Abstract
Exaggerated exercise blood pressure (BP) is linked to cardiovascular disease (CVD). Although evening chronotypes have greater CVD risk than morning (Morn) types, it is unknown if exercise BP differs in intermediate (Int) types. Adults with obesity were classified as either Morn [n = 23 (18 females), Morning-Eveningness Questionnaire (MEQ) = 63.96 ± 1.0, 54.74 ± 1.4 yr, 33.7 ± 0.6 kg/m2] or Int [n = 23 (19 females), MEQ = 51.36 ± 1.1, 55.96 ± 1.8 yr, 37.2 ± 1.2 kg/m2] chronotype per MEQ. A graded, incremental treadmill test to maximal aerobic capacity (V̇o2max) was conducted. Systolic (SBP) and diastolic (DBP) blood pressure and mean arterial pressure (MAP), rate pressure product (RPP), heart rate (HR), and rate of perceived intensity (RPE) were determined at baseline, 4 min, 6 min, and maximal stages. HR recovery (HRR; maximum postexercise) was determined at 1 and 2 min postexercise. Preexercise fasted aortic waveforms (applanation tonometry), plasma leptin, nitrate/nitrite (nitric oxide bioavailability), and body composition (dual X-ray, DXA) were also collected. Int had lower V̇o2max and plasma nitrate (both P ≤ 0.02) than Morn. No difference in preexercise BP, aortic waveforms, or body composition were noted between groups, although higher plasma leptin was seen in Int compared with Morn (P = 0.04). Although Int had higher brachial DBP and MAP across exercise stages (both P ≤ 0.05) and higher HR, RPE, and RPP at 6 min of exercise (all P ≤ 0.05), covarying for V̇o2max nullified the BP, but not HR or RPE, difference. HRR was greater in Morn independent of V̇o2max (P = 0.046). Fasted leptin correlated with HR at exercise stage 4 (r = 0.421, P = 0.041) and 6 min (r = 0.593, P = 0.002). This observational study suggests that Int has exaggerated BP and HR responses to exercise compared with Morn, although fitness abolished BP differences.
NEW & NOTEWORTHY This study compares blood pressure and heart rate responses with graded, incremental exercise between morning and intermediate chronotype adults with obesity. Herein, blood pressure responses to exercise were elevated in intermediate compared with morning chronotype, although V̇o2max abolished this observation. However, heart rate responses to exercise were higher in intermediate vs. morning chronotypes independent of fitness. Collectively, this exercise hemodynamic response among intermediate chronotype may be related to reduced aerobic fitness, altered nitric oxide metabolism, and/or elevated aortic waveforms.
Keywords: aortic waveforms, blood pressure, circadian rhythm, nitric oxide, obesity
INTRODUCTION
Hypertension, or high blood pressure (BP), is a leading risk factor in cardiovascular disease (CVD) progression (1). In fact, high BP is associated with type 2 diabetes (T2D), myocardial infarction, stroke, and heart failure (1, 2). According to recent statistics, nearly 47% of adults in the United States have hypertension, and only ∼24% of those diagnosed have their condition under control (3). Obesity is associated with increased CVD risk that may contribute to the progression of hypertension (4). Indeed, people with obesity have a 3.5-fold increased likelihood of hypertension diagnosis (4). Although standard practice assesses BP under resting conditions, BP responses to submaximal exercise independently predicts future hypertension even in people with normal resting BP (5–8). This is clinically relevant as exercise BP is thought to predict future CVD-related mortality (9).
Chronotype is defined as the internal circadian rhythmicity of an individual and influences the time of day in which people prefer to perform activities (i.e., sleep/wake cycles, physical activity, etc.) (10). Chronotype encompasses a range of classifications to identify a 24-h period including morning (Morn), intermediate (Int), evening chronotype. Evening chronotype is when an individual prefers later bedtimes with delayed times to daily peak alertness. Although it is not inherently problematic, evening chronotypes are associated with a greater risk for obesity (11), insulin resistance (12), and hypertension (10, 13) compared with morning chronotypes (i.e., early to bed, early to rise). This increased disease risk in evening types is potentially due to reduced physical activity (14), altered sleeping habits (15), and/or poor dietary intake (10, 16). Though there is increasing evidence demonstrating heightened disease risk in evening types compared with morning types, limited work is available in intermediate types. This is an important population of interest since nearly 50% or more of the United States population identifies as intermediate chronotypes based on daily sleep and social patterns (17–19). It is valuable to recognize that chronotype classification falls along a broad spectrum with intermediate chronotypes lying between morning and evening types. Individuals who identify as intermediate chronotypes tend to fluctuate in preferences of more morningness to more eveningness aspects (i.e., early to bed-late to rise, late to bed, early to rise, etc.).
Prior work in healthy adult males demonstrated that exaggerated exercise SBP was closely associated with endothelial dysfunction and reduced aortic compliance (5). This was of interest as we recently reported that insulin infusion reduced forward pressure waveforms more so in early compared with later chronotypes with obesity (13). This might be relevant as central blood pressure disturbances could influence the exercise BP response (20). However, few data are available assessing hemodynamic responses to exercise across chronotypes. Indeed, one prior study in young healthy men demonstrated that early chronotypes had elevated heart rate (HR) during morning handgrip stress testing compared with evening chronotypes, which showcased higher HR in the afternoon compared with morning chronotype (21). This suggests exercise timing aligned with one’s chronotype may produce elevated hemodynamic responses to resistance exercise compared with when contraction is performed outside of alignment. However, if we consider the diurnal pattern of blood pressure, surges in blood pressure occur upon waking with peak values occurring midday (22). It is unknown how chronotype may alter blood pressure regulation during this morning to midday time period. Furthermore, whether aerobic exercise would induce differences in BP or heart rate between intermediate (Int) and morning (Morn) chronotypes in individuals with obesity is unknown. Therefore, we tested the hypothesis that exercise BP and heart rate responses would be exaggerated in Int chronotypes when compared with Morn counterparts with obesity. We further hypothesized that altered basal central hemodynamics or disturbances in nitric oxide metabolism would relate to greater exercise BP responses.
MATERIALS AND METHODS
Study Design and Participants
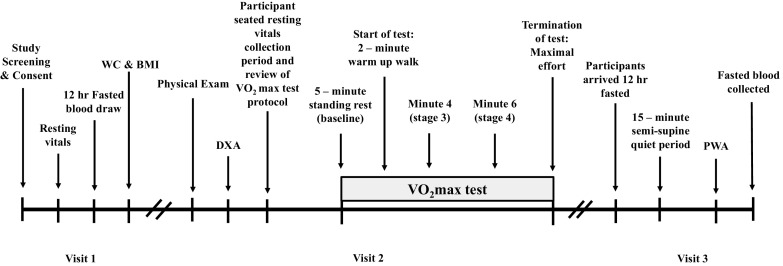
Forty-six sedentary participants with obesity according to BMI (≥30.0 kg/m2) were recruited for this cross-sectional study via social media and/or newspaper flyers from the Charlottesville, VA, and/or New Brunswick, NJ, communities. On the basis of the Morning-Eveningness Questionnaire (MEQ) as previously described (12), participants were classified as either morning (Morn; i.e., scores: 59–86) or intermediate (Int; i.e., scores: 42–58). Some data from current participants (n = 39) were previously reported (13) but are shown herein given relevance to interpretations. The Epworth Sleepiness Scale and Pittsburgh Sleep Quality Index (PSQI) were also used as self-report methods in addition to assess the likelihood of dozing or falling asleep during specific daily events (i.e., watching tv, sitting inactive, in a car while stopped, etc.), as well as a measure of one’s sleep quality and patterns of sleep across a 1-mo time span (23, 24), respectfully. Individuals were included if 40–70 yr, nonsmoking, physically inactive (exercise <60 min/wk, and ≤1.5 metabolic equivalent of tasks expended per activity) (25), and weight stable (<2 kg weight change during last 3 mo). Preceding exercise testing, participants underwent a physical examination including overnight 12-h fasted blood/urine chemistries and resting electrocardiogram (ECG; visit 1) to determine eligibility. Participants were excluded from the study if currently taking medications known to influence insulin sensitivity (e.g., biguanides, GLP-1 agonists, etc.) or affect vasodilatory function (e.g., calcium channel blockers, α-blockers, β-blockers, nitrates, etc.). Female participants were asked to indicate status of menses (Table 1) and were noted if currently on oral contraceptives (OC) or use of hormone replacement treatment (HRT) (Morn: OC, 0; HRT, 1; Int: OC, 0; HRT, 1). A study timeline may be found in Fig. 1 and visits were ∼7 days apart. Participants were provided with written and verbal informed consent before participation, as approved by the Institutional Review Board. This study is part of a larger clinical trial (Clinical Trials Registration No. NCT03355469).
