Abstract
Physical activity, including walking, has numerous health benefits in older adults, supported by a plethora of observational and interventional studies. Walking decreases the risk or severity of various health outcomes such as cardiovascular and cerebrovascular diseases, type 2 diabetes mellitus, cognitive impairment and dementia, while also improving mental well-being, sleep, and longevity. Dose-response relationships for walking duration and intensity are established for adverse cardiovascular outcomes. Walking’s favorable effects on cardiovascular risk factors are attributed to its impact on circulatory, cardiopulmonary, and immune function. Meeting current physical activity guidelines by walking briskly for 30 min per day for 5 days can reduce the risk of several age-associated diseases. Additionally, low-intensity physical exercise, including walking, exerts anti-aging effects and helps prevent age-related diseases, making it a powerful tool for promoting healthy aging. This is exemplified by the lifestyles of individuals in Blue Zones, regions of the world with the highest concentration of centenarians. Walking and other low-intensity physical activities contribute significantly to the longevity of individuals in these regions, with walking being an integral part of their daily lives. Thus, incorporating walking into daily routines and encouraging walking-based physical activity interventions can be an effective strategy for promoting healthy aging and improving health outcomes in all populations. The goal of this review is to provide an overview of the vast and consistent evidence supporting the health benefits of physical activity, with a specific focus on walking, and to discuss the impact of walking on various health outcomes, including the prevention of age-related diseases. Furthermore, this review will delve into the evidence on the impact of walking and low-intensity physical activity on specific molecular and cellular mechanisms of aging, providing insights into the underlying biological mechanisms through which walking exerts its beneficial anti-aging effects.
Keywords: Cardiovascular disease, Cerebrovascular disease, Blood pressure, Mortality, Healthy aging, Aging, Walking
Introduction
The Western world is experiencing a significant demographic shift as the population ages. According to the United Nations, the number of people aged 60 years or older is expected to more than double by 2050, reaching 2.1 billion [1]. With this increase in the aging population comes a greater focus on healthy aging, which involves maintaining physical and mental health as people age [2–4].
One area of research that has gained attention in recent years is the study of determinants of healthy aging in the Blue Zones, regions of the world where people live longer, healthier lives than anywhere else [5–7]. Researchers have identified five Blue Zones around the world, including Okinawa in Japan, Sardinia in Italy, Nicoya in Costa Rica, Icaria in Greece, and the Seventh-day Adventist community in Loma Linda, California. These regions have the highest concentration of centenarians, people who have lived beyond 100 years, and the lifestyles of the people living in these regions have been studied to determine the factors contributing to their longevity and healthy aging. This research has identified several factors that contribute to this phenomenon, including diet, social connectedness, and physical activity [5–7].
One of the key lifestyle characteristics of Blue Zone populations is their high levels of physical activity, which includes regular walking in addition to other low-intensity physical activities. These populations engage in physical activity as part of their daily routine, such as walking to work or for daily errands, gardening, and performing other manual labor activities. Numerous studies have evaluated the evidence linking walking and physical activity in addition to other lifestyle factors in Blue Zones to healthy aging and longevity. In the Nicoya Peninsula of Costa Rica, physical activity is an integral part of daily life. The region’s terrain is hilly, and the residents often walk long distances to work or to visit friends and family. This continuous movement, coupled with a healthy diet rich in whole grains, fruits, and vegetables, contributes to the long and healthy lives of the Nicoyans. Similarly, on the Greek island of Ikaria, where the terrain is rugged, residents engage in extensive walking and often participate in physical labor activities such as farming and goat herding. In Sardinia, walking and other physical activities also play a crucial role in healthy aging.
To promote healthy aging, health promotion programs should focus on the determinants of healthy aging identified in the Blue Zones. Regular physical activity, including walking, is a fundamental aspect of a healthy lifestyle and is associated with numerous health benefits, particularly in the context of healthy aging and longevity in the Blue Zones. Therefore, health promotion programs designed to promote healthy aging should prominently include recommendations for walking in addition to other forms of regular physical activity, as a way to improve overall health and well-being.
Though the terms “physical activity” and “exercise” are commonly used interchangeably, they are not necessarily the same. Physical activity is defined as any bodily movement produced by skeletal muscles that requires energy expenditure and includes exercise as well as usual occupational and/or domestic activity [8]. In contrast, exercise is intentional physical activity and can include aerobic training, high-intensity interval training, or resistance training [9]. The evidence supporting the health benefits of physical activity and exercise training is extensive and consistent. Regular physical activity is linked to a reduced risk or severity of adverse vascular outcomes, such as cardiovascular disease (CVD) and type 2 diabetes (T2D), as well as non-vascular outcomes, including various cancers, osteoarthritis, and infectious diseases [10–12]. Importantly, the beneficial health effects of physical activity are irrespective of age, sex, ethnicity, or the presence of comorbidities [13]. Furthermore, regular physical activity and exercise training are also well documented to increase levels of cardiorespiratory fitness (CRF) [14, 15], which is one of the strongest predictors of adverse cardiovascular outcomes [16–18]. Cardiorespiratory fitness is often characterized as maximal oxygen uptake (VO2max) or peak VO2 (VO2peak).
Promoting physical activity has been an important strategy to specifically reduce the prevalence and incidence of common cardiometabolic conditions all over the world. Components of physical activity include frequency, duration, and intensity, which together comprise the volume. To derive the maximal benefits of physical activity, an appropriate intensity, frequency, and duration are required.
Physical activity can also be classified based on the level of intensity: light, moderate, and vigorous. Despite current physical activity guideline recommendations, which state that adults should engage in at least 150–300 min of moderate-intensity physical activity or 75–150 min of vigorous-intensity physical activity per week or an equivalent combination of both types of physical activity per week [8, 9, 19], there is a research gap regarding the dose-response association between volume and intensity of physical activity and health outcomes [8, 9].
Walking is the most commonly reported physical activity and is often classified as light or brisk. Light walking is classified as low-intensity physical activity and brisk walking as moderate intensity physical activity. While the cardiovascular benefits of walking are widely acknowledged, there is uncertainty regarding the ideal “dose” required to reap cardioprotective benefits, as well as the impact of walking on non-vascular outcomes. Conflicting data also suggests that the intensity of physical activity may be associated with greater benefits than the quantity [20, 21].
The objective of this review is to provide a comprehensive summary of the extensive literature on the health benefits of walking in older adults, including the cardiovascular benefits and postulated biologic mechanisms underlying the associations between walking and health outcomes. Additionally, this review aims to examine the implications for clinical practice and population health and to provide recommendations for future research directions. Specifically, this review will explore the role of walking in promoting healthy aging and improving health outcomes in older adults, with a focus on the specific recommendations that should be included in health promotion programs targeting physical activity, particularly walking. Furthermore, the review will investigate the anti-aging effects of walking, offering valuable insights into the potential contribution of walking to healthy aging.
Health benefits of walking
Methods
We conducted a thorough search for observational studies, including prospective cohort, nested case-control, case-cohort or retrospective cohort studies, randomized controlled trials (RCTs), and non-RCTs from MEDLINE and EMBASE up to May 2023. Our search focused on the cardiovascular benefits of walking, with a particular emphasis on robust systematic reviews and meta-analyses of these study designs when available, according to the hierarchy of evidence [22]. Our search terms included a range of keywords related to “walking” and cardiovascular health, such as “cardiovascular disease,” “coronary heart disease,” “sudden cardiac death,” “heart failure,” “hypertension,” and “blood pressure,” as well as keywords related to other health outcomes, including “dementia,” “depression,” “anxiety,” “pulmonary disease,” “sleep,” “fracture,” “mortality,” “lipids,” “inflammation,” “oxidative stress,” “arterial stiffness,” “arterial compliance,” and “intima media thickness.” We restricted our review to studies conducted in human populations, reported in English, and in adults.
While “steps per day” is a commonly used metric for quantifying physical activity, it captures all types of activities involving “a movement made by lifting your foot and putting it down in a different place,” including walking and running [23]. As such, this review did not specifically focus on studies that used the measure of “steps per day.” However, we included studies that captured walking activities using accelerometer-derived daily step count, such as 40 steps/min or faster defined as intentional walking or purposeful movement or 100 steps/min defined as moderate walking pace or brisk walk [24]. We excluded studies that examined walking in combination with other physical activity types such as cycling. Additionally, cross-sectional studies were not included as they do not address temporality. An important goal of this review was to provide a comprehensive overview of the existing evidence on the cardiovascular and other health benefits of walking and to explore the biologic mechanisms underlying these associations in promoting healthy aging.
Cardiovascular outcomes
Cardiovascular risk factors
Several observational cohort and interventional studies have explored the impact of walking on cardiovascular risk factors. However, the results of these individual studies have been conflicting, leading to several systematic reviews and meta-analyses on the topic. One meta-analysis conducted by Kelley and colleagues [25] evaluated the effects of walking on resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) by pooling data from 16 RCTs and non-RCTs. The study showed that walking exercise programs led to mean reductions in SBP and DBP of 3 and 2 mmHg, respectively. Another meta-analysis by Murphy and colleagues [26] pooled data from 24 RCTs to quantify the effect of walking interventions on selected risk factors, including aerobic fitness, blood pressure, and measures of body composition. The study showed that walking interventions increased aerobic fitness and decreased body weight, body mass index (BMI), percent body fat, and resting DBP in sedentary adults. Other meta-analyses have shown that walking significantly decreased glycated hemoglobin (A1c), BMI, and DBP, and increased VO2max, while having no effect on high-density lipoprotein cholesterol (HDL-C) or low-density lipoprotein cholesterol (LDL-C) levels [27–32]. Overall, these findings suggest that walking is associated with significant improvements in cardiovascular risk factors and also has the potential to be used as a therapeutic tool for individuals with T2D [33].
Regular exercise, including walking, has a profound impact on endothelial function, which plays a critical role in cardiovascular health [34–38]. Endothelial cells line the inner surface of blood vessels and are responsible for regulating vascular tone and thereby blood pressure, maintaining vascular integrity, regulating hemostasis, and platelet aggregation. The preservation of endothelial health is paramount in preventing the development of atherosclerosis and pathological vascular remodeling in large vessels. Equally important is the maintenance of microvascular endothelial health, as it plays a crucial role in preserving capillary architecture, regulating the tone of resistance arteries to ensure adequate nutrient and oxygen delivery to tissues, and maintaining barrier function, such as the integrity of the blood-brain barrier. Additionally, microvascular endothelial cells are involved in modulating the exchange of molecules, regulation of immune cell function, including leukocyte extravasation, and supporting the maintenance of stem cell niches. Aging is commonly associated with generalized endothelial dysfunction, which negatively impacts the proper functioning of both the large vessels and the microcirculation [39–42]. This age-related endothelial dysfunction compromises the overall health and disrupts the homeostasis of various tissues and organ systems. Age-related endothelial dysfunction contributes to the development of macrovascular (atherosclerotic diseases, including stroke, coronary artery disease, peripheral artery disease) and microvascular diseases (including microvascular pathologies affecting the heart brain, kidneys, skeletal muscle) [39–42]. Exercise promotes endothelial health in aging by promoting an anti-inflammatory, anti-atherogenic gene expression profile, stimulating the production and release of vasodilator nitric oxide (NO), and promoting angiogenesis [43–46]. Exercise-induced shear stress plays a crucial role in regulating various aspects of endothelial function and phenotype. Increased blood flow and shear stress during exercise trigger the release of endothelial-derived NO, leading to vasodilation, lowering blood pressure, and improving tissue perfusion [45–48]. This vasodilatory effect not only enhances oxygen and nutrient delivery, but also facilitates the removal of waste products. Shear stress can modulate the expression of pro-atherogenic and anti-atherogenic genes and activates various intracellular signaling pathways, including those involved in antioxidant defense and vascular remodeling. These mechanisms collectively contribute to the maintenance of a youthful endothelial phenotype and the prevention of endothelial dysfunction and atherosclerotic plaque formation. By promoting favorable shear stress and regulating gene expression, exercise serves as a powerful modulator of endothelial function. By enhancing endothelial function, exercise promotes optimal cardiovascular function and vascular health in older adults and reduces the risk of endothelial dysfunction-associated conditions such as hypertension, atherosclerosis, and CVD.
Hypertension
The blood pressure–lowering effect of walking has been widely investigated in observational cohort studies [27, 31, 32, 49, 50] and RCTs, as discussed in the previous section on cardiovascular risk factors. Prospective cohort studies have also reported associations between walking and the risk of hypertension. For instance, a study of 6017 Japanese men (aged 35–60 years) without a history of hypertension or diabetes at baseline found that walking for longer durations was associated with a reduced risk of hypertension [51]. Specifically, compared to a walk of 10 min or less, an 11- to 20-min walk and a walk of 21 min or more were associated with a 12% and 29% lower risk of hypertension, respectively [51]. Similarly, a study of 15,357 university graduates initially free of chronic disease or hypertension found that a normal, brisk, or very brisk walking pace was each associated with a reduced risk of hypertension compared to a slow walking pace, after adjusting for established risk factors [52]. Moreover, a recent prospective analysis of 83,435 postmenopausal women (aged 50–79 years) without known hypertension, heart failure, coronary heart disease (CHD), or stroke found that walking at guideline-recommended volumes (>7.5 MET hours per week) and at faster speeds (≥2 miles per hour) was associated with a lower risk of hypertension [53].
Cardiovascular and cerebrovascular diseases
Several prospective epidemiological studies have been conducted to investigate the associations between walking and CVD outcomes [54]. In 2008, Hamer and Chida conducted the first systematic review and meta-analysis of these studies [55], which included 18 prospective studies comprising 459,833 participants free from CVD at baseline with 19,249 CVD cases at follow-up. The authors found that comparing the highest versus the lowest walking category was associated with a 31% reduced risk of CVD. Another meta-analysis by Zheng and colleagues in 2009 found that an increase of approximately 30 minutes of normal walking a day for 5 days a week was associated with a 19% reduction in CHD risk, with no evidence of a difference between men and women [56]. Other studies have reported dose-response reductions in CVD risk with higher walking duration, distance, energy expenditure, and pace [57, 58]. Additionally, walking at a brisk/fast pace was associated with a 24% and 21% reduced risk of CVD mortality, respectively, compared with walking at a slow pace [59]. Other studies reached similar conclusions. In analysis of the UK Biobank comprising 318,185 participants, Celis-Morales and colleagues [54] investigated the associations between usual walking pace and a range of health outcomes. In fully adjusted models, compared to slow pace walkers, men and women with a brisk walking pace had a 38% and 53% reduced risk of CVD mortality, respectively [54]. In another analysis of the UK Biobank cohort, slow walking pace compared with average walking pace was associated with a higher risk of stroke (hazard ratio, HR = 1.45) in the overall study population of 363,137 participants [60]. In a subgroup analysis, the association was only existent among participants aged ≥65 years (HR = 1.42) [60]. In pooled analysis of 8 prospective cohort studies that examined the association between walking pace and stroke risk, individuals in the fastest walking-pace category had a 44% lower risk of stroke compared to individuals in the slowest walking-pace category [61]. In dose-response analysis, every 1 km/h increment in baseline walking pace was associated with a 13% decreased risk of stroke [61]. In addition to walking pace (intensity), a number of individual studies have reported incremental dose-response reductions in the risk of adverse cardiovascular outcomes in relation to increasing walking duration or distance and higher energy expenditure from walking [62–68]. In a recent analysis of the UK Biobank in which data on accelerometer-measured daily step count was available for 78,500 individuals, more daily steps (including purposeful or intentional walking steps) were associated with lower CVD incidence and mortality [69].
