Abstract
Upper limb motor function is a potential new biomarker of cognitive impairment and may aid discrimination from healthy ageing. However, it remains unclear which assessments to use. This study aimed to explore what methods have been used and to describe associations between upper limb function and cognitive impairment. A scoping review was conducted using PubMed, CINAHL and Web of Science. A systematic search was undertaken, including synonyms for key concepts ‘upper limb’, ‘motor function’ and ‘cognitive impairment’. Selection criteria included tests of upper limb motor function and impaired cognition in adults. Analysis was by narrative synthesis. Sixty papers published between 1998 and 2022, comprising 41,800 participants, were included. The most common assessment tasks were finger tapping, Purdue Pegboard Test and functional tasks such as writing. Protocols were diverse in terms of equipment used and recording duration. Most participants were recruited from clinical settings. Alzheimer’s Disease was the most common cause of cognitive impairment. Results were mixed but, generally, slower speed, more errors, and greater variability in upper limb movement variables was associated with cognitive impairment. This review maps the upper limb motor function assessments used and summarises the available evidence on how these associate with cognitive impairment. It identifies research gaps and may help guide protocols for future research. There is potential for upper limb motor function to be used in assessments of cognitive impairment.
Keywords: Upper limb, Motor function, Cognitive impairment, Dementia, Mild cognitive impairment
Introduction
The underlying brain pathology for most types of dementia develops over decades, prior to the cognitive symptoms emerging [1]. Motor changes related to this neuropathology have shown potential as non-invasive biomarkers [1–3]. In 2020, The 5th Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD) recommended that assessment of motor function should be included in dementia investigations as there is strong evidence that it can aid detection of cognitive impairment or dementia risk in older adults [2]. Motor biomarkers provide a low cost and accessible method for identifying early-stage cognitive impairment [4] and predict transition from mild cognitive impairment (MCI) to dementia [3, 4]. This may facilitate referral to specialised clinics, early risk modification and recruitment to intervention trials [5–7].
Gait has been the most studied motor biomarker with strong evidence showing cognitive impairment associates with impairments in gait [1, 5, 8, 9]. From gait studies, we know that the premotor cortex plays a key role in controlling and coordinating the neural activity in areas of the brain (such as the basal ganglia, brainstem and cerebellum) that are involved in planning and execution of movement [10]. The higher level control of the prefrontal cortex is further implicated when a cognitive task is performed while walking (dual-task). Damage to the prefrontal cortex caused by stroke or neurodegenerative disease is associated with gait impairment such as slowed walking speed and greater step time variability [2, 10–12]. Although the neurocognitive mechanisms underpinning the upper limb motor function (ULMF) changes seen with cognitive impairment are not fully understood yet, it would seem likely that they are comparable to those for gait.
Assessment of ULMF may provide additional benefits as many subtle measures of gait are undetectable by clinical observation and require electronic gait analysis systems which limits widespread access [13, 14]. In addition, gait analysis poses challenges for remote assessment and in people who have ambulatory difficulties. In contrast, analysis of ULMF is generally more accessible as it can be assessed using readily available mobile phones and computers and tests can be performed seated.
Emerging evidence shows that a range of ULMFs change in cognitive impairment and may aid discrimination from healthy ageing, but this has been less explored than gait [15–20]. It remains unclear what tasks of ULMF to use, how best to measure these and what movement variables associate with cognitive impairment. This hinders integration of ULMF assessments into investigations of cognitive impairment. This review thus aimed to address the question: ‘What methods of assessing ULMF have been used to investigate the association of ULMF with cognitive impairment in adults?’ via four sub questions:
What tests (including the task, equipment, protocol, and movement variables) of ULMF have been used to investigate cognitive impairment in adults?
What conditions/diseases with resultant cognitive impairment have been studied?
What were the major participant recruitment settings?
How does ULMF associate with cognitive impairment?
Methods
A scoping review was conducted using JBI methodology for Scoping Reviews and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses—extension for scoping reviews (PRISMA-ScR) guidelines [21, 22]. A protocol was designed to define the questions and clarify methods and reporting (published in Figshare [23]).
We searched PubMed, CINAHL and Web of Science databases for studies published in English up to March 2022. Search terms included synonyms for the three main concepts: 1. Cognitive impairment, 2. Upper limb, 3. Motor function. We included terms describing specialised tasks of the hands and upper limbs such as writing, drawing and grasping. Appendix 1 shows the keywords and Medical Subject Headings (MeSH) terms used for the PubMed search.
Eligibility criteria
All human research studies and systematic reviews examining the association between ULMF and cognitive impairment (caused by any disease/condition) in adults (≥ 18 years) were included. Books, theses, research protocols, and blogs were excluded. Eligible studies required at least one test of cognition and inclusion of participants with cognitive impairment. All tests involving dynamic and volitional functions of the upper limb were eligible, except for grip strength. Evidence from many other studies shows grip strength is associated with cognitive impairment and its measurement is recommended by the fifth Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD5) on early non-cognitive markers of dementia [2]. Furthermore, it could be argued that grip strength is not kinematic/dynamic, but rather a kinetic/isometric contraction. Studies of movement analysis in sleep were also excluded as these movements are considered involuntary. All methods of assessing ULMF were eligible.
Data extraction process
Search results were exported to Covidence software, and duplicates removed. Titles and abstracts were screened independently by two reviewers (KR plus JA, MC or KL) against the eligibility criteria. Full texts of selected articles were retrieved and independently screened by two reviewers for inclusion; disagreements were resolved through consensus of the two reviewers and, when required, a third reviewer. As recommended by JBI manual for evidence synthesis [22], a draft data extraction table was developed, piloted, and revised by all authors before it was created in Covidence. This extraction table structured the researchers approach to ensure they extracted the same sets of data from each study and provided a logical summary of results based on the questions of the scoping review [22]. One author (KR) used this to extract data on each study’s design, recruitment setting and characteristics, disease or condition resulting in cognitive impairment, tests (including ULMF task, equipment, protocol, and movement variables) and key findings.
Results
Selection of sources of evidence
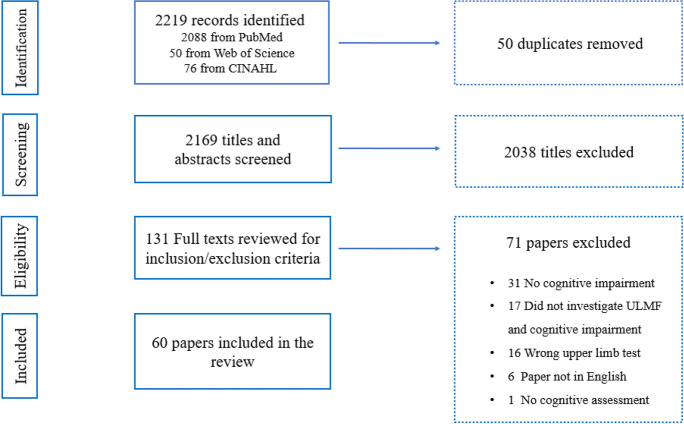
2,219 records were initially identified and, after removing duplicates, 2,169 sources remained. Sixty papers met all selection criteria. Figure 1 shows the flow of information through the steps of this review.