Table 1.
Participant demographics
| Morning | Intermediate | P Value | Cohen’s d Effect Size | |
|---|---|---|---|---|
| Participant characteristics | ||||
| n (male/female) | 23 (5/18) | 23 (4/19) | ||
| Age, yr | 54.7 ± 1.4 | 56.0 ± 1.8 | 0.590 | 0.160 |
| Body mass, kg | 94.6 ± 2.1 | 102.7 ± 4.4 | 0.121 | 0.484 |
| BMI, kg/m2 | 33.7 ± 0.6 | 37.2 ± 1.2 | 0.017 | 0.754 |
| Menopausal status, n | ||||
| Pre | 3 | 2 | ||
| Peri | 2 | 4 | ||
| Post | 13 | 13 | ||
| Race/ethnicity, n | ||||
| African American | 2 | 4 | ||
| Hispanic/Latino | 3 | 0 | ||
| White | 18 | 19 | ||
| MEQ score | 64.0 ± 1.0 | 51.4 ± 1.1 | <0.001 | 2.508 |
| Epworth sleepiness score | 6.52 ± 0.8 | 6.95 ± 0.9 | 0.717 | 0.113 |
| PSQI total score | 6.27 ± 0.5 | 5.35 ± 0.6 | 0.207 | 0.414 |
| PSQI 3-factor sleep efficiency | 1.4 ± 0.37 | 1.1 ± 0.21 | 0.456 | 0.234 |
| PSQI 3-factor perceived sleep quality | 3.28 ± 0.52 | 2.32 ± 0.50 | 0.192 | 0.422 |
| PSQI 3-factor daily disturbances | 2.67 ± 0.24 | 2.26 ± 0.19 | 0.192 | 0.401 |
| Body composition | ||||
| FFM, kg | 53.0 ± 1.3 | 50.6 ± 1.9 | 0.311 | 0.364 |
| Body fat, % | 43.5 ± 1.2 | 44.4 ± 1.0 | 0.584 | 0.179 |
| VAT volume, cm3 | 904.0 ± 43.9 | 981.3 ± 54.3 | 0.273 | 0.396 |
| Aerobic fitness | ||||
| V̇o2max, L/min | 2.3 ± 0.1 | 2.2 ± 0.1 | 0.438 | 0.234 |
| V̇o2max, mL/kg/min | 24.5 ± 0.9 | 21.0 ± 0.8 | 0.004 | 0.888 |
| V̇o2max, mL/FFM kg/min | 43.1 ± 1.4 | 37.9 ± 1.1 | 0.005 | 0.968 |
| V̇emax, L/min | 71.5 ± 2.2 | 71.0 ± 3.7 | 0.911 | 0.034 |
| HRmax, beats/min | 163.6 ± 2.4 | 157.3 ± 2.6 | 0.080 | 0.294 |
| RERmax, AU | 1.1 ± 0.02 | 1.1 ± 0.02 | 0.684 | 0.124 |
| RPEmax, AU | 17.4 ± 0.3 | 18.0 ± 0.3 | 0.182 | 0.399 |
| Cardiometabolic disease risk | ||||
| ATP III score | 3.5 ± 0.1 | 3.5 ± 0.2 | 0.910 | 0.021 |
| WC, cm | 107.8 ± 2.1 | 114.1 ± 3.0 | 0.094 | 0.505 |
| SBP, mmHg | 129.6 ± 3.1 | 133.8 ± 1.5 | 0.229 | 0.364 |
| DBP, mmHg | 77.9 ± 2.2 | 81.2 ± 1.7 | 0.251 | 0.347 |
| FPG, mmol/L* | 95.8 ± 2.8 | 100.0 ± 2.6 | 0.286 | 0.326 |
| TG, mmol/L | 137.2 ± 11.8 | 152.0 ± 10.9 | 0.362 | 0.282 |
| HDL-c, mmol/L | 46.4 ± 2.2 | 48.0 ± 1.8 | 0.579 | 0.169 |
| Fasting biochemistries | ||||
| Leptin, ng/mL* | 37.4 ± 5.4 | 57.4 ± 6.8 | 0.042 | 0.468 |
| Nitrate, mmol/L* | 32.5 ± 3.9 | 21.4 ± 2.3 | 0.022 | 0.901 |
| Nitrite, mmol/L* | 0.03 ± 0.004 | 0.02 ± 0.004 | 0.202 | 0.501 |
| Sodium, mmol/L | 140.1 ± 0.4 | 139.9 ± 0.4 | 0.647 | 0.137 |
| Chloride, mmol/L | 105.3 ± 0.4 | 105.0 ± 0.3 | 0.588 | 0.161 |
| Potassium, mmol/L | 4.1 ± 0.06 | 4.1 ± 0.07 | 0.635 | 0.150 |
| Hemoglobin, g/dL | 14.0 ± 0.3 | 13.8 ± 0.3 | 0.536 | 0.184 |
| Hematocrit, % | 42.2 ± 0.5 | 42.3 ± 0.7 | 0.600 | 0.158 |
Values are means ± SE; n, number of participants. Sample size and sex distribution between group differences are shown [Morn, n = 23 (18 females); and Int, n = 23 (19 females)]. ATP III score, adult treatment panel III quantified risk of metabolic syndrome; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-c, high-density lipoprotein; MEQ score, Morning-Eveningness questionnaire score; RERmax, maximal respiratory exchange ratio; RPEmax, maximally achieved rate of perceived exertion based on the borg scale; SBP, systolic blood pressure; TG, triglycerides; VAT, visceral adipose tissue; V̇emax, maximal exercise ventilation; V̇o2max, aerobic capacity relative to mean body weight (kg) and fat free mass (FFM); WC, waist circumference.
*Reflects blood analytes measured during visit 3. All other biochemistries were obtained during visit 1. Independent sample t test was used to identify group differences between morning and intermediate chronotype. Effect size was calculated via Cohen’s d.
Figure 1.
Study timeline. Participants arrived at the Clinical Research Center (CRC) between 6:00 am and 12:00 pm to complete study visits. Visit 1 included review of the written and verbal informed consent. Upon participant agreement to enroll in the study, resting vitals (i.e., heart rate, blood pressure, and resting electrocardiogram), fasting biochemistries, waist circumference (WC), and body mass index (BMI), were obtained to confirm eligibility. Visit 2 included physical examination completed by the study physician that was then followed by completion of dual X-ray (DXA) assessment and maximal aerobic fitness assessment (V̇o2max). Visit 3 included a resting pulse waveform analysis (PWA) measurement in the semi-supine position and additional blood work.