Cognitive function and dementia
The evidence regarding the relationship between physical activity and adverse cognitive outcomes such as cognitive impairment, Alzheimer’s disease (AD), and dementia has been inconsistent. While some studies have reported decreased dementia risk with higher physical activity [70, 71], others have found no association [72–74]. Similar inconsistencies have been found in individual studies of walking with cognitive outcomes. However, a meta-analysis of 17 prospective cohort studies evaluating the association of walking pace with the risk of cognitive decline and dementia among elderly populations found that comparing the lowest to the highest category of walking pace was associated with an increased risk of cognitive decline (relative risk, RR = 1.89) and dementia (RR = 1.66) [75]. Moreover, with every 1 dm/s (360 m/h) decrement in walking pace, the risk of dementia increased by 13% [75]. Another study assessed the dose-response association of daily step count and intensity with the incidence of all-cause dementia among adults [76]. It found that approximately 9800 steps per day may be optimal to reduce the risk of dementia; a minimum dose of 3800 steps per day was associated with a 25% lower risk of dementia [76]. In addition, steps performed at higher intensity resulted in stronger associations [76]. While the evidence is not yet definitive, these studies suggest that walking and higher levels of physical activity may be beneficial for cognitive health. There is increasing evidence that microvascular pathologies play a critical role in the pathogenesis of cognitive impairment and dementia [77–84]. While the exact mechanisms are still being studied, it is becoming increasingly clear that walking and other forms of physical activity have a more profound effect on endothelial cell physiology and the genesis of vascular cognitive impairment (VCI) than on amyloid pathologies within the brain parenchyma. As such, it may be important for future studies to separately investigate the effects of walking on VCI and AD, in order to better understand the complex relationships between physical activity, brain health, and cognitive outcomes.
Type 2 diabetes mellitus
In a 2007 meta-analysis of 10 prospective studies that investigated the association between moderate-intensity physical activity and the risk of T2D, the pooled analysis of 5 studies specifically evaluating the role of walking showed that regular walking (approximately ≥2.5 h/week) was associated with a 30% reduced risk of T2D compared with almost no walking [85]. In 2020, Ballin and colleagues [86] examined the association between daily step count (assessed with accelerometer with activity threshold set to >100 counts/min) and incident diabetes in 3055 community-dwelling 70 year olds. Participants who took ≥ 4500 steps/day had a 59% lower risk of diabetes compared to those taking fewer steps. Furthermore, the dose-response analysis indicated a steep decline in the risk of diabetes until around 6000 steps/day, with the risk decreasing at a slower rate until it levelled off at around 8000 steps/day [86]. In a recent analysis of 162,155 UK Biobank participants, both average and slow walking pace were each associated with a higher risk of incident T2D compared to brisk walking in both men and women, independent of major confounding factors [87]. Furthermore, recent results from the population-based prospective cohort Hispanic Community Health Study/Study of Latinos, which included 6634 adults, demonstrated that accumulating more daily steps (including purposeful walking steps or brisk walk) and greater step intensity were associated with a reduced risk of diabetes [88].
All-cause mortality
In the study by Hamer and Chida [55], which investigated the relationship between walking and the risk of all-cause mortality, the highest versus the lowest walking category was associated with a 32% reduced risk of mortality. Similar to the findings for CVD, the results were not significantly different for men and women, with walking pace being a stronger independent predictor compared to walking volume [55]. High walking volume or intensity was associated with the strongest risk reduction [55]. In a pooled analysis of 14 prospective cohort studies, Kelly and colleagues [50] showed an incremental reduction in the risk of all-cause mortality with high walking volume, with a standardized dose of 11.25 MET-hours per week being associated with an 11% risk reduction.
Stamatakis and colleagues found that walking at an average or brisk/fast pace was associated with a 20% and 24% reduced risk of all-cause mortality, respectively, compared to walking at a slow pace [59]. In an analysis of the UK Biobank cohort, Celis-Morales and colleagues found that men and women with a brisk walking pace had a 21% and 27% reduced risk of all-cause mortality, respectively, compared to slow pace walkers [54]. Furthermore, more daily steps, including purposeful or intentional walking steps, up to approximately 10,000 steps, were associated with a lower risk of all-cause mortality in the UK Biobank analysis [69]. A study of 17,466 women (aged 62–101 years) found that approximately 4400 steps per day was associated with a 41% reduction in mortality rate compared with approximately 2700 steps per day, with a steady decline in mortality rates up to approximately 7500 steps per day, beyond which mortality rates levelled [89]. However, the time spent at a stepping rate of 40 steps/min or faster (intentional walking) was not clearly related to mortality risk.
In a recent meta-analysis of 15 international cohorts investigating the associations of daily step count and stepping intensity with all-cause mortality, it was demonstrated that taking more steps per day was associated with progressively lower risk, up to a level that varied by age: 6000–8000 steps per day among adults aged 60 years and older and 8000-10,000 steps per day among adults younger than 60 years [24]. However, the time spent walking at 40 steps/min or faster (intentional walking) and 100 steps/min or faster (defined as moderate rate walking pace) was not found to be significantly associated with mortality [24].
Cancer
Stamatakis and colleagues [59] conducted a prospective pooled analysis of 11 population-based baseline surveys in England and Scotland in 2018. Their findings reported no evidence of an association between walking pace and cancer mortality. Similarly, Celis-Morales and colleagues [54] found no evidence of associations between walking pace and all-cause cancer, colorectal, and breast cancer in their analysis of the UK Biobank cohort; however, brisk walking was associated with a higher risk of prostate cancer. On the other hand, a recent analysis of the UK Biobank cohort, which measured accelerometer-based daily step count in 78,500 individuals, showed that accruing more daily steps, including intentional walking steps, was associated with a lower risk of incident cancer and mortality due to cancer [69].
Respiratory pathologies
Celis-Morales and colleagues [54] found, in their analysis of the UK Biobank cohort, that brisk walking was associated with reduced risk of respiratory disease in both men and women. Compared to slow pace walkers, men and women with a brisk walking pace had a 34% lower risk of respiratory disease. The corresponding risk reduction for chronic obstructive pulmonary disease was even greater, at 65% and 72%, respectively. Furthermore, several prospective studies have reported that daily walking habits are associated with a reduced risk of pneumonia-related mortality in older people, with risk reductions ranging from 10 to 42% [90–92]. Although two of the studies did not consider other forms of physical activity besides walking, one study demonstrated that daily walking alone was sufficient to reduce pneumonia-related mortality among older people who do not engage in other exercise habits regularly [90].
Bone health
Regular physical activity and exercise have been shown to have a positive impact on bone health, reducing the rate of bone loss, conserving bone tissue, increasing bone mineral density (BMD), and lowering the risk of fractures [93, 94]. Weight-bearing endurance activities, muscle-strengthening physical activity, balance exercise, and resistance exercise are recommended in various guidelines to preserve bone health and reduce the risk of falls [94, 95]. However, there is uncertainty about the type and intensity of exercise that is beneficial for bone health. Although some systematic reviews and meta-analyses of RCTs have shown no significant effect of regular walking on BMD in perimenopausal and postmenopausal women [96, 97], others have demonstrated a positive effect on lumbar BMD but not on the femur or calcaneus [98]. One conclusion is that walking alone is not sufficient for those at risk of osteoporosis, and that other forms of exercise in addition to walking should be incorporated [98]. Nevertheless, a recent study suggested that a training program comprising fast walking and running exercises may increase or preserve BMD at the femoral neck in postmenopausal women [99]. Pooled analysis of results from RCTs and quasi-RCTs of adults with chronic musculoskeletal pain showed that walking was associated with significant improvements in pain and function, but the longer-term effectiveness was uncertain [100]. Analysis of data from the Nurses’ Health Study and the Women’s Health Initiative prospective cohort study showed that walking was associated with a lower risk of hip fracture among postmenopausal women [101, 102]. However, in a 5-year follow-up of an Australian population-based prospective study comprising postmenopausal women and men aged 50 years or older, individuals who walked more than 3 h per week had an increased risk of fractures compared with those who reported no walking [103]. Overall, while walking may have some positive effects on bone health, it is important to consider incorporating other types of exercise to optimize bone health outcomes.
Sleep health
Regular physical activity has been shown to improve sleep quality and duration, but there is ongoing debate regarding the types of physical activity that are most effective in promoting better sleep [104, 105]. Wilbur et al. conducted a RCT to evaluate the impact of a 24-week, home-based, moderate-intensity walking intervention on various menopausal symptoms, including sleep, in 173 sedentary midlife women (aged 45–65 years) [106]. The study found that the frequency of adherence to walking significantly influenced a positive change in sleep symptoms. In a longitudinal study of 103 midlife women (average age = 53, range 40–60 years), increased activity levels during the day were associated with an increase in total sleep time at night, with a stronger protective effect observed in overweight and obese women [106]. A recent 4-week RCT that assessed the effect of walking on sleep quality and duration in 59 healthy participants (average age of 49 years) observed a positive relationship between daily active minutes and sleep quality, but not duration. Women who were more active and took more steps also reported better sleep quality compared to those who were less active [107].
Mental health conditions and quality of life
Physical activity and exercise have well-documented mental health benefits, even at levels below public health recommendations [108–111]. Studies have shown that physical activity is associated with a reduced risk of depression, with evidence suggesting a causal relationship [112]. In addition to the physical health benefits of walking, it has the potential to enhance emotional and psychological well-being, improve mood, and reduce the risk of various mental conditions. Recent research has demonstrated the effectiveness of walking in reducing the symptoms of depression compared to non-walking interventions such as social support, stretching, and cognitive interventions [113]. Sessions ranged from 20 to 50 min per day to 5 times per week over 6.2 days to 6 months [113]. In another study, brisk walking was found to improve mood state [114], and walking has also been shown to boost creative inspiration. People’s creative output increased by an average of 60% while walking compared to sitting, according to experiments by Oppezzo and Schwartz [115]. Furthermore, walking has been positively linked to various aspects of health-related quality of life [116, 117].
Cellular and molecular pathways contributing to the anti-aging health benefits of low-intensity exercise and walking
Endocrine and metabolic pathways
The health benefits of physical activity or exercise training are well documented and observed across multiple organ systems including the cardiovascular system. These benefits are achieved through several mechanisms, such as improvements in intermediate or cardiovascular risk factors including BMI, blood pressure, endothelial function, blood glucose, and insulin resistance [27, 29] (Fig. 1). This is consistent with some studies of the associations between walking and adverse cardiovascular outcomes which have reported incomplete attenuations of the associations following adjustment for cardiovascular risk factors such as BMI [58]. However, evidence suggests that these pathways may not completely account for the effects of physical activity or exercise on cardiometabolic health. Emerging evidence indicates that exercise triggers the release of exerkines, which exert their effects through endocrine, paracrine, and/or autocrine pathways [118]. Exerkines have potential roles in improving cardiovascular, metabolic, immune, and neurological health. For instance, exerkines produced by the cardiovascular system could mitigate systemic inflammation and ischemia, while those produced in adipose tissue enhance lipolysis, thermogenesis, and glucose metabolism. Extracellular vesicles have also been implicated in mediating the systemic benefits, including anti-aging effects, of exercise [119–128]. These vesicles encompass all membranous structures that cells secrete and were proposed as mediators of intercellular communication in both physiological and pathological conditions [129–131]. Although their exact function is not yet well understood, they may modulate immune responses, metabolism, angiogenesis, tissue maintenance, and repair [119–128, 132] through cell non-autonomous mechanisms. The observation that senescent cells exhibit an increased release of extracellular vesicles, coupled with an altered compositional profile, posits a compelling implication in their role as mediators of paracrine senescence during the aging process [133–138]. During exercise, muscle and other tissues increase the release of extracellular vesicles with a cargo that may contribute to the mediation of systemic effects of exercise [119–128, 139]. Physical activity is known to have favorable effects on lipid metabolism, reducing levels of serum triglycerides and LDL-C and increasing levels of HDL-C. It has been reported that 3.5–7 h of moderate to vigorous physical activity per week or 30–60 min of exercise on most days could reduce triglycerides by up to 50%, reduce LDL-C by up to 5% and increase HDL-C by 5–10% [13]. However, the evidence collected so far suggests that walking may not have significant effects on lipid profiles [27, 29]. This observation may be related to the intensity of physical activity, as walking may not provide enough intensity to improve lipid profiles, especially in those with hypercholesterolemia or hypertriglyceridemia [13]. The cardiovascular benefits of walking may also be influenced by confounding or interaction with other physical activity types, given that those who walk may also engage in other types of physical activity that have a protective effect on cardiovascular risk [140]. Furthermore, walking is an enjoyable physical activity that can reduce stress, enhance psychological well-being and trigger the release of endorphins, which promote relaxation and improve mood [141]. It is well known that ongoing stress is associated with an increased risk of physical ailments including CVD and cancer, as well as mental health issues and adverse effects on overall health [142].
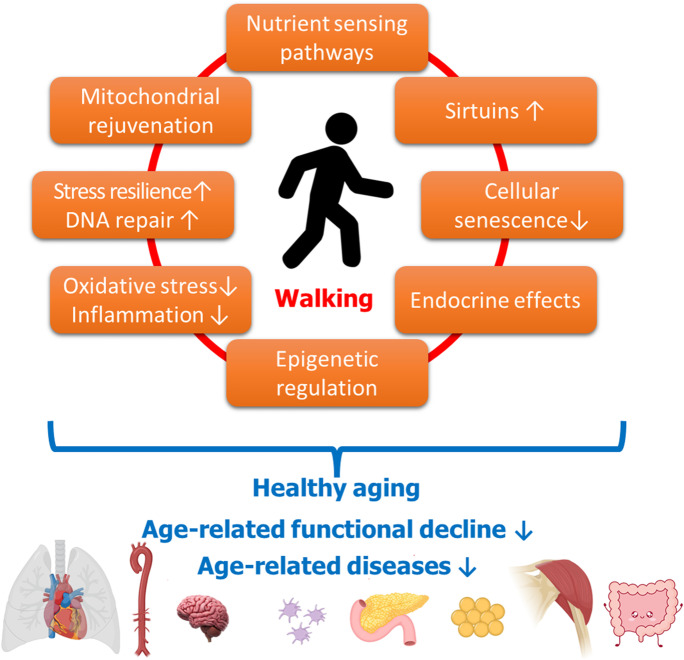
Fig. 1.
