Abstract
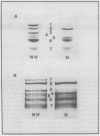
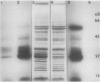
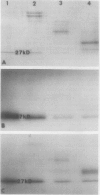
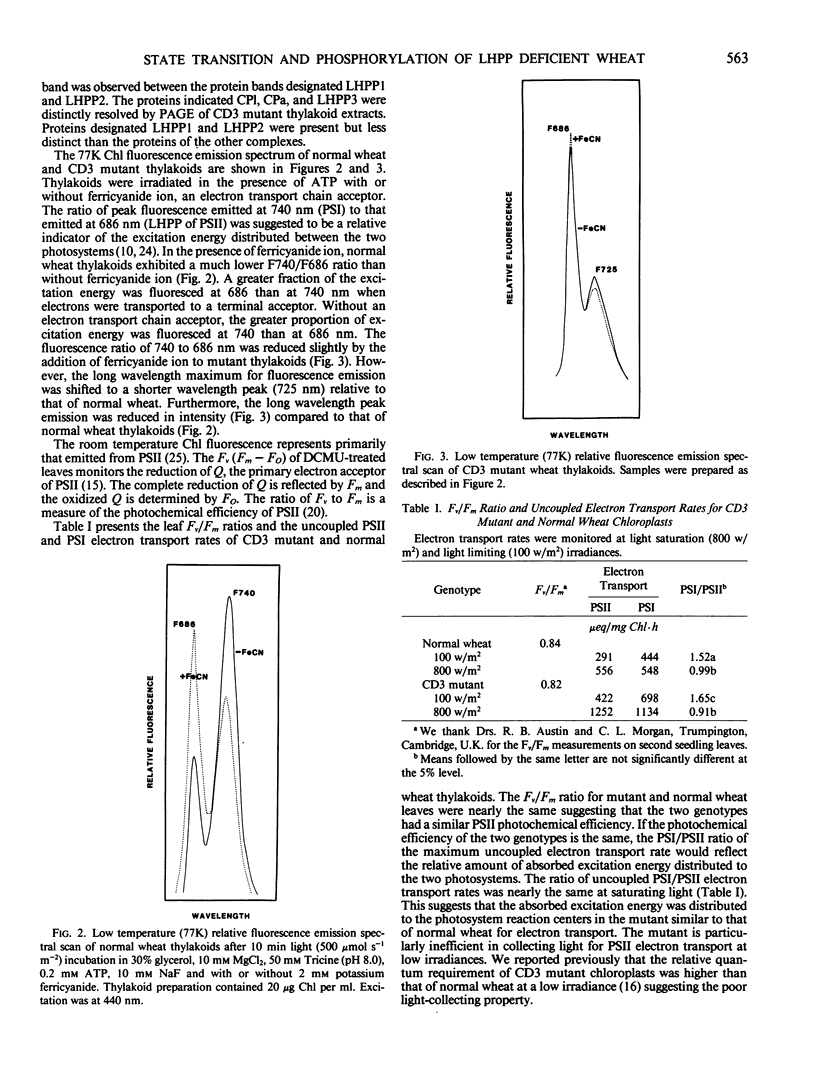
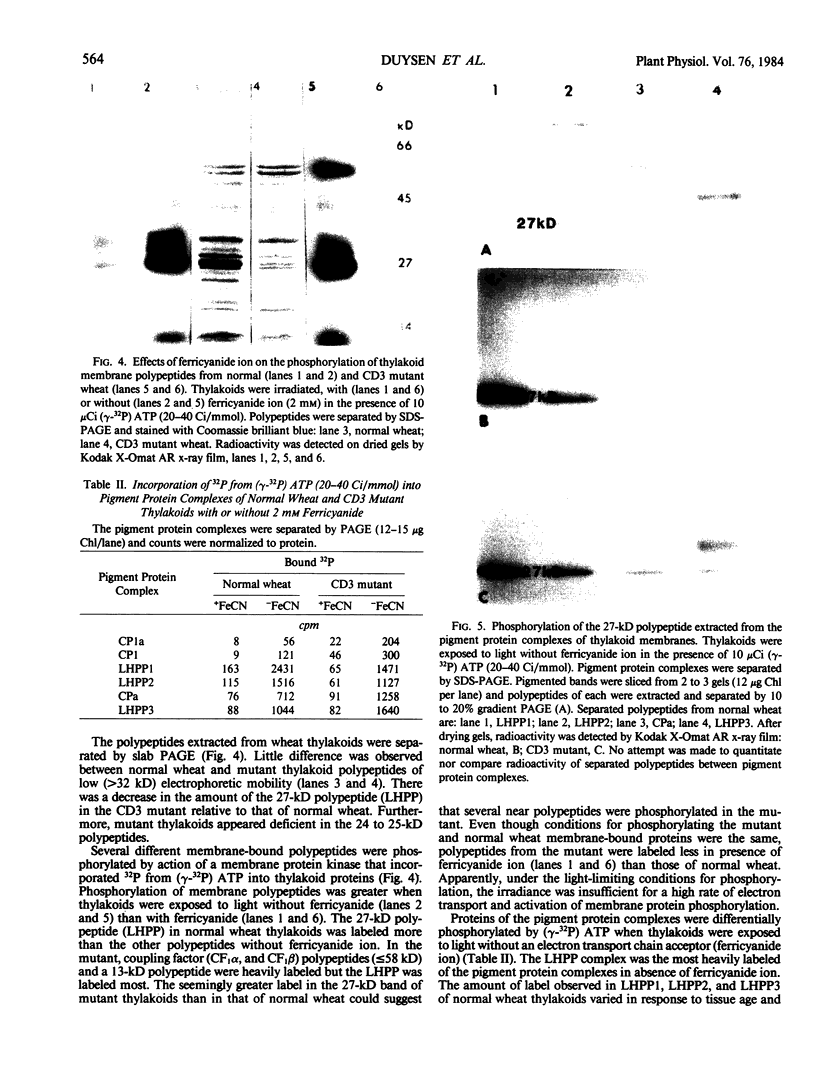
Chloroplasts of the CD3 wheat mutant were deficient primarily in chlorophyll of light harvesting pigment proteins (LHPP) 1 and 2 and CP1a. The reduced level of protein associated with chlorophyll of LHPP1 and LHPP2 and the reduced level of low molecular weight polypeptides between 23 and 29 kilodaltons confirmed that the CD3 mutant was deficient in the LHPP complex. The high fluorescence emission ratio at 740 (F740) to 686 nanometers (F686) observed from chloroplasts of normal wheat following light induced phosphorylation of the LHPP complex was not noted from mutant chloroplasts. The long wavelength peak fluorescence emission (F740) was shifted to a shorter wavelength peak (F725) and was reduced in intensity compared to that of normal wheat thylakoids. The ratio of variable fluorescence to maximum fluorescence, a measure of PSII photochemical efficiency, was the same for the normal wheat and mutant leaves. The ratios of uncoupled photosystem I/photosystem II electron transport rates for mutant and normal wheat chloroplasts were similar at saturating light suggesting that absorbed excitation energy was distributed to the two photosystem reaction centers of the mutant in a similar manner as in the normal wheat. Proteins of the LHPP complex were differentially phosphorylated by action of a membrane protein kinase when both normal wheat and CD3 mutant thylakoids were irradiated without an electron transport chain acceptor. Even though the F740/F686 ratio was low in mutant thylakoids, the phosphorylation of the 27-kilodalton LHPP polypeptide was consistent with the mutant being in a state II condition. The data gave rise to the suggestion that the F740/F686 ratio might not indicate excitation energy distribution to the two photosystems in the mutant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Anderson J. M., Ryrie I. J. Transbilayer organization of the chlorophyll-proteins of spinach thylakoids. Eur J Biochem. 