Graded Exercise Testing
Participants were asked to arrive at the Clinical Research Center (CRC) between 06:00 am and 10:00 am to complete a physical examination with the study physicians and aerobic fitness assessment. If scheduling constraints occurred because of participants’ availability, participants were able to extend the time up to 12:00 pm. This was done to minimize stress on participants. In addition, participants were instructed to avoid engagement in strenuous physical activity as well as ingestion of alcohol, caffeine, and medications 24 h before the study visits. After collection of seated systolic (SBP) and diastolic (DBP) blood pressure and heart rate (HR) conducted after a 5-min resting period, participants were instructed on the use of the Borg’s rating of perceived exertion scale (i.e., RPE of 6–20). All blood pressure readings were collected via manual auscultation by a single laboratory staff member for the duration of the test. Participants completed a continuous incremental treadmill exercise test with indirect calorimetry (CareFusion, Vmax CART, Yorba Linda, CA) to assess maximal oxygen consumption (V̇o2max) using standard criteria by our laboratory (26). The treadmill protocol began with an initial standing rest stage that was conducted for 5 min to allow for the assessment of basal oxygen consumption and hemodynamic outcomes. Participants then underwent a warm-up stage marked as the first 2 min of the exercise test where a self-selected speed was chosen. Self-selected speed was advised to illicit a perceived moderate intensity determined once participants achieved ∼50% age-predicted maximal heart rate, and it was then maintained for the duration of the exercise test. Incremental incline increased by 2.5% every 2 min until participants reached V̇o2max. Blood pressure (BP) was measured during the last 30 s of rest (baseline), minute 4 (stage 3), minute 6 (stage 4), and maximal. HR was measured continuously throughout the test using 12-lead ECG up to maximal (HRmax). Rate pressure product (RPP = SBP × HR) and RPE were calculated at each stage. Furthermore, while participants were brought down to a 0% incline and 1.5 mph speed, HR recovery (HRR) was calculated (HRmax – recovery) at 1 and 2 min postexercise to estimate parasympathetic activity.
Body Composition
Participants total body mass (in kg) was measured at each visit via a digital scale determined to the nearest 0.10 kg with participants wearing minimal clothing and no shoes while height (in m) was measured during visit 1 with a stadiometer determined to the nearest 0.10 cm. Body mass index (BMI; kg/m2) was then computed. In addition, waist circumference (WC) was measured 2 cm above the umbilicus using a tape measure to the nearest 0.10 cm twice and averaged. Participants completed assessment of body fat and fat-free mass (FFM) during visit 2 using dual-energy X-ray absorptiometry (Horizon DXA System, Marlborough, MA) following an appropriate 4-h fast of food and beverage, including water, and refraining a minimum 4 h from engaging in physical exercise.
Pulse Waveform Analysis
Aortic waveform and hemodynamic measurements were recorded during visit 3 upon initial arrival to the CRC in the fasted state between 06:00 am and 10:00 am using applanation tonometry via the SphygmoCor XCEL system (AtCor Medical, Itasca, IL). Measurements were collected after 15 min with participants resting quietly in a semi-supine position in a temperature-controlled room (range, 22–23°C). A blood pressure cuff was set on the participants’ upper left arm, and three measures were recorded. Data were averaged to provide augmentation index corrected for standardized heart rate of 75 beats/min (AIx75), augmentation pressure (AP), brachial systolic (bSBP) and diastolic (bDBP) blood pressure, heart rate (HR), central systolic (cSBP) and diastolic (cDBP) blood pressure, brachial (bPP) and central (cPP) pulse pressure, and mean arterial pressure (MAP). Central forward pressure (Pf), backward pressure (Pb), and wave reflection magnitude (Pb/Pf × 100) (27) were characterized through deconvolution analysis. Pulse pressure amplification (PPA) was also calculated as a ratio (bPP/cPP) (28). Blood was then drawn from an intravenous line in the antecubital region for glucose, leptin, nitrate, and nitrite.
Biochemical Analysis
Fasting measures of sodium, chloride, potassium, hemoglobin, and hematocrit were analyzed as surrogates of hydration/blood volume status, and creatinine levels were analyzed to estimate renal function during visit 1 (via LabCorp). Fasted plasma glucose was analyzed instantly after collection by a glucose oxidase assay (YSI Instruments 2700, Yellow Springs, OH), whereas fasted leptin, nitrate, and nitrite whole blood samples were centrifuged at 4°C for 10 min at 3,000 rpm and stored at −80°C until later analysis during visit 3. Plasma leptin was assessed using an enzyme-linked immunosorbent assay (EMD Millipore, Billerica, MA). Plasma nitrate and nitrite were assessed separately using the chemiluminescent technique to determine nitric oxide (NO) bioavailability. Nitrites were measured at room temperature using glacial acetic acid and potassium iodide (KI) to selectively reduce nitrite to nitric oxide (NO) without reduction of higher oxides such as nitrates. For nitrates, vanadium trichloride (VCl3−) in 1 M HCL at 95°C was used to reduce nitrates, as well as lower oxides, i.e., nitrite and all R-(X)-NO species. Standards were prepared using serial dilutions of sodium nitrite/nitrate (Sigma-Aldrich) in Picopure water. Volumes of 20 µL of standards, 50 µL of sample (for nitrite), and 10 µL of sample (for nitrate) were injected into the reaction vessel containing the appropriate reducing agent. Argon gas was used to carry free NO gas from the reaction vessel into the reaction chamber of the Sievers Nitric Oxide Analyzer 280 where ozone (O3) combines with free NO. The reaction of NO and O3 causes proportional emission of light that is detected by the photomultiplier and recorded by the Zysense NOAnalyzer-1.0 software (29). Off-line analysis was conducted using R v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Areas under the curve for standards and samples were calculated using functions from the Miscellaneous Esoteric Statistical Scripts package (30). Sample concentrations were calculated from a regression line from standard concentrations.
Statistical Analysis
Data were analyzed using SPSS (IBM, v.28.0). All data were normally distributed as assessed using interquartile range. Demographics, biochemistries, and aortic waveforms were compared with independent, two-tailed unpaired t tests. A two-way (group × time) repeated-measures ANOVA was used to determine the effects of chronotype on hemodynamic exercise responses. If group × time interaction effects were observed, then Tukey honestly significant difference (HSD) assessment was conducted for post hoc analysis. Since fitness differed between chronotypes, V̇o2max (mL/kg/min) was used as a covariate in the two-way repeated measures (i.e., ANCOVA). Because of technical issues, n = 3 Morn and n = 5 Int pulse wave analysis were not able to be obtained. Cohen’s d effect size was also calculated to assess physiological relevance between groups when completing independent, two-tailed unpaired t tests. Partial η2 effect size was calculated when completing ANOVA and/or ANCOVA tests. Relevance of Cohen’s d was interpreted as small d = 0.2, medium d = 0.5, or large d = 0.8, whereas relevance of partial η2 was interpreted as small η2 = 0.01, medium η2 = 0.06, or large η2 = 0.14, respectively. Pearson correlations were used to assess relationships. Data are means ± SE, and significance was denoted as P ≤ 0.05.
