Abstract
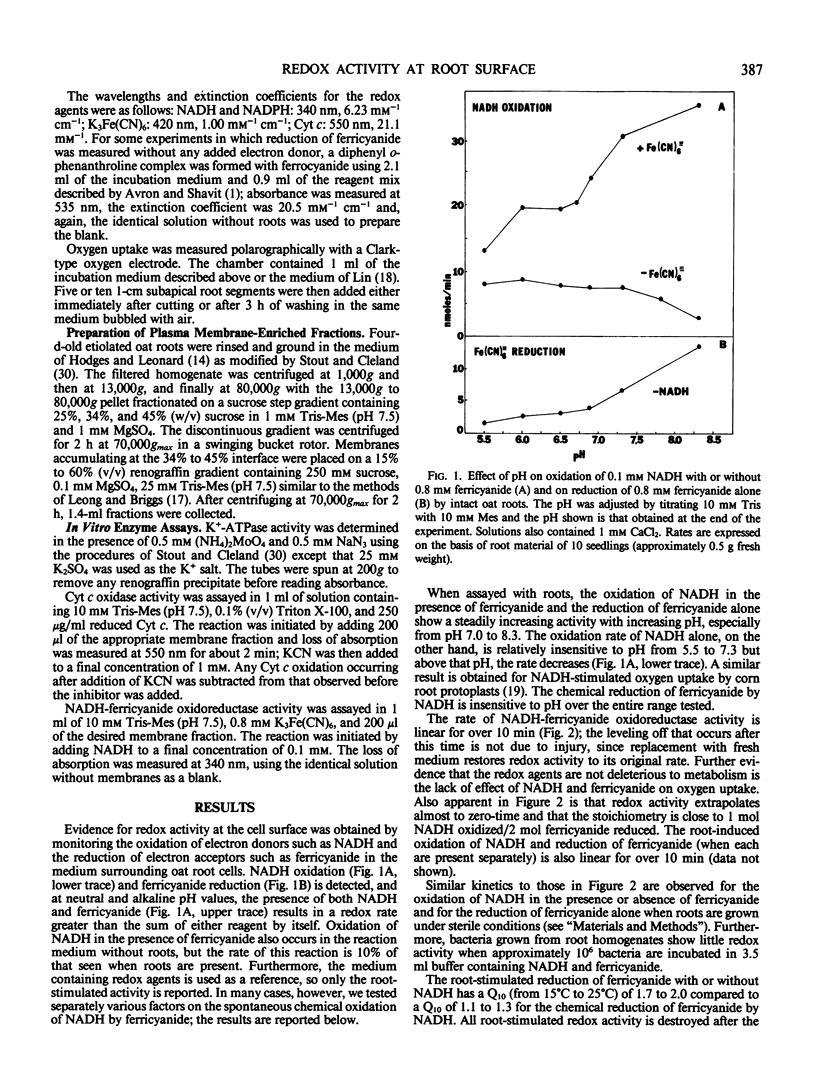
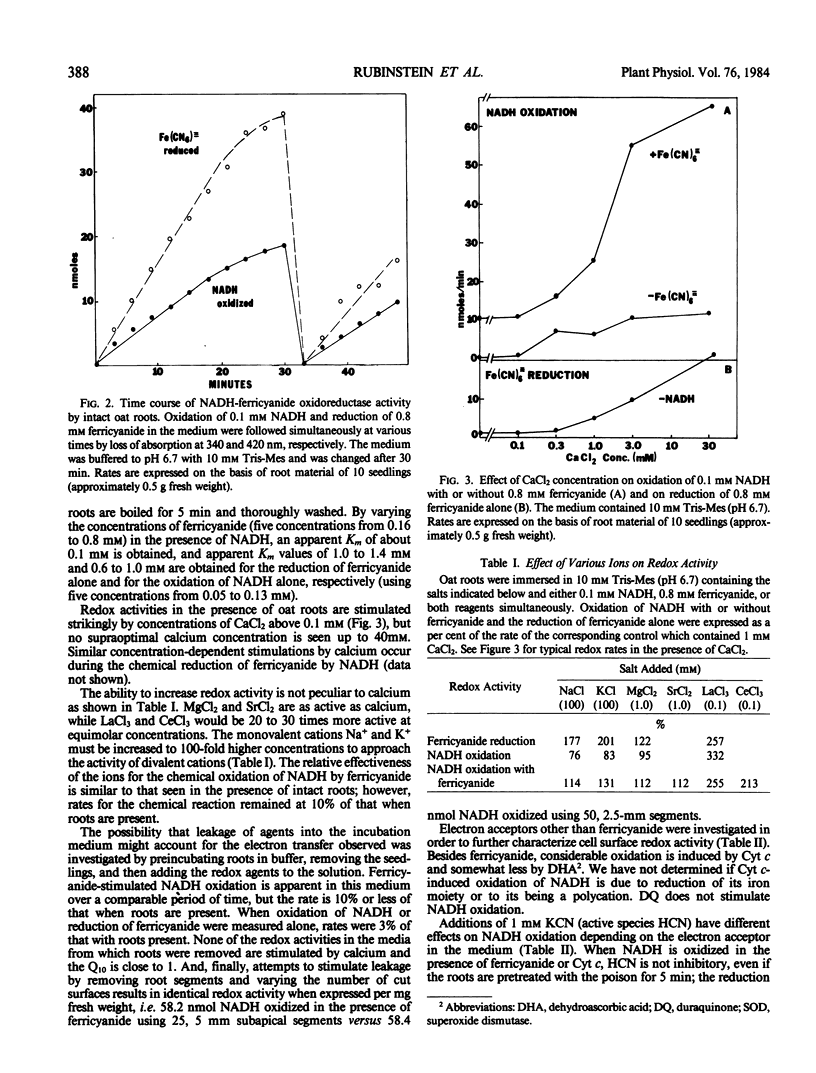
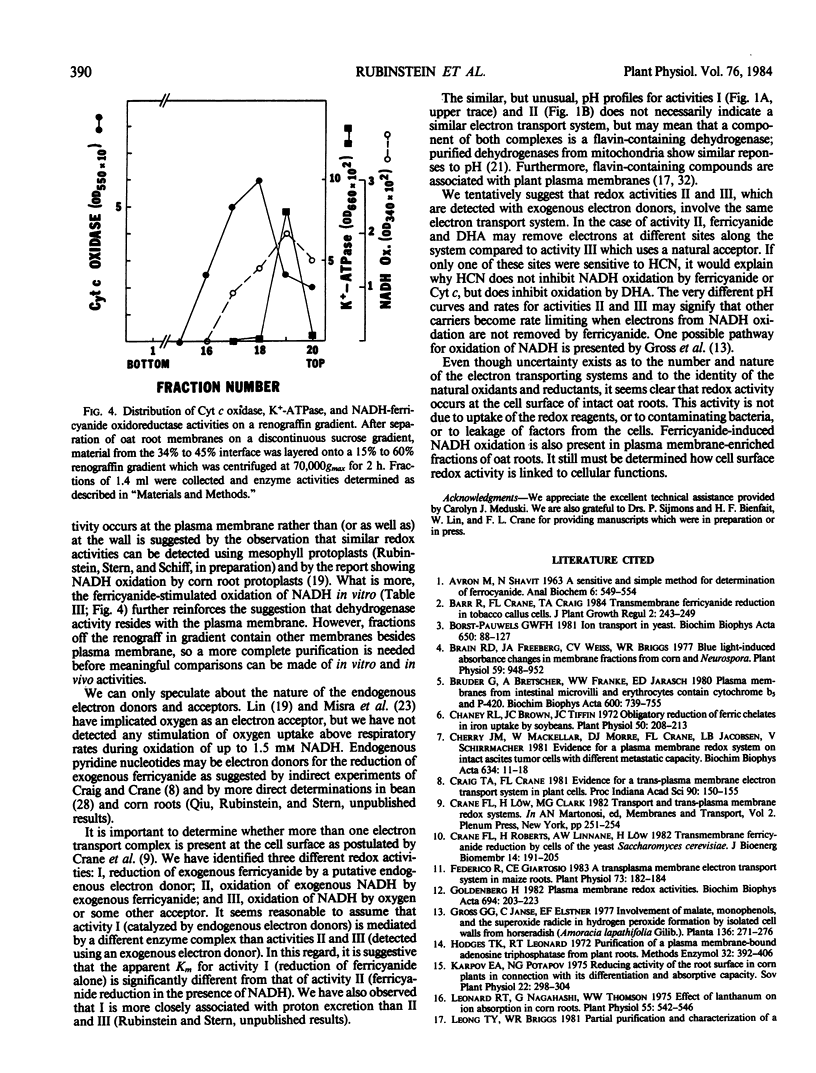
Electron transport activity at the cell surface of intact oat seedlings (Avena sativa L. cv Garry) was examined by measuring the oxidation and/or reduction of agents in the medium bathing the roots. Oxidation of NADH with or without added electron acceptors and reduction of ferricyanide by an endogenous electron donor were detected. The activities appear to be due to electron transfer at, or across, the plasma membrane and not due to reagent uptake or leakage of oxidants or reductants. NADH-ferricyanide oxidoreductase activity was also detected in plasma membrane-enriched preparations from Avena roots. Based on redox responses to pH, various ions, and to a variety of electron donors and acceptors, the results indicate that more than one electron transport system is present at the plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Borst-Pauwels G. W. Ion transport in yeast. Biochim Biophys Acta. 1981 Dec;650(2-3):88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- Brain R. D., Freeberg J. A., Weiss C. V., Briggs W. R. Blue light-induced Absorbance Changes in Membrane Fractions from Corn and Neurospora. Plant Physiol. 1977 May;59(5):948–952. doi: 10.1104/pp.59.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G., Bretscher A., Franke W. W., Jarasch E. D. Plasma membranes from intestinal microvilli and erythrocytes contain cytochromes b5 and P-420. Biochim Biophys Acta. 1980 Aug 14;600(3):739–755. doi: 10.1016/0005-2736(80)90477-0. [DOI] [PubMed] [Google Scholar]