Mechanisms mediating the anti-aging health benefits of light physical exercise and walking. This figure illustrates how light physical exercise and walking contribute to healthy organismal aging by potentially reversing or attenuating underlying cellular and molecular mechanisms of aging. By preventing or delaying age-related functional decline and the onset of age-related diseases in multiple organ systems, exercise and walking promote overall anti-aging effects
Physical activity plays a crucial role in influencing hormonal changes associated with aging, particularly in relation to insulin-like growth factor 1 (IGF-1) [143–146]. Epidemiological studies have consistently shown that circulating IGF-1 levels decline with age, which has been linked to the development of various age-related conditions and diseases [147–150]. Interestingly, centenarians have been found to have higher levels of IGF-1 compared to younger individuals [151]. Animal studies further support the pleiotropic anti-aging effects of IGF-1 [151, 152], including its atheroprotective, microvascular protective, and neuroprotective properties [79, 151, 153–160]. While genetic IGF-1 deficiency in humans is associated with shortened lifespan [161], certain genetically modified mouse models with IGF-1 deficiency show lifespan extension due to its anti-cancer growth action [162]. In preclinical models of aging, exercise has been shown to enhance skeletal muscle mass, exercise capacity, metabolism indicators, and protein synthesis, while reducing oxidative stress and apoptosis through the activation of the IGF-1 pathway [146]. However, studies on the effect of physical activity on IGF-1 levels in humans have yielded mixed results [143, 145, 163–166], with some suggesting that physical activity increases circulating IGF-1 levels [143, 167]. The specific effects of walking on IGF-1 levels are less well understood and require further investigation [167, 168].
Age-related changes in sex hormone levels, such as testosterone and estrogen, are also well-documented phenomena of aging [169]. In men, declining levels of androgens, including testosterone, are associated with decreased muscle mass and strength, reduced bone mineralization, and increased central body fat [169]. Resistance training has been shown to increase testosterone levels [169]. As for walking, studies suggest that individuals who take more than 4000 steps daily are less likely to have low testosterone levels, with an approximate increase of 7 ng/dL for every additional 1000 steps taken [170].
In women, estrogen levels decrease with age, which can impact longevity [169]. Estrogen plays a role in antioxidant activity, membrane stabilization, and maintenance of bone mass [169]. Physical activity, including exercise programs, can help counterbalance the decline in estrogen levels observed in postmenopausal women [169]. A 12-week exercise program, for example, has been shown to improve estradiol levels in postmenopausal women, with anaerobic exercises potentially having a more substantial effect compared to aerobic exercises [171]. Further research is needed to investigate the specific effects of walking interventions on estrogen levels in older females.
Understanding the intricate relationship between physical activity, hormonal changes, and the aging process is essential for developing comprehensive interventions to promote healthy aging and prevent age-related diseases. Further studies are warranted to explore the mechanisms and differential effects of walking interventions on hormone levels in older adults, with the ultimate goal of optimizing health outcomes and improving overall well-being.
While the health benefits of physical activity and nutrition are often studied separately, it is widely recognized that they are both vital aspects of a healthy lifestyle and contribute to healthy aging. Integrating nutrition and physical activity can yield more substantial positive health outcomes compared to approaches that solely focus on one or the other [172]. This comprehensive approach recognizes that optimal nutrition plays a crucial role in facilitating exercise performance and enhancing the beneficial effects of exercise. Incorporating natural food components with physiological actions, often referred to as “functional foods” [173, 174], can provide essential nutrients, improve performance and endurance, enhance muscle strength, prevent injury and fatigue, and maintain immunity [175]. Moreover, nutrition therapy has emerged as a promising approach to increasing cardiorespiratory fitness levels among diverse populations with exercise limitations, including those with chronic obstructive pulmonary disease, heart failure, obesity, sarcopenia, and frailty [14, 176–181]. By combining physical activity and optimal nutrition, individuals can optimize their overall health, promote healthy aging, and enhance their quality of life [182–191].
Effects on cellular and molecular mechanisms of aging
In this section, we explore the effects of low intensity exercise on the fundamental molecular and cellular mechanisms of aging, drawing primarily from experimental studies conducted on rodent models with different exercise paradigms in laboratory settings. Wherever available, we will also discuss relevant human data on the effects of exercise on these mechanisms. These studies have shed light on potential mechanisms through which exercise may exert its beneficial effects on aging. However, it is important to acknowledge that while we review these mechanisms for the benefit of the reader, the direct extrapolation of findings from rodent studies to the effects of walking in humans is still speculative and warrants further investigation. Additionally, extrapolating results on the effects of different exercise regimens from human studies to the specific context of walking can also be challenging, given the unique characteristics and physiological responses associated with this specific mode of physical activity.
The current view is that, in general, exercise exerts multifaceted effects on synergistic cellular and molecular mechanisms that underlie the aging process, targeting various hallmarks of aging.
First, exercise may promote DNA repair and maintenance, enhancing genome stability and reducing the accumulation of DNA damage over time [192–195]. It activates DNA repair enzymes and increases the expression of proteins involved in DNA damage response pathways [196, 197]. Additionally, exercise can modulate telomere length and telomerase activity, which are associated with cellular senescence and aging [198–205].
Second, exercise mitigates oxidative stress [36, 45, 206], a key contributor to cellular aging and the pathogenesis of age-related diseases in various organ systems [42, 82, 84, 207–221]. It enhances antioxidant defenses, increases the expression of endogenous antioxidant enzymes, improves mitochondrial function, and reduces the production of reactive oxygen species (ROS) [222–224].
Third, exercise influences cellular senescence and inflammation, two interconnected hallmarks of aging. Cellular senescence refers to a state of irreversible cell cycle arrest that occurs in response to various stressors, such as DNA damage. Senescent cells accumulate with age and are implicated in tissue dysfunction and the pathogenesis of age-related diseases [80, 225–232]. Cellular senescence is characterized by the secretion of a complex mixture of pro-inflammatory molecules, known as the senescence-associated secretory phenotype (SASP) [233]. The SASP includes a variety of inflammatory cytokines, chemokines, growth factors, and enzymes involved in remodeling of the extracellular matrix (i.e., matrix metalloproteinases), which can promote local and systemic inflammation. This chronic low-grade sterile inflammation, often referred to as “inflammaging,” contributes to the development of a wide range of age-related diseases and tissue dysfunction. Exercise has been shown to attenuate the accumulation of senescent cells [234, 235] and reduce the production of pro-inflammatory cytokines [236–239], thereby promoting an anti-inflammatory environment and dampening inflammaging.
Fourth, exercise modulates metabolism and energy homeostasis, regulating key nutrient sensing pathways such as insulin/IGF-1 signaling, the mammalian target of rapamycin (mTOR) pathway [240–244], and activating sirtuins and AMP-activated protein kinase (AMPK) [245–249]. These pathways play a crucial role in the regulation of aging by sensing the availability of nutrients and energy levels in cells and modulating various cellular processes including metabolism, mitochondrial function, protein synthesis, and stress responses and cellular resilience.
Fifth, exercise also exerts beneficial effects on the mitochondria. Members of the sirtuin family of NAD+-dependent deacylases (SIRT-1, SIRT-3) play a critical role in regulation of mitochondrial biogenesis and bioenergetics, cellular resilience, and organismal lifespan [83, 219, 250–263]. Aging is associated with a decline in mitochondrial function, partially driven by mitochondrial DNA damage, dysregulation of mitochondrial gene expression, decline in SIRT-1 and SIRT-3 activity, and uncoupling of the electron transport chain [264–270]. This reduction in mitochondrial function can promote various age-related conditions, including sarcopenia and cardiovascular and cerebrovascular diseases [84, 207, 212, 264, 269, 271–273], due to increased mitochondria-derived production of reactive oxygen species (ROS), impaired cellular energetics, decreased cellular adenosine triphosphate levels, increased apoptosis, and cellular injury. Importantly, exercise has been shown to counteract these processes and improve mitochondrial function [196, 222, 223, 274–276]. Protective mechanisms induced by physical activity include the activation of the PGC-1α-dependent pathway, which promotes mitochondrial biogenesis, the reduction of mitochondrial ROS production and activation of autophagy, and the mitochondrial unfolded protein response [222, 223, 274–283]. Aerobic training sessions have been shown to upregulate sirtuins in skeletal muscle and other tissues, which in turn activates biogenesis and mitochondrial oxidative capacity [248, 262, 284–290]. Additionally, studies have demonstrated that resistance training can increase the activity of complex IV enzymes, which is associated with improved oxidative capacity. These findings have been observed in both animal and human studies, indicating the positive impact of exercise on mitochondrial function. While in-depth studies investigating the effects of walking on mitochondrial function are limited, initial studies have shown promising effects of walking interventions on mitochondrial function [291]. Future studies should determine how regular walking affect mitochondrial biogenesis and mitochondrial function in older adults in different tissues. Importantly, the quality of mitochondrial function can also influence an individual’s ability to engage in physical activity. Thus, there is a reciprocal relationship between physical activity and mitochondrial function, reinforcing the importance of exercise as a means to promote healthy aging and maintain optimal cellular metabolism.
Sixth, exercise affects cellular and tissue regeneration, promoting the maintenance and functionality of stem cells [292, 293]. It stimulates the release of growth factors and cytokines that support tissue repair and regeneration [145, 294–298].
Finally, exercise exerts a significant influence on the epigenetic regulation of aging processes, encompassing DNA methylation [299, 300], sirtuin activation, and histone acetylation [285, 287], thereby modulating gene expression patterns associated with aging and contributing to the maintenance of a youthful cellular phenotype. Various aging clocks have been developed to estimate biological age by measuring specific molecular and cellular biomarkers, including DNA methylation patterns and gene expression profiles. Exercise has emerged as a promising modality to positively influence biological age [300]. Studies have demonstrated that regular physical activity, including both aerobic and resistance exercise, is associated with a slower rate of biological aging as measured by different aging clocks. However, further research is needed to fully understand the extent to which exercise in general and walking interventions in particular can influence and reverse biological aging and to explore the potential of exercise interventions as a means to target and modify the trajectory of biological age.
By targeting these evolutionarily conserved mechanisms of aging, exercise exerts a holistic and profound impact on the aging process, promoting enhanced cellular function, tissue health, and overall longevity. Exercise paradigms serve as powerful interventions capable of delaying cellular aging processes, postponing the onset of age-related diseases and fostering healthy aging, as supported by a wealth of evidence from both preclinical and clinical studies. Future research is warranted to elucidate the extent to which uncomplicated, self-directed walking interventions can confer comparable benefits [237, 238, 301–303], as this knowledge holds great potential for promoting accessible and effective strategies for healthy aging.
Adverse effects of walking
Despite the substantial health benefits associated with regular physical activity, vigorous-intensity physical activity may act as a trigger for cardiovascular outcomes such as ventricular arrhythmias, sudden cardiac arrest, sudden cardiac death, and acute coronary syndromes such as myocardial ischemia and myocardial infarction, transient ischemic attacks (TIAs), and cerebrovascular accidents strokes [13]. The risk of these outcomes is greatest in athletes and in people who do not habitually perform such intense physical activity. In athletes, it appears the intensities and volumes of these vigorous-intensity physical activity regimens far exceed those proposed by guideline recommendations [8, 9, 304]. Nevertheless, there is unequivocal evidence that the benefits of physical activity outweigh its potential adverse effects in healthy individuals. Walking is described as a low- to moderate-intensity physical activity; there is currently little evidence to suggest an increase in injuries or serious adverse events due to walking apart from a few isolated reports of calf injuries and falls, which occurred in people with conditions that put them at risk of these events [28]. However, too much walking could also be harmful especially in individuals who are not properly conditioned. If new to walking, it is essential to start slowly and gradually build up your duration and intensity.
Optimizing physical activity: from brisk walking to step goals and health benefits
There is irrefutable evidence that adherence to current physical activity guideline recommendations [8, 9, 19] can reduce the risk of chronic diseases such as CVD and T2D and contribute to overall health. Brisk walking is an example of a moderate-intensity physical activity that counts towards the weekly recommended physical activity goals. While any type of walking can be beneficial, walking at a faster pace is associated with better cardiovascular and overall health compared to walking at a slow pace. A brisk walk of at least 30 min per day for 5 days allows one to meet the current physical activity guideline recommendations of at least 150–300 min of moderate-intensity aerobic physical activity per week [8, 9, 19]. Emerging data from prospective studies suggest that the cardiovascular and mortality benefits of physical activity can be achieved through both concentrated and spread-out patterns of activity [305]. The so-called weekend warrior pattern, with physical activity concentrated in 1 or 2 sessions per week, may be suitable for individuals with busy lifestyles who cannot meet the recommended physical activity levels. However, it should be noted that some beneficial effects of physical activity, such as reductions in blood pressure and lipids, are acute and need to be sustained by chronic regular physical activity [306, 307]. Additionally, the weekend warrior pattern may be more likely to be associated with musculoskeletal injuries and may not be suitable for people with comorbidities or musculoskeletal disorders.
It has been proposed that physical activity recommendations should be translated into step- or pedometer-based guidelines, as this could increase the clinical and public health impact of physical activity promotion [69, 76, 308]. A recent review of objectively measured physical activity types with clinical outcomes demonstrated that step count was the strongest and most consistently associated with a wide range of clinical outcomes [309]. In a study that sought to convert physical activity recommendations into a pedometer-based step goal, moderate-intensity walking was estimated to be approximately equal to at least 100 steps/min [308]. To achieve physical activity recommendations of at least 150 min per week of moderate-intensity physical activity, individuals needed to achieve the goal of walking a minimum of 3000 steps in 30 min for 5 days per week [308]. A goal of 10,000 steps per day has been widely promoted for decades as being the number associated with optimal health benefits [89, 310]. Recent evidence suggests that aiming for 8000 to 10,000 steps per day can substantially reduce the risk of CVD, diabetes, dementia, and premature death, and this goal is more attainable than the widely promoted reference of 10,000 steps per day [24, 69, 76, 86, 89]. Research suggests that the relationship between step count and health outcomes follows a curvilinear pattern, indicating that the benefits associated with increasing step count may be more pronounced for individuals with lower step volumes [76]. As step count increases, the protective effects on health outcomes tend to attenuate [76]. However, it is important to note that these recommendations may differ for aging adults. Specifically, a study has shown that even a modest increase in step count can have a significant impact on all-cause mortality in older adults aged 60 or more. In this study, it was found that taking as few as 6000 steps per day was associated with a reduction in mortality risk [24]. This highlights the importance of encouraging regular physical activity, such as walking, among aging individuals to improve their overall health and longevity. Another aspect to consider in relation to step count is cadence, which refers to the number of steps taken per minute. A study conducted in elderly patients found that those with a cadence of 100 steps or more per minute had a 21% lower risk of all-cause mortality compared to individuals with slower cadences [311]. Furthermore, for each ten-step increase in cadence, there was an additional 4% reduction in mortality risk. This suggests that not only the overall step count but also the pace or cadence at which one walks may have implications for health outcomes, particularly in older adults. These findings highlight the importance of considering both step count and cadence in promoting physical activity among individuals of different age groups. Encouraging individuals to increase their step count, especially those with lower step volumes, can have significant health benefits. Additionally, emphasizing the importance of maintaining a brisk walking pace or higher cadence may further enhance the positive impact on health outcomes, particularly in the older adult population.
Although the dose-response relationships between walking and cardiovascular risk factors have not been well quantified, pooled analysis of RCTs that have evaluated walking interventions for a minimum of 4 weeks has reported clinically important reductions in SBP and DBP of approximately 4–5 and 2 mmHg, respectively [25, 29, 32]. These blood pressure reduction effects are more pronounced in adults with high baseline blood pressure [49]. A 2-mmHg reduction in SBP could reduce mortality from stroke and vascular causes by 10% and 7%, respectively [312]; SBP reductions of 5–7 mmHg among individuals with hypertension translate to a 20–30% reduced risk of CVD [313], and a 2-mmHg reduction in DBP could reduce the risk of CHD by 6% and stroke and TIAs by 15% [314].