1982 Apr 1;123(2):465–472. doi: 10.1111/j.1432-1033.1982.tb19790.x. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J., Briantais J. M., Vernotte C. Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch Biochem Biophys. 1976 Jul;175(1):54–63. doi: 10.1016/0003-9861(76)90484-7. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Moya I. Intersystem excition transfer in isolated chloroplasts. Biochim Biophys Acta. 1973 Dec 14;325(3):530–538. doi: 10.1016/0005-2728(73)90212-0. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Ditto C. L., Arntzen C. J. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978 Apr 15;187(1):252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Armond P. A., Gross E. L., Arntzen C. J. Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch Biochem Biophys. 1976 Jul;175(1):64–70. doi: 10.1016/0003-9861(76)90485-9. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Malkin S., Armond P. A., Mooney H. A., Fork D. C. Photosystem II Photosynthetic Unit Sizes from Fluorescence Induction in Leaves : CORRELATION TO PHOTOSYNTHETIC CAPACITY. Plant Physiol. 1981 Mar;67(3):570–579. doi: 10.1104/pp.67.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin S., Siderer Y. The effect of salt concentration on the fluorescence parameters of isolated chloroplasts. Biochim Biophys Acta. 1974 Dec 19;368(3):422–431. doi: 10.1016/0005-2728(74)90187-x. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Miller G. J., McIntyre K. R. The light-harvesting chlorpohyll-protein complex of photosystem II. Its location in the photosynthetic membrane. J Cell Biol. 1976 Nov;71(2):624–638. doi: 10.1083/jcb.71.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. A developmental study of photosystem I peripheral chlorophyll proteins. Plant Physiol. 1980 May;65(5):823–827. doi: 10.1104/pp.65.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Ryrie I. J., Anderson J. M., Goodchild D. J. The role of the light-harvesting chlorophyll a/b protein complex in chloroplast membrane stacking. Cation-induced aggregation of reconstituted proteoliposomes. Eur J Biochem. 1980 Jun;107(2):345–354. doi: 10.1111/j.1432-1033.1980.tb06035.x. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]