Abstract
Background: The aim of this study was to explore the combined efficacy ofeffective-component compatibility of Bufei Yishen formula III (ECC-BYF III) and exercise rehabilitation (ER) in inhibiting airway mucus hypersecretion in a chronic obstructive pulmonary disease (COPD) rat model.
Methods: A total of 48 SD rats were divided into control, model, acetylcysteine (NAC), ECC-BYF III, ER, and ECC-BYF III + ER groups (n=8). COPD rats were exposed to cigarette smoke and bacteria for 8 weeks and administered various treatments over the next eight weeks. Rats were euthanized at week 17 after pulmonary function testing. Pathological examination of lung tissues was performed. IL-6 and IL-10 levels were measured in bronchoalveolar lavage fluid (BALF) and protein levels of MUC5AC, MUC5B, AQP-5, EGFR, ERK, JNK, and p38 were measured in lung tissues.
Results: Improved pulmonary function and pathological changes were observed in ECC-BYF III, ECC-BYF III + ER, and NAC groups. ECC-BYF III and ECC-BYF III + ER had greater mean alveolar number (MAN) compared with NAC. Lung inflammation and goblet cell generation were reduced and MUC5AC, MUC5B and AQP-5 expressions were lower in all treatment groups. ECC-BYF III has more significant effect on MUC5AC than ER and NAC. ECC-BYFIII + ER had a greater effect on suppressing IL-6 in BALF compared with other treatments. ECC-BYFIII, ER, and ECC-BYF III + ER reduced EGFR, ERK, JNK, and p38 phosphorylated protein levels. ECC-BYFIII+ER had a greater effect on p-JNK and p-p38 than ECC-BYFIII and NAC.
Conclusion: ECC-BYF III, ER, and ECC-BYF III + ER have efficacy in inhibiting airway mucus hypersecretion with improved pulmonary function and pathological changes. ECC-BYF III had a greater effect in improving MAN and MUC5AC in lung tissue. ECC-BYF III+ER had a greater effect in alleviating pulmonary pathology and inflammation. These effects may be mediated by inhibition of the EGFR/MAPK pathway.
Keywords: Airway mucus hypersecretion, Chinese medicine, Chronic obstructive pulmonary disease, Exercise Rehabilitation
Introduction
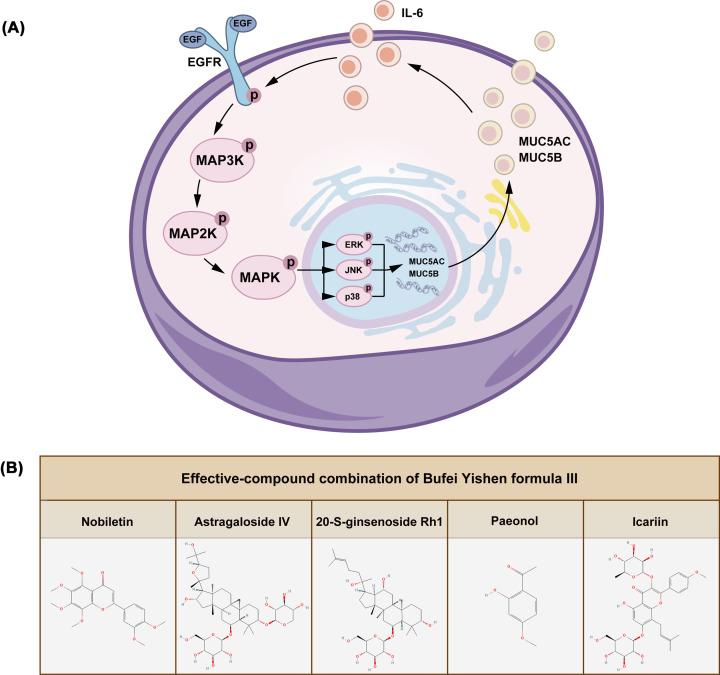
Chronic obstructive pulmonary disease (COPD) is characterized by persistent respiratory symptoms and airflow limitation [1] and represents a serious hazard to public health. Accordingly, there is an urgent need for novel clinical treatments due to the high morbidity and mortality of COPD [2]. Airway mucus hypersecretion (AMH) is an independent risk factor and contributes to the pathophysiology of COPD. COPD is primarily caused by an increase in goblet cells and the enlargement of submucosal glands. Oxidative stress and inflammation, which are caused by cigarette smoke (CS) and harmful substances, lead to airway epithelial injury, mucus hypersecretion, thereby leading to chronic cough and bronchitis. Excessive mucus blockage of airways can lead to airflow restriction and dyspnea, further exacerbating COPD [3,4]. Therefore, the inhibition of mucus hypersecretion and the promotion of effective sputum excretion represent effective strategies for reducing acute exacerbations of COPD and delaying disease progression. EGFR/MAPK signaling is involved in regulating airway mucus secretion and increasing airway goblet cell hyperplasia and mucus hypersecretion [5]. The synthesis of MUC5AC is regulated by the EGFR signaling pathway, with the EGFR pathway known to be activated by direct damage to airway epithelial cells by oxidants and arachidonic acid metabolites in smoke [6]. A variety of ligands have been shown to bind to EGFR, promote EGFR phosphorylation, and activate multiple downstream pathways including the MAPK pathway with corresponding biological effects. ERK, JNK, and p38, as the key mediators of the MAPK pathway, are closely related to AMH (Figure 1A) [7]. Accordingly, inhibition of the EGFR/MAPK pathway represents a major therapeutic target for inhibiting mucus hypersecretion.
Figure 1. EGFR/MAPK mechanism and chemical structures of ECC-BYF III.
(A) Schematic diagram of EGFR/MAPK. (B) The chemical structure of the five compounds in ECC-BYF III.
