Abstract
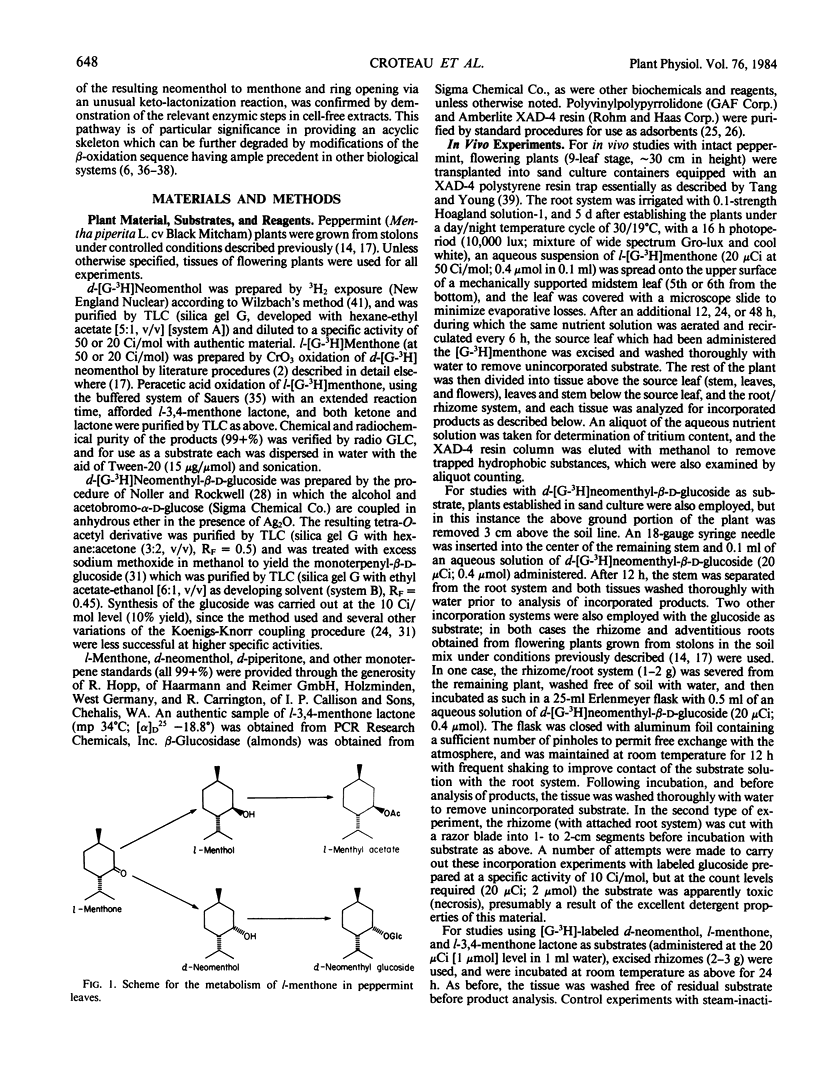
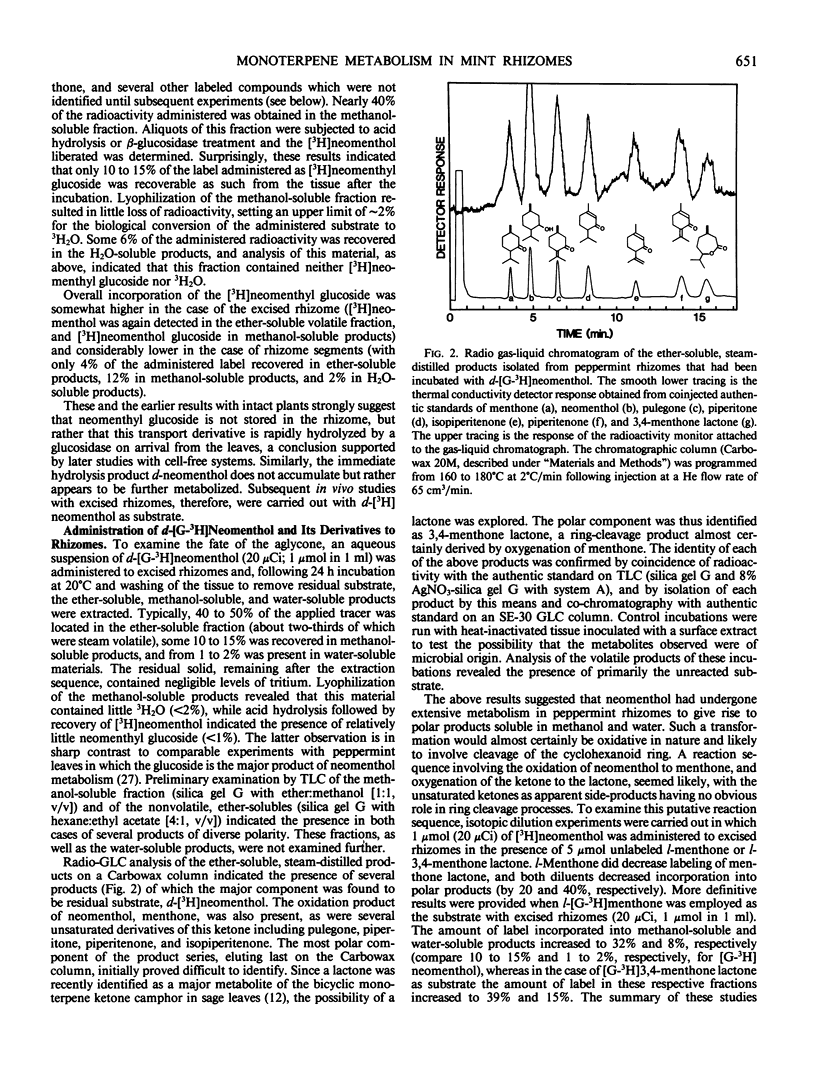
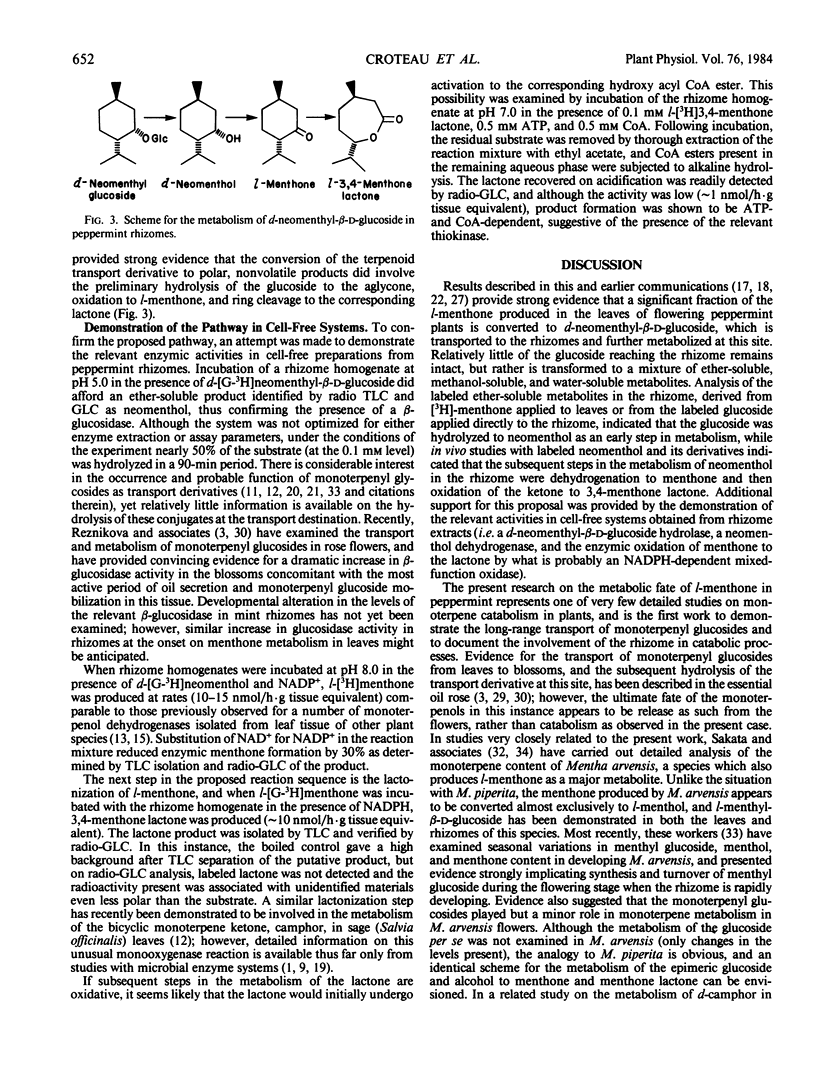
Previous studies have shown that the monoterpene ketone l-[G-3H] menthone is reduced to the epimeric alcohols l-menthol and d-neomenthol in leaves of flowering peppermint (Mentha piperita L.), and that a portion of the menthol is converted to menthyl acetate while the bulk of the neomenthol is transformed to neomenthyl-β-d-glucoside which is then transported to the rhizome (Croteau, Martinkus 1979 Plant Physiol 64: 169-175). Analysis of the disposition of l-[G-3H]menthone applied to midstem leaves of intact flowering plants allowed the kinetics of synthesis and transport of the monoterpenyl glucoside to be determined, and gave strong indication that the glucoside was subsequently metabolized in the rhizome. Studies with d-[G-3H]neomenthyl-β-d-glucoside as substrate, using excised rhizomes or rhizome segments, confirmed the hydrolysis of the glucoside as an early step in metabolism at this site, and revealed that the terpenoid moiety was further converted to a series of ether-soluble, methanol-soluble, and water-soluble products. Studies with d-[G-3H]neomenthol as the substrate, using excised rhizomes, showed the subsequent metabolic steps to involve oxidation of the alcohol back to menthone, followed by an unusual lactonization reaction in which oxygen is inserted between the carbonyl carbon and the carbon bearing the isopropyl group, to afford 3,4-menthone lactone. The conversion of menthone to the lactone, and of the lactone to more polar products, were confirmed in vivo using l-[G-3H]menthone and l-[G-3H]-3,4-menthone lactone as substrates. Additional oxidation products were formed in vivo via the desaturation of labeled neomenthol and/or menthone, but none of these transformations appeared to lead to ring opening