RESULTS
Participant Demographics
There were no significant differences identified between Int and Morn with age, sleep, or indices of drowsiness (Table 1). In addition, there was no significant difference in the timing of exercise testing between groups (Morn, 11:34 ± 0.03 am vs. Int, 11:00 ± 0.03 am min; t test, P = 0.626) despite participants self-selecting appointment timing. Though Int had a higher BMI (P = 0.017), body fat, FFM, and visceral fat were comparable between groups (Table 1). Similarly, no differences in cardiometabolic disease risk factors, electrolytes, hemoglobin, or hematocrit levels were observed between chronotypes (Table 1). Furthermore, no differences were observed between groups with fasted creatinine levels (Morn, 0.800 ± 0.0.03 vs. Int, 0.858 ± 0.04 mg/dL; t test, P = 0.234). However, Int did present with low V̇o2max scaled to body weight or FFM (t test; P = 0.004 and P = 0.005, respectively, Table 1) despite no difference in oxygen consumption across baseline (Morn, 0.821 ± 0.08 vs. Int, 0.809 ± 0.09 L/min), 4 min (Morn, 1.165 ± 0.08 vs. Int, 1.185 ± 0.10 L/min), 6 min (Morn, 1.239 ± 0.04 vs. Int, 1.256 ± 0.09 L/min), or peak (Morn, 2.351 ± 0.10 vs. Int, 2.172 ± 0.12 L/min; interaction effect, P = 0.712). No group differences were observed either for maximal HR, RPE, or RER (Table 1).
Aortic Waveforms and NO Bioavailability
Baseline aortic waveform components were not different between groups (Table 2). However, Int had significantly higher baseline HR than Morn (60.8 ± 1.0 vs. 65.7 ± 1.9 beats/min; P = 0.031). Furthermore, fasting nitrate levels were significantly lower in Int compared with Morn (P = 0.022, Table 1), although no difference was seen with nitrite levels.
Table 2.
Baseline aortic waveforms
| Morning | Intermediate | P Value | Cohen’s d Effect Size | |
|---|---|---|---|---|
| Brachial, mmHg | ||||
| SBP | 132.0 ± 2.3 | 133.4 ± 2.1 | 0.673 | 0.128 |
| DBP | 81.5 ± 1.4 | 79.4 ± 1.8 | 0.386 | 0.276 |
| MAP | 99.9 ± 1.9 | 97.5 ± 1.4 | 0.307 | 0.323 |
| PP | 50.0 ± 1.5 | 53.9 ± 1.6 | 0.087 | 0.529 |
| Central, mmHg | ||||
| SBP | 123.0 ± 2.2 | 122.4 ± 1.9 | 0.846 | 0.059 |
| DBP | 82.5 ± 1.4 | 80.1 ± 1.8 | 0.329 | 0.317 |
| MAP | 96.4 ± 1.7 | 93.5 ± 1.3 | 0.174 | 0.451 |
| PP | 39.9 ± 1.4 | 42.4 ± 1.4 | 0.224 | 0.372 |
| HR, beats/min | 60.8 ± 1.0 | 65.7 ± 1.9 | 0.031 | 0.681 |
| PPA | 1.3 ± 0.01 | 1.3 ± 0.02 | 0.428 | 0.242 |
| AP, mmHg | 12.5 ± 0.8 | 12.6 ± 0.9 | 0.943 | 0.022 |
| AIx75, % | 27.1 ± 2.0 | 26.7 ± 1.7 | 0.881 | 0.044 |
| Pf, mmHg | 27.2 ± 1.3 | 28.3 ± 1.2 | 0.522 | 0.195 |
| Pb, mmHg | 17.9 ± 0.6 | 18.1 ± 0.5 | 0.783 | 0.084 |
Values are means ± SE. Sample size and sex distribution between groups for all aortic waveform data include the following: Morn, n = 20 (16 females); and Int, n = 18 (16 females). AP, augmentation pressure; Aix75, augmentation index; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; Pb, backward wave reflection; Pf, forward wave reflection; PP, pulse pressure; PPA, pulse pressure amplification; SBP, systolic blood pressure. Independent sample t test was used to identify group differences between morning and intermediate chronotype within a subset analysis. Effect size was calculated via Cohen’s d.
Graded Exercise Blood Pressure and Heart Rate
There was no difference in fasting BP between chronotypes. Despite SBP, DBP, and MAP increasing in both groups (time effect, P ≤ 0.001), Int had greater SBP and MAP responses throughout exercise testing (group effect, both P ≤ 0.050) than Morn (Fig. 2, A–C). Nevertheless, covarying for V̇o2max attenuated this DBP (group effect, P = 0.152, η2 = 0.059) and MAP (group effect, P = 0.307, η2 = 0.032) response. Regardless, Morn had a lower submaximal HR response to exercise than Int (interaction effect, P = 0.002, Fig. 2D). Groups HR responses per stages of exercise intensity were as follows: baseline HR (Morn, 77.5 ± 1.9 vs. Int, 78.0 ± 2.3 beats/min), minute 4 HR (Morn, 109.6 ± 1.6 vs. Int, 113.8 ± 2.8 beats/min), minute 6 HR (Morn, 117.6 ± 1.6 vs. Int, 125.2 ± 2.8 beats/min), and HRmax (Morn, 163.6 ± 2.4 vs. Int, 157.3 ± 2.6 beats/min). Specifically, HR at minute 6 was significantly lower in Morn (P = 0.024) than Int, and this observation was independent of fitness (interaction effect, P = 0.029, η2 = 0.076). In addition, Int had a greater RPP with exercise (interaction effect, P = 0.045, Fig. 2E), which occurred primarily at minute 6 of exercise testing (P = 0.009) compared with Morn (Morn, 17,274.9 ± 590.3 vs. Int, 19,844.4 ± 724.3 mmHg × beats/min). Like DBP, covarying for V̇o2max nullified this RPP observation (interaction effect, P = 0.131, η2 = 0.051). Not surprisingly, Int reported higher RPEs (group effect, P = 0.011, η2 = 0.160, Fig. 2F). Interestingly, after covarying for V̇o2max, Int still had higher RPE with submaximal exercise (interaction effect, P = 0.021, η2 = 0.099; and group effect, P = 0.048, η2 = 0.120). This difference was specifically observed at minute 6 of exercise testing (Morn, 11.6 ± 0.3 vs. Int, 12.6 ± 0.3 AU; P = 0.048). No difference was observed in energy expenditure between chronotype, though after covarying for V̇o2max Int had less energy expenditure at maximal efforts only (Morn, 11.9 ± 0.5 vs. Int, 10.7 ± 0.6 kcal/min; P = 0.013; Fig. 2G).
Figure 2.