Fig. 1.
PRISMA flow diagram of the study selection in the review
Characteristics of the evidence
Table 1 summarises the characteristics of 60 included articles. The papers were dated from 1995 to February 2022 and comprised 41,800 participants. Most studies (55%) were conducted in the United States, Germany, Japan, China, and the United Kingdom. There were 54 cross-sectional studies (90%), five longitudinal studies (8%) and one systematic review. Five of the cross-sectional studies were sub-studies of longitudinal cohorts.
Sub-question 1. What tests of ULMF have been used to investigate cognitive impairment in adults?
Table 1.
Characteristics of included papers
| Author (year) | Country | Setting | Design | Population | Test(s) of cognition |
|---|---|---|---|---|---|
| Ott (1995) [24] | USA | Clinic | Cross-sectional |
25 AD 25 HC |
MMSE |
| Welch (1997) [25] | USA | Clinic | Cross-sectional |
42 with KS 14 alcohol dependents without KS 26 HC |
Wechsler Memory Scale-Revised |
| Camicioli (1998) [26] | USA | Community |
Longitudinal (minimum 1.2 years) |
85 at baseline: - 18 developed CI - 67 remained cognitively intact |
Clinical Dementia Rating Scale |
| Goldman (1998) [27] | USA | Research | Cross-sectional |
58 PD without dementia 22 PD with questionable dementia 43 HC |
Comprehensive neuropsychological assessment |
| Willis (1998) [28] | USA | Clinic | Cross-sectional |
26 AD 42 HC |
MMSE |
| Goldman (1999) [29] | USA | Clinic | Cross-sectional |
60 mild AD 43 HC |
Wechsler Memory Scale |
| Schroter (2003) [30] | Germany | Clinic | Cross-sectional |
35 AD 39 MCI 39 Major depression 40 HC |
MMSE |
| Amieva (2004) [31] | France | Clinic |
Longitudinal (2 years); a sub-study of a multicentre double-blind RCT |
90 MCI at baseline: - 29 progressed to dementia - 61 remained dementia free |
MMSE |
| Muhlack (2006) [32] | Germany | Clinic | Cross-sectional |
12 AD 12 MCI 12 HC |
MMSE |
| Bramell-Risberg (2010) [33] | Sweden | Community |
Cross-sectional (part of a longitudinal study—Good Ageing in Skane) |
301 CI 419 intermediate CI 1,207 HC |
MMSE (grouped based on 3-word recall test) |
| Buracchio (2010) [6] | USA | Community |
Longitudinal (mean 9 years) |
204 at baseline: - 95 converted to MCI - 109 remained cognitively normal |
MMSE |
| Ameli (2011) [34] | Germany | Clinic | Cross-sectional |
8 MCI 8 AD |
Comprehensive neuropsychological assessment |
| Rousseaux (2012) [35] | France | Clinic | Cross-sectional |
31 AD 38 HC |
MMSE |
| Rabinowitz (2014) [36] | Israel | Community | Cross-sectional |
170 participants: - 97 with CI, - 73 without CI |
MMSE |
| Henley (2014) [37] | UK | Clinic | Cross-sectional |
20 bvFTD, 11 semantic PPA 4 non-fluent PPA 8 AD 31 HC |
Comprehensive neuropsychological assessment |
| Johnen (2015) [38] | Germany | Clinic | Cross-sectional |
20 AD 20 bvFTD 20 HC |
MMSE |
| Ward (2015) [39] | Brazil | Clinic | Cross-sectional |
52 AD 45 MCI 39 HC |
MMSE |
| Nagahama (2015) [40] | Japan | Clinic | Cross-sectional |
74 DLB 100 AD 52 VaD 75 HC |
MMSE |
| Lin (2016) [41] | China | Clinic | Cross-sectional |
10 AD 10 HC |
MMSE |
| Toosizadeh (2016) [42] | USA | Clinic | Cross-sectional |
10 CI 57 HC |
MMSE MoCA |
| Fritz (2016) [43] | USA | Clinic | Cross-sectional |
21 LBD 21 AD 21 PD 11 DLB 10 PDD |
MMSE |
| Souza (2016) [44] | Brazil | Clinic | Cross-sectional |
41 PD-AD 19 PD-MCI 41 PD 88 HC |
MMSE |
| Dahdal (2016) [45] | Switzerland | Clinic | Cross-sectional |
20 PD-MCI 31 PD-cognitively normal |
MMSE |
| Darweesh (2017) [19] | Netherlands | Community |
Longitudinal (Median 9.2 years) A sub-study of a prospective population-based Rotterdam Study |
4856 at baseline: - 227 developed dementia, - 50 developed parkinsonism |
MMSE |
| Kay (2017) [46] | USA | Clinic | Cross sectional |
24 aMCI 41 APOEε4 carriers HC 65 non-carriers HC |
MMSE |
| Kueper (2017) [3] | Canada | NA | Systematic review | NA | NA |
| Bartoli (2017) [47] | Italy | Clinic | Cross-sectional |
20 CI 20 HC |
MMSE |
| Sanin (2017) [48] | Austria | Clinic | Cross-sectional |
45 AD 38 MCI 50 HC |
MMSE |
| Garre-Olmo (2017) [49] | Spain | Clinic | Cross-sectional |
23 AD 12 MCI 17 HC |
Cambridge Cognitive Examination Revised |
| Suzumura (2018) [50] | Japan | Clinic | Cross-sectional |
31 AD 15 MCI 48 HC |
MMSE |
| Roalf (2018) [51] | USA | Clinic | Cross-sectional |
131 AD 46 PD 63 MCI 62 HC |
MMSE |
| Gupta (2018) [52] | India | Clinic | Cross-sectional | 90 alcohol abstinent patients | MMSE |
| Rycroft (2018) [53] | USA | Community | Cross-sectional (Part of the Boston Rehabilitative Impairment Study of the Elderly (RISE)) |
68 aMCI 15 naMCI 98 mdMCI 249 HC |
Comprehensive neuropsychological assessment |