of the p-menthane skeleton. Each step in the main reaction sequence, from hydrolysis of neomenthyl glucoside to lactonization of menthone, was demonstrated in cell-free extracts from the rhizomes of flowering mint plants. The lactonization step is of particular significance in providing a means of cleaving the p-menthane ring to afford an acyclic carbon skeleton that can be further degraded by modifications of the well-known β-oxidation sequence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton L. N., Markovetz A. J. A novel ketone monooxygenase from Pseudomonas cepacia. Purification and properties. J Biol Chem. 1977 Dec 10;252(23):8561–8566. [PubMed] [Google Scholar]
- Bugorskii I. S., Reznikova S. A., Zaprometov M. N. O vzaimosviazi gliukozidnosviazannykh i svobodnykh spirtov v protsesse obrazovaniia efirnogo masla v lepestkakh rozy. Biokhimiia. 1979 Jun;44(6):1068–1073. [PubMed] [Google Scholar]
- Burbott A. J., Loomis W. D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967 Jan;42(1):20–28. doi: 10.1104/pp.42.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbott A. J., Loomis W. D. Evidence for metabolic turnover of monoterpenes in peppermint. Plant Physiol. 1969 Feb;44(2):173–179. doi: 10.1104/pp.44.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN P. J., MEERMAN G., GUNSALUS I. C. THE MICROBIOLOGICAL TRANSFORMATION OF FENCHONE. Biochem Biophys Res Commun. 1965 Jun 18;20:104–108. doi: 10.1016/0006-291x(65)90955-1. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., GUNSALUS I. C. An enzyme system for cyclic ketone lactonization. Biochem Biophys Res Commun. 1961 Nov 29;6:293–297. doi: 10.1016/0006-291x(61)90382-5. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., NAMTVEDT M. J., GUNSALUS I. C. MIXED FUNCTION OXIDATION. II. SEPARATION AND PROPERTIES OF THE ENZYMES CATALYZING CAMPHOR LACTONIZATION. J Biol Chem. 1965 Jan;240:495–503. [PubMed] [Google Scholar]