Mucolytics are the most important drugs used to manage AMH and its sequelae including COPD and bronchiectasis and function by enhancing the clearance of the mucus layer of the respiratory tract [1,8]. Mucolytics including N-acetylcysteine, carbocysteine, erdosteine, and dornase alfa have antioxidative and anti-inflammatory properties in addition to the mucolytic action described above. However, many mucolytics are associated with adverse effects such as vomiting, diarrhea, laryngitis, gastric ulceration, and chest pain. Further, overdose of mucolytics can lead to thrombocytopenia, hemolysis, and acute renal failure [4]. In recent years, Traditional Chinese Medicine (TCM) is playing an increasingly important role in treating AMH and COPD due to efficacy in alleviating symptoms, reducing the frequency of acute exacerbations frequency, and improving overall health [9,10]. Our previous study demonstrated that Bufei Yishen formula (BYF; patent ZL.201110117578.1) can restore protease/antiprotease and reduce oxidative stress and airway inflammation, thereby improving the clinical symptoms and quality of life of patients with COPD and reducing the number of acute exacerbations [11,12]. Effective-component compatibility of Bufei Yishen formula I (ECC-BYF I) and effective-component compatibility of ECC-BYF II (ECC-BYF II) were developed following network pharmacologic analysis of the active components of BYF, in vitro experiments involving multiple cell models, and in vivo verification and optimization [13,14]. ECC-BYF III was obtained by further optimization with the addition of astragaloside IV and 20-S-ginsenoside Rh1 among other components. ECC-BYF III has a simple composition with same efficacy and safety as BYF [15]. ECC-BYF III has been shown to have efficacy in a rat model of COPD by enhancing pulmonary function, reducing oxidative stress, inflammation, and mucin secretion [15,16]; however, the mechanisms underlying the effects of ECC-BYF III on AMH and COPD remains unclear.
Pulmonary rehabilitation exercise and acupuncture have achieved good results in clinical studies of COPD [17,18]. Exercise rehabilitation (ER) represents an effective means of disease treatment and rehabilitation and is an important component of pulmonary rehabilitation programs [1]. Proper exercise can effectively improve pulmonary function and respiratory muscle endurance in patients with COPD, thereby improving dyspnea and exercise tolerance and reducing hospital admissions and mortality [19,20]; however, the mechanisms underlying the effects of ER remain unclear. Herein, we evaluated the efficacy of ECC-BYF III and ER in inhibiting AMH in a rat model of COPD and evaluated the mechanisms underlying these effects with a particular focus on the EGFR/MAPK signaling pathway.
Materials and methods
Animal husbandry
Sprague–Dawley (SD) rats aged 6–7 weeks and weighing 220 ± 20 g were procured from Beijing Vital River Laboratory Animal Technology Co., Ltd (Special Pathogen Free, No. 110011211105823815). All animal experiments took place at Henan University of Chinese Medicine. The procedures of the present study were approved by the Experimental Animal Care and Ethics Committees of the First Affiliated Hospital of Henan University of Chinese Medicine with the ethical review approval number YFYDW2019031. Rats were killed by intraperitoneal injection of 2% pentobarbital sodium at 40 mg/kg.
Drug preparation
ECC-BYF III is composed of astragaloside IV and 20-S-ginsenoside Rh1, etc. (Chengdu Must BioTech Co., Ltd., Chengdu, China) (Figure 1B). The purity of these compounds was verified to be more than 98% by high-performance liquid chromatography. The acetylcysteine (NAC) is a kind of effective mucolytic recommended by Global Initiative for Chronic Obstructive Lung Disease (GOLD). So, we chose NAC (Zambon S.p.A, Italia; 600 mg/tablet) as the control drug.
Establishment of a rat model of COPD
A total of 48 SD rats were housed under standardized environment conditions and given food and water ad libitum for 7 days, then were randomly assigned to six experimental groups as follows: normal control group, disease model group, ECC-BYF III group, ER group, ECC-BYF III + ER group, and the NAC group (n=8 for each). The COPD rat model was established by exposing rats to CS (Hongqi Canal® filter cigarettes; Henan Tobacco Industry, Zhengzhou, China) and Klebsiella pneumoniae (KP) (0.1 ml, 6 × 108 CFU/ml; ID: 46,117-5a1; National Centre for Medical Culture Collection, Beijing, China) [21]. From weeks 1 to 8, SD rats were exposed to CS at a concentration of 3000 ± 500 ppm for 40 min twice a day in all groups except the control group. KP solution was dropped into alternating nostrils once every 5 days. Normal saline was used instead of bacterial solution in the normal control group.
From weeks 9 to 16, rats in the normal control and disease model groups were intragastrically administered 10 ml/kg sodium carboxymethyl cellulose. Rats in the NAC group were administered 54 mg/kg/d NAC suspension. Rats in the ECC-BYF III group were administered 5.5 mg/kg/d ECC-BYF III. Rats in the ER group were provided exercise by running at 8 m per min on an experimental treadmill for 20 min per day. The experimental schema was validated in a preliminary experiment (Supplementary Material S1). Rats in the ECC-BYF III + ER group were treated with ECC-BYF III combined with exercise using the same drug dosage and exercise regimen as provided to the ECC-BYF III and ER groups, respectively. The COPD rat model was evaluated according to symptoms, lung function, and pulmonary pathology.
Dosages of NAC were calculated using the following formula [22,23]:
Where D is the dose and K is the body shape index defined as K = A/W2/3 where A is the surface area in m2 and W is the weight in kg.
At week 17, rats were killed by intraperitoneal injection of 2% pentobarbital sodium at 40 mg/kg.
Pulmonary function tests
Peak expiratory flow (PEF) and expiratory flow at 50% tidal volume (EF50) were measured every 4 weeks from week 0 to week 16 using whole-body plethysmography (Buxco, NC, U.S.A.).
Lung and bronchus tissue morphology
Lung tissues were cut into slices of 4 mm thickness and fixed with 4% paraformaldehyde with conventional dehydration and embedding in paraffin. Sections were stained with hematoxylin and eosin or Alcian Blue/periodic acid-Schiff (AB-PAS). Images were taken using an optical microscope (Olympus, Japan) and used to calculate the mean linear intercept (MLI), mean alveolar number (MAN) and airway wall thickness.