Additionally, walking has been demonstrated to increase levels of CRF [16–18], a strong predictor of adverse cardiovascular outcomes [315, 316], and middle-aged individuals who meet current recommendations for moderate-intensity physical activity (such as walking) are more likely to achieve at least moderate levels of CRF [317, 318].
Conclusion and perspectives
In conclusion, the evidence overwhelmingly supports walking as a powerful anti-aging intervention that can reduce the risk of chronic age-related diseases such as CVD, hypertension, T2D, and cancer. Walking also improves pain and function in musculoskeletal disorders, promotes sleep and mental health and increases resilience. A brisk walk for at least 30 min, 5 days a week, is recommended to meet physical activity guidelines. Emerging data suggest that both concentrated and spread-out physical activity patterns can provide similar cardiovascular and mortality benefits. Step count is a strong and consistent predictor of clinical outcomes, and aiming for 8000 to 10,000 steps per day could substantially reduce the risk of a range of age-related diseases. Although some benefits of physical activity are acute, sustained and regular physical activity is necessary to maintain these effects. Overall, walking is a simple and effective intervention that can be easily integrated into daily routines to promote healthy aging and prevent chronic age-related diseases. Although it is not as high intensity as other physical activity types such as running, its health benefits are substantial and are irrespective of age, sex, race, or geographical location. Incorporating regular walking into daily routines should be encouraged as a key strategy for healthy aging and disease prevention.
Despite established physical activity guidelines and targets in most countries, and the World Health Organization’s recommendation that all nations implement policies to facilitate physical activity regardless of age or disability, global participation in physical activity has not improved over the last two decades. Recent estimates indicate that one in four adults do not meet aerobic exercise recommendations [319].
Walking-based interventions have the potential to improve health outcomes and promote healthy aging in a variety of populations, including employees at sedentary jobs at the workplace, older adults, individuals with chronic conditions, and those at risk for age-related diseases. One key advantage of walking-based interventions is their accessibility and affordability. Walking requires no special equipment or facilities and can be done at any time of day, making it an ideal form of physical activity for people of all ages and abilities. Furthermore, walking can be incorporated into daily routines, such as commuting to work, running errands, or taking leisurely strolls, making it an easy and convenient way to increase physical activity levels. Walking-based interventions have been shown to be effective in a variety of settings, including workplace health promotion programs [320], community-based programs [321], and clinical settings [322]. In particular, workplace walking interventions have been associated with improved productivity [323], reduced absenteeism [324], increased organizational commitment [320], improved job motivation [320], and lowered healthcare costs [325], while clinical walking interventions have been shown to improve functional status [326], reduce falls [327], and enhance quality of life [326, 328] in individuals with chronic conditions.
Substantial inequalities in physical activity participation persist across demographic factors such as age, sex, disability, socioeconomic status, and geographic location [329, 330]. These data underscore the urgent need for tailored walking-based interventions that effectively address the root causes of these disparities to maximize the potential of physical activity to improve health outcomes.
There is an urgent need to invest in services and interventions that promote walking across all populations. Promising target populations include sedentary, less active, and obese individuals who are unable to engage in vigorous-intensity physical activity, those who do not have access to exercise facilities, and individuals who are just not aware of the health benefits of walking. Interventions, supports, and programs that have been documented to promote and increase walking include outdoor walking groups [28], community-based walking programs, use of pedometers [331], computer- or mobile phone-based interventions [332, 333], transportation walking [334], and school and workplace initiatives [332]. Physicians specialized in preventive medicine, lifestyle medicine, or longevity medicine and health professionals have a key role to play in prescribing walking to their patients, especially those individuals who are unable to engage in vigorous-intensity physical activities.
To advance the field of geroscience, preventive medicine, and public health, future research should prioritize several areas of inquiry. First, research should quantify the frequency, duration, intensity, and volume of walking required to improve risk factors for CVD and other age-related diseases. Second, there should be a focus on describing the dose-response relationships between walking and various health outcomes, including the identification of thresholds for optimal benefit. Third, it is important to identify and evaluate other strategies for promoting and sustaining participation in walking over the long term. Finally, physical activity guideline recommendations based on step-counts for various populations, including different occupational groups, need to be developed. Such research will provide valuable insights into the role of walking as an effective intervention for promoting healthy aging and preventing chronic age-associated diseases. Studies investigating the age-specific effects of exercise and walking on health outcomes are also warranted [335]. As older adults experience age-related declines in immune function, they are at increased risk of severe illness and death from infectious diseases. As the COVID-19 pandemic swept the globe, older adults were identified as a particularly vulnerable population due to their increased risk for severe illness and death from the SARS-CoV-2 virus [179, 180, 336–348]. As a result, there has been growing interest in developing interventions to boost the immune function of older adults and improve their overall health and resilience [349, 350]. Walking-based interventions and exercise programs have been identified as a promising approach for contributing to these goals [351–355]. Future research should continue to explore the potential of walking as a low-cost and accessible intervention for improving immune function and other health outcomes in older adults. Comprehensive healthy aging programs containing walking-based interventions are important for improving societal resilience to future pandemics and promoting healthy aging for all.
Author contribution
SKK: conceptualization, methodology, data curation, formal analysis, investigation, writing — review and editing; ZU, AC, and VFP: conceptualization, investigation, writing — review and editing.
Funding
Drs. Anna Csiszar and Zoltan Ungvari were supported by the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528). Project no. TKP2021-NKTA-47 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme. Funding for the project through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) was provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund. Project no. 135784 has also been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme. This work was also supported by grants from the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1). Dr. Setor K. Kunutsor is funded by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and Leicester NIHR Biomedical Research Centre (BRC). The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declarations
Competing interests
Dr. Anna Csiszar serves as Associate Editor for GeroScience. Dr. Zoltan Ungvari serves as Editor in Chief for GeroScience.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zoltan Ungvari, Email: zoltan-ungvari@ouhsc.edu.
Setor K. Kunutsor, Email: skk31@cantab.net
References
- 1.Aging in the United States. Population Reference Bureau, 2021, https://www.prb.org/aging-unitedstates-fact-sheet/. Accessed 05/09/2023.
- 2.Health and well-being and the 2030 Agenda for Sustainable Development in the WHO European Region: an analysis of policy development and implementation. Report of the first survey to assess Member States’ activities in relation to the WHO European Region Roadmap to Implement the 2030 Agenda for Sustainable Development. Copenhagen: WHO Regional Office for Europe; 2021.
- 3.WHO’s work on the UN Decade of Healthy Ageing (2021-2030). https://www.who.int/initiatives/decade-of-healthy-ageing (accessed on 03/15/2023).
- 4.World Health Organization. Decade of healthy ageing 2020-2030, Update 5, 2020. https://www.who.int/initiatives/decade-of-healthy-ageing. Accessed 15 Mar 2023.
- 5.Buettner D, Skemp S. Blue Zones: lessons from the world’s longest lived. Am J Lifestyle Med. 2016;10:318–321. doi: 10.1177/1559827616637066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrysohoou C, Pitsavos C, Lazaros G, Skoumas J, Tousoulis D, Stefanadis C, Ikaria Study I Determinants of all-cause mortality and incidence of cardiovascular disease (2009 to 2013) in older adults: the Ikaria study of the Blue Zones. Angiology. 2016;67:541–548. doi: 10.1177/0003319715603185. [DOI] [PubMed] [Google Scholar]
- 7.Poulain M, Herm A, Errigo A, Chrysohoou C, Legrand R, Passarino G, Stazi MA, Voutekatis KG, Gonos ES, Franceschi C, Pes GM. Specific features of the oldest old from the longevity Blue Zones in Ikaria and Sardinia. Mech Ageing Dev. 2021;198:111543. doi: 10.1016/j.mad.2021.111543. [DOI] [PubMed] [Google Scholar]
- 8.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunutsor SK, Makikallio TH, Seidu S, de Araujo CGS, Dey RS, Blom AW, Laukkanen JA. Physical activity and risk of venous thromboembolism: systematic review and meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35:431–442. doi: 10.1007/s10654-019-00579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunutsor SK, Seidu S, Laukkanen JA. Physical activity reduces the risk of pneumonia: systematic review and meta-analysis of 10 prospective studies involving 1,044,492 participants. Geroscience. 2022;44:519–532. doi: 10.1007/s11357-021-00491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunutsor SK, Seidu S, Makikallio TH, Dey RS, Laukkanen JA. Physical activity and risk of atrial fibrillation in the general population: meta-analysis of 23 cohort studies involving about 2 million participants. Eur J Epidemiol. 2021;36:259–274. doi: 10.1007/s10654-020-00714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M, Group ESCSD ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2020;2021(42):17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 14.Billingsley H, Rodriguez-Miguelez P, Del Buono MG, Abbate A, Lavie CJ, Carbone S. Lifestyle interventions with a focus on nutritional strategies to increase cardiorespiratory fitness in chronic obstructive pulmonary disease, heart failure, obesity, sarcopenia, and frailty. Nutrients. 2019;11:2849. doi: 10.3390/nu11122849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4:e002014. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisloff U, American Heart Association Physical Activity Committee of the Council on L, Cardiometabolic H, Council on Clinical C, Council on E, Prevention, Council on C, Stroke N, Council on Functional G, Translational B and Stroke C Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circ. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 17.Laukkanen JA, Isiozor NM, Kunutsor SK. Objectively assessed cardiorespiratory fitness and all-cause mortality risk: an updated meta-analysis of 37 cohort studies involving 2,258,029 participants. Mayo Clin Proc. 2022;97:1054–1073. doi: 10.1016/j.mayocp.2022.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Laukkanen JA, Kunutsor SK, Yates T, Willeit P, Kujala UM, Khan H, Zaccardi F. Prognostic relevance of cardiorespiratory fitness as assessed by submaximal exercise testing for all-cause mortality: a UK Biobank prospective study. Mayo Clin Proc. 2020;95:867–878. doi: 10.1016/j.mayocp.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 19.UK chief medical officers’ physical activity guidelines 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf. Accessed 25 Nov 2023.
- 20.Dempsey PC, Rowlands AV, Strain T, Zaccardi F, Dawkins N, Razieh C, Davies MJ, Khunti KK, Edwardson CL, Wijndaele K, Brage S, Yates T. Physical activity volume, intensity and incident cardiovascular disease. Eur Heart J. 2022;43(46):4789–4800. doi: 10.1093/eurheartj/ehac613. [DOI] [PubMed] [Google Scholar]
- 21.Hamer M, O’Donovan G, Lee IM, Stamatakis E. The ‘weekend warrior’ physical activity pattern: how little is enough? Br J Sports Med. 2017;51:1384–1385. doi: 10.1136/bjsports-2017-097538. [DOI] [PubMed] [Google Scholar]
- 22.OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 10 Aug 2023.