- Cantwell S. G., Lau E. P., Watt D. S., Fall R. R. Biodegradation of acyclic isoprenoids by Pseudomonas species. J Bacteriol. 1978 Aug;135(2):324–333. doi: 10.1128/jb.135.2.324-333.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., El-Bialy H., El-Hindawi S. Metabolism of monoterpenes: lactonization of (+)-camphor and conversion of the corresponding hydroxy acid to the glucoside-glucose ester in sage (Salvia officinalis). Arch Biochem Biophys. 1984 Feb 1;228(2):667–680. doi: 10.1016/0003-9861(84)90037-7. [DOI] [PubMed] [Google Scholar]
- Croteau R., Hooper C. L., Felton M. Biosynthesis of monoterpenes. Partial purfication and characterization of a bicyclic monoterpenol dehydrogenase from sage (Salvia officinalis). Arch Biochem Biophys. 1978 May;188(1):182–193. doi: 10.1016/0003-9861(78)90371-5. [DOI] [PubMed] [Google Scholar]
- Croteau R., Hooper C. L. Metabolism of Monoterpenes: Acetylation of (-)-Menthol by a Soluble Enzyme Preparation from Peppermint (Mentha piperita) Leaves. Plant Physiol. 1978 May;61(5):737–742. doi: 10.1104/pp.61.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Karp F. Biosynthesis of monoterpenes: enzymatic concersion of neryl pyrophosphate to 1,8-cineole, alpha-terpineol, and cyclic monoterpene hydrocarbons by a cell-free preparation from sage (Salvia officinalis). Arch Biochem Biophys. 1976 Oct;176(2):734–746. doi: 10.1016/0003-9861(76)90217-4. [DOI] [PubMed] [Google Scholar]
- Croteau R., Martinkus C. Metabolism of Monoterpenes: Demonstration of (+)-Neomenthyl-beta-d-Glucoside as a Major Metabolite of (-)-Menthone in Peppermint (Mentha Piperita). Plant Physiol. 1979 Aug;64(2):169–175. doi: 10.1104/pp.64.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R. Site of Monoterpene Biosynthesis in Majorana hortensis Leaves. Plant Physiol. 1977 Mar;59(3):519–520. doi: 10.1104/pp.59.