Under microscopy (×200), six visual field were taken in each slice, and the alveolar number and the linear intercept in a fixed area of visual field were measured. MAN (/mm2) = Na/A. Na is the number of pulmonary alveoli in each visual field. A is the area of the visual field. Then, we made a cross(+) under the visual field and counted the number of alveolar septum on the cross. MLI (μm) = L/Ns. Ns is the number of alveolar septum. L is total length of the cross. Under microscopy (×200), three visual field which include airway (100–300 μm in diameter) were taken in each slice, and select three internal diameters and corresponding external diameters of the airways, respectively, subtract and average value, then divide by 2, and this means the airway wall thickness.
Immunohistochemistry staining
Six slices of lung tissues in each group were immunostained with a MUC5AC polyclonal antibody (E-AB-40037, 1:200, Elabscience) or a MUC5B polyclonal antibody (E-AB-15988, 1:500, Elabscience) and imaged under a microscope at 200 times magnification. Integral optical density was determined using IPP 6.0 software.
Immunofluorescence
Lung slices were incubated with an AQP-5 antibody (sc-74402, 1:1000, Santa Cruz Biotechnology) overnight at 4°C. Slices were then incubated with secondary antibody (Goat Anti-Mouse IgG (H + L) Fluor594-conjugated, S0005, 1:1000, Affinity) for 20 min and counterstained with DAPI for 10 min.
Measurements of IL-6 and IL-10 in bronchoalveolar lavage fluid
For bronchoalveolar lavage, 3 ml of 4°C normal saline was injected into the left bronchus and then pumped back into a centrifuge tube. This procedure was repeated three times. Samples were centrifuged at 3500 rpm for 15 min to obtain the liquid supernatant before storage at −80°C.
IL-6 and IL-10 levels in bronchoalveolar lavage fluid (BALF) were detected using a commercial ELISA kit (BD Bioscience pharmingen, U.S.A.) and quantified according to the manufacturer’s protocol.
Western blotting
Phosphorylated EGFR (p-EGFR), phosphorylated ERK (p-ERK), phosphorylated JNK (p-JNK), phosphorylated p38 (p-p38), ERK, JNK, and p38 were detected by Western blotting using p-EGFR (3777S, 1:1000, CST), p-ERK (28733-1-AP, 1:1000, Proteintech), p-JNK (4668S, 1:1000, CST), p-p38 (4511, 1:1000, CST), ERK (16443-1-AP, 1:1000, Proteintech), JNK (9252, 1:1000, CST), p38 (8690, 1:1000, CST), and GAPDH (10494-1-AP, 1:3000, Proteintech) antibodies. Denatured proteins were subjected to electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% skim milk in 1× TBST. Membranes were incubated with primary antibody at 4°C overnight and then the corresponding secondary antibody. Protein bands were detected using enhanced chemiluminescence reagents.
Statistical analysis
Data are presented as mean ± standard error. Data were analyzed using IBM SPSS 22.0 software. One-way analysis of variance with an appropriate post hoc test was used to compare data with a parametric distribution. If the variances were homogeneous, LSD method was performed. If the variances were inconsistent, Dunnett T3 test was performed. P-values <0.05 were considered statistically significant.
Results
ECC-BYF III and ER improve pulmonary function in COPD rats
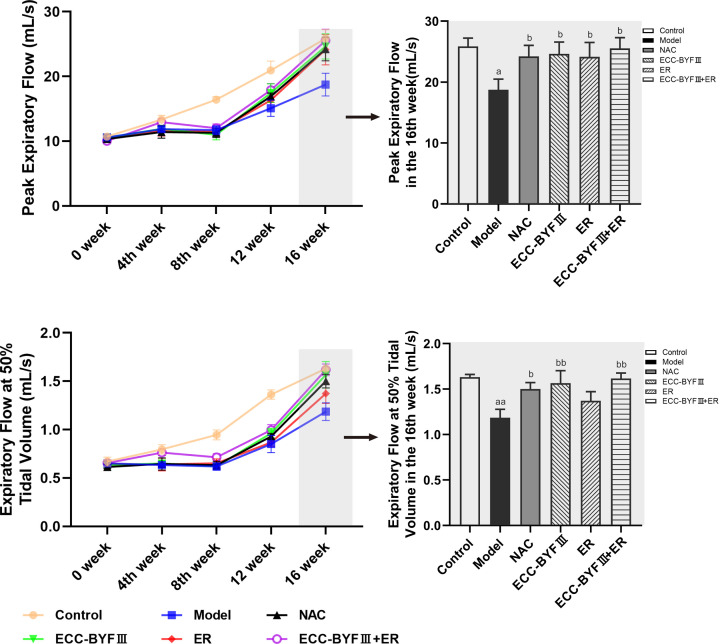
Pulmonary function testing is the main method of measuring airflow restriction and diagnosing COPD. During the experimental period, lung function increased significantly in the normal control group and decreased significantly in the disease model group. Lung function in the disease model group was significantly reduced compared with the normal control group, while lung function recovered significantly in all of the treatment groups. After week 16, PEF and EF50 in disease model group were significantly decreased compared with the disease model group (P<0.05 and P<0.01, respectively). PEF and EF50 were significantly increased in the ECC-BYF III, ECC-BYF III + ER, and NAC groups (P<0.05 and P<0.01, respectively). There was a trend toward increased EF50 in the ER group; however, this difference did not reach statistical significance (Figure 2).
Figure 2. Pulmonary function changes (PEF and EF50) in all groups.
The values are expressed as mean ± SE, (n=6). aP<0.05 vs. the normal control group, aaP<0.01 vs. the normal control group, bP<0.05 vs. the disease model group, and bbP<0.01 vs. the disease model group.
