10.1016/0024-3205(82)90554-9. [DOI] [PubMed] [Google Scholar]