- 23.Bassett DR, Jr, Toth LP, LaMunion SR, Crouter SE. Step counting: a review of measurement considerations and health-related applications. Sports Med. 2017;47:1303–1315. doi: 10.1007/s40279-016-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Jefferis BJ, Kraus WE, Lee IM, Matthews CE, Omura JD, Patel AV, Pieper CF, Rees-Punia E, Dallmeier D, Klenk J, Whincup PH, Dooley EE, Pettee Gabriel K, Palta P, Pompeii LA, Chernofsky A, Larson MG, Vasan RS, Spartano N, Ballin M, Nordstrom P, Nordstrom A, Anderssen SA, Hansen BH, Cochrane JA, Dwyer T, Wang J, Ferrucci L, Liu F, Schrack J, Urbanek J, Saint-Maurice PF, Yamamoto N, Yoshitake Y, Newton RL, Jr, Yang S, Shiroma EJ, Fulton JE, Steps for Health C Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7:e219–e228. doi: 10.1016/S2468-2667(21)00302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley GA, Kelley KS, Tran ZV. Walking and resting blood pressure in adults: a meta-analysis. Prev Med. 2001;33:120–127. doi: 10.1016/S0091-7435(01)80008-6. [DOI] [PubMed] [Google Scholar]
- 26.Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev Med. 2007;44:377–385. doi: 10.1016/j.ypmed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PLoS One. 2014;9:e109767. doi: 10.1371/journal.pone.0109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson S, Jones A. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br J Sports Med. 2015;49:710–715. doi: 10.1136/bjsports-2014-094157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murtagh EM, Nichols L, Mohammed MA, Holder R, Nevill AM, Murphy MH. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta-analysis of randomised control trials. Prev Med. 2015;72:34–43. doi: 10.1016/j.ypmed.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Bemelmans RH, Blommaert PP, Wassink AM, Coll B, Spiering W, van der Graaf Y, Visseren FL. The relationship between walking speed and changes in cardiovascular risk factors during a 12-day walking tour to Santiago de Compostela: a cohort study. BMJ Open. 2012;2:e000875. doi: 10.1136/bmjopen-2012-000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oja P, Kelly P, Murtagh EM, Murphy MH, Foster C, Titze S. Effects of frequency, intensity, duration and volume of walking interventions on CVD risk factors: a systematic review and meta-regression analysis of randomised controlled trials among inactive healthy adults. Br J Sports Med. 2018;52:769–775. doi: 10.1136/bjsports-2017-098558. [DOI] [PubMed] [Google Scholar]
- 32.Lee LL, Mulvaney CA, Wong YKY, Chan ES, Watson MC, Lin HH. Walking for hypertension. Cochrane Database Syst Rev. 2021;2:CD008823. doi: 10.1002/14651858.CD008823.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghetti P, Balducci S, Guidetti L, Mazzuca P, Rossi E, Schena F, Italian Society of D, Italian Association of Medical D, Italian Society of M and Sports S Walking for subjects with type 2 diabetes: a systematic review and joint AMD/SID/SISMES evidence-based practical guideline. Nutr Metab Cardiovasc Dis. 2020;30:1882–1898. doi: 10.1016/j.numecd.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/S0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 35.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. Aust J Phys. 2019;597:4901–4914. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, Irukayama-Tomobe Y, Yokota T, Ohmori H, Matsuda M. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 38.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106:1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari Z. Role of endothelial NAD(+) deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi C, Song X, Wang H, Yan Y, Liu B. The role of exercise-induced myokines in promoting angiogenesis. Front Physiol. 2022;13:981577. doi: 10.3389/fphys.2022.981577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross M, Kargl CK, Ferguson R, Gavin TP, Hellsten Y. Exercise-induced skeletal muscle angiogenesis: impact of age, sex, angiocrines and cellular mediators. Eur J Appl Physiol. 2023;123:1415–1432. doi: 10.1007/s00421-022-05128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet-induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. Aust J Phys. 2021;599:911–925. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray KO, Mahoney SA, Venkatasubramanian R, Seals DR, Clayton ZS. Aging, aerobic exercise, and cardiovascular health: barriers, alternative strategies and future directions. Exp Gerontol. 2023;173:112105. doi: 10.1016/j.exger.2023.112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinloog JPD, Mensink RP, Roodt JO, Thijssen DHJ, Hesselink MKC, Joris PJ. Aerobic exercise training improves not only brachial artery flow-mediated vasodilatation but also carotid artery reactivity: a randomized controlled, cross-over trial in older men. Phys Rep. 2022;10:e15395. doi: 10.14814/phy2.15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivgulam ME, Liu H, Schwartz BD, Langley JE, Bray NW, Kimmerly DS, O’Brien MW. Impact of exercise training interventions on flow-mediated dilation in adults: an umbrella review. Sports Med. 2023;53:1161–1174. doi: 10.1007/s40279-023-01837-w. [DOI] [PubMed] [Google Scholar]
- 49.Mandini S, Conconi F, Mori E, Myers J, Grazzi G, Mazzoni G. Walking and hypertension: greater reductions in subjects with higher baseline systolic blood pressure following six months of guided walking. PeerJ. 2018;6:e5471. doi: 10.7717/peerj.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly P, Kahlmeier S, Gotschi T, Orsini N, Richards J, Roberts N, Scarborough P, Foster C. Systematic review and meta-analysis of reduction in all-cause mortality from walking and cycling and shape of dose response relationship. Int J Behav Nutr Phys Act. 2014;11:132. doi: 10.1186/s12966-014-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi T, Tsumura K, Suematsu C, Okada K, Fujii S, Endo G. Walking to work and the risk for hypertension in men: the Osaka Health Survey. Ann Intern Med. 1999;131:21–26. doi: 10.7326/0003-4819-131-1-199907060-00005. [DOI] [PubMed] [Google Scholar]
- 52.Etzig C, Gea A, Martinez-Gonzalez MA, Sullivan MF, Jr, Sullivan E, Bes-Rastrollo M. The association between self-perceived walking pace with the incidence of hypertension: the ‘Seguimiento Universidad de Navarra’ cohort. J Hypertens. 2021;39:1188–1194. doi: 10.1097/HJH.0000000000002788. [DOI] [PubMed] [Google Scholar]
- 53.Miller CR, Wactawski-Wende J, Manson JE, Haring B, Hovey KM, Laddu D, Shadyab AH, Wild RA, Bea JW, Tinker LF, Martin LW, Nguyen PK, Garcia L, Andrews CA, Eaton CB, Stefanick ML, LaMonte MJ. Walking volume and speed are inversely associated with incidence of treated hypertension in postmenopausal women. Hypertension. 2020;76:1435–1443. doi: 10.1161/HYPERTENSIONAHA.120.15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Celis-Morales CA, Gray S, Petermann F, Iliodromiti S, Welsh P, Lyall DM, Anderson J, Pellicori P, Mackay DF, Pell JP, Sattar N, Gill JMR. Walking pace is associated with lower risk of all-cause and cause-specific mortality. Med Sci Sports Exerc. 2019;51:472–480. doi: 10.1249/MSS.0000000000001795. [DOI] [PubMed] [Google Scholar]
- 55.Hamer M, Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. Br J Sports Med. 2008;42:238–243. doi: 10.1136/bjsm.2007.039974. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Orsini N, Amin J, Wolk A, Nguyen VT, Ehrlich F. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol. 2009;24:181–192. doi: 10.1007/s10654-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 57.LaMonte MJ, Manson JE, Chomistek AK, Larson JC, Lewis CE, Bea JW, Johnson KC, Li W, Klein L, LaCroix AZ, Stefanick ML, Wactawski-Wende J, Eaton CB. Physical activity and incidence of heart failure in postmenopausal women. JACC Heart Fail. 2018;6:983–995. doi: 10.1016/j.jchf.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boone-Heinonen J, Evenson KR, Taber DR, Gordon-Larsen P. Walking for prevention of cardiovascular disease in men and women: a systematic review of observational studies. Obes Rev. 2009;10:204–217. doi: 10.1111/j.1467-789X.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stamatakis E, Kelly P, Strain T, Murtagh EM, Ding D, Murphy MH. Self-rated walking pace and all-cause, cardiovascular disease and cancer mortality: individual participant pooled analysis of 50 225 walkers from 11 population British cohorts. Br J Sports Med. 2018;52:761–768. doi: 10.1136/bjsports-2017-098677. [DOI] [PubMed] [Google Scholar]
- 60.Hayes S, Forbes JF, Celis-Morales C, Anderson J, Ferguson L, Gill JMR, Gray S, Hastie C, Iliodromoti S, Lyall D, Pellicori P, Sattar N, Welsh CE, Pell J. Association between walking pace and stroke incidence: findings from the UK Biobank prospective cohort study. Stroke. 2020;51:1388–1395. doi: 10.1161/STROKEAHA.119.028064. [DOI] [PubMed] [Google Scholar]
- 61.Quan M, Xun P, Wang R, He K, Chen P. Walking pace and the risk of stroke: a meta-analysis of prospective cohort studies. J Sport Health Sci. 2020;9:521–529. doi: 10.1016/j.jshs.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is “no pain, no gain” passe? JAMA. 2001;285:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 63.Noda H, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A, Group JS Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol. 2005;46:1761–1767. doi: 10.1016/j.jacc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 64.Smith TC, Wingard DL, Smith B, Kritz-Silverstein D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. J Clin Epidemiol. 2007;60:309–317. doi: 10.1016/j.jclinepi.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee IM, Paffenbarger RS., Jr Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.STR.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 66.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 67.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 68.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 69.Del Pozo CB, Ahmadi MN, Lee IM, Stamatakis E. Prospective associations of daily step counts and intensity with cancer and cardiovascular disease incidence and mortality and all-cause mortality. JAMA Intern Med. 2022;182:1139–1148. doi: 10.1001/jamainternmed.2022.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of physical activity on cognitive decline, dementia, and its subtypes: meta-analysis of prospective studies. Biomed Res Int. 2017;2017:9016924. doi: 10.1155/2017/9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J. The relationship between physical activity and dementia: a systematic review and meta-analysis of prospective cohort studies. J Gerontol Nurs. 2018;44:22–29. doi: 10.3928/00989134-20180814-01. [DOI] [PubMed] [Google Scholar]
- 72.Kivimaki M, Singh-Manoux A, Pentti J, Sabia S, Nyberg ST, Alfredsson L, Goldberg M, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Nordin M, Oksanen T, Strandberg T, Suominen SB, Theorell T, Vahtera J, Vaananen A, Virtanen M, Westerholm P, Westerlund H, Zins M, Seshadri S, Batty GD, Sipila PN, Shipley MJ, Lindbohm JV, Ferrie JE, Jokela M, consortium IP-W. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ. 2019;365:l1495. doi: 10.1136/bmj.l1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabia S, Dugravot A, Dartigues JF, Abell J, Elbaz A, Kivimaki M, Singh-Manoux A. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi: 10.1136/bmj.j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kunutsor SK, Laukkanen JA, Kauhanen J, Willeit P. Physical activity may not be associated with long-term risk of dementia and Alzheimer’s disease. Eur J Clin Investig. 2021;51:e13415. doi: 10.1111/eci.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quan M, Xun P, Chen C, Wen J, Wang Y, Wang R, Chen P, He K. Walking pace and the risk of cognitive decline and dementia in elderly populations: a meta-analysis of prospective cohort studies. J Gerontol A Biol Sci Med Sci. 2017;72:266–270. doi: 10.1093/gerona/glw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Del Pozo CB, Ahmadi M, Naismith SL, Stamatakis E. Association of daily step count and intensity with incident dementia in 78 430 adults living in the UK. JAMA Neurol. 2022;79:1059–1063. doi: 10.1001/jamaneurol.2022.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang X, Crumpler RF, Thomas KN, Mazique JN, Roman RJ, Fan F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol Int. 2022;109(1):20–30. doi: 10.1556/2060.2022.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owens CD, Mukli P, Csipo T, Lipecz A, Silva-Palacios F, Dasari TW, Tarantini S, Gardner AW, Montgomery PS, Waldstein SR, Kellawan JM, Nyul-Toth A, Balasubramanian P, Sotonyi P, Csiszar A, Ungvari Z, Yabluchanskiy A. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am J Physiol Heart Circ Physiol. 2022;322(6):H924–H935. doi: 10.1152/ajpheart.00616.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–2394. doi: 10.1007/s11357-021-00405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–2440. doi: 10.1007/s11357-021-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balasubramanian P, Kiss T, Tarantini S, Nyul-Toth A, Ahire C, Yabluchanskiy A, Csipo T, Lipecz A, Tabak A, Institoris A, Csiszar A, Ungvari Z. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–H761. doi: 10.1152/ajpheart.00736.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–546. doi: 10.1007/s11357-020-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 86.Ballin M, Nordstrom P, Niklasson J, Alamaki A, Condell J, Tedesco S, Nordstrom A. Daily step count and incident diabetes in community-dwelling 70-year-olds: a prospective cohort study. BMC Public Health. 2020;20:1830. doi: 10.1186/s12889-020-09929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boonpor J, Ho FK, Gray SR, Celis-Morales CA. Association of self-reported walking pace with type 2 diabetes incidence in the UK Biobank prospective cohort study. Mayo Clin Proc. 2022;97:1631–1640. doi: 10.1016/j.mayocp.2022.02.028. [DOI] [PubMed] [Google Scholar]
- 88.Cuthbertson CC, Moore CC, Sotres-Alvarez D, Heiss G, Isasi CR, Mossavar-Rahmani Y, Carlson JA, Gallo LC, Llabre MM, Garcia-Bedoya OL, Farelo DG, Evenson KR. Associations of steps per day and step intensity with the risk of diabetes: the Hispanic Community Health Study / Study of Latinos (HCHS/SOL) Int J Behav Nutr Phys Act. 2022;19:46. doi: 10.1186/s12966-022-01284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee IM, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med. 2019;179:1105–1112. doi: 10.1001/jamainternmed.2019.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ikeda T, Inoue S, Konta T, Murakami M, Fujimoto S, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Narita I, Kondo M, Shibagaki Y, Kasahara M, Asahi K, Watanabe T. Can daily walking alone reduce pneumonia-related mortality among older people? Sci Rep. 2020;10:8556. doi: 10.1038/s41598-020-65440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inoue Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H, Tamakoshi A. Risk and protective factors related to mortality from pneumonia among middleaged and elderly community residents: the JACC Study. J Epidemiol. 2007;17:194–202. doi: 10.2188/jea.17.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ukawa S, Zhao W, Yatsuya H, Yamagishi K, Tanabe N, Iso H, Tamakoshi A. Associations of daily walking time with pneumonia mortality among elderly individuals with or without a medical history of myocardial infarction or stroke: findings from the Japan Collaborative Cohort Study. J Epidemiol. 2019;29:233–237. doi: 10.2188/jea.JE20170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carter MI, Hinton PS. Physical activity and bone health. Mo Med. 2014;111:59–64. [PMC free article] [PubMed] [Google Scholar]
- 94.Brooke-Wavell K, Skelton DA, Barker KL, Clark EM, De Biase S, Arnold S, Paskins Z, Robinson KR, Lewis RM, Tobias JH, Ward KA, Whitney J, Leyland S. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med. 2022;56:837–846. doi: 10.1136/bjsports-2021-104634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR, American College of Sports M American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.MSS.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 96.Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43:521–531. doi: 10.1016/j.bone.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Ma D, Wu L, He Z. Effects of walking on the preservation of bone mineral density in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Menopause. 2013;20:1216–1226. doi: 10.1097/GME.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 98.Palombaro KM. Effects of walking-only interventions on bone mineral density at various skeletal sites: a meta-analysis. J Geriatr Phys Ther. 2005;28:102–107. doi: 10.1519/00139143-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Pellikaan P, Giarmatzis G, Vander Sloten J, Verschueren S, Jonkers I. Ranking of osteogenic potential of physical exercises in postmenopausal women based on femoral neck strains. PLoS One. 2018;13:e0195463. doi: 10.1371/journal.pone.0195463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Connor SR, Tully MA, Ryan B, Bleakley CM, Baxter GD, Bradley JM, McDonough SM. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96:724–734. doi: 10.1016/j.apmr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 102.LaMonte MJ, Wactawski-Wende J, Larson JC, Mai X, Robbins JA, LeBoff MS, Chen Z, Jackson RD, LaCroix AZ, Ockene JK, Hovey KM, Cauley JA, Women’s Health I Association of physical activity and fracture risk among postmenopausal women. JAMA Netw Open. 2019;2:e1914084. doi: 10.1001/jamanetworkopen.2019.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikander R, Gagnon C, Dunstan DW, Magliano DJ, Ebeling PR, Lu ZX, Zimmet PZ, Shaw JE, Daly RM. Frequent walking, but not total physical activity, is associated with increased fracture incidence: a 5-year follow-up of an Australian population-based prospective study (AusDiab) J Bone Miner Res. 2011;26:1638–1647. doi: 10.1002/jbmr.363. [DOI] [PubMed] [Google Scholar]