ECC-BYF III and ER ameliorate lung injury in COPD rats
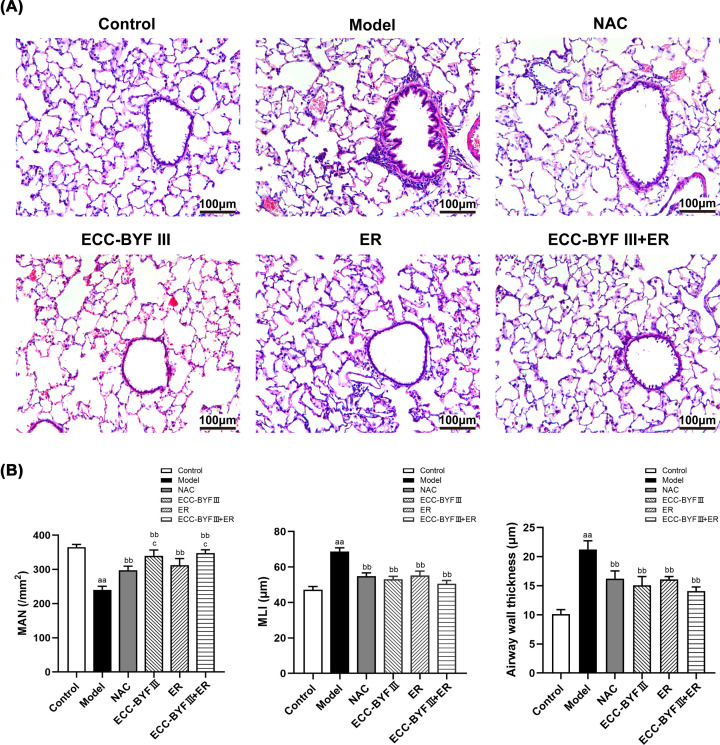
Histopathological changes are important characteristics of COPD and predominantly manifest as dilation of the alveolar cavities with inflammatory cell infiltration and airway wall thickening. We evaluated histopathological changes in lung tissues from COPD rats. Compared with the normal control group, the lung tissues in disease model group had severe pathological changes including decreased MAN, increased MLI, and increased airway wall thickness (P<0.01). These changes were significantly improved in the NAC, ECC-BYF III, ER, and ECC-BYF III + ER (P<0.01). This trend was even more pronounced in the ECC-BYF III and ECC-BYF III + ER compared with the NAC group (P<0.05, Figure 3).
Figure 3. Lung tissue histopathology changes in the lung tissues in all groups.
(A) Photographs of lung tissue histopathology (HE, ×200); (B) Changes of MAN, MLI and airway wall thickness. The values are expressed as the mean ± SE, (n=6). aaP<0.01 vs. the normal control group; bbP<0.01 vs. the disease model group; cP<0.05 vs. the NAC group.
ECC-BYF III and ER ameliorated airway inflammation and increases in goblet cell number in COPD rats
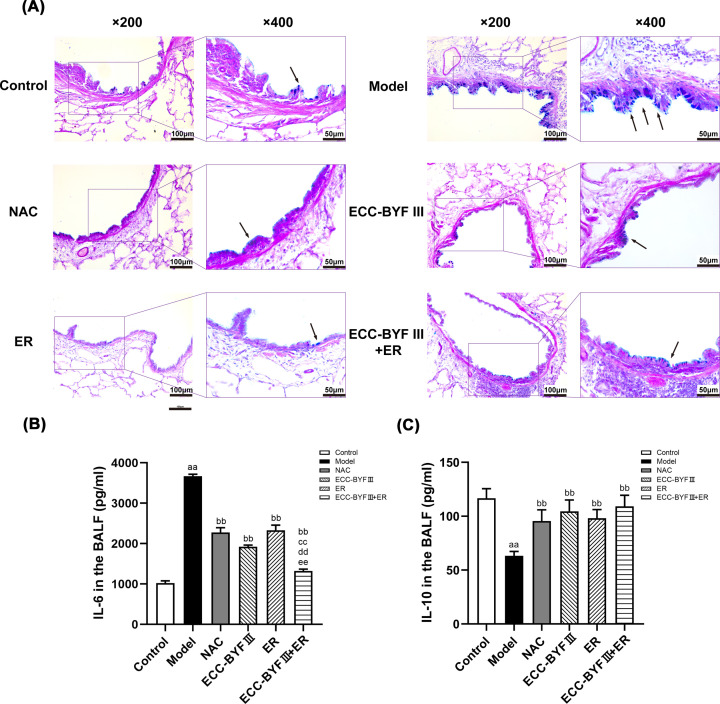
We examined lung and bronchus tissue morphology and airway mucin expression to determine the effects of ECC-BYF III and ER on AMH in COPD rats. Harmful substances such as CS repeatedly stimulate the airways causing an inflammatory response, excessive hyperplasia of goblet cells and mucous glands, and airway epithelial cell damage leading to mucus hypersecretion in COPD. AB-PAS staining demonstrated few blue-stained goblet cells in the airway epithelium of rats from the normal control group and numerous, blue-stained goblet cells in the disease model group. The treatment groups had fewer goblet cells compared with the disease model group, which had higher numbers induced by CS and bacterial inoculation (Figure 4A).
Figure 4. Changes of goblet cells in airway and changes of IL-6 and IL-10 in all groups.
(A) AB-PAS staining photos of the airway in all groups (AB-PAS; ×200, ×400).→: goblet cells. (B) Changes of IL-6 in all groups. The data are expressed as the mean ± SE, (n=6). aaP<0.01 vs. the normal control group; bbP<0.01 vs. the disease model group; ccP<0.01 vs. the NAC group; ddP<0.01 vs. the ECC-BYF III group; eeP<0.01 vs. the ER group. (C) Changes of IL-10 in all groups. The data are expressed as the mean ± SE (n=6). aaP<0.01 vs. the normal control group; bbP<0.01 vs. the disease model group.
