Abstract
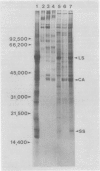
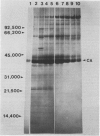
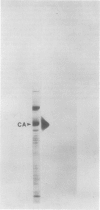
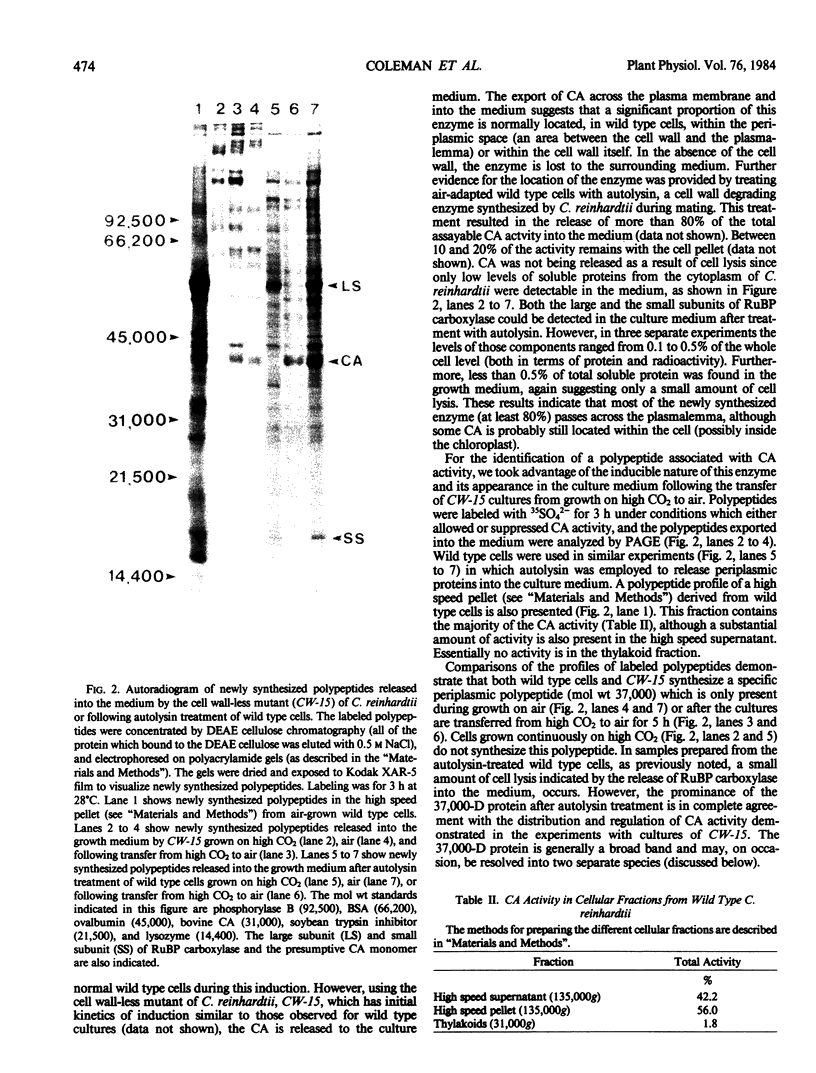
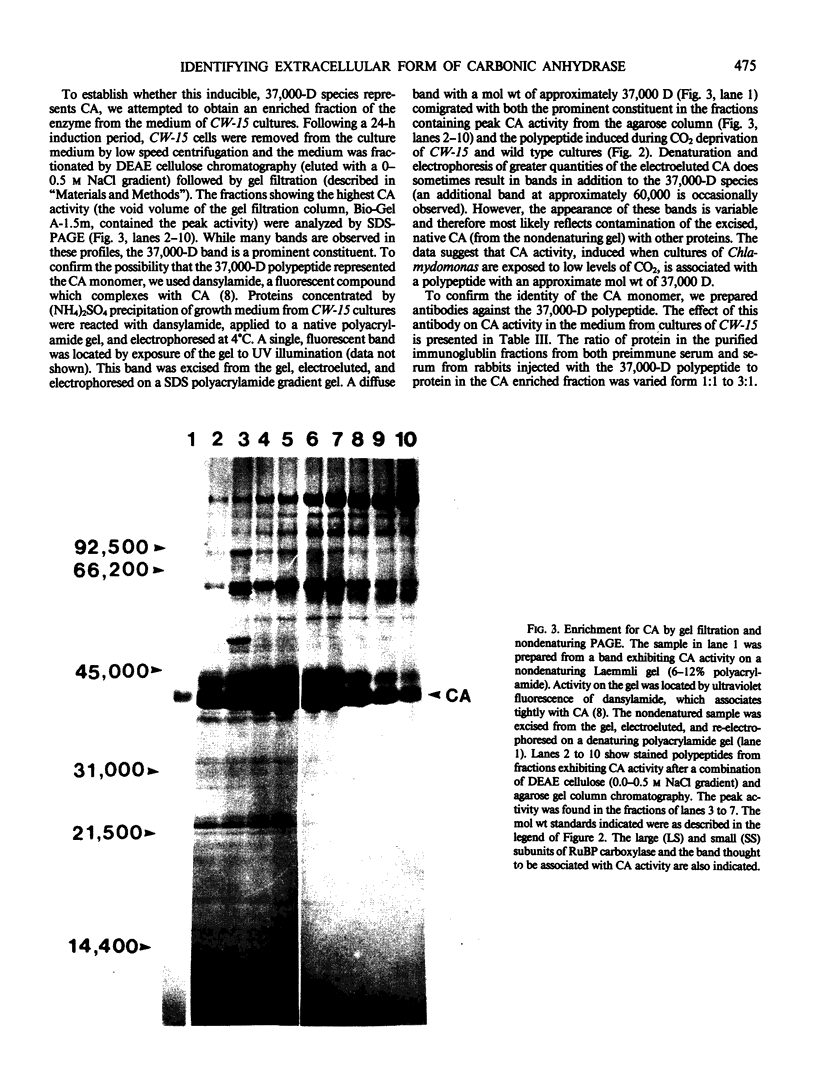
We have examined the induction of carbonic anhydrase activity in Chlamydomonas reinhardtii and have identified the polypeptide responsible for this activity. This polypeptide was not synthesized when the alga was grown photoautotrophically on 5% CO2, but its synthesis was induced under low concentrations of CO2 (air levels of CO2). In CW-15, a mutant of C. reinhardtii which lacks a cell wall, between 80 and 90% of the carbonic anhydrase activity of air-adapted cells was present in the growth medium. Furthermore, between 80 and 90% of the carbonic anhydrase is released if wild type cells are treated with autolysin, a hydrolytic enzyme responsible for cell wall degradation during mating of C. reinhardtii. These data extend the work of Kimpel, Togasaki, Miyachi (1983 Plant Cell Physiol 24: 255-259) and indicate that the bulk of the carbonic anhydrase is located either in the periplasmic space or is loosely bound to the algal cell wall. The polypeptide associated with carbonic anhydrase activity has a molecular weight of approximately 37,000. Several lines of evidence indicate that this polypeptide is responsible for carbonic anhydrase activity: (a) it appears following the transfer of C. reinhardtii from growth on 5% CO2 to growth on air levels of CO2, (b) it is located in the periplasmic space or associated