| Gulde (2018) [54] | Germany | Clinic | Cross-sectional |
11 AD 15 HC |
MMSE |
| Jeppesen Kragh (2018) [55] | Denmark | Clinic | Cross-sectional |
17 AD 19 FTD 13 DLB 15 HC |
MMSE ACE |
| Zhang (2018) [56] | China | Community | Cross-sectional |
20 MCI/no Tai Chi 20 MCI/Tai Chi 30 HC/no Tia Chi 30 HC/Tai Chi |
MoCA |
| Carment (2018) [17] | France | Clinic | Cross-sectional |
11 CI HC groups: - 10 young adults - 8 middle-aged adults - 11 older adults |
MMSE |
| Fadda (2019) [57] | Italy | Clinic | Cross sectional |
10 DLB 10 HC |
MMSE FAB |
| Toosizadeh (2019) [58] | USA | Clinic | Cross-sectional |
22 AD 24 MCI 35 HC |
MMSE MoCA |
|
Mollica (2019) [59] |
Spain | Clinic | Cross-sectional |
15 AD/Amyloid β + 20 HC/Amyloid β + 37 HC/Amyloid β - |
Comprehensive neuropsychological assessment |
| Tomita (2020) [60] | Japan | Community | Cross-sectional |
60 CI 42 CH |
MoCA |
| Bologna (2020) [61] | Italy | Clinic | Cross-sectional |
20 mild to moderate AD 20 HC |
Comprehensive neuropsychological assessment MMSE |
| Liou (2020) [62] | Taiwan | Clinic | Cross-sectional |
11,935 mild dementia 20,883 moderate to severe dementia |
FUNDES-Adult |
| San Martin-Valenzuela (2020) [63] | Spain | Clinic | Cross-sectional |
28 MHE 38 without MHE |
MMSE |
| Hesseberg (2020) [18] | Norway | Community | Cross-sectional (part of a 1-year longitudinal study) |
38 dementia 60 MCI |
MMSE |
| Ntracha (2020) [64] | Greece | Community | Cross-sectional |
11 MCI 12 HC |
MMSE |
| Ehsani (2020) [65] | USA | Community | Cross-sectional |
16 early-stage AD 30 aMCI 35 HC |
MMSE MoCA |
| Zhang (2021) [66] | China | Research | Cross-sectional |
20 MCI 41 HC |
MoCA |
| Paixao (2021) [67] | Portugal | Community | Cross-sectional |
22 dementia (institutionalised) 28 dementia (community dwelling) 26 HC |
ACE |
| Mancioppi (2021) [68] | France | Clinic | Cross-sectional |
17 MCI 27 HC |
MMSE |
| Nagahama (2021) [69] | Japan | Clinic | Cross-sectional |
162 AD 103 DLB |
MMSE |
| Uwa-Agbonikhena (2021) [70] | Ukraine | Clinic | Cross-sectional | 86 participants 1-year post-stroke |
MMSE MoCA |
| Beeri (2021) [71] | USA | Community |
Longitudinal (mean 7.3 years) |
1160 with no CI at baseline 166 developed AD |
MMSE |
| Zhao (2021) [72] | China | Community | Cross-sectional |
35 AD/no exercise habits 35 AD/exercise habits 35 HC/no exercise habits 35 HC/exercise habits |
MMSE MoCA |
| Suzumura (2021) [73] | Japan | Clinic | Cross-sectional |
44 AD 20 MCI |
MMSE |
| Colella (2021) [74] | Italy | Clinic | Cross-sectional |
14 aMCI 16 HC |
MoCA |
| Cosgrove (2021) [75] | UK | Clinic | Cross-sectional |
22 PD-normal cognition 23 PD-MCI 10 PD-Dementia 19 HC-normal cognition 10 HC-MCI |
MoCA |
| Davoudi (2021) [76] | USA | Research | Cross-sectional |
29 AD 27 VaD 175 HC |
Comprehensive neuropsychological assessment MMSE |
| Kutz (2022) [77] | Germany | Research | Cross-sectional (part of the SENDA study) |
66 MCI 80 pMCI 79 HC |
MoCA |
| Schmidt (2022) [78] | Germany | Clinic | Cross-sectional | 47 PD | MMSE |
Papers are presented chronologically according to publication date. MMSE Mini Mental State Examination, AD Alzheimer’s Disease, HC healthy controls, PPA primary progressive aphasia, CI cognitive impairment, RCT Randomised Clinical Trial, KS Korsakoff’s Syndrome, LBD Lewy Body dementia, PD Parkinson’s Disease, MCI mild cognitive impairment, CCT cube copying test, bvFTD behavioural variant frontotemporal dementia, DLB dementia with Lewy body, VaD vascular dementia, MoCA Montreal Cognitive Assessment, PDD Parkinson’s disease with dementia, PD-AD Parkinson’s disease with Alzheimer’s Disease, PD-MCI Parkinson’s disease with mild cognitive impairment, aMCI amnestic MCI, naMCI non-amnestic MCI, mdMCI multi-domain MCI, Amyloid β + Amyloid Beta positive, Amyloid β - Amyloid Beta negative, ACE Addenbrooke’s Cognitive Examination, FAB Frontal Assessment Battery, FTD frontotemporal dementia, FUNDES-Adult Functional Disability Evaluation Scale-Adults, MHE Minimal Hepatic Encephalopathy, pMCI possible MCI, SENDA study Sensor-based systems for early detection of dementia, NA not applicable
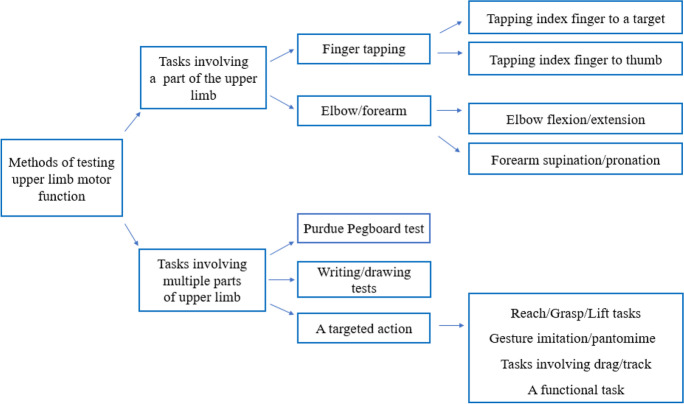
Table 2 and Fig. 2 outline the tests of ULMF in the included papers. The narrative synthesis considers the 4 main components of motor function tests: the task, equipment, protocol and movement variables. We recognise there are many ways to group upper limb assessments and that, some may argue that there are better ways to group the tests, especially for those that are new/experimental. In this review, and for ease of classification, we grouped the tasks based on the number of parts of the upper limb that are involved in completing the ULMF assessment.
Table 2.