Chronic respiratory inflammation is one of the major pathological features of COPD. To observe the suppressive effects of ECC-BYF III, ER, and ECC-BYF III + ER on airway inflammation in COPD rats, we measured the levels of representative inflammatory factors (IL-6 and IL-10) using ELISA. The ECC-BYF III, ER, ECC-BYF III + ER, and NAC groups had lowers IL-6 levels and higher IL-10 levels in BALF compared with the disease model group (P<0.01), which indicated ECC-BYF III, ER, the combination of ECC-BYF III and ER, and NAC inhibited airway inflammation in COPD rats. The ECC-BYF III + ER group had greater suppression of IL-6 levels in BALF compared with other treatment groups (P<0.01; Figure 4B,C).
ECC-BYF III and ER ameliorate mucoprotein levels in lung tissues from COPD rats
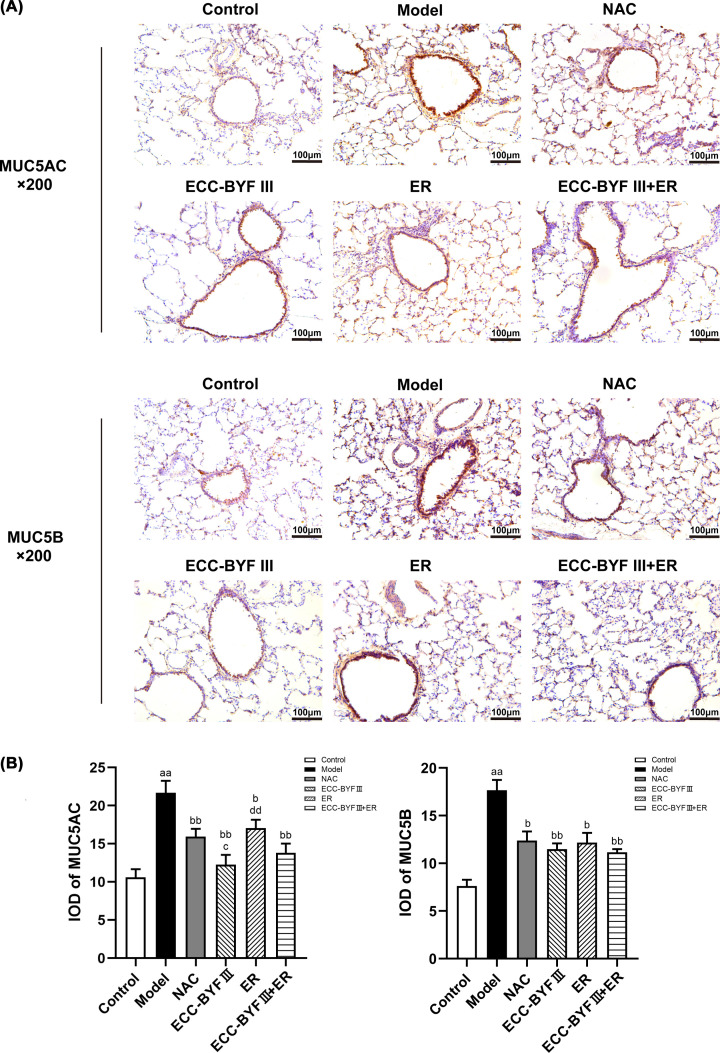
As the major macromolecular component, MUC5AC and MUC5B are predominantly responsible for the biophysical properties of airway mucus. Immunohistochemistry demonstrated that rats in the disease model group had increased MUC5AC and MUC5B levels in lung tissues compared with the normal control group (P<0.01). MUC5AC and MUC5B levels were ameliorated in the treatment groups (P<0.05, P<0.01). MUC5AC levels were significantly lower in the ECC-BYF III group compared with the ER and NAC groups (P<0.05 and P<0.01, respectively, Figure 5).
Figure 5. Expression of MUC5AC and MUC5B in the airway.
(A) The expression level of MUC5AC and MUC5B in the airway tested using immunohistochemistry (×200). (B) Values are the mean ± SE, (n=8). aaP<0.01 vs. the normal control group; bP<0.05 vs. the disease model group, bbP<0.01 vs. the disease model group; cP<0.05 vs. the NAC group; ddP<0.01 vs. the ECC-BYF III group.
ECC-BYF III and ER ameliorate increased AQP-5 levels in lung tissues from COPD rats
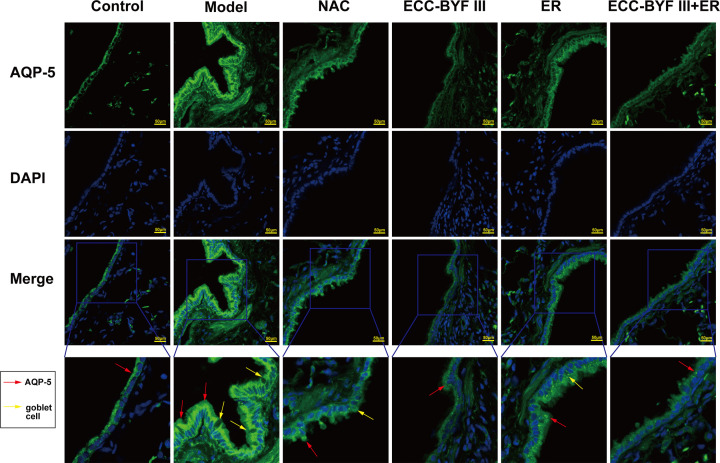
Excessive mucin synthesis causes airway inflammation which stimulates goblet cell proliferation and induces AMH [24]. In addition to increases in the absolute amount of mucin, COPD mucus hypersecretion also results in an imbalance in the ratio of mucin to water and salt ratio, with aquaporin 5 (AQP-5) known to regulate the mucin/water–salt ratio. Immunofluorescence demonstrated that rats in disease model group had increased AQP-5 levels in lung tissue compared with the normal control group, with numerous goblet cells observed. The increased AQP-5 levels were ameliorated in the treatment groups (Figure 6).
Figure 6. Expression of AQP-5 in the airway.
The expression level of AQP-5 in the airway tested using immunofluorescence (×400).