- 104.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38:427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 105.Kubitz KA, Landers DM, Petruzzello SJ, Han M. The effects of acute and chronic exercise on sleep. A meta-analytic review. Sports Med. 1996;21:277–291. doi: 10.2165/00007256-199621040-00004. [DOI] [PubMed] [Google Scholar]
- 106.Wilbur J, Miller AM, McDevitt J, Wang E, Miller J. Menopausal status, moderate-intensity walking, and symptoms in midlife women. Res Theory Nurs Pract. 2005;19:163–180. doi: 10.1891/rtnp.19.2.163.66799. [DOI] [PubMed] [Google Scholar]
- 107.Sullivan Bisson AN, Robinson SA, Lachman ME. Walk to a better night of sleep: testing the relationship between physical activity and sleep. Sleep Health. 2019;5:487–494. doi: 10.1016/j.sleh.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pearce M, Garcia L, Abbas A, Strain T, Schuch FB, Golubic R, Kelly P, Khan S, Utukuri M, Laird Y, Mok A, Smith A, Tainio M, Brage S, Woodcock J. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:550–559. doi: 10.1001/jamapsychiatry.2022.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, Fleck MP, Carvalho AF, Stubbs B. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175:631–648. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- 110.Guo Z, Li R, Lu S. Leisure-time physical activity and risk of depression: a dose-response meta-analysis of prospective cohort studies. Medicine. 2022;101:e29917. doi: 10.1097/MD.0000000000029917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aylett E, Small N, Bower P. Exercise in the treatment of clinical anxiety in general practice - a systematic review and meta-analysis. BMC Health Serv Res. 2018;18:559. doi: 10.1186/s12913-018-3313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC. Smoller JW and Major Depressive Disorder Working Group of the Psychiatric Genomics C. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry. 2019;76:399–408. doi: 10.1001/jamapsychiatry.2018.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcia SR, Bund SJ. Nitric oxide modulation of coronary artery myogenic tone in spontaneously hypertensive and Wistar-Kyoto rats. Clin Sci (Colch). 1998;94:225–229. doi: 10.1042/cs0940225. [DOI] [PubMed] [Google Scholar]
- 114.Edwards MK, Loprinzi PD. Experimental effects of brief, single bouts of walking and meditation on mood profile in young adults. Health Promot Perspect. 2018;8:171–178. doi: 10.15171/hpp.2018.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oppezzo M, Schwartz DL. Give your ideas some legs: the positive effect of walking on creative thinking. J Exp Psychol Learn Mem Cogn. 2014;40:1142–1152. doi: 10.1037/a0036577. [DOI] [PubMed] [Google Scholar]
- 116.Lin YY, Liu MF, Tzeng JI, Lin CC. Effects of walking on quality of life among lung cancer patients: a longitudinal study. Cancer Nurs. 2015;38:253–259. doi: 10.1097/NCC.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 117.Yang S, Kim H. Effects of a walking exercise-focused health promotion program for middle-aged women in the Korean community. Int J Environ Res Public Health. 2022;19:14947. doi: 10.3390/ijerph192214947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee CH, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020;32:101508. doi: 10.1016/j.redox.2020.101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Catitti G, De Bellis D, Vespa S, Simeone P, Canonico B, Lanuti P. Extracellular vesicles as players in the anti-inflammatory inter-cellular crosstalk induced by exercise training. Int J Mol Sci. 2022;23:14098. doi: 10.3390/ijms232214098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chong MC, Silva A, James PF, Wu SSX, Howitt J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell. 2022;21:e13647. doi: 10.1111/acel.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McIlvenna LC, Whitham M. Exercise, healthy ageing, and the potential role of small extracellular vesicles. J Physiol. 2022 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 123.Pierdona TM, Martin A, Obi PO, Seif S, Bydak B, Labouta HI, Eadie AL, Brunt KR, McGavock JM, Senechal M, Saleem A. Extracellular vesicles as predictors of individual response to exercise training in youth living with obesity. Front Biosci. 2022;27:143. doi: 10.31083/j.fbl2705143. [DOI] [PubMed] [Google Scholar]
- 124.Zhao H, Chen X, Hu G, Li C, Guo L, Zhang L, Sun F, Xia Y, Yan W, Cui Z, Guo Y, Guo X, Huang C, Fan M, Wang S, Zhang F, Tao L. Small extracellular vesicles from brown adipose tissue mediate exercise cardioprotection. Circ Res. 2022;130:1490–1506. doi: 10.1161/CIRCRESAHA.121.320458. [DOI] [PubMed] [Google Scholar]
- 125.Lisi V, Senesi G, Bertola N, Pecoraro M, Bolis S, Gualerzi A, Picciolini S, Raimondi A, Fantini C, Moretti E, Parisi A, Sgro P, Di Luigi L, Geiger R, Ravera S, Vassalli G, Caporossi D, Balbi C. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biol. 2023;63:102737. doi: 10.1016/j.redox.2023.102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maggio S, Canonico B, Ceccaroli P, Polidori E, Cioccoloni A, Giacomelli L, Ferri Marini C, Annibalini G, Gervasi M, Benelli P, Fabbri F, Del Coco L, Fanizzi FP, Giudetti AM, Lucertini F, Guescini M. Modulation of the circulating extracellular vesicles in response to different exercise regimens and study of their inflammatory effects. Int J Mol Sci. 2023;24:3039. doi: 10.3390/ijms24033039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siqueira IR, Batabyal RA, Freishtat R, Cechinel LR. Potential involvement of circulating extracellular vesicles and particles on exercise effects in malignancies. Front Endocrinol. 2023;14:1121390. doi: 10.3389/fendo.2023.1121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Liu Y, Zhang S, Li N, Xing C, Wang C, Wang J, Wei M, Yang G, Yuan L. Exercise improves metabolism and alleviates atherosclerosis via muscle-derived extracellular vesicles. Aging Dis. 2023;14:952–965. doi: 10.14336/AD.2022.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 130.Lionetti V, Barile L. Fndc5/irisin-enriched extracellular vesicles: a new hormonal relay in the regular race against vascular ageing. Eur Heart J. 2022;43:4596–4598. doi: 10.1093/eurheartj/ehac517. [DOI] [PubMed] [Google Scholar]
- 131.Smith JA, Leonardi T, Huang B, Iraci N, Vega B, Pluchino S. Extracellular vesicles and their synthetic analogues in aging and age-associated brain diseases. Biogerontology. 2015;16:147–185. doi: 10.1007/s10522-014-9510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Panagiotou N, Wayne Davies R, Selman C, Shiels PG. Microvesicles as vehicles for tissue regeneration: changing of the guards. Curr Pathobiol Rep. 2016;4:181–187. doi: 10.1007/s40139-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17:e12734. doi: 10.1111/acel.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fafian-Labora JA, Rodriguez-Navarro JA, O’Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab. 2020;32:71–86. doi: 10.1016/j.cmet.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Melidoni A. Small extracellular vesicles combat senescence. Nat Rev Mol Cell Biol. 2020;21:498–499. doi: 10.1038/s41580-020-0271-7. [DOI] [PubMed] [Google Scholar]
- 136.Mensa E, Guescini M, Giuliani A, Bacalini MG, Ramini D, Corleone G, Ferracin M, Fulgenzi G, Graciotti L, Prattichizzo F, Sorci L, Battistelli M, Monsurro V, Bonfigli AR, Cardelli M, Recchioni R, Marcheselli F, Latini S, Maggio S, Fanelli M, Amatori S, Storci G, Ceriello A, Stocchi V, De Luca M, Magnani L, Rippo MR, Procopio AD, Sala C, Budimir I, Bassi C, Negrini M, Garagnani P, Franceschi C, Sabbatinelli J, Bonafe M, Olivieri F. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J Extracell Vesicles. 2020;9:1725285. doi: 10.1080/20013078.2020.1725285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wallis R, Mizen H, Bishop CL. The bright and dark side of extracellular vesicles in the senescence-associated secretory phenotype. Mech Ageing Dev. 2020;189:111263. doi: 10.1016/j.mad.2020.111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh C, Koh D, Jeon HB, Kim KM. The role of extracellular vesicles in senescence. Mol Cell. 2022;45:603–609. doi: 10.14348/molcells.2022.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Panagiotou N, Neytchev O, Selman C, Shiels PG. Extracellular vesicles, ageing, and therapeutic interventions. Cells. 2018;7:110. doi: 10.3390/cells7080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rafferty AP, Reeves MJ, McGee HB, Pivarnik JM. Physical activity patterns among walkers and compliance with public health recommendations. Med Sci Sports Exerc. 2002;34:1255–1261. doi: 10.1097/00005768-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 141.Ball K, Timperio A, Salmon J, Giles-Corti B, Roberts R, Crawford D. Personal, social and environmental determinants of educational inequalities in walking: a multilevel study. J Epidemiol Community Health. 2007;61:108–114. doi: 10.1136/jech.2006.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 143.Stein AM, Silva TMV, Coelho FGM, Arantes FJ, Costa JLR, Teodoro E, Santos-Galduroz RF. Physical exercise, IGF-1 and cognition A systematic review of experimental studies in the elderly. Dement Neuropsychol. 2018;12:114–122. doi: 10.1590/1980-57642018dn12-020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Norling AM, Gerstenecker AT, Buford TW, Khan B, Oparil S, Lazar RM. The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. Geroscience. 2019;42(1):141–158. doi: 10.1007/s11357-019-00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arazi H, Babaei P, Moghimi M, Asadi A. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021;21:50. doi: 10.1186/s12877-020-01937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li B, Feng L, Wu X, Cai M, Yu JJ, Tian Z. Effects of different modes of exercise on skeletal muscle mass and function and IGF-1 signaling during early aging in mice. J Exp Biol. 2022;225:jeb244650. doi: 10.1242/jeb.244650. [DOI] [PubMed] [Google Scholar]
- 147.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/S0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 149.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(Pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vitale G, Pellegrino G, Vollery M, Hofland LJ. ROLE of IGF-1 system in the modulation of longevity: controversies and new insights from a centenarians’ perspective. Front Endocrinol. 2019;10:27. doi: 10.3389/fendo.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. doi: 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–911. doi: 10.1007/s11357-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age. 2016;38:239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, Yabluchanskiy A, Koller A, Orsi G, Perlaki G, Schwarcz A, Buki A, Ungvari Z, Toth PJ. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience. 2022;44:2771–2783. doi: 10.1007/s11357-022-00623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fazekas-Pongor V, Peterfi A, Major D, Szarvas Z, Fekete M, Tabak AG, Csiszar A, Sonntag WE, Austad SN, Ungvari ZI. Decreased lifespan in female “Munchkin” actors from the cast of the 1939 film version of The Wizard of Oz does not support the hypothesis linking hypopituitary dwarfism to longevity. Geroscience. 2022;44:2527–2539. doi: 10.1007/s11357-022-00680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DK, Bartke A. Studies of aging in ames dwarf mice: effects of caloric restriction. J Am Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.de Alcantara BD, da Silva AE, Rosa JPP, Facundo LA, Costa CMA, Silva AC, Narciso FV, Silva A, de Mello MT. Can IGF-1 serum levels really be changed by acute physical exercise? A systematic review and meta-analysis. J Phys Act Health. 2020;17:575–584. doi: 10.1123/jpah.2019-0453. [DOI] [PubMed] [Google Scholar]
- 164.Stein AM, da Silva TMV, Coelho FGM, Rueda AV, Camarini R, Galduroz RFS. Acute exercise increases circulating IGF-1 in Alzheimer’s disease patients, but not in older adults without dementia. Behav Brain Res. 2021;396:112903. doi: 10.1016/j.bbr.2020.112903. [DOI] [PubMed] [Google Scholar]
- 165.Sullivan BP, Weiss JA, Nie Y, Garner RT, Drohan CJ, Kuang S, Stout J, Gavin TP. Skeletal muscle IGF-1 is lower at rest and after resistance exercise in humans with obesity. Eur J Appl Physiol. 2020;120:2835–2846. doi: 10.1007/s00421-020-04509-z. [DOI] [PubMed] [Google Scholar]
- 166.Zebrowska A, Sikora M, Konarska A, Zwierzchowska A, Kaminski T, Robins A, Hall B. Moderate intensity exercise in hypoxia increases IGF-1 bioavailability and serum irisin in individuals with type 1 diabetes. Ther Adv Endocrinol Metab. 2020;11:2042018820925326. doi: 10.1177/2042018820925326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim T, Chang JS, Kim H, Lee KH, Kong ID. Intense walking exercise affects serum IGF-1 and IGFBP3. J Lifestyle Med. 2015;5:21–25. doi: 10.15280/jlm.2015.5.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wakai K, Suzuki K, Ito Y, Watanabe Y, Inaba Y, Tajima K, Nakachi K, Tamakoshi A. Time spent walking or exercising and blood levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3): a large-scale cross-sectional study in the Japan Collaborative Cohort study. Asian Pac J Cancer Prev. 2009;10(Suppl):23–27. [PubMed] [Google Scholar]
- 169.Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol: Series A. 2012;67:1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Del Giudice F, Glover F, Belladelli F, De Berardinis E, Sciarra A, Salciccia S, Kasman AM, Chen T, Eisenberg ML. Association of daily step count and serum testosterone among men in the United States. Endocrine. 2021;72:874–881. doi: 10.1007/s12020-021-02631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Razzak ZA, Khan AA, Farooqui SI. Effect of aerobic and anaerobic exercise on estrogen level, fat mass, and muscle mass among postmenopausal osteoporotic females. Int J Health Sci. 2019;13:10–16. [PMC free article] [PubMed] [Google Scholar]
- 172.Shao T, Verma HK, Pande B, Costanzo V, Ye W, Cai Y, Bhaskar L. Physical activity and nutritional influence on immune function: an important strategy to improve immunity and health status. Front Physiol. 2021;12:751374. doi: 10.3389/fphys.2021.751374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lionetti V, Tuana BS, Casieri V, Parikh M, Pierce GN. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur Heart J. 2019;40:575–582. doi: 10.1093/eurheartj/ehy597. [DOI] [PubMed] [Google Scholar]
- 174.Svezia B, Cabiati M, Matteucci M, Passino C, Pe ME, Lionetti V, Del Ry S. Tuscany Sangiovese grape juice imparts cardioprotection by regulating gene expression of cardioprotective C-type natriuretic peptide. Eur J Nutr. 2020;59:2953–2968. doi: 10.1007/s00394-019-02134-x. [DOI] [PubMed] [Google Scholar]
- 175.Aoi W, Naito Y, Yoshikawa T. Exercise and functional foods. Nutr J. 2006;5:15. doi: 10.1186/1475-2891-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fazekas-Pongor V, Fekete M, Balazs P, Arva D, Penzes M, Tarantini S, Urban R, Varga JT. Health-related quality of life of COPD patients aged over 40 years. Physiol Int. 2021;108(2):261–273. doi: 10.1556/2060.2021.00017. [DOI] [PubMed] [Google Scholar]
- 177.Fekete M, Fazekas-Pongor V, Balazs P, Tarantini S, Szollosi G, Pako J, Nemeth AN, Varga JT. Effect of malnutrition and body composition on the quality of life of COPD patients. Physiol Int. 2021;108(2):238–250. doi: 10.1556/2060.2021.00170. [DOI] [PubMed] [Google Scholar]
- 178.Fekete M, Kerti M, Fazekas-Pongor V, Balazs P, Csizmadia Z, Nemeth AN, Tarantini S, Varga JT. Effect of interval training with non-invasive ventilation in severe chronic obstructive pulmonary disease-a prospective cohort study with matched control group. Ann Palliat Med. 2021;10:5289–5298. doi: 10.21037/apm-21-378. [DOI] [PubMed] [Google Scholar]