Summary of tests of upper limb motor function
| Author (year) |
Hand/upper limb assessed | Task | Equipment | Protocol | Movement variables |
|---|---|---|---|---|---|
| Ott (1995) [24] | Both hands | FT (index-target tapping) | Computer keyboard | Tapping any key of their choice at a fast pace for 8 s once with their index finger and then alternately tapped the index-middle finger | Number of taps (average of the five trials) |
| Welch (1997) [25] | Both hands | FT (index-target tapping) | Not specified | Finger tapping test as part of a neuropsychological battery (Halstead-Reitan manual) after 3 weeks of alcohol abstinence | Tapping speed Mean tapping score |
| Camicioli (1998) [26] | Both hands | FT (index-thumb tapping) | Not mentioned |
Tapping index finger to thumb for 10 s (right and left hand) |
Total number of taps for each hand |
| Goldman (1998) [27] | Both hands, dominant hand first | FT (index-target tapping) | An electronic device which is not specified | Tapping index finger for 10 s in 3 positions for each hand: wrist and elbow restrained, elbow restrained, and no restraint | Number of taps |
| Willis (1998) [28] | Both hands |
1. FT (index-thumb tapping) 2. Forearm, supination/ pronation |
Not specified |
1. Tapping index fingers to thumbs at a fast pace for 10 s 2. Fast-paced supination/pronation of the dominant hand for 10 s |
Number of correct cycles completed |
| Goldman (1999) [29] | Both hands, dominant hand first | FT (index-target tapping) | An electronic device which is not specified | Tapping index finger for 10 s in 3 positions for each hand: wrist and elbow restrained, elbow restrained, and no restraint | Number of taps |
| Schroter (2003) [30] | Dominant and non-dominant hand | Writing | Digitising tablet and a pressure-sensitive inking stylus |
Drawing concentric circles on a digitising tablet as fast as possible for 30 s, then repeating the task while performing an additional distraction task with the nondominant hand for 10 s |
Peak velocity SD of velocity Number of changes in direction |
| Amieva (2004) [31] | Not specified | FT (index-target tapping) | Computer keyboard | No details provided | Speed |
| Muhlack (2006) [32] |
Both hands separately (dominant hand first) |
PPT | 25-hole computer-based contact pegboard |
Transferring pegs from a rack into one of 25 holes in the board individually and as quickly as possible |
Time taken to complete the task for each hand Total time |
| Bramell-Risberg (2010) [42 | Both forearms | Forearm, supination/pronation | An optical shaft encoder (Hewlett Packard HEDS5701-A00) connected to a microcontroller (Microchip PIC 16C84) sending the data to the computer |
Supinating and pronating each forearm separately for 10 s while gripping the handle of the shaft and bend their elbow approximately 90° |
Number of supination/ pronation Speed |
| Buracchio (2010) [6] | Both hands (index fingers) | FT (index-target tapping) | A counting machine with a lever |
Tapping a lever with an attached counter using the index finger of each hand for 10 s |
Mean speed value of three trials |
| Ameli (2011) [34] | Dominant hand | Research/Grasp/Lift | A cylindrical and cordless object, mounted on top of an opaque plastic box which was either empty (400 g total) or contained an added 200 g mass (600 g total) |
Lifting the object about 5 cm above the supporting table and holding for 4 s before putting it down under two conditions: with and without visual cues on weight of the object |
Linear acceleration in three dimensions Peak acceleration |
| Rousseaux (2012) [35] | Both hands | Lille gestural apraxia test | Photos and various tools and objects as required | Imitating meaningless and symbolic gestures, pantomiming complex actions |
Individual scores of each subtest Total score |
| Rabinowitz (2014) [36] | Dominant hand | FT (index-target tapping) | A touchpad mounted on a pressure transducer (FSR, InterLink Electronics, Camarillo, CA, USA) |
Tapping index finger on a pressure pad for 15 s at a self-selected pace |
Touch time Time off touch pad Touch cycle Touch SD Coefficient of variation |
| Henley (2014) [37] | Dominant hand | FT (index-target tapping) | Superlab on a computer |
Tapping index finger on a computer key once in pace with the beat of a series of tones and once tapping in pace with a series of tones after the sounds ceased |
Inter-response interval Inter-response interval variance Time variance Motor variance Response interval drift Response interval absolute drift |
| Johnen (2015) [38] | Both hands |
The Cologne Apraxia Screening (CAS) Ideomotor Apraxia Test (IAT) The Monster Apraxia Items (MI) |
Pictures of gestures |
Pantomiming object use and commonly used gestures, and imitating hand and finger postures and face postures |
Total score for each test Apraxia subdomain scores (% correct gestures) |
| Ward (2015) [39] | Dominant hand | Eight functional tasks from the Cambridge Cognitive Examination | Not specified |
Drawings of pentagon, spiral, house, clock, and inserting a sheet of paper into an envelope, waving goodbye, cutting a sheet of paper with a pair of scissors, and brushing teeth |
Sub-items scores Total score |
| Nagahama (2015) [40] | Both hands | Gesture imitation | NA |
Imitating gestures after watching demonstration by examiner. Maximum 10 s was allowed to imitate each gesture |
Accuracy scores Time taken to imitate each gesture Average scores of both hands |
| Lin (2016) [41] | Dominant hand | Trail making | A custom-made test device with a wooden board and 16 electronic plates with lights connected to a computer. A pencil-like stick was connected to the board by a wire |
Striking lit target sensors with the pencil-like stick as fast as possible in three predetermined sequences: fixed pattern from left to right, random pattern and a fixed pattern while counting backward |
Reaction time |
| Toosizadeh (2016) [42] | Dominant arm | Elbow flexion/extension | Wearable sensors: A tri-axial gyroscope and accelerometer sensor (BioSensics LLC, Boston, MA, USA) | Flexing and extending elbow at fast pace for 20 s. Then performed this test while counting numbers backward by one |
Speed Flexibility Power Rise time Moment Speed reduction Speed variability Flexion number |
| Fritz (2016) [43] | Both (dominant then non-dominant hand) | PPT | 9-hole Purdue Pegboard |
Placing pegs into holes in a board and then removing them |
Time |
| Souza (2016) [44] | Both hands | ILFT | NA |
Imitating bimanual non-symbolic gestures after demonstration by examiner |
Individual scores Total score |
| Dahdal (2016) [45] | Right hand | Functional tasks of upper limb such as holding the stylus inside a 5.8-mm-wide well for 20 s without touching the rim | An electric stylus |
Holding the stylus and performing various tasks to test steadiness, precision, tapping, dexterity and aiming A total of 5 tasks were performed, each taking 20 s |
Number of errors Number of taps Time to complete tasks |
| Darweesh (2017) [19] | Both hands (each hand separately and both hands together) | PPT | 25-hole Purdue Pegboard |
Placing as many cylindrical metal pegs as possible into one of 25 holes in a pegboard as possible in 30 s |
Average score of three trials |
| Kay (2017) [46] | Right hand | FT (index-target tapping) |