ECC-BYF III and ER inhibit AMH in COPD rats by regulating the EGFR/EGFR signaling pathway
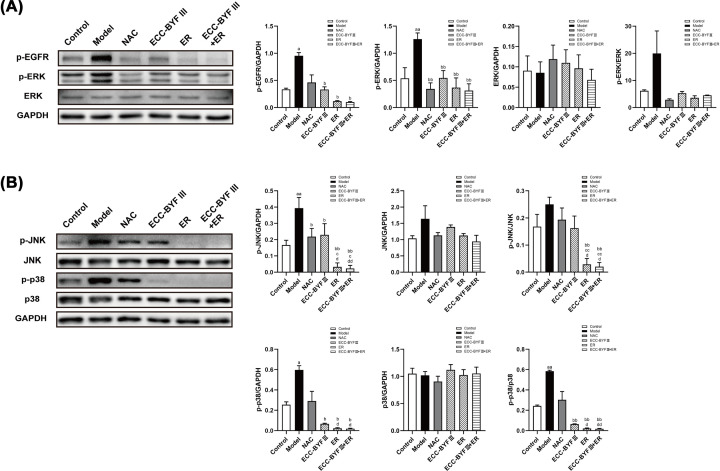
EGFR can activate multiple downstream pathways, including MAPK, which promote goblet cell proliferation and AMH. To further investigate, the potential effects of ECC-BYF III and ER on AMH in COPD rats, we examined the levels of proteins related to the EGFR/MAPK signaling pathway. Western blotting demonstrated that protein levels of phosphorylated EGFR, ERK, JNK, and p38 in the rats from the disease model group were increased compared with the normal control group (P<0.05, P<0.01), with significantly lower levels in the ECC-BYF III, ER, and ECC-BYF III + ER groups compared with the normal control group (P<0.05, P<0.01). p-JNK and p-JNK/JNK levels were higher in the ER and ECC-BYF III + ER groups compared with the NAC and ECC-BYF III groups (P<0.05, P<0.01). p-p38 and p-p38/p38 levels were higher in the ER and ECC-BYF III + ER groups compared with the NAC group (P<0.05). There were trends toward increased levels of phosphorylated EGFR and p38 in the disease model groups and trends toward decreased levels of phosphorylated EGFR and p38 in the NAC group; however, there differences did not reach statistical significance. There was no significant change in protein levels of ERK, JNK, or p38 between groups. These findings indicate that EGFR/MAPK signaling may affect AMH through changes in protein phosphorylation (Figure 7).
Figure 7. Protein expression of EGFR, ERK, JNK, and p38 in the airway of COPD rats.
The protein expression of p-EGFR, p-ERK, ERK, p-JNK, JNK, p-p38, and p38. Values are the mean ± SE, (n=3). aP<0.05 vs. the normal control group; aaP<0.01 vs. the normal control group; bP<0.05 vs. the disease model group; bbP<0.01 vs. the disease model group; cP<0.05 vs. the NAC group; ccP<0.01 vs. the NAC group; dP<0.05 vs. the ECC-BYF III group; ddP<0.01 vs. the ECC-BYF III group.
Discussion
COPD is a major disease with substantial effects on human health. In recent years, TCM for the treatment of COPD has received increasing attention due to its efficacy and safety. As TCM has been shown to be an effective and safe treatment for lung distention (Feizhang disease), TCM may also represent a treatment option for COPD. Previous multi-center clinical studies have demonstrated that BYF has positive effects in patients with stable COPD by ameliorating clinical symptoms, reducing the frequency of acute episodes, and improving athletic ability and quality of life [11]. Further, BHY was found to be effective in treating COPD rats by enhancing pulmonary function, reducing oxidative stress and inflammation, and regulating protease–antiprotease imbalance [12]. Based on these previous studies of BYF, we screened for the active components of BYF using systematic pharmacology and cell models with repeated in vitro and in vivo experiments, with the active component identified and termed ECC-BYF I [13]. We then obtained ECC-BYF II by evaluating the therapeutic effects of ECC-BYF I [14]. After further component reduction, we identified five effective components and named them ECC-BYF III. ECC-BYF III can improve AMH; however, the mechanisms underlying this effect remain unclear [16]. Recent studies have demonstrated the positive effects of ER in clinic settings. Reasonable exercise training has positive effects on patients with COPD by improving pulmonary function, enhancing skeletal muscle stamina, improving quality of life, and reducing the cost of treatment [19,25]. Previous studies have shown that ER can improve clinical symptoms, 6-min walking distance, and quality of life in patients with COPD [26]. ECC-BYF III + ER have also been shown to improve pulmonary function and pathological injury in addition to regulating inflammation and immunity in COPD rats [27].
The pathogenesis of COPD is complex. AMH is an independent risk factor for COPD and is associated with disease progression and unfavorable prognosis [14]. AMH has been shown to result in airflow restriction and progressive decline in lung function [28]. The findings of the present study indicates that NAC, ECC-BYF III, and the combination of ECC-BYF III and ER can improve lung function and lung injury, with ECC-BYF III and the combination of ECC-BYF III and ER having greater efficacy in improving MAN than NAC. Goblet cell metaplasia and increased mucin production are important manifestations of AMH. In the present study, we observed increased numbers of goblet cells and increased protein levels of MUC5AC, MUC5B, and AQP-5 in COPD rats. Further, reduced goblet cell numbers and decreased MUC5AC, MUC5B, and AQP-5 levels were observed in rats treated with NAC, ECC-BYF III, ER, or a combination of ECC-BYF III and ER. ECC-BYF III had a greater effect on MUC5AC levels than ER and NAC. Airway inflammation is promoted by external hazardous substances and causes proliferation of goblet cells and mucous glands, with damage to airway epithelial cells contributing to AMH [29]. To further explore the mechanisms underlying the pathogenesis of AMH, we measured inflammatory markers in BALF and demonstrated increased IL-6 levels and decreased IL-10 levels in COPD rats. All treatments evaluated in the present study affected the levels of inflammatory markers in BALF, with the combination of ECC-BYF III and ER found to have a greater suppressive effect on IL-6 levels than other treatments.