- 179.Fekete M, Szarvas Z, Fazekas-Pongor V, Feher A, Dosa N, Lehoczki A, Tarantini S, Varga JT. COVID-19 infection in patients with chronic obstructive pulmonary disease: from pathophysiology to therapy. Mini-review. Physiol Int. 2022;109(1):9–19. doi: 10.1556/2060.2022.00172. [DOI] [PubMed] [Google Scholar]
- 180.Fekete M, Szollosi G, Tarantini S, Lehoczki A, Nemeth AN, Bodola C, Varga L, Varga JT. Metabolic syndrome in patients with COPD: causes and pathophysiological consequences. Physiol Int. 2022;109(1):90–105. doi: 10.1556/2060.2022.00164. [DOI] [PubMed] [Google Scholar]
- 181.Szarvas Z, Fekete M, Horvath R, Shimizu M, Tsuhiya F, Choi HE, Kup K, Fazekas-Pongor V, Pete KN, Cserjesi R, Bakos R, Gobel O, Kovacs O, Gyongyosi K, Pinter R, Kovats Z, Ungvari Z, Tarantini S, Horvath G, Muller V, Varga JT. Cardiopulmonary rehabilitation programme improves physical health and quality of life in post-COVID syndrome. Ann Palliat Med. 2023;12(3):549. doi: 10.21037/apm-22-1143. [DOI] [PubMed] [Google Scholar]
- 182.Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: summary. J Gerontol A Biol Sci Med Sci. 2001;56 Spec No 2:89–94. doi: 10.1093/gerona/56.suppl_2.89. [DOI] [PubMed] [Google Scholar]
- 183.Cavill JL, Jancey JM, Howat P. Review and recommendations for online physical activity and nutrition programmes targeted at over 40s. Glob Health Promot. 2012;19:44–53. doi: 10.1177/1757975912441227. [DOI] [PubMed] [Google Scholar]
- 184.Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. 2016;75:174–180. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 185.Shad BJ, Wallis G, van Loon LJ, Thompson JL. Exercise prescription for the older population: the interactions between physical activity, sedentary time, and adequate nutrition in maintaining musculoskeletal health. Maturitas. 2016;93:78–82. doi: 10.1016/j.maturitas.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 186.Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, Reginster JY, Chapurlat R, Chan DC, Bruyere O, Rizzoli R, Cooper C, Dennison EM, Group I-ESW Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Balan E, Decottignies A, Deldicque L. Physical activity and nutrition: two promising strategies for telomere maintenance? Nutrients. 2018;10:1942. doi: 10.3390/nu10121942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gronstedt H, Vikstrom S, Cederholm T, Franzen E, Seiger A, Wimo A, Faxen-Irving G, Bostrom AM. A study protocol of Older Person’s Exercise and Nutrition Study (OPEN) - a sit-to-stand activity combined with oral protein supplement - effects on physical function and independence: a cluster randomized clinical trial. BMC Geriatr. 2018;18:138. doi: 10.1186/s12877-018-0824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dominguez LJ, Veronese N, Vernuccio L, Catanese G, Inzerillo F, Salemi G, Barbagallo M. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients. 2021;13:4080. doi: 10.3390/nu13114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lorbergs AL, Prorok JC, Holroyd-Leduc J, Bouchard DR, Giguere A, Gramlich L, Keller H, Tang A, Racey M, Ali MU, Fitzpatrick-Lewis D, Sherifali D, Kim P, Muscedere J. Nutrition and physical activity clinical practice guidelines for older adults living with frailty. J Frailty Aging. 2022;11:3–11. doi: 10.14283/jfa.2021.51. [DOI] [PubMed] [Google Scholar]
- 191.Neil-Sztramko SE, Teggart K, Moore C, Sherifali D, Fitzpatrick-Lewis D, Coletta G, Phillips SM, Newbold KB, Alvarez E, Kuspinar A, Kennedy CC, Santaguida PL, Ganann R. Community-based group physical activity and/or nutrition interventions to promote mobility in older adults: an umbrella review. BMC Geriatr. 2022;22:539. doi: 10.1186/s12877-022-03170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dimauro I, Sgura A, Pittaluga M, Magi F, Fantini C, Mancinelli R, Sgadari A, Fulle S, Caporossi D. Regular exercise participation improves genomic stability in diabetic patients: an exploratory study to analyse telomere length and DNA damage. Sci Rep. 2017;7:4137. doi: 10.1038/s41598-017-04448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hernandez-Alvarez D, Rosado-Perez J, Gavia-Garcia G, Arista-Ugalde TL, Aguiniga-Sanchez I, Santiago-Osorio E, Mendoza-Nunez VM. Aging, physical exercise, telomeres, and sarcopenia: a narrative review. Biomedicines. 2023;11:598. doi: 10.3390/biomedicines11020598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ludlow AT, Gratidao L, Ludlow LW, Spangenburg EE, Roth SM. Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle. Exp Physiol. 2017;102:397–410. doi: 10.1113/EP086189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vilela TC, de Andrade VM, Radak Z, de Pinho RA. The role of exercise in brain DNA damage. Neural Regen Res. 2020;15:1981–1985. doi: 10.4103/1673-5374.282237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goh J, Wong E, Soh J, Maier AB, Kennedy BK. Targeting the molecular & cellular pillars of human aging with exercise. FEBS J. 2021;290(3):649–668. doi: 10.1111/febs.16337. [DOI] [PubMed] [Google Scholar]
- 197.Radak Z, Suzuki K, Higuchi M, Balogh L, Boldogh I, Koltai E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic Biol Med. 2016;98:187–196. doi: 10.1016/j.freeradbiomed.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 198.Fernandez de la Puente M, Hernandez-Alonso P, Canudas S, Marti A, Fito M, Razquin C, Salas-Salvado J. Modulation of telomere length by Mediterranean diet, caloric restriction, and exercise: results from PREDIMED-Plus Study. Antioxidants. 2021;10:1596. doi: 10.3390/antiox10101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim JH, Ko JH, Lee DC, Lim I, Bang H. Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause. 2012;19:1109–1115. doi: 10.1097/gme.0b013e3182503e97. [DOI] [PubMed] [Google Scholar]
- 200.LaRocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech Ageing Dev. 2010;131:165–167. doi: 10.1016/j.mad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ludlow AT, Witkowski S, Marshall MR, Wang J, Lima LC, Guth LM, Spangenburg EE, Roth SM. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J Gerontol A Biol Sci Med Sci. 2012;67:911–926. doi: 10.1093/gerona/gls002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Osthus IB, Sgura A, Berardinelli F, Alsnes IV, Bronstad E, Rehn T, Stobakk PK, Hatle H, Wisloff U, Nauman J. Telomere length and long-term endurance exercise: does exercise training affect biological age? A pilot study. PLoS One. 2012;7:e52769. doi: 10.1371/journal.pone.0052769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sanchez-Gonzalez JL, Sanchez-Rodriguez JL, Martin-Vallejo J, Martel-Martel A, Gonzalez-Sarmiento R. Effects of physical exercise on cognition and telomere length in healthy older women. Brain Sci. 2021;11:1417. doi: 10.3390/brainsci11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Valente C, Andrade R, Alvarez L, Rebelo-Marques A, Stamatakis E, Espregueira-Mendes J. Effect of physical activity and exercise on telomere length: systematic review with meta-analysis. J Am Geriatr Soc. 2021;69:3285–3300. doi: 10.1111/jgs.17334. [DOI] [PubMed] [Google Scholar]
- 205.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Bauersachs J, Thum T, Pfreundschuh M, Muller P, Haendeler J, Bohm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 206.Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging. 2016;8:2897–2914. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–748. doi: 10.1007/s11357-020-00180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, Ungvari Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019;41:711–725. doi: 10.1007/s11357-019-00102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z, Csiszar A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience. 2019;41:727–738. doi: 10.1007/s11357-019-00107-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly (ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–542. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41:619–630. doi: 10.1007/s11357-019-00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–617. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130(8):518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 221.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 222.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Phys Regul Integr Comp Phys. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 223.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Phys Regul Integr Comp Phys. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 224.Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 225.Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2014;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. 2018;320:1319–1320. doi: 10.1001/jama.2018.12440. [DOI] [PubMed] [Google Scholar]
- 229.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Valentijn FA, Falke LL, Nguyen TQ, Goldschmeding R. Cellular senescence in the aging and diseased kidney. J Cell Commun Signal. 2018;12:69–82. doi: 10.1007/s12079-017-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–444. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, Wiley G, Garman L, Ungvari Z, Csiszar A. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience. 2022;44:661–681. doi: 10.1007/s11357-022-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, Lecy DR, Yousefzadeh MJ, Schafer MJ, White TA, Atkinson EJ, LeBrasseur NK. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell. 2021;20:e13415. doi: 10.1111/acel.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 237.Brown M, McClean CM, Davison GW, Brown JCW, Murphy MH. The acute effects of walking exercise intensity on systemic cytokines and oxidative stress. Eur J Appl Physiol. 2018;118:2111–2120. doi: 10.1007/s00421-018-3930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang Q, Guo M, Chen T, Cheng H, Yang Q, Zhao Z, She R, Yang X, Xiao W, Yang X, Li L. Walking and taking vitamin C alleviates oxidative stress and inflammation in overweight students, even in the short-term. Front Public Health. 2022;10:1024864. doi: 10.3389/fpubh.2022.1024864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Weyh C, Krüger K, Strasser B. Physical activity and diet shape the immune system during aging. Nutrients. 2020;12:622. doi: 10.3390/nu12030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen K, Zheng Y, Wei JA, Ouyang H, Huang X, Zhang F, Lai CSW, Ren C, So KF, Zhang L. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv. 2019;5:eaaw1888. doi: 10.1126/sciadv.aaw1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kido K, Sase K, Yokokawa T, Fujita S. Enhanced skeletal muscle insulin sensitivity after acute resistance-type exercise is upregulated by rapamycin-sensitive mTOR complex 1 inhibition. Sci Rep. 2020;10:8509. doi: 10.1038/s41598-020-65397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li M, Verdijk LB, Sakamoto K, Ely B, van Loon LJ, Musi N. Reduced AMPK-ACC and mTOR signaling in muscle from older men, and effect of resistance exercise. Mech Ageing Dev. 2012;133:655–664. doi: 10.1016/j.mad.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Reynolds TH, IV, Reid P, Larkin LM, Dengel DR. Effects of aerobic exercise training on the protein kinase B (PKB)/mammalian target of rapamycin (mTOR) signaling pathway in aged skeletal muscle. Exp Gerontol. 2004;39:379–385. doi: 10.1016/j.exger.2003.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O’Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, Hornberger TA, Spriet LL, Heigenhauser GJ, Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep. 2017;7:5028. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Campos JC, Marchesi Bozi LH, Krum B, Grassmann Bechara LR, Ferreira ND, Arini GS, Albuquerque RP, Traa A, Ogawa T, van der Bliek AM, Beheshti A, Chouchani ET, Van Raamsdonk JM, Blackwell TK, Ferreira JCB. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc Natl Acad Sci U S A. 2023;120:e2204750120. doi: 10.1073/pnas.2204750120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, Kemp BE, Raha S, Steinberg GR, Tarnopolsky MA. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. doi: 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu W, Wang Z, Xia Y, Kuang H, Liu S, Li L, Tang C, Yin D. The balance of apoptosis and autophagy via regulation of the AMPK signal pathway in aging rat striatum during regular aerobic exercise. Exp Gerontol. 2019;124:110647. doi: 10.1016/j.exger.2019.110647. [DOI] [PubMed] [Google Scholar]
- 248.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yoon KJ, Zhang D, Kim SJ, Lee MC, Moon HY. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem Biophys Res Commun. 2019;512:604–610. doi: 10.1016/j.bbrc.2019.03.086. [DOI] [PubMed] [Google Scholar]
- 250.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 251.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD(+) -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075–1085. doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–3412. doi: 10.1093/eurheartj/ehv290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Benigni A, Cassis P, Conti S, Perico L, Corna D, Cerullo D, Zentilin L, Zoja C, Perna A, Lionetti V, Giacca M, Trionfini P, Tomasoni S, Remuzzi G. Sirt3 deficiency shortens life span and impairs cardiac mitochondrial function rescued by Opa1 gene transfer. Antioxid Redox Signal. 2019;31:1255–1271. doi: 10.1089/ars.2018.7703. [DOI] [PubMed] [Google Scholar]
- 255.Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco A, Kaiser TA, Waltz TB, Zhang N, Ellis JL, Elliott PJ, Frederick DW, Bohr VA, Schmidt MS, Brenner C, Sinclair DA, Sauve AA, Baur JA, de Cabo R. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 2018;27:667–676. doi: 10.1016/j.cmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP. Mitochondrial SIRT3 and heart disease. Cardiovasc Res. 2010;88:250–256. doi: 10.1093/cvr/cvq250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol. 2013;48:634–639. doi: 10.1016/j.exger.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 261.Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017;16:4–16. doi: 10.1111/acel.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhou L, Pinho R, Gu Y, Radak Z. The role of SIRT3 in exercise and aging. Cells. 2022;11:2596. doi: 10.3390/cells11162596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gonzalez-Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci. 2015;70:1334–1342. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305(4):H459–H476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Horan MP, Pichaud N, Ballard JW. Quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci. 2012;67(10):1022–1035. doi: 10.1093/gerona/glr263. [DOI] [PubMed] [Google Scholar]
- 269.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Silaghi CN, Farcas M, Craciun AM. Sirtuin 3 (SIRT3) pathways in age-related cardiovascular and neurodegenerative diseases. Biomedicines. 2021;9:1574. doi: 10.3390/biomedicines9111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Daussin FN, Boulanger E, Lancel S. From mitochondria to sarcopenia: role of inflammaging and RAGE-ligand axis implication. Exp Gerontol. 2021;146:111247. doi: 10.1016/j.exger.2021.111247. [DOI] [PubMed] [Google Scholar]
- 272.Del Campo A, Contreras-Hernandez I, Castro-Sepulveda M, Campos CA, Figueroa R, Tevy MF, Eisner V, Casas M, Jaimovich E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging. 2018;10:34–55. doi: 10.18632/aging.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Coen PM, Musci RV, Hinkley JM, Miller BF. Mitochondria as a target for mitigating sarcopenia. Front Physiol. 2018;9:1883. doi: 10.3389/fphys.2018.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cordeiro AV, Peruca GF, Braga RR, Bricola RS, Lenhare L, Silva VRR, Anaruma CP, Katashima CK, Crisol BM, Barbosa LT, Simabuco FM, da Silva ASR, Cintra DE, de Moura LP, Pauli JR, Ropelle ER. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. Geroscience. 2020;43:1513–1518. doi: 10.1007/s11357-020-00246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr. 2009;89:467S–471S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab. 2009;297:E92–103. doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 278.Gill JF, Santos G, Schnyder S, Handschin C. PGC-1alpha affects aging-related changes in muscle and motor function by modulating specific exercise-mediated changes in old mice. Aging Cell. 2018;17:e12697. doi: 10.1111/acel.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Halling JF, Jessen H, Nohr-Meldgaard J, Buch BT, Christensen NM, Gudiksen A, Ringholm S, Neufer PD, Prats C, Pilegaard H. PGC-1alpha regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am J Physiol Endocrinol Metab. 2019;317:E513–E525. doi: 10.1152/ajpendo.00059.2019. [DOI] [PubMed] [Google Scholar]