A box with a push button E-Prime software (Psychology Software Tools, Pittsburgh, PA) |
Participants tapped a button with their right index finger for 12 s in synchrony with a visual flashing yellow and black checkerboard | Inter-tap intervals |
| Kueper (2017) [3] | NA | Finger tapping | NA | Not specified | Not specified |
| Bartoli (2017) [47] | Both hands (starting with the dominant hand) | Target tracking | A haptic interface (Omni®, Sensable) controlled by a custom-made software | Participants followed the target movement when it moved continuously on a computer screen, abruptly and when remained stationary. There were 22 trials, each lasting 20 s |
Reaction times Mean absolute error with and without feedback |
| Sanin (2017) [48] | Both hands | Gesture imitation | NA |
Imitating gestures/fingers configurations and hand movements |
Individual scores of each test Total score |
| Garre-Olmo (2017) [49] | Dominant hand | Writing a sentence, drawing a clock face, and copying a house, pentagons, and a spiral | A commercial Intuos WACOM series 4 size L digitising tablet and a pressure sensitive Intuos ink pen |
Writing sentences and drawing circles with a wireless electronic stylus on a paper fastened to the digitising tablet |
Pressure Time Speed Acceleration |
| Suzumura (2018) [50] | Both hands | FT (index-target tapping) | JustTouch screen |
Tapping marks on the screen using the index finger of right, left and then both hands for 15 s at a fast pace and in pace with sound signals |
Mean lag time Lag-time SD Mean inter-tap time Intertap-time SD Contact time Contact time SD Inter-hand phase difference Inter-hand phase SD |
| Roalf (2018) [51] | Both hands | FT (index-target tapping) | Light Beam Finger and Foot Tapper Test (NeuroCognitive Engineering, 1995) |
Tapping index finger through a light beam device for 10 s with dominant, non-dominant and then both hands. PD participants performed test while on medication |
Total tap count Intra-individual variability Inter-tap interval |
| Gupta (2018) [52] | Both hands | FT (index-target tapping) | A custom-made software running on a laptop computer |
Tapping index finger on a computer key for 10 s |
Frequency |
| Rycroft (2018) [53] | Both forearms | Forearm, supination/pronation | NA |
Supinating and pronating forearm of each arm separately for 20 s at a self-selected (two trials) and at a fast pace (two trials) |
Number of accurate movements |
| Gulde (2018) [54] | Dominant hand | Gesture imitation | Pictures of objects | Imitating meaningless finger and hand gestures and performing pantomimes of object use | Correct imitations and pantomimes |
| Jeppesen Kragh (2018) [55] | Both hands (right and left hand individually) |
1. FT (index-target tapping) 2. Forearm supination/ pronation 3. Reach/grasp/Lift |
Force transducer (Mini-40, ATI Industrial Automation, Apex, NC, USA) |
1. Tapping the force transducer for 10 s with the index finger at a fast pace, 2. Tapping the force transducer with the palm and back of their hands at a fast pace, 3. Grasping and lifting the force transducer and held it stable for 20 s at a height of 10 cm |
1 & 2 Frequency Mean inter-onset interval Inter-onset interval SD 3 Mean orientation index Mean position index |
| Zhang (2018) [56] | Both hands | FT (index-target tapping) | An infrared photoelectric sensor connected to a computer | Placing index finger within the frame and tapping at a fast pace for 8 s | Frequency |
| Carment (2018) [14] | Dominant hand | FT (index-target tapping) | Finger Force Manipulandum; a device with force sensitive pistons |
Matching the applied index finger force to the target force using the visual feedback and then using auditory cues |
Frequency Variability |
| Fadda (2019) [57] | Both arms | Hand to Mouth movement | Wearable sensors: Markers were placed on upper limb bony landmarks. An 8-cameras motion-capture system (SMART-D, BTS Bioengineering, Italy) | Reaching, touching mouth and returning to initial position. Arms were tested separately |
Time events (total and each phase) Speed, Smoothness Accuracy variables |
| Toosizadeh (2019) [58] | Dominant arm | Elbow flexion/extension |
Wearable sensor: A tri-axial gyroscope and accelerometer sensor (BioSensics LLC, Boston, MA, USA) |
Flexing/extending the elbow under two conditions: fast-paced for 20 s, and self-pace for 60 s. Self-selected pace was performed as a single-task and under two dual-tasks |
Speed Rise time Flexion number Range of motion Variability |
|
Mollica (2019) [59] |
Both hands | FT (index-target tapping) | Computer keyboard (E-Prime 2.0 (Psychology Software Tools Inc., Pittsburgh, PA) | Tapping computer's spacebar with their index finger as fast as they could for 10 s while looking at a fixation point on the monitor. Hands were tested separately |
Speed Intrasubject variability |
| Tomita (2020) [60] | Both hands | FT (index-target tapping) | Wearable sensors: Magnetic sensors were placed on the thumb and index finger of both hands. Magnetic sensors (UB-2; Hitachi Maxell, Tokyo, Japan) |
Tapping index fingers to thumbs at a fast pace for 15 s once in-phase and once alternately Instruction: open fingers 5 cm while tapping |
Amplitude (movement distance between the thumb and index finger) Total tap count Rhythm |
| Bologna (2020) [61] | Right hand | FT (index-thumb tapping) | Wearable sensors: Reflective markers attached to the tips of the index finger and thumb and detected via an optoelectronic system (SMART motion system, BTS Engineering, Italy) |
Tapping index finger to thumb at a self-selected pace for 15 s |
Amplitude Velocity Movement slope Rhythm |
| Liou (2020) [62] | Both hands | Domain 8 of FUNDES-Adult assessment | FUNDES-Adult assessment records | Assessment included pen-holding, buttoning, and knotting | Level of assistance required to complete each task |
| San Martin-Valenzuela (2020) [63] | Both hands | FT (index-target tapping) | Not specified |
Tapping a key at a fast pace using the index finger of each hand separately and then both hands simultaneously for 10 s |
Number of finger-taps |
| Hesseberg (2020) [18] | Dominant hand | FT (index to target tapping) | A counting machine | Tapping index finger of the dominant hand at a fast pace for 10 s on a counting machine | Mean tap number of the five trials |
| Ntracha (2020) [64] | Not specified | Typing on a virtual keyboard | Custom-made virtual keyboard on an app on Smartphones | Typing down up to 4 short texts, around a paragraph in length about familiar topics. No time limit. Phones’ autocorrection feature were disabled | Number of errors Keystroke timing |
| Ehsani (2020) [65] | Dominant arm | Elbow flexion/extension | Wearable motion sensors (tri-axial gyroscope sensors (BioSensics LLC, Cambridge, MA) |
Flexing/extending elbow at a self-selected pace for 60 s under one single-task and two dual-task conditions |
Entropy Angular velocity |
| Zhang (2021) [66] | Dominant hand | Drag and Drop Test | A Huawei M5 touch screen tablet |
Dragging blocks one by one from a start area and dropping them to a target area within 60 s without hitting a partition in between the start and target areas or dropping the blocks in the start area |
Number of successful and failed dragged blocks Time taken to move a block Average speed Speed SD |