Goblet cell hyperplasia and impairment of mucociliary scavenging are the major mechanisms underlying the pathogenesis of AMH, with both regulated by multiple signaling pathways including the EGFR/MAPK pathway. In the present study, we observed significantly decreased levels of phosphorylated EGFR and significant increased levels of phosphorylated p38, ERK, and JNK in the disease model group indicating that the EGFR/MAPK pathway is activated in COPD rats. Interestingly, ECC-BYF III, ER, and the combination of ECC-BYF III and ER reduced the protein levels of phosphorylated EGFR, ERK, JNK, and p38, with the combination of ECC-BYF III and ER have greater effect than ECC-BYF III and NAC in decreasing the levels of p-JNK and p-p38. Accordingly, ECC-BYF III, ER, and the combination of ECC-BYF III and ER may improve AMH in COPD rats through inhibiting EGFR and downstream MAPK pathways including ERK, JNK, and p38 signaling. Further, the combination of ECC-BYF III and ER having greater effects on suppressing levels of phosphorylated JNK and p38 protein.
In conclusion, ECC-BYF III, ER, and the combination of ECC-BYF III and ER were found to have efficacy in treating AMH in COPD rats. Each treatment resulted in improved lung function and lung tissue injury, reduced inflammatory cell infiltration, reduced goblet cell number, and decreased levels of MUC5AC and MUC5B in lung tissues. ECC-BYF III and the combination of ECC-BYF III and ER had greater effects on improving MAN, with ECC-BYF III treatment leading to higher levels of MUC5AC in BALF compared with treatment with ER. The combination of ECC-BYF III and ER had the greatest suppressive effect on IL-6 compared with other treatments. The effects of ECC-BYF III and ER on AMH in COPD rats may be mediated by inhibition of the EGFR/MAPK signaling pathway.
Supplementary Material
Acknowledgements
This study was supported by Henan Key Laboratory of Chinese Medicine for Respiratory Disease, Co-construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of P.R., the authors are grateful to lab members for their insightful comments on the work.
Abbreviations
- AB-PAS
Alcian Blue/periodic acid-Schiff
- AMH
airway mucus hypersecretion
- AQP-5
aquaporin 5
- BALF
bronchoalveolar lavage fluid
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- ECC-BYF I
effective-component compatibility of Bufei Yishen formula I
- ECC-BYF II
effective-component compatibility of Bufei Yishen formula II
- ER
exercise rehabilitation
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- KP
Klebsiella pneumoniae
- MAN
mean alveolar number
- MLI
mean linear intercept
- NAC
acetylcysteine
- PEF
peak expiratory flow
- p-EGFR
phosphorylated EGFR
- p-ERK
phosphorylated ERK
- p-JNK
phosphorylated JNK
- SD
Sprague–Dawley
- TCM
Traditional Chinese Medicine
Data Availability
Data for the present study can be obtained from the corresponding author according to the rules.
Competing Interests
This is no financial or commercial conflicts of interest among the contributors. While acceptance by your prestigious publication is being considered, the contents of the work had not been submitted or published in any institution,
Funding
This study was supported by the National Natural Sciences Foundation of China [grant numbers 82074406, 81973822, and 82004267], Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases co-constructed by Henan province and Education Ministry of P.R. China (Collaborative Innovation Center [2002]002).
CRediT Author Contribution
Kexin Xu: Conceptualization, Data curation, Formal analysis, Validation, Writing—original draft, Writing—review & editing. Jindi Ma: Writing—review & editing. Ruilong Lu: Validation. Xuejie Shao: Validation. Yakun Zhao: Validation. Lili Cui: Validation. Zhiguang Qiu: Validation. Yange Tian: Resources, Funding acquisition, Writing—review & editing. Jiansheng Li: Conceptualization, Resources, Supervision, Funding acquisition, Methodology.
Ethics Approval and Consent to Participate
All experimental protocols were approved by the Experimental Animal Care and Ethics Committees of the First Affiliated Hospital at Henan University of Traditional Chinese Medicine (Animal ethical approval No: YFYDW2019031), Zhengzhou, China.
Consent for Publication
Not applicable.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (2022) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. report [Online] http://www.goldcopd.org [Google Scholar]
- 2.Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W.et al. (2019) Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 394, 1145–1158 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy J.V. and Dickey B.F. (2010) Airway mucus function and dysfunction. N. Engl. J. Med. 363, 2233–2247 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R. and Wadhwa R. (2022) Mucolytic medications. StatPearls, p. 2022, StatPearls Publishing, Treasure Island (FL) [PubMed] [Google Scholar]
- 5.Song W.Y., Song Y.S., Ryu H.W., Oh S.R., Hong J. and Yoon D.Y. (2017) Tilianin inhibits MUC5AC expression mediated via down-regulation of EGFR-MEK-ERK-Sp1 signaling pathway in NCI-H292 human airway cells. J. Microbiol. Biotechnol. 27, 49–56 10.4014/jmb.1610.10012 [DOI] [PubMed] [Google Scholar]
- 6.Mao L., Bai C.X., Zhang M., Wang Y.H. and Chen J. (2004) Expression of epidermal growth factor receptor and MUC5AC on human airway with chronic obstructive pulmonary disease. Chin. J. Tubere Respir. Dis. 9, 12–15 [PubMed] [Google Scholar]
- 7.Nie Y.C., Wu H., Li P.B., Xie L.M., Luo Y.L., Shen J.G.et al. (2012) Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IκB-NF-κB signaling pathways. Eur. J. Pharmacol. 690, 207–213 10.1016/j.ejphar.2012.06.040 [DOI] [PubMed] [Google Scholar]