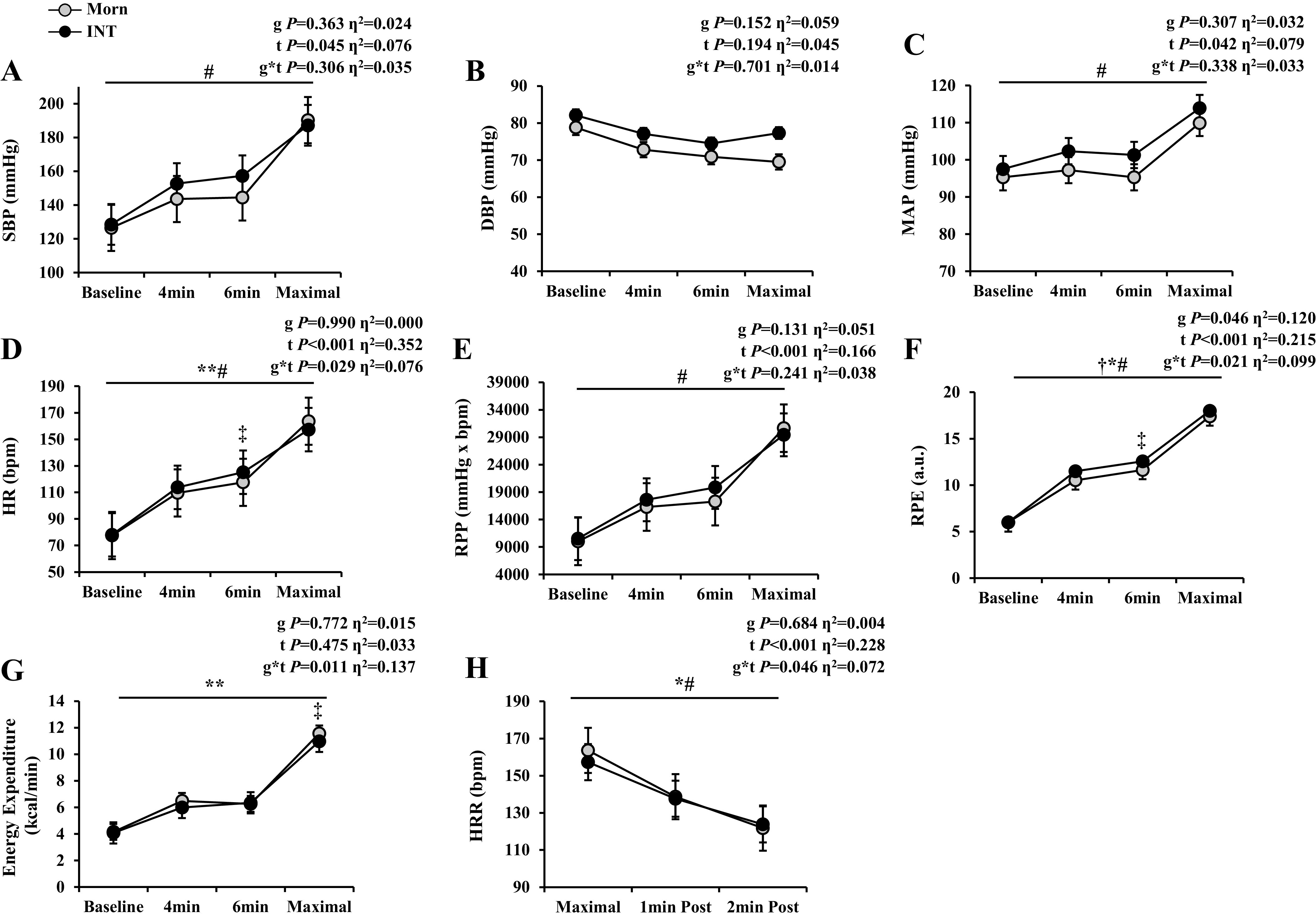
Exercise hemodynamics. Data are represented as means ± SE. Two-way (group × time) ANCOVAs were used to determine the effect of graded exercise testing with fitness as a covariate. A–G: exercise hemodynamics at baseline, minutes 4 and 6, and maximal exertion: systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B), mean arterial pressure (MAP; C), heart rate (HR; D), rate pressure product (RPP; E), rate of perceived exertion via BORG scale (RPE; F), and energy expenditure (G). Sample size and sex distribution between groups for all exercise hemodynamic data include the following: Morn, n = 23 (18 females); Int, n = 23 (19 females). H: heart rate recovery (HRR) after maximal exercise testing and 1 and 2 min postexercise: Morn, n = 22 (18 females); Int, n = 22 (18 females). Significance denoted represents results after covarying for V̇o2max. †P ≤ 0.05, significant group effect. #P ≤ 0.05, significant time effect. *P ≤ 0.05 or **P ≤ 0.01, significant group × time effect. ‡P ≤ 0.05, post hoc analysis. Effect size was calculated via partial η2.
Heart Rate Recovery
Int have a blunted HRR response following 2 min postexercise (interaction effect, P = 0.036, Fig. 2H). Group HR responses are as such per 2 min recovery period: HRR 1 min (Morn, 138.7 ± 3.0 vs. Int, 137.6 ± 3.2 beats/min) and HRR 2 min (Morn, 121.8 ± 2.4 vs. Int, 123.8 ± 3.2 beats/min). Interestingly, Int maintained blunted HRR responses to graded exercise testing even after covarying for fitness (interaction effect, P = 0.046, η2 = 0.072).
Correlations
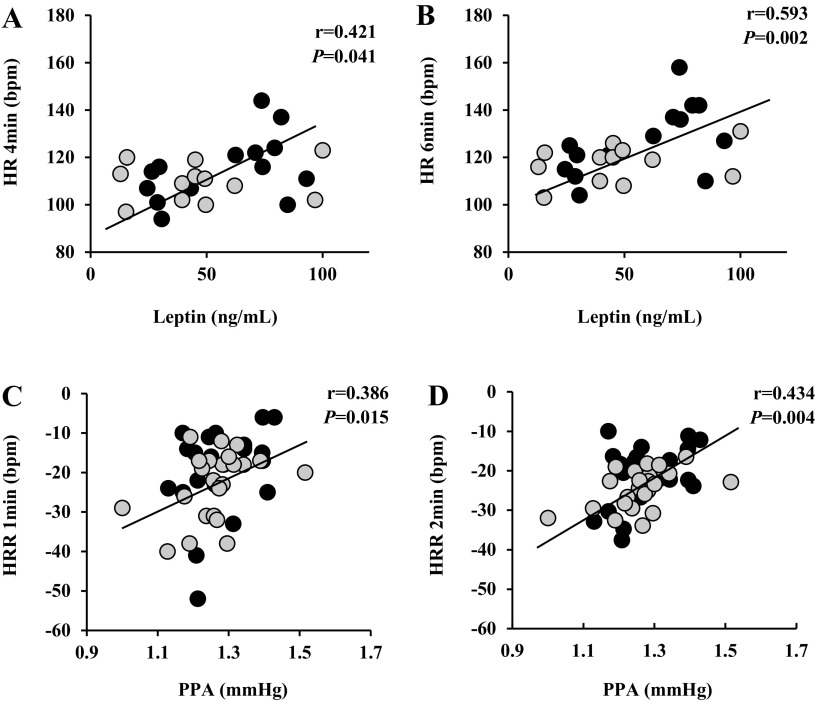
Higher MEQ scores were associated with V̇o2max (r = 0.375, P = 0.019). Although no correlations were noted with aortic waveforms or nitrite metabolism and submaximal hemodynamic responses to exercise, fasting leptin correlated with submaximal HR responses (fasting leptin vs. stage 3 HR, r = 0.421, P = 0.041; and stage 4 HR, r = 0.593, P = 0.002) (Fig. 3, A and B). Interestingly, greater HRmax is positively associated with fasted nitrate levels (r = 0.447, P = 0.015). Furthermore, blunted HRRs at 1-min (r = 0.386, P = 0.015; Fig. 3C) and 2-min postexercise (r = 0.434, P = 0.004; Fig. 3D) were both associated with greater basal PPA.
Figure 3.
Correlations of submaximal heart rate responses to exercise and heart rate recovery (HRR) at 1- and 2-min postexercise between fasting plasma leptin and aortic waveform components of pulse pressure amplification (PPA). A–D: heart rate (HR) 4 min vs. fasting leptin (A), HR 6 min vs. fasting leptin (B), HRR 1 min vs. PPA (C), and HRR 2 min vs. PPA (D). Sample size and sex distribution between groups within a subset analysis for correlations of leptin: Morn, n = 12 (10 females); Int, n = 14 (13 females); and PPA [Morn, n = 23 (18 females); Int, n = 21 (17 females)]. Black circles, intermediate chronotypes; gray circles, morning chronotypes.