| Paixao (2021) [67] | Both hands | Grocery Shelving Task (putting grocery cans on a shelf) | Adjustable shelf and twenty 420-g grocery cans divided into two grocery bags | Standing up from a chair, walking 1 m toward the shelf, and placing all the cans in the shopping bags on the shelf as quickly as possible, one can in each hand | Time |
| Mancioppi (2021) [68] | Dominant hand | FT (index-target tapping) | Wearable sensors, based on microelectromechanical sensors composed by the SensHand |
Tapping index finger at a self-selected pace for 15 s, under single- and dual-task conditions |
Number of taps Dual-task cost Opening velocity Velocity SD |
| Nagahama (2021) [69] | Both hands | Gesture imitation | Not specified |
Imitating gestures after watching examiner’s hand with each hand separately and then simultaneously |
Time taken to imitate Accuracy of imitation |
| Uwa-Agbonikhena (2021) [70] | Both hands | Various tasks such as hook grasp, cylinder grasp, elbow flexion/extension, forearm supination and pronation, etc | The Fugl-Meyer assessment | As per Fugl-Meyer upper extremity assessment of sensorimotor function protocol |
Subtest scores Total motor function score |
| Beeri (2021) [71] | Both hands |
1. PPT 2. FT (index-target tapping) |
1. 25-hole Purdue Pegboard, 2. an electronic tapper (Western Psychological Services, Los Angeles, CA) |
1. Inserting as many pegs as possible in 30 s 2. Tapping the tapper with their index finger at a fast pace for 10 s |
1. Average score of two trials for each hand 2. Average score of two trials for each hand (number of taps) |
| Zhao (2021) [72] | Both hands) | FT (index-target tapping) | An infrared photoelectric sensor | Tapping index finger for 8 s at a fast pace within the device's frame first with the right and then with the left hand | Frequency |
| Suzumura (2021) [73] | Both hands | FT (index-thumb tapping) |
A FT device with magnetic sensors (UB-2, Maxell Holdings, Ltd, Tokyo, Japan) Colour-coded cables were attached to the dorsum of participants' index fingers and thumbs |
Tapping index finger to thumb at a fast pace for 15 s under four conditions: a single hand at a time, both hands simultaneously and both hands alternately |
36 variables such as: Max amplitude Max velocity SD of velocity Accretion SD of inter-tapping interval Inter-tapping interval variability |
| Colella (2021) [74] | Dominant hand | FT (index-thumb tapping) | Wearable sensors: An optoelectronic system (SMART motion, BTS Technology, Italy) |
Tapping index finger on their thumb as widely as possible at a fast pace for 15 s in three trials |
Amplitude Velocity Rhythm |
| Cosgrove (2021) [75] | Both hands (trials alternated between hands) | Reach/Grasp/Lift | The object was a cylindrical Philips Imageo rechargeable candle made of Perspex. Movements were recorded using a Polhemus Patriot electromagnetic tracking device | Grasping the object, lifting it vertically and then placing it back on the table. Five trials for each hand completed under four conditions: normal lighting (self- and fast-paced) and self-selected pace (darkened room and eyes closed) |
Reaction time Movement time |
| Davoudi (2021) [76] | Dominant hand | Drawing (digital Clock Drawing Test) | Digital pen | Drawing a clockface with hands to 10 after 11 on paper using a digital pen |
37 variables including: Number of strokes Completion time Velocity Average pen pressure on paper |
| Kutz (2022) [77] | Dominant hand (dominant index finger) | FT (index-target tapping) | A force transducer (manufacturer: Measurement Specialties Inc., Hampton, VA, USA; Model: FX-1901–0001-50 L) |
Tapping index finger on the force transducer for 15 s and under two different conditions: at a self-selected pace (three trials) and at a fast pace (two trials) |
Tapping cycle time and its components (time on the device and time off) Maximum tapping force |
| Schmidt (2022) [78] | Both hands | ILFT | Camera to take photos of gestures for subsequent evaluation |
Imitating bimanual non-symbolic gestures after demonstration by examiner |
Individual scores Total score |
FT finger tapping; PPT Purdue Pegboard Test; ILFT Interlocking Finger Test, SD Standard Deviation
Fig. 2.
Task groups used for testing upper limb motor function. There were two major groups of tasks: 1) tasks involving a part of the upper limb, e.g., finger tapping or elbow flexion, and 2) tasks involving multiple parts of the upper limb, such as writing/drawing tests or Purdue Pegboard Test (PPT)
Tasks involving a part of the upper limb
Finger tapping
Finger tapping (FT) was the most common task with twenty-seven (45%) studies using it as the main task, or one of the tasks. Nearly all studies analysed FT frequency or number of finger-taps. Protocols have evolved with advances in technology, allowing more precise recording with more recent papers including analysis of additional variables such as time between taps and rhythm fluctuations. FT was performed either by tapping a key/lever with the index finger (index-target tapping) or tapping the index finger to the thumb (index-thumb tapping):
Index-target tapping
Twenty-two (37%) studies used this task. The first, published in 1995 [24], used a computer keyboard to count the number of fast-paced finger-taps. Since then, eight studies used fast-paced tapping of a computer key or lever [6, 18, 27, 29, 55, 59, 63, 71]. Movement variables such as number of taps and tapping speed were extracted. Three studies measured self-paced and fast-paced FT for 15 s, using a force transducer [36, 77] or touchpad [50]. Three studies employed ‘cued’ FT protocols requiring tapping to defined frequencies paced by auditory cues [51] or visual cues [46].
More recent studies used infrared-light sensor technologies to measure FT: two used photoelectric sensors arranged around a frame to measure fast-paced FT over 8 s [51] and one required participants to tap their index finger through an infrared light beam for 10 s [51]. Another study [68], used wearable electromechanical sensors on the index finger during 15 s of comfortable pace tapping on the table.
Index-thumb tapping
In total, five (8%) studies used index-thumb tapping; the first, published in 1998, measured the number of taps in 10 s performed by people with Alzheimer’s Disease (AD) [28]. In four recent papers (since 2020) participants were asked to tap at a fast pace for 15 s while wearing reflective markers or magnetic sensors on their thumb and index finger [60, 61, 73, 74]. Extracted movement variables included speed, amplitude, and variabilities in time and speed of a finger-tap cycle [60, 61, 73, 74].
Elbow/forearm movements
The first study of forearm movement in cognitive impairment was in1998 [28] and researchers visually counted the number of correct supination/pronation cycles in 10 s. The next study was 12 years later [33] using an optical shaft encoder to measure the number of fast-paced supination/pronation cycles in 10 s. Since then, two studies used wearable 3-D gyroscopes to measure additional variables such as speed, rise time and speed variability of self-paced and fast-paced elbow flexion movements in 20 s [42, 58] and one used the same device to assess speed and variability of self-paced elbow flexions over 60 s [65].