- 8.Bateman E.D., Rennard S., Barnes P.J., Dicpinigaitis P.V., Gosens R., Gross N.J.et al. (2009) Alternative mechanisms for tiotropium. Pulm. Pharmacol. Ther. 22, 533–542 10.1016/j.pupt.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Liu W., Zhang X., Mao B. and Jiang H. (2020) Systems pharmacology-based study of Tanreqing injection in airway mucus hypersecretion. J. Ethnopharmacol. 249, 112425 10.1016/j.jep.2019.112425 [DOI] [PubMed] [Google Scholar]
- 10.Wang H.F., Zhang H.L., Li J.S., Xueqing Y., Suyun L., Bin L.et al. (2015) Effectiveness and safety of traditional Chinese medicine on stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement. Ther. Med. 23, 603–611 10.1016/j.ctim.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 11.Li S.Y., Li J.S., Wang M.H., Xie Y., Yu X.Q., Sun Z.K.et al. (2012) Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement. Altern. Med. 12, 197 10.1186/1472-6882-12-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J.S., Zhao P., Li Y., Tian Y.G. and Wang Y.H. (2015) Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci. Rep. 5, 15290 10.1038/srep15290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J.S., Liu X.F., Dong H.R., Zheng W.C., Feng S.X., Tian Y.G.et al. (2020) Effective-constituent compatibility-based analysis of Bufei Yishen formula, a traditional herbal compound as an effective treatment for chronic obstructive pulmonary disease. J. Integr. Med. 18, 351–362 10.1016/j.joim.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Li J.S., Ma J.D., Tian Y.G., Zhao P., Liu X.F., Dong H.R.et al. (2020) Effective-component compatibility of Bufei Yishen formula II inhibits mucus hypersecretion of chronic obstructive pulmonary disease rats by regulating EGFR/PI3K/mTOR signaling. J. Ethnopharmacol. 257, 112796 10.1016/j.jep.2020.112796 [DOI] [PubMed] [Google Scholar]
- 15.Liu L., Qin Y.Q., Cai Z.H., Tian Y.G., Liu X.F., Li J.S.et al. (2021) Effective-components combination improves airway remodeling in COPD rats by suppressing M2 macrophage polarization via the inhibition of mTORC2 activity. Phytomedicine 92, 153759 10.1016/j.phymed.2021.153759 [DOI] [PubMed] [Google Scholar]
- 16.Qin Y.Q., Li M.Y., Tian Y.G., Zhao P., Li K.C., Li C.C.et al. (2021) Compatibility characteristics of Bufei Yishen formula III in regulating chronic obstructive pulmonary disease mucus hypersecretion. Chin. Crit. Care Med. 33, 1309–1314 [DOI] [PubMed] [Google Scholar]
- 17.Garvey C., Bayles M.P., Hamm L.F., Hill K., Holland A., Limberg T.M.et al. (2016) Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: an official statement from the American Association of cardiovascular and pulmonary rehabilitation. J. Cardiopulm Rehabil. Prev. 36, 75–83 10.1097/HCR.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 18.Liu Q., Duan H., Lian A., Zhuang M., Zhao X. and Liu X. (2021) Rehabilitation effects of acupuncture on the diaphragm dysfunction in chronic obstructive pulmonary disease: a systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 16, 2023–2037 10.2147/COPD.S313439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrianopoulos V., Klijn P., Franssen F.M. and Spruit M.A. (2014) Exercise training in pulmonary rehabilitation. Clin. Chest. Med. 35, 313–322 10.1016/j.ccm.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y.Q., Jiang F., Chen Y., Chen P. and Cai S. (2018) Exercise assessments and trainings of pulmonary rehabilitation in COPD: a literature review. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 2013–2023 10.2147/COPD.S167098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Li S.Y., Li J.S., Deng L., Tian Y.G., Jiang S.L.et al. (2012) A rat model for stable chronic obstructive pulmonary disease induced by cigarette smoke inhalation and repetitive bacterial infection. Biol. Pharm. Bull. 35, 1752–1760 10.1248/bpb.b12-00407 [DOI] [PubMed] [Google Scholar]
- 22.Huang J.-H., Huang X.-H., Chen Z.-Y., Zheng Q.-S. and Sun R.-Y. (2004) Dose conversion among different animals and healthy volunteers in pharmacological study. Chin. J. Clin. Pharm. Ther. 9, 1069–1072, ISSN:1009-2501 [Google Scholar]
- 23.Zhang L.X., Tian Y.G., Zhao P., Feng S.X., Han X.X. and Li J.S. (2022) Network pharmacology analysis uncovers the effect on apoptotic pathway by Bu-Fei formula for COPD treatment. J. Ethnopharmacol. 289, 115022 10.1016/j.jep.2022.115022 [DOI] [PubMed] [Google Scholar]
- 24.Wee P. and Wang Z. (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9, 52 10.3390/cancers9050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widyastuti K., Makhabah D.N., Setijadi A.R., Sutanto Y.S., Suradi and Ambrosino N. (2018) Benefits and costs of home pedometer assisted physical activity in patients with COPD. A preliminary randomized controlled trial. Pulmonology 24, 211–218 10.1016/j.pulmoe.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 26.Zhang H.L., Li J.S., Yu X.Q., Li S.Y., Halmurat U., Xie Y.et al. (2017) An evaluation of activity tolerance, patient-reported outcomes and satisfaction with the effectiveness of pulmonary daoyin on patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 2333–2342 10.2147/COPD.S117461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J.S., Zhu L.H., Tian Y.G., Liu S., Jin F.L., Chen K.et al. (2022) Effect of effective-component compatibility of Bufei Yishen Formula III combined with exercise rehabilitation in chronic obstructive pulmonary disease rats. Chin. Crit. Care Med. 42, 987–995 [Google Scholar]
- 28.Ramos F.L., Krahnke J.S. and Kim V. (2014) Clinical issues of mucus accumulation in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan N.S., Tang X., Ding H., Yan Y.X., Zhuang Y., Qi C.et al. (2021) Influence of coexistence of mild OSA on airway mucus hypersecretion in patients with COPD. J. Breath Res. 15, 2 10.1088/1752-7163/abd52e [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the present study can be obtained from the corresponding author according to the rules.