- Chaney R. L., Brown J. C., Tiffin L. O. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972 Aug;50(2):208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Mackellar W., Morré D. J., Crane F. L., Jacobsen L. B., Schirrmacher V. Evidence for a plasma membrane redox system on intact ascites tumor cells with different metastatic capacity. Biochim Biophys Acta. 1981 Jan 14;634(1):11–18. doi: 10.1016/0005-2728(81)90123-7. [DOI] [PubMed] [Google Scholar]
- Crane F. L., Roberts H., Linnane A. W., Löw H. Transmembrane ferricyanide reduction by cells of the yeast Saccharomyces cerevisiae. J Bioenerg Biomembr. 1982 Jun;14(3):191–205. doi: 10.1007/BF00745020. [DOI] [PubMed] [Google Scholar]
- Federico R., Giartosio C. E. A transplasmamembrane electron transport system in maize roots. Plant Physiol. 1983 Sep;73(1):182–184. doi: 10.1104/pp.73.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg H. Plasma membrane redox activities. Biochim Biophys Acta. 1982 Oct 20;694(2):203–223. doi: 10.1016/0304-4157(82)90025-9. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Nagahashi G., Thomson W. W. Effect of lanthanum on ion absorption in corn roots. Plant Physiol. 1975 Mar;55(3):542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T. Y., Briggs W. R. Evidence from studies with acifluorfen for participation of a flavin-cytochrome complex in blue light photoreception for phototropism of oat coleoptiles. Plant Physiol. 1982 Sep;70(3):875–881. doi: 10.1104/pp.70.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T. Y., Briggs W. R. Partial purification and characterization of a blue light-sensitive cytochrome-flavin complex from corn membranes. Plant Physiol. 1981 May;67(5):1042–1046. doi: 10.1104/pp.67.