Tasks involving multiple parts of the upper limb
Purdue Pegboard Test (PPT)
The PPT involves placing a series of pegs into holes on a board as fast as possible and has been utilised in four studies. Three used the 25-hole PPT [19, 32, 71] and one used a 9-hole pegboard [43]. Studies used various protocols: two measured the number of pegs inserted into holes of a 25-hole pegboard in 30 s [19, 71] and two timed participants inserting pegs and removing them from a 25-hole pegboard [32] and a 9-hole pegboard respectively [43].
Writing/drawing tasks
Three studies [30, 49, 76] used writing or drawing tasks to investigate whether kinematic measures (such as speed and smoothness) of digital pen movements on a digitising tablet or paper could differentiate between people with cognitive impairment—including MCI, AD and Vascular dementia (VaD)—and healthy controls (HC). In one study [76], participants drew a clockface on paper using a digitising pen. In another, participants drew concentric circles on a digitising tablet, at a fast pace [30]. In a study [49] which included sentence writing tasks too, participants drew circles on a digitising tablet at a self-selected pace.
Reach/Grasp/Lift tasks
Three studies used tasks involving reach/grasp/lift of an object [34, 55, 75]. In one study [34], participants with MCI and AD lifted objects with different weights and held them for 4 s. In another [55], participants with dementia (various types) lifted an object for 20 s. Both studies analysed steadiness and speed. One study [75] assessed reaching for an object at self-selected and fast paces, under various visual conditions in HC and Parkinson’s Disease (PD), some with cognitive impairment, measuring the time to complete the task.
Gesture imitation
Six studies analysed the ability of participants to imitate bimanual hand gestures after watching a demonstration by the examiner and recorded the number of correct performances and number of errors [35, 38, 40, 48, 54, 69]. In one study, the examiner demonstrated gestures sitting next to the participants to reduce perceptual complexities [48]. In the rest, examiners demonstrated gestures in front of participants [44, 78]. Two studies used the Interlocking Finger Test (ILFT) [44, 78] in which the examiner demonstrates specific shapes with their hands one at a time, and then asks the participants to imitate those gestures, as accurately as possible—for example, interlocking the fingers in a particular manner.
Dragging or tracking tasks
One study used a robotic haptic interface to measure reaction times and mean error of tracking movements in participants with cognitive impairment [47]. The device guided the hand to a target position and gave real-time visual feedback about the hand position as it tracked the target.
Another study used a custom-made electronic board to measure target-tracking reaction times in AD and HC [41]. An electric pen has been used to measure tracking movement variables (such as number of errors and total time) of various upper limb tasks such as hitting targets or guiding the pen through a narrow space [45]. In a recent study, participants used a computer tablet to drag virtual blocks to a target without dropping them in the wrong area [66]; the number of successful and failed attempts within 60 s, and time taken to move a block were analysed.
Tasks resembling day-to-day upper limb functions
One study explored ULMF in people with dementia by measuring the time taken to shelve groceries [67]. Another analysed “hand to mouth” movement variables such as time, speed, and smoothness, in dementia with Lewy bodies [57]. One study used a smartphone app custom-made keyboard to analyse characteristics of virtual key presses (such as keystroke timing) during typing [64].
Three studies analysed participants’ movements as they followed a specific protocol of various functional tasks, and scores were given by observation of their performance. One used parts of the Cambridge Cognitive Examination involving tasks such as putting paper into an envelope, waving goodbye, cutting paper with scissors and brushing teeth [39]. One study used part of the Functional Disability Evaluation Scale-Adult version (FUNDES-Adult), which includes pen-holding, buttoning, and knotting tasks [62]. While another chose the Fugl-Meyer assessment [70].
Sub-question 2. What conditions/diseases with resultant cognitive impairment have been studied?
Table 3 summarises the conditions or diseases leading to cognitive impairment included in this review. Of 54 cross-sectional studies, 37 included participants with dementia—26 of them with AD diagnosis—and 22 had a group with MCI. Eight studies investigated participants with PD and cognitive impairment, one recruited participants with Minimal Hepatic Encephalopathy [63]. Two studies recruited participants with cognitive decline related to excessive alcohol consumption [25, 52]. Participants with no known cognitive impairment were recruited in seven studies and after testing for cognitive impairment allocated to groups with and without cognitive impairment. Of five longitudinal studies, four recruited people with no known cognitive impairment at baseline [6, 19, 26, 71] and one recruited participants with MCI [31].
Sub-question 3. What were the major recruitment settings?
Table 3.
Diseases or conditions leading to cognitive impairment
| Disease/conditions causing cognitive impairment | Number of papers |
|---|---|
| Alzheimer’s Disease | Twenty-six [21, 25, 27, 28, 30, 34, 36, 39, 43, 46, 48, 51, 52, 54, 55, 59, 60, 63, 65, 66, 68, 73, 75, 77, 78] |
| Parkinson’s Disease | Eight [24, 30, 31, 33, 37, 53, 73, 74] |
| Dementia with Lewy Bodies (DLB) or Lewy Body Dementia (LBD) | Four [28, 39, 69, 75] |
| Frontotemporal Dementia (FTD) | Three [46, 51, 75] |
| Alcohol or drug related | Two [28, 33] |
| Vascular Dementia (VaD) | Three [22, 49] |
| Minimal Hepatic Encephalopathy | One [41] |
| Stroke | One [62] |
| Depression | One [63] |
Forty-two studies recruited participants from clinical settings (e.g., neurological/ memory/cognition clinics, hospitals or rehabilitation centres), fourteen from community settings such as primary health services, day centres and exercise classes [6, 18, 19, 26, 33, 36, 53, 56, 60, 64, 65, 67, 71, 72], and four from research settings (e.g., from other research cohorts) [27, 66, 76, 77].
Sub-question 4. How does ULMF associate with cognitive impairment?
Most studies of index-target tapping found significant differences between HC and people with dementia [24, 25, 37, 51, 59, 71, 72] and MCI [6, 17, 26, 36, 46, 51, 56, 63, 77]. Generally, MCI and dementia were both associated with slower, less rhythmic and lower frequency finger-taps. However, two studies found no association between tapping frequency and cognitive impairment [27, 29].
Studies of index-thumb tapping had mixed results too. Three studies reported associations between cognitive impairment and lower frequency, and increased variability, of FT [26, 60, 73]. However, two studies using wearable sensors, found no differences between FT frequency and amplitude in MCI or AD compared to HC [61, 74] although one found FT in MCI was less rhythmic [74]. The systematic review [3] concluded that FT was not associated with incident dementia in people with MCI.
For studies of forearm supination/pronation, slower speed and increased variability were associated with cognitive impairment [28, 33, 53, 55]. The three studies of elbow flexion [42, 58, 65] found no differences under single-task condition between participants with cognitive impairment (MCI and AD) and HC but with dual-task conditions (elbow flexion and a cognitive task), there were significant associations.