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Winters J. N. Demonstration of the Intercellular Compartmentation of l-Menthone Metabolism in Peppermint (Mentha piperita) Leaves. Plant Physiol. 1982 Apr;69(4):975–977. doi: 10.1104/pp.69.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue N. A., Norris D. B., Trudgill P. W. The purification and properties of cyclohexanone oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur J Biochem. 1976 Mar 16;63(1):175–192. doi: 10.1111/j.1432-1033.1976.tb10220.x. [DOI] [PubMed] [Google Scholar]
- Kjonaas R., Martinkus-Taylor C., Croteau R. Metabolism of Monoterpenes: Conversion of l-Menthone to l-Menthol and d-Neomenthol by Stereospecific Dehydrogenases from Peppermint (Mentha piperita) Leaves. Plant Physiol. 1982 May;69(5):1013–1017. doi: 10.1104/pp.69.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Martinkus C., Croteau R. Metabolism of Monoterpenes : EVIDENCE FOR COMPARTMENTATION OF l-MENTHONE METABOLISM IN PEPPERMINT (MENTHA PIPERITA) LEAVES. Plant Physiol. 1981 Jul;68(1):99–106. doi: 10.1104/pp.68.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEUBERT W. Degradation of isoprenoid compounds by micro-organisms. I. Isolation and characterization of an isoprenoid-degrading bacterium, Pseudomonas citronellolis n. sp. J Bacteriol. 1960 Mar;79:426–434. doi: 10.1128/jb.79.3.426-434.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEUBERT W., FASS E. UNTERSUCHUNGEN UEBER DEN BAKTERIELLEN ABBAU VON ISOPRENOIDEN. V. DER MECHANISMUS DES ISOPRENOIDABBAUES. Biochem Z. 1964 Dec 7;341:35–44. [PubMed] [Google Scholar]
- Tang C. S., Young C. C. Collection and Identification of Allelopathic Compounds from the Undisturbed Root System of Bigalta Limpograss (Hemarthria altissima). Plant Physiol. 1982 Jan;69(1):155–160. doi: 10.1104/pp.69.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]