with the cell wall, like the bulk of the carbonic anhydrase activity, (c) it binds dansylamide, an inhibitor of the enzyme which fluoresces upon illumination with ultraviolet light, (d) antibodies which inhibit carbonic anhydrase activity only cross-react with this 37,000 dalton species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S., Mishima H., Miyachi Y. Levels of cyclic-AMP, cyclic-GMP and betamethasone in the aqueous humor following topical administration of betamethasone in rabbit eyes. Hiroshima J Med Sci. 1983 Sep;32(3):301–304. [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Coleman J. R., Colman B. Measurement of photorespiration in algae. Plant Physiol. 1982 Jan;69(1):259–262. doi: 10.1104/pp.69.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Drescher D. G. Purification of blood carbonic anhydrases and specific detection of carbonic anhydrase isoenzymes on polyacrylamide gels with 5-dimethylaminonaphthalene-1-sulfonamide (DNSA). Anal Biochem. 1978 Oct 1;90(1):349–358. doi: 10.1016/0003-2697(78)90037-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcus Y., Harel E., Kaplan A. Adaptation of the Cyanobacterium Anabaena variabilis to Low CO(2) Concentration in Their Environment. Plant Physiol. 1983 Jan;71(1):208–210. doi: 10.1104/pp.71.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Reduced Inorganic Carbon Transport in a CO(2)-Requiring Mutant of Chlamydomonas reinhardii. Plant Physiol. 1983 Oct;73(2):273–276. doi: 10.1104/pp.73.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]