All studies using the PPT found dementia was associated with slower movements compared to MCI [18, 19, 32, 43]. All studies analysing writing/drawing kinematics found increased irregularity of movements, variability in speed and decreased accuracy differentiated HC participants from AD [30, 39, 49, 76] and from MCI [30, 39, 49]. One study [76] that compared measures of clock drawing in AD and VaD found that VaD drew more slowly (having slower speed and taking longer to draw).
Using reach/grasp/lift tasks, one study [34] found no differences between those with MCI or dementia, but another found dementia was associated with more variability than MCI [18]. A study of PD reported that those with dementia [75] had longer reaching reaction times. Studies employing gestures found significant imitation impairment in participants with dementia [35, 40, 48, 54, 69]. The inability in correct imitation of gestures in the ILFT was also correlated with cognitive impairment [44, 78]. Studies using functional tasks found variables of ULMF (such as increased time to complete the task, decreased smoothness, and less accuracy of movements) correlated with cognitive impairment [45, 57, 62, 67, 70]. Studies measuring tracking abilities found that participants with MCI and AD had more errors and slower reaction times than HC [41, 47]. Using digital tests, two studies [64, 66] showed differences between MCI and HC: one [66] reported reduced speed of dragging virtual blocks and another [64] identified more errors in virtual keyboard presses.
Among the longitudinal studies, two [6, 26] found that slower baseline FT in HC was associated with cognitive impairment at follow up, but another [31] found no such association. One study [19] found lower PPT scores were associated with higher risk of developing dementia at follow up. A study [71], using both PPT and index-target tapping concluded that lower performance scores of both tests were associated with risk of MCI and dementia at follow up.
Fifteen studies additionally investigated how ULMF associates with individual cognitive domains. Eight studies, using index-target tapping, found significant associations between FT variables (tapping cycle time, tapping rate and time variability) and memory (working and episodic), verbal fluency and executive function [18, 24, 27, 36, 37, 46, 59, 63]. One study found slowed index-thumb tapping speed associated with verbal fluency and executive function but not with delayed memory [25].
One study of elbow flexion found associations between speed and rhythm fluctuations with executive function [42]. Lower PPT scores correlated with impaired attention, visuo-spatial and executive function [18]. Using gesture tasks, studies found imitation accuracy associated with verbal fluency, attention [44, 69, 78] and executive function [44] but another did not [78]. Tracking ability was correlated with memory and visuospatial domains [47] and functional tasks were associated with attention, visuospatial and executive function [70].
Discussion
Sixty studies published between 1995 and 2022, and comprising 41,800 participants, met the criteria to inform this review. To our knowledge, this is the first review investigating the association of ULMF with cognitive impairment. The studies used a diverse range of ULMF tasks from a simple movement, such as FT or elbow flexion, to more complex movements such as writing/drawing. Studies also used a range of protocols (self-paced, fast-paced, dual-task etc.), test durations (ranging from 8 to 60 s), and equipment. With technology advancements over time, the precision of data collection equipment has progressed, so analyses have evolved from counting the number of repetitions to detailed quantification of rhythm, amplitude and speed. The recruitment settings were mostly clinical, and the conditions included were predominantly AD, MCI and PD. Many studies found that, compared to age-matched older adults, people with cognitive impairment had slower speeds, longer reaction times and more errors and variability in their ULMF performance. However, these associations were not universal, especially among the FT studies. FT (index-target or index-thumb tapping) was the most common ULMF test, but protocols, durations and equipment varied significantly among these studies which may be the reason why FT studies had mixed results. Studies of elbow flexion, writing/drawing tasks and the PPT had no conflicting results, although there were fewer studies compared to the large number that assessed FT.
With no limitation in dates, this review provides a broad view of how ULMF assessments in the context of cognitive impairment have evolved since conception about 25 years ago. We systematically searched published literature using established guidelines and published our protocol in advance in an open access repository (Figshare). It is important to acknowledge that we excluded studies measuring hands/arms strength, such as grip strength, and excluded studies with only healthy participants which may have excluded some of the ULMF tests. We also acknowledge that it is possible relevant studies without linked keywords may have inadvertently been excluded.
This review highlights that ULMF assessments hold potential to be used in cognitive impairment investigations as many (but not all) of the studies found associations between ULMF and cognitive impairment. However, it also revealed a major gap in the current literature and that is the lack of consistency between the experimental methods used to assess ULMF. It remains unclear whether one specific type of test is superior to others, and it remains unclear how many repetitions of a task, or what test duration, should be used to balance sensitivity with potential effects of fatigue. The review demonstrated that, in a similar way to how gait analysis now has some recommended standard protocols [14], there remains a need to also standardise ULMF assessment methods—in terms of test durations and protocols (fast-paced vs. self-selected pace); this would substantially aid comparison of studies and clarify which tests are most discriminatory.
Most ULMF studies used 10 to 15 s as the test duration which seems to be a pragmatic balance between capturing enough data for robust analysis of movements whilst minimising the effects of fatigue. Several studies measured ULMF performance at various paces (self-selected pace vs fast pace) or under different conditions (single task and dual task). These approaches, as well as analysing multiple component measures of movements (such as frequency, speed, amplitude and rhythm) appeared to be more sensitive to cognitive impairment than testing just one movement under one condition and/or few movement components. Future research should consider analysing frequency, speed, and variability of ULMF as the core measures as these have repeatedly been shown to associate with cognitive impairment.
As most studies of ULMF have compared healthy controls to just one group who had clinically-manifested cognitive impairment, especially Alzheimer’s Disease (AD) dementia, it remains unclear how ULMF changes across the dementia continuum. Furthermore, there have been relatively few studies of other types of dementia. Future research should therefore aim to recruit participants with earlier stages of dementia pathology, such as subjective cognitive impairment, MCI, and early-stage dementia to provide richer insights into the changes related to disease progression. It was noteworthy that most studies classified participants according to screening tool cut-off scores rather than a more comprehensive cognitive assessment using established diagnosis criteria; we would recommend that future researchers aim to ascertain a more rigorous evaluation of the various domains of cognitive impairment as this would allow a more granular comparison with ULMF features and the opportunity to explore whether certain underlying pathologies have specific ULMF motor signatures.
For ULMF assessment to be included in CCCDTD as a recommended motor function assessment in dementia investigations, it is necessary to know how best to assess ULMF that is significantly associated with cognitive impairment and dementia. We are still learning about the association between ULMF and cognitive impairment and methods of testing ULMF are yet to be fully explored. This review shows that despite some inconclusive results, there is emerging evidence to support including ULMF in cognitive impairment investigations.
Conclusion
In this scoping review, we summarised the current available evidence on the association of ULMF and cognitive impairment and also the tests, protocols, recruitment settings and conditions used to assess this association. Of the identified methods of ULMF assessment, FT was the most commonly used test followed by functional tasks of upper limb, PPT and elbow/forearm movement. