Abstract
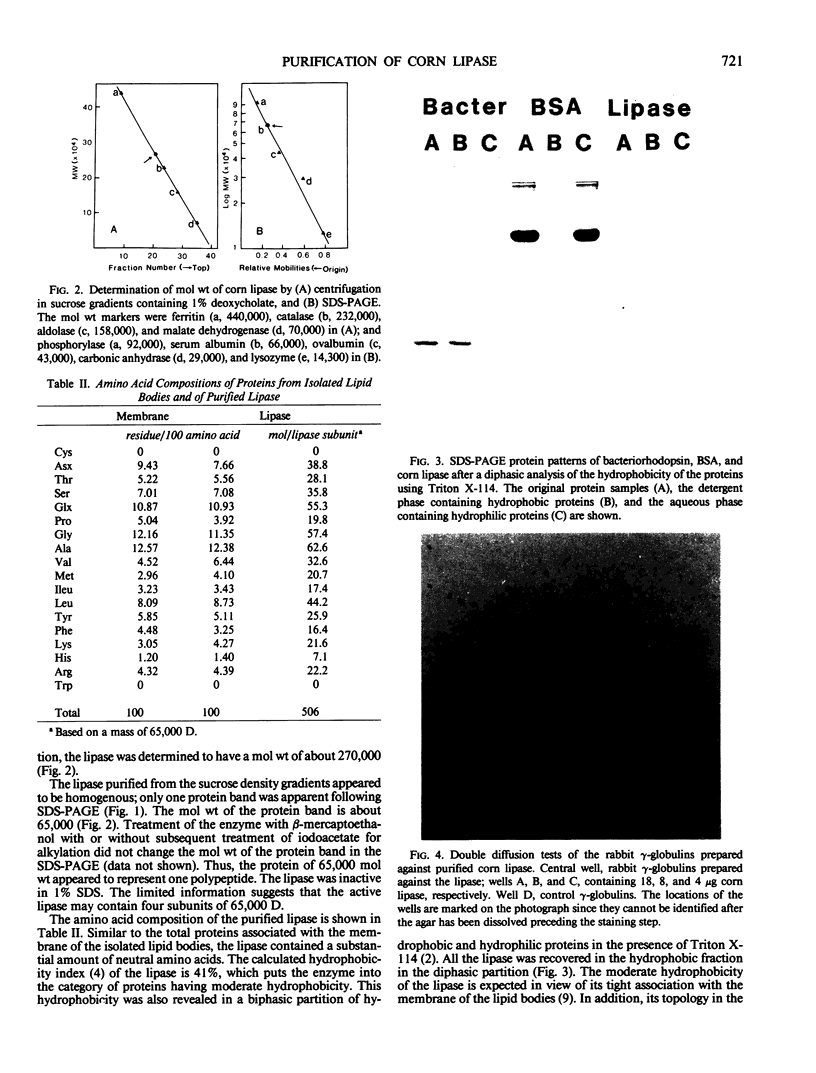
The lipase from the scutella of corn (Zea mays) MO-17 seedlings was purified 272-fold to apparent homogeneity as evidenced by sodium dodecyl sulfate polyacrylamide gel electrophoresis and double immunodiffusion. The procedure involved isolation of the lipid bodies, extraction with diethyl ether, DE-52 ion exchange chromatography, and sucrose density gradient centrifugation. The enzyme had an approximate molecular weight of 270,000 daltons after sucrose density gradient centrifugation, and 65,000 daltons after sodium dodecyl sulfate polyacrylamide gel electrophoresis. The lipase contained no cysteine and its molecular weight in sodium dodecyl sulfate was not reduced by β-mercaptoethanol. The amino acid composition as well as a biphasic partition using Triton X-114 revealed the enzyme to be a hydrophobic protein. Rabbit γ-globulin containing antibodies raised against the purified lipase formed one precipitin line with the lipase in a double diffusion test, and precipitated all the lipase activity from a solution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H., Wimer L. T., Huang A. H. Lipase in the Lipid Bodies of Corn Scutella during Seedling Growth. Plant Physiol. 1983 Oct;73(2):460–463. doi: 10.1104/pp.73.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R. A., Liu K. D., Huang A. H. Spherosomes of Castor Bean Endosperm: MEMBRANE COMPONENTS, FORMATION, AND DEGRADATION. Plant Physiol. 1980 Jun;65(6):1176–1180. doi: 10.1104/pp.65.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon M., Chan S. H. A simple and sensitive colorimetric method for the determination of long-chain free fatty acids in subcellular organelles. Anal Biochem. 1979 Sep 1;97(2):403–409. doi: 10.1016/0003-2697(79)90093-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]