DISCUSSION
It is reasonable to anticipate that chronotypes may interact with exercise on hemodynamic exercise responses given body temperature and urinary catecholamine matches chronotype preference (21). The present study demonstrated in people with obesity that Int chronotypes have exaggerated BP and HR responses to incremental exercise when compared with Morn chronotypes. However, the differences in aerobic fitness status may underline these BP responses since covarying for V̇o2max nullified statistical significance between chronotypes. Independent of fitness, however, HR remained lower in Morn compared with Int counterparts during exercise, as were HRR responses. This highlights that Morn chronotypes experience lower HR during submaximal exercise and quicker recovery from maximal effort than Int chronotypes. Furthermore, Morn chronotype self-report lower RPE independent of fitness status, which follows lower observed HR and suggests less-perceived effort to exercise. Interestingly, our findings are somewhat in contrast with prior work in young healthy men of early chronotypes status who presented with elevated HR during morning handgrip stress testing compared with evening chronotypes (21). It is difficult to reconcile such differences between this prior work and the current study since the age of the individuals (young adult vs. middle-aged/older), body mass (lean vs. obese), and/or exercise modality (strength vs. aerobic) could impact observations. In either case, the present study adds to the scope of literature showcasing Int chronotype with obesity has a differential hemodynamic and stress response to submaximal exercise, and this may help with exercise considerations for CVD risk management.
There are several potential explanations for why Int chronotypes experienced differential BP and HR responses. Low cardiorespiratory fitness is independently associated with increased CVD incidents and all-cause mortality (31). Greater aerobic fitness is associated with vascular function via reduced vascular resistance, as well as improved vasodilation promoting enhanced blood flow to support attenuated BP responses to incremental exercise (32). Observational data from our group (33) and others (14) suggest that Morn chronotypes engage in more physical activity and less sedentary behavior throughout the day. Not surprisingly, then, we have shown Morn chronotypes tend to have higher aerobic fitness. This suggests either greater cardiac output and/or oxygen extraction may influence hemodynamic responses to exercise (34). Indeed, once blood pressure was covaried for fitness in the current study, we note no difference in BP between chronotypes across exercise intensities. This indicates that the exaggerated BP in Int is likely related to abilities to either deliver or use oxygen. Consistent with the hypothesis of low oxygen use is the finding that later chronotypes in our previous work relied more on carbohydrate than early chronotypes at rest and submaximal exercise (33). Here, we observed no submaximal oxygen consumption differences during the incremental exercise test. Thus, we expand on our prior exercise work (33) and show that HR remained low independent of fitness in Morn compared with Int chronotype. Given that cardiac output is the product of stroke volume and HR, our work suggests chronotype may have unique effects on HR regulation. The precise explanation for how chronotype influences the regulation of HR is beyond the scope of this work, but neural control is one possibility. Indeed, exercise raises sympathetic neural activation to raise cardiac output through rises in HR for adequate blood perfusion and oxygen supply to working tissues (35). This is simultaneously matched with what is understood as parasympathetic withdrawal initiated by exercise, therefore further increasing sympathetic stimulation relative to exercise intensity (36). Indeed, parasympathetic withdrawal may mediate a rapid increase in HR through higher brain centers and arterial baroreflex reset allowing for a greater buffering response and higher operating point to accommodate the increased exercise stimuli (36). This is of interest as Int responded to exercise with higher HR responses compared with Morn despite no statistical differences and low effect size in maximal HR. In line with this, HRR is often used as an indirect estimate of parasympathetic activity. In the current work, Morn chronotypes demonstrated quicker HRR than Int, thereby suggesting parasympathetic reactivation (36). HRR positively correlated with PPA in the present work, too. Taken together, our work highlights that Int chronotypes may have increased cardiac workload relative to peripheral regulation (37), through in part, impaired vagal tone (38) and/or elevated waveform reflections/arterial stiffness (39).
Another possibility explaining exercise HR differences independent of fitness between Morn and Int is obesity (40). In particular, increased adiposity is associated with progression of inflammation and endothelial dysfunction in the development of arterial stiffness (41). If Int chronotype presented with more body fat, it would be reasonable to ascribe it to HR differences than to adiposity. However, the present study did not observe differences between chronotype in total body fat or visceral adiposity. This implies that fat mass in people with obesity is not likely an explanatory factor of exercise HR responses between Int and Morn types. Secretion of inflammatory markers from adipose tissue (i.e., adipokines) though could still be relevant. Of interest, people with Int had elevated fasting levels of leptin, and we observed that fasting leptin correlated with submaximal HR responses. Since leptin resistance is associated with increased risk of arterial stiffness (42, 43), our work suggests adipose function among Int chronotypes may be a potential mechanism of HR dysregulation with graded exercise. It is important to acknowledge leptin exhibits diurnal variation and prior work suggests later chronotypes have higher leptin in the morning than earlier chronotypes in relation to insulin resistance (44), which is consistent with our prior work (12). As such, leptin (and/or insulin) may have contributed to higher sympathetic and lower parasympathetic activity (45) such that it influenced exercise hemodynamics.
Nitrate and nitrite are precursor molecules that can be converted in tissues to nitric oxide. This is relevant as nitric oxide is thought to be the primary stimulator of vasodilation that can reduce vascular resistance and promote oxygen extraction for transformation of energy liberation. Exercise promotes vasodilation and a decline in systemic vascular resistance, in part, by raising endothelial shear stress activation of the PI3K pathway for nitric oxide production (46, 47). Interestingly, Int presented with reduced resting plasma nitrate as well as had moderate effect sizes for nitrite concentrations. Why Int had lower nitrate and nitrite levels is difficult to address within the current design, but it likely has to do with either less availability or storage. Regarding availability, dietary sources of nitrate (e.g., beets, green leafy vegetables) interact not only in general circulation to raise nitric oxide bioavailability but also through oral bacteria via digestion to raise nitrite levels, which, in turn, is acted upon by mammalian nitrite reductase in the stomach to form nitric oxide (48). In our study, participants were provided with a 24-h standardized diet consisting of mixed meal (55% CHO, 15% PRO, 30% FAT) before the overnight fasted blood draw and aortic waveform measures, thus limiting variability of nitrate/nitrite dense foods between groups. Since dietary nitrate stores within skeletal muscle (i.e., reservoir) are considered important for whole body nitric oxide bioavailability at rest and during exercise (49, 50), we interpret the present work to suggest that chronotype may influence the conversion and/or storage of nitrate for optimal endothelial function. This could have relevance as the lower nitrate and/or nitrite might contribute to reduced vasomotor tone of blood pressure during exercise and lower muscle oxygen extraction. Although Int chronotype did not experience less energy expenditure than Morn chronotypes at submaximal aerobic efforts in this protocol, alterations in oxygen extraction could help explain our previous finding of reduced fat oxidation in later versus early chronotypes (33).