- 280.Koh JH, Pataky MW, Dasari S, Klaus KA, Vuckovic I, Ruegsegger GN, Kumar AP, Robinson MM, Nair KS. Enhancement of anaerobic glycolysis - a role of PGC-1alpha4 in resistance exercise. Nat Commun. 2022;13:2324. doi: 10.1038/s41467-022-30056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kristensen CM, Brandt CT, Ringholm S, Pilegaard H. PGC-1alpha in aging and lifelong exercise training-mediated regulation of UPR in mouse liver. Exp Gerontol. 2017;98:124–133. doi: 10.1016/j.exger.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 282.Liang J, Zhang H, Zeng Z, Wu L, Zhang Y, Guo Y, Lv J, Wang C, Fan J, Chen N. Lifelong aerobic exercise alleviates sarcopenia by activating autophagy and inhibiting protein degradation via the AMPK/PGC-1alpha signaling pathway. Metabolites. 2021;11:323. doi: 10.3390/metabo11050323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Neto IVS, Pinto AP, Munoz VR, de Cassia MR, Pauli JR, Ropelle ER, Silva A. Pleiotropic and multi-systemic actions of physical exercise on PGC-1alpha signaling during the aging process. Ageing Res Rev. 2023;87:101935. doi: 10.1016/j.arr.2023.101935. [DOI] [PubMed] [Google Scholar]
- 284.Vargas-Ortiz K, Perez-Vazquez V, Macias-Cervantes MH. Exercise and sirtuins: a way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019;20:2717. doi: 10.3390/ijms20112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 286.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vassilopoulos A, Pennington JD, Andresson T, Rees DM, Bosley AD, Fearnley IM, Ham A, Flynn CR, Hill S, Rose KL, Kim HS, Deng CX, Walker JE, Gius D. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal. 2014;21:551–564. doi: 10.1089/ars.2013.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brandauer J, Andersen MA, Kellezi H, Risis S, Frosig C, Vienberg SG, Treebak JT. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front Physiol. 2015;6:85. doi: 10.3389/fphys.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23:128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Osler ME, Fritz T, Caidahl K, Krook A, Zierath JR, Wallberg-Henriksson H. Changes in gene expression in responders and nonresponders to a low-intensity walking intervention. Diabetes Care. 2015;38:1154–1160. doi: 10.2337/dc14-2606. [DOI] [PubMed] [Google Scholar]
- 292.Liu L, Kim S, Buckley MT, Reyes JM, Kang J, Tian L, Wang M, Lieu A, Mao M, Rodriguez-Mateo C, Ishak HD, Jeong M, Wu JC, Goodell MA, Brunet A, Rando TA. Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging. Cell Stem Cell. 2023;30:689–705. doi: 10.1016/j.stem.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Soendenbroe C, Dahl CL, Meulengracht C, Tamas M, Svensson RB, Schjerling P, Kjaer M, Andersen JL, Mackey AL. Preserved stem cell content and innervation profile of elderly human skeletal muscle with lifelong recreational exercise. Aust J Phys. 2022;600:1969–1989. doi: 10.1113/JP282677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 295.Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361:eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Marais L, Stein DJ, Daniels WM. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis. 2009;24:587–597. doi: 10.1007/s11011-009-9157-2. [DOI] [PubMed] [Google Scholar]
- 298.Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/S0169-328X(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 299.Haupt S, Niedrist T, Sourij H, Schwarzinger S, Moser O. The impact of exercise on telomere length, DNA methylation and metabolic footprints. Cells. 2022;11:153. doi: 10.3390/cells11010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jokai M, Torma F, McGreevy KM, Koltai E, Bori Z, Babszki G, Bakonyi P, Gombos Z, Gyorgy B, Aczel D, Toth L, Osvath P, Fridvalszky M, Teglas T, Posa A, Kujach S, Olek R, Kawamura T, Seki Y, et al. DNA methylation clock DNAmFitAge shows regular exercise is associated with slower aging and systemic adaptation. Geroscience. 2023 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 301.Prasertsri P, Phoemsapthawee J, Kuamsub S, Poolpol K, Boonla O. Effects of long-term regular continuous and intermittent walking on oxidative stress, metabolic profile, heart rate variability, and blood pressure in older adults with hypertension. J Environ Public Health. 2022;2022:5942947. doi: 10.1155/2022/5942947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Stenbäck V, Mutt SJ, Leppäluoto J, Gagnon DD, Mäkelä KA, Jokelainen J, Keinänen-Kiukaanniemi S, Herzig KH. Association of physical activity with telomere length among elderly adults - the Oulu Cohort 1945. Front Physiol. 2019;10:444. doi: 10.3389/fphys.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kammire MS, Deal AM, Damone EM, Rosen V, Nyrop KA, Mitin N, Muss HB. Does walking during chemotherapy impact p16(INK4a) levels in women with early breast cancer. J Clin Lab Anal. 2022;36:e24753. doi: 10.1002/jcla.24753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Laukkanen JA, Kunutsor SK, Ozemek C, Makikallio T, Lee DC, Wisloff U, Lavie CJ. Cross-country skiing and running’s association with cardiovascular events and all-cause mortality: a review of the evidence. Prog Cardiovasc Dis. 2019;62:505–514. doi: 10.1016/j.pcad.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 305.Kunutsor SK, Jae SY, Laukkanen JA. “Weekend warrior” and regularly active physical activity patterns confer similar cardiovascular and mortality benefits: a systematic meta-analysis. Eur J Prev Cardiol. 2022;30(3):e7–10. doi: 10.1093/eurjpc/zwac246. [DOI] [PubMed] [Google Scholar]
- 306.Hagberg JM, Montain SJ, Martin WH., 3rd Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1987;63:270–276. doi: 10.1152/jappl.1987.63.1.270. [DOI] [PubMed] [Google Scholar]
- 307.Gyntelberg F, Brennan R, Holloszy JO, Schonfeld G, Rennie MJ, Weidman SW. Plasma triglyceride lowering by exercise despite increased food intake in patients with type IV hyperlipoproteinemia. Am J Clin Nutr. 1977;30:716–720. doi: 10.1093/ajcn/30.5.716. [DOI] [PubMed] [Google Scholar]
- 308.Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, Macera CA, Ainsworth BE. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36:410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 309.Ramsey KA, Meskers CGM, Maier AB. Every step counts: synthesising reviews associating objectively measured physical activity and sedentary behaviour with clinical outcomes in community-dwelling older adults. Lancet Healthy Longev. 2021;2:e764–e772. doi: 10.1016/S2666-7568(21)00203-8. [DOI] [PubMed] [Google Scholar]
- 310.Torjesen I. Sixty seconds on . . . exercise. BMJ. 2018;362:k3006. doi: 10.1136/bmj.k3006. [DOI] [PubMed] [Google Scholar]
- 311.Brown JC, Harhay MO, Harhay MN. Walking cadence and mortality among community-dwelling older adults. J Gen Intern Med. 2014;29:1263–1269. doi: 10.1007/s11606-014-2926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective SC. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 313.Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, Macko RF, McTiernan A, Pate RR, Piercy KL, Physical Activity Guidelines Advisory C Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51:1314–1323. doi: 10.1249/MSS.0000000000001943. [DOI] [PubMed] [Google Scholar]
- 314.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. doi: 10.1001/archinte.1995.00430070053006. [DOI] [PubMed] [Google Scholar]
- 315.Lavie CJ, Sanchis-Gomar F, Ozemek C. Fit is it for longevity across populations. J Am Coll Cardiol. 2022;80:610–612. doi: 10.1016/j.jacc.2022.05.030. [DOI] [PubMed] [Google Scholar]
- 316.O’Keefe JH, Lavie CJ. Run for your life ... at a comfortable speed and not too far. Heart. 2013;99:516–519. doi: 10.1136/heartjnl-2012-302886. [DOI] [PubMed] [Google Scholar]
- 317.Myers J, Kokkinos P, Arena R, LaMonte MJ. The impact of moving more, physical activity, and cardiorespiratory fitness: why we should strive to measure and improve fitness. Prog Cardiovasc Dis. 2021;64:77–82. doi: 10.1016/j.pcad.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 318.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 319.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 320.Haslam C, Kazi A, Duncan M, Clemes S, Twumasi R. Walking works wonders: a tailored workplace intervention evaluated over 24 months. Ergonomics. 2019;62:31–41. doi: 10.1080/00140139.2018.1489982. [DOI] [PubMed] [Google Scholar]
- 321.Baker G, Gray SR, Wright A, Fitzsimons C, Nimmo M, Lowry R, Mutrie N, the Scottish Physical Activity Research C The effect of a pedometer-based community walking intervention “Walking for Wellbeing in the West” on physical activity levels and health outcomes: a 12-week randomized controlled trial. Int J Behav Nutr Phys Act. 2008;5:44. doi: 10.1186/1479-5868-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Harris T, Kerry SM, Limb ES, Victor CR, Iliffe S, Ussher M, Whincup PH, Ekelund U, Fox-Rushby J, Furness C, Anokye N, Ibison J, DeWilde S, David L, Howard E, Dale R, Smith J, Cook DG. Effect of a primary care walking intervention with and without nurse support on physical activity levels in 45- to 75-year-olds: the Pedometer And Consultation Evaluation (PACE-UP) Cluster Randomised Clinical Trial. PLoS Med. 2017;14:e1002210. doi: 10.1371/journal.pmed.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Puig-Ribera A, McKenna J, Gilson N, Brown WJ. Change in work day step counts, wellbeing and job performance in Catalan university employees: a randomised controlled trial. Promot Educ. 2008;15:11–16. doi: 10.1177/1025382308097693. [DOI] [PubMed] [Google Scholar]
- 324.Santos IL, Miragaia D. Physical activity in the workplace: a cost or a benefit for organizations? A systematic review. Int J Workplace Heal. 2023;16:108–135. doi: 10.1108/IJWHM-04-2021-0076. [DOI] [Google Scholar]
- 325.Diaz-Benito VJ, Vanderhaegen F, Moro MIB. Physical activity and health promotion programs in the workplace: a meta-analysis of effectiveness in European organizations. J Workplace Behav He. 2020;35:232–255. doi: 10.1080/15555240.2020.1720515. [DOI] [Google Scholar]
- 326.Gordon CD, Wilks R, McCaw-Binns A. Effect of aerobic exercise (walking) training on functional status and health-related quality of life in chronic stroke survivors: a randomized controlled trial. Stroke. 2013;44:1179–1181. doi: 10.1161/STROKEAHA.111.000642. [DOI] [PubMed] [Google Scholar]
- 327.Voukelatos A, Merom D, Rissel C, Sherrington C, Watson W, Waller K. The effect of walking on falls in older people: the ‘Easy Steps to Health’ randomized controlled trial study protocol. BMC Public Health. 2011;11:888. doi: 10.1186/1471-2458-11-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, Hodges JS. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34:2174–2179. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shibuya J, Ohyanagi M, Iwasaki T. Enhanced myogenic response in resistance small arteries from spontaneously hypertensive rats: relationship to the voltage-dependent calcium channel. Am J Hypertens. 1998;11:767–773. doi: 10.1016/S0895-7061(98)00055-7. [DOI] [PubMed] [Google Scholar]
- 330.Myers J, Harber MP, Johnson L, Arena R, Kaminsky LA. Current state of unhealthy living characteristics in White, African American and Latinx populations. Prog Cardiovasc Dis. 2022;71:20–26. doi: 10.1016/j.pcad.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 331.Kang M, Marshall SJ, Barreira TV, Lee JO. Effect of pedometer-based physical activity interventions: a meta-analysis. Res Q Exerc Sport. 2009;80:648–655. doi: 10.1080/02701367.2009.10599604. [DOI] [PubMed] [Google Scholar]
- 332.Sternfeld B, Block C, Quesenberry CP, Jr, Block TJ, Husson G, Norris JC, Nelson M, Block G. Improving diet and physical activity with ALIVE: a worksite randomized trial. Am J Prev Med. 2009;36:475–483. doi: 10.1016/j.amepre.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 333.Yamawaki K, Oka K, Nakamura Y. Effect of a walking program with the e-mail function of cellular phone. Int J Sport Health Sci. 2009;6:264–273. doi: 10.5432/ijshs.IJSHS20070275. [DOI] [Google Scholar]
- 334.Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, Mutrie N, Scottish Physical Activity Research C Interventions to promote walking: systematic review. BMJ. 2007;334:1204. doi: 10.1136/bmj.39198.722720.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Michalis M, Finn KJ, Podstawski R, Gabnai S, Koller A, Cziraki A, Szanto M, Alfoldi Z, Ihasz F. Differences in cardiorespiratory responses of young and senior male endurance athletes to maximal graded exercise test. Physiol Int. 2020;107:444–454. doi: 10.1556/2060.2020.00032. [DOI] [PubMed] [Google Scholar]
- 336.Gado K, Kovacs AK, Domjan G, Nagy ZZ, Bednarik GD. COVID-19 and the elderly. Physiol Int. 2022 (Epub ahead of print). [DOI] [PubMed]
- 337.Feher A, Szarvas Z, Lehoczki A, Fekete M, Fazekas-Pongor V. Co-infections in COVID-19 patients and correlation with mortality rate. Minireview. Physiol Int. 2022;109(1):1–8. doi: 10.1556/2060.2022.00015. [DOI] [PubMed] [Google Scholar]
- 338.Peterfi A, Meszaros A, Szarvas Z, Penzes M, Fekete M, Feher A, Lehoczki A, Csipo T, Fazekas-Pongor V. Comorbidities and increased mortality of COVID-19 among the elderly: a systematic review. Physiol Int. 2022 (Epub ahead of print). [DOI] [PubMed]
- 339.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kuo CL, Pilling LC, Atkins JL, Masoli JAH, Delgado J, Tignanelli C, Kuchel GA, Melzer D, Beckman KB, Levine ME. Biological aging predicts vulnerability to COVID-19 severity in UK Biobank participants. J Gerontol A Biol Sci Med Sci. 2021;76:e133–e141. doi: 10.1093/gerona/glab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Moccia F, Gerbino A, Lionetti V, Miragoli M, Munaron LM, Pagliaro P, Pasqua T, Penna C, Rocca C, Samaja M, Angelone T. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian Society of Cardiovascular Researches. Geroscience. 2020;42:1021–1049. doi: 10.1007/s11357-020-00198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pence BD. Severe COVID-19 and aging: are monocytes the key? Geroscience. 2020;42:1051–1061. doi: 10.1007/s11357-020-00213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Salimi S, Hamlyn JM. COVID-19 and crosstalk with the hallmarks of aging. J Gerontol A Biol Sci Med Sci. 2020;75:e34–e41. doi: 10.1093/gerona/glaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Santesmasses D, Castro JP, Zenin AA, Shindyapina AV, Gerashchenko MV, Zhang B, Kerepesi C, Yim SH, Fedichev PO, Gladyshev VN. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19:e13230. doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wang XQ, Song G, Yang Z, Chen RJ, Zheng YL, Hu HY, Su X, Chen PJ. Association between ageing population, median age, life expectancy and mortality in coronavirus disease (COVID-19) Aging. 2020;12:24570–24578. doi: 10.18632/aging.104193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhang J, Wang H, Wei M, Zhang H, Xia B, Wang X, Pei Y, Dong L, Li Y. Incidence of cerebrovascular disease as a comorbidity in patients with COVID-19: a meta-analysis. Aging. 2020;12:23450–23463. doi: 10.18632/aging.104086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhou C, Zhang T, Ren H, Sun S, Yu X, Sheng J, Shi Y, Zhao H. Impact of age on duration of viral RNA shedding in patients with COVID-19. Aging. 2020;12:22399–22404. doi: 10.18632/aging.104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Gado K, Khodier M, Virag A, Domjan G, Dornyei G. Anemia of geriatric patients. Physiol Int. 2022;109:119–134. doi: 10.1556/2060.2022.00218. [DOI] [PubMed] [Google Scholar]
- 349.da Silveira MP, da Silva Fagundes KK, Bizuti MR, Starck É, Rossi RC, de Resende ESDT. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2021;21:15–28. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 351.Lee J, Ryu H. Usability of a new digital walking program for older adults: a pilot study. BMC Geriatr. 2023;23:193. doi: 10.1186/s12877-023-03739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Arazi H, Falahati A, Suzuki K. Moderate intensity aerobic exercise potential favorable effect against COVID-19: the role of renin-angiotensin system and immunomodulatory effects. Front Physiol. 2021;12:747200. doi: 10.3389/fphys.2021.747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Baker FL, Zuniga TM, Smith KA, Batatinha H, Kulangara TS, Seckeler MD, Burgess SC, Katsanis E, Simpson RJ. Exercise mobilizes diverse antigen specific T-cells and elevates neutralizing antibodies in humans with natural immunity to SARS CoV-2. Brain Behav Immun Health. 2023;28:100600. doi: 10.1016/j.bbih.2023.100600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Codella R, Chirico A, Lucidi F, Ferrulli A, La Torre A, Luzi L. The immune-modulatory effects of exercise should be favorably harnessed against COVID-19. J Endocrinol Investig. 2021;44:1119–1122. doi: 10.1007/s40618-020-01403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Supriya R, Gao Y, Gu Y, Baker JS. Role of exercise intensity on Th1/Th2 immune modulations during the COVID-19 pandemic. Front Immunol. 2021;12:761382. doi: 10.3389/fimmu.2021.761382. [DOI] [PMC free article] [PubMed] [Google Scholar]