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Responses of corn root protoplasts to exogenous reduced nicotinamide adenine dinucleotide: Oxygen consumption, ion uptake, and membrane potential. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3773–3776. doi: 10.1073/pnas.79.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw H., Crane F. L. Redox function in plasma membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):141–161. doi: 10.1016/0304-4157(78)90002-3. [DOI] [PubMed] [Google Scholar]
- MINAKAMI S., RINGLER R. L., SINGER T. P. Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem. 1962 Feb;237:569–576. [PubMed] [Google Scholar]
- Miner C., López-Burillo S., García-Sancho J., Herreros B. Plasma membrane nadh dehydrogenase and Ca2+-dependent potassium transport in erythrocytes of several animal species. Biochim Biophys Acta. 1983 Jan 19;727(2):266–272. doi: 10.1016/0005-2736(83)90412-1. [DOI] [PubMed] [Google Scholar]
- Misra P. C., Craig T. A., Crane F. L. A link between transport and plasma membrane redox system(s) in carrot cells. J Bioenerg Biomembr. 1984 Apr;16(2):143–152. doi: 10.1007/BF00743045. [DOI] [PubMed] [Google Scholar]
- Muñoz V., Butler W. L. Photoreceptor Pigment for Blue Light in Neurospora crassa. Plant Physiol. 1975 Feb;55(2):421–426. doi: 10.1104/pp.55.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Römheld V., Marschner H. Mechanism of iron uptake by peanut plants : I. Fe reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983 Apr;71(4):949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., van den Briel W., Bienfait H. F. Cytosolic NADPH is the electron donor for extracellular fe reduction in iron-deficient bean roots. Plant Physiol. 1984 May;75(1):219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]