Limitations
The present study has limitations. Although study participants were asked to schedule exercise testing sessions within the hours of 6:00 am and 10:00 am, testing ranged up to 12:00 pm to accommodate participant scheduling conflicts and/or adjustments in laboratory availability. However, individuals self-selected their exercise testing time, and there was no difference in exercise time between groups. Although this likely minimized potential physiological/psychological stress, further investigation into exercise testing alignment with chronotype awaits to be seen. Furthermore, it is unknown how these individuals would perform if tested during afternoon/evening time of day. Heart rate variability (HRV) is an effective noninvasive method of assessment of the autonomic nervous system and vagal tone (51), as it reflects the variance from beat to beat of the heart across a specific time period. The present study uses HRR as a surrogate measure of parasympathetic activity, though it should be noted that previous work has demonstrated this approach relates to cardiac vagal tone and is a predictor of cardiovascular morbidity/mortality independent of exercise workload (52). Endogenous nitric oxide production is modulated by peripheral clock genes, suggesting a diurnal variation of nitric oxide bioavailability (53). This diurnal variation may present with a direct influence over morning BP responses, as nitric oxide bioavailability is generally lower during earlier morning hours and progressively increases throughout the day in relation to physical activity/dietary consumption of nitrate-based foods. The Epworth and PSQI sleep history questionnaires were provided to participants to assess habitual sleep habits, as sleep has transient effects on endothelial function and blood pressure regulation (54). Though habitual sleep patterns were not different between groups (Table 1), acute sleep patterns were not collected the night before exercise testing. Finally, menstrual status has been noted as a prominent influence on cardiovascular response to graded exercise (55) with postmenopausal women experiencing greater BP responses to increasing exercise intensity when compared with women who are eumenorrheic. However, in our study, there were equal numbers of post-, peri-, and premenopausal women between Morn and Int groups. This indicates menses status is unlikely to have impacted our conclusions regarding chronotype difference. Dehydration has been indicated as a potential propagator of increased heart rate responses to graded exercise (56). However, we performed clinical analysis of electrolytes and proteins reflective of dehydration and interpreted these results to suggest hydration status is unlikely to explain the observed chronotype differences in hemodynamic responses to exercise.
Conclusion
In conclusion, individuals with obesity who self-identify as Int chronotype had exaggerated blood pressure responses to incrementally graded exercise than Morn chronotypes. However, controlling for V̇o2max abolished statistical differences in blood pressure, suggesting that fitness is a factor influencing blood pressure regulation. Int though had higher HR responses to submaximal exercise and a blunted HRR following exercise independent of aerobic fitness. This finding highlights potentially altered sympathovagal balance in Int compared with Morn counterparts. Future work should further examine the mechanism(s) by which circadian rhythm/alignment influences vascular responses to exercise across chronotypes to enhance CVD risk reduction.
DATA AVAILABILITY
These data have not been made publicly available. However, the corresponding author can provide further information on the data upon reasonable request.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant RO1-HL130296 (to S.K.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.M. conceived and designed research; M.-M.E.R., J.K.D., and S.K.M. performed experiments; M.-M.E.R. analyzed data; M.-M.E.R. and S.K.M. interpreted results of experiments; M.-M.E.R. prepared figures; M.-M.E.R. drafted manuscript; M.-M.E.R., J.K.D., G.P., A.J.G., A.M.S., and S.K.M. edited and revised manuscript; S.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research assistants of the Applied Metabolism and Physiology Laboratory for their work and all participants for their efforts. We thank Dr. Eugene J. Barrett for medical oversight, the nursing staff of the Clinical Research Center for technical assistance, and Lisa Farr from the Exercise Physiology Core Laboratory for fitness testing.
REFERENCES
- 1. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension 75: 285–292, 2020. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliveros E, Patel H, Kyung S, Fugar S, Goldberg A, Madan N, Williams KA. Hypertension in older adults: assessment, management, and challenges. Clin Cardiol 43: 99–107, 2020. doi: 10.1002/clc.23303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Facts About Hypertension | cdc.gov. Centers for Disease Control and Prevention (Online). Published October 14, 2022. https://www.cdc.gov/bloodpressure/facts.htm.
- 4. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 122: 1–7, 2017. doi: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 5. Tzemos N, Lim PO, Mackenzie IS, MacDonald TM. Exaggerated exercise blood pressure response and future cardiovascular disease. J Clin Hypertens (Greenwich) 17: 837–844, 2015. doi: 10.1111/jch.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz MG, Picone DS, Nikolic SB, Williams AD, Sharman JE. Exaggerated blood pressure response to early stages of exercise stress testing and presence of hypertension. J Sci Med Sport 19: 1039–1042, 2016. doi: 10.1016/j.jsams.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 7. Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high-normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high-normal blood pressure. J Am Coll Cardiol 36: 1626–1631, 2000. doi: 10.1016/s0735-1097(00)00903-7. [DOI] [PubMed] [Google Scholar]
- 8. Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol 51: 29–35, 1998. doi: 10.1016/s0895-4356(97)00223-0. [DOI] [PubMed] [Google Scholar]
- 9. Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 121: 2109–2116, 2010. doi: 10.1161/CIRCULATIONAHA.109.895292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makarem N, Paul J, Giardina EGV, Liao M, Aggarwal B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol Int 37: 673–685, 2020. doi: 10.1080/07420528.2020.1732403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruiz-Lozano T, Vidal J, de Hollanda A, Canteras M, Garaulet M, Izquierdo-Pulido M. Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int J Obes (Lond) 40: 1550–1557, 2016. doi: 10.1038/ijo.2016.116. [DOI] [PubMed] [Google Scholar]
- 12. Remchak ME, Heiston EM, Ballantyne A, Dotson BL, Stewart NR, Spaeth AM, Malin SK. Insulin sensitivity and metabolic flexibility parallel plasma TCA Levels in early chronotype with metabolic syndrome. J Clin Endocrinol Metab 107: e3487–e3496, 2022. doi: 10.1210/clinem/dgac233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Remchak MME, Heiston EM, Ballantyne A, Dotson BL, Malin SK. Aortic waveform responses to insulin in late versus early chronotype with metabolic syndrome. Physiol Rep 10: e15473, 2022. doi: 10.14814/phy2.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nauha L, Jurvelin H, Ala-Mursula L, Niemelä M, Jämsä T, Kangas M, Korpelainen R. Chronotypes and objectively measured physical activity and sedentary time at midlife. Scand J Med Sci Sports 30: 1930–1938, 2020. doi: 10.1111/sms.13753. [DOI] [PubMed] [Google Scholar]
- 15. Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, Csako G, Cizza G; Sleep Extension Study Group. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One 8: e56519, 2013. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maukonen M, Kanerva N, Partonen T, Kronholm E, Konttinen H, Wennman H, Männistö S. The associations between chronotype, a healthy diet and obesity. Chronobiol Int 33: 972–981, 2016. doi: 10.1080/07420528.2016.1183022. [DOI] [PubMed] [Google Scholar]
- 17. Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—influence of age and sex. PLoS One 12: e0178782, 2017. doi: 10.1371/journal.pone.0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montaruli A, Castelli L, Mulè A, Scurati R, Esposito F, Galasso L, Roveda E. Biological rhythm and chronotype: new perspectives in health. Biomolecules 11: 487, 2021. doi: 10.3390/biom11040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chronotypes. Sleep Foundation (Online). Published January 8, 2021. https://www.sleepfoundation.org/how-sleep-works/chronotypes [2022 Nov 23].
- 20. Ryuzaki M, Morimoto S, Niiyama M, Seki Y, Yoshida N, Oshima Y, Mizuguchi Y, Watanabe D, Ando T, Ichihara A. The relationships between the differences in the central blood pressure and brachial blood pressure and other factors in patients with essential hypertension. Intern Med 56: 587–596, 2017. doi: 10.2169/internalmedicine.56.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nebel LE, Howell RH, Krantz DS, Falconer JJ, Gottdiener JS, Gabbay FH. The circadian variation of cardiovascular stress levels and reactivity: relationship to individual differences in morningness/eveningness. Psychophysiology 33: 273–281, 1996. doi: 10.1111/j.1469-8986.1996.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 22. White WB. Importance of blood pressure control over a 24-hour period. J Manag Care Pharm 13: 34–39, 2007. doi: 10.18553/jmcp.2007.13.s8-b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crook S, Sievi NA, Bloch KE, Stradling JR, Frei A, Puhan MA, Kohler M. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax 74: 390–396, 2019. doi: 10.1136/thoraxjnl-2018-211959. [DOI] [PubMed] [Google Scholar]
- 24. Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep 29: 112–116, 2006. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 25. Katzmarzyk PT, Powell KE, Jakicic JM, Troiano RP, Piercy K, Tennant B; 2018 Physical Activity Guidelines Advisory Committee*. Sedentary behavior and health: update from the 2018 Physical Activity Guidelines Advisory Committee. Med Sci Sports Exerc 51: 1227–1241, 2018. doi: 10.1249/MSS.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dotson BL, Heiston EM, Miller SL, Malin SK. Insulin stimulation reduces aortic wave reflection in adults with metabolic syndrome. Am J Physiol Heart Circ Physiol 320: H2305–H2312, 2021. doi: 10.1152/ajpheart.00975.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone. Hypertension 48: 595–601, 2006. doi: 10.1161/01.HYP.0000238330.08894.17. [DOI] [PubMed] [Google Scholar]
- 28. Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, Salvi P, Smulyan H, Safar ME. Pulse pressure amplification: a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol 55: 1032–1037, 2010. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 29. Nagababu E, Rifkind JM. Measurement of plasma nitrite by chemiluminescence. In: Methods in Molecular Biology: Free Radicals and Antioxidant Protocols, edited by Uppu RM, Murthy SN, Pryor WA, Parinandi NL.. Totowa, NJ: Humana Press, 2010, p. 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ekstrom CT. MESS: Miscellaneous Esoteric Statistical Scripts version 0.5.9 from CRAN. MESS: Miscellaneous Esoteric Statistical Scripts. R package version 0.5.6 (Online). Published 2019. https://CRAN.R-project.org/package=MESS.
- 31. Després JP. Physical activity, sedentary behaviours, and cardiovascular health: when will cardiorespiratory fitness become a vital sign? Can J Cardiol 32: 505–513, 2016. doi: 10.1016/j.cjca.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 32. Fagard R. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol 33: 853–856, 2006. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- 33. Malin SK, Remchak MME, Smith AJ, Ragland TJ, Heiston EM, Cheema U. Early chronotype with metabolic syndrome favours resting and exercise fat oxidation in relation to insulin-stimulated non-oxidative glucose disposal. Exp Physiol 107: 1255–1264, 2022. doi: 10.1113/EP090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee D-C, Earnest CP, Church TS, O'Keefe JH, Milani RV, Blair SN. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 117: 207–219, 2015. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol (1985) 97: 731–738, 2004. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 36. Michael S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front Physiol 8: 301, 2017. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension. Hypertension 54: 375–383, 2009. [Erratum in Hypertension 58: e30, 2011]. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 38. Eleftheriadou I, Drosos GC, Tentolouris A, Konstantonis G, Sfikakis PP, Protogerou AD, Tentolouris N. Pulse pressure amplification and cardiac autonomic dysfunction in patients with type 2 diabetes mellitus. J Hum Hypertens 32: 531–539, 2018. doi: 10.1038/s41371-018-0070-1. [DOI] [PubMed] [Google Scholar]
- 39. Pichler G, Martinez F, Vicente A, Solaz E, Calaforra O, Redon J. Pulse pressure amplification and its determinants. Blood Press 25: 21–27, 2016. doi: 10.3109/08037051.2015.1090713. [DOI] [PubMed] [Google Scholar]
- 40. Shariq OA, McKenzie TJ. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg 9: 80–93, 2020. doi: 10.21037/gs.2019.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leopold JA. Cellular and molecular mechanisms of arterial stiffness associated with obesity. Hypertension 62: 1003–1004, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D'Elia L, Giaquinto A, De Luca F, Strazzullo P, Galletti F. Relationship between circulating leptin levels and arterial stiffness: a systematic review and meta-analysis of observational studies. High Blood Press Cardiovasc Prev 27: 505–513, 2020. doi: 10.1007/s40292-020-00404-y. [DOI] [PubMed] [Google Scholar]
- 43. D'Elia L, Giaquinto A, Iacone R, Russo O, Strazzullo P, Galletti F. Serum leptin is associated with increased pulse pressure and the development of arterial stiffening in adult men: results of an eight-year follow-up study. Hypertens Res 44: 1444–1450, 2021. doi: 10.1038/s41440-021-00718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baldanzi G, Hammar U, Fall T, Lindberg E, Lind L, Elmståhl S, Theorell-Haglöw J. Evening chronotype is associated with elevated biomarkers of cardiometabolic risk in the EpiHealth cohort: a cross-sectional study. Sleep 45: zsab226, 2022. doi: 10.1093/sleep/zsab226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russo B, Menduni M, Borboni P, Picconi F, Frontoni S. Autonomic nervous system in obesity and insulin-resistance—the complex interplay between leptin and central nervous system. Int J Mol Sci 22: 5187, 2021. doi: 10.3390/ijms22105187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakellariou XM, Papafaklis MI, Domouzoglou EM, Katsouras CS, Michalis LK, Naka KK. Exercise-mediated adaptations in vascular function and structure: beneficial effects in coronary artery disease. World J Cardiol 13: 399–415, 2021. doi: 10.4330/wjc.v13.i9.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kolluru GK, Sinha S, Majumder S, Muley A, Siamwala JH, Gupta R, Chatterjee S. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of eNOS: a basis for shear stress mediated angiogenesis. Nitric Oxide 22: 304–315, 2010. doi: 10.1016/j.niox.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 48. Keller RM, Beaver L, Prater MC, Hord NG. Dietary nitrate and nitrite concentrations in food patterns and dietary supplements. Nutr Today 55: 218–226, 2020. doi: 10.1097/NT.0000000000000253. [DOI] [Google Scholar]
- 49. Kadach S, Piknova B, Black MI, Park JW, Wylie LJ, Stoyanov Z, Thomas SM, McMahon NF, Vanhatalo A, Schechter AN, Jones AM. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 121: 1–10, 2022. doi: 10.1016/j.niox.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radic Biol Med 38: 1164–1169, 2005. doi: 10.1016/j.freeradbiomed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 51. Tiwari R, Kumar R, Malik S, Raj T, Kumar P. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev 17: e160721189770, 2021. doi: 10.2174/1573403X16999201231203854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gourine AV, Ackland GL. Cardiac vagus and exercise. Physiology (Bethesda) 34: 71–80, 2019. doi: 10.1152/physiol.00041.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Man AWC, Li H, Xia N. Circadian rhythm: potential therapeutic target for atherosclerosis and thrombosis. Int J Mol Sci 22: 676, 2021. doi: 10.3390/ijms22020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cherubini JM, Cheng JL, Williams JS, MacDonald MJ. Sleep deprivation and endothelial function: reconciling seminal evidence with recent perspectives. Am J Physiol Heart Circ Physiol 320: H29–H35, 2021. doi: 10.1152/ajpheart.00607.2020. [DOI] [PubMed] [Google Scholar]
- 55. Park J, Kim MS, Nho H, Kim KA, Kim JK, Choi HM. The effect of cardiovascular responses after aerobic exercise in menstrual cycle. Health (NY) 9: 425–434, 2017. doi: 10.4236/health.2017.93030. [DOI] [Google Scholar]
- 56. Barr SI. Effects of dehydration on exercise performance. Can J Appl Physiol 24: 164–172, 1999. doi: 10.1139/h99-014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data have not been made publicly available. However, the corresponding author can provide further information on the data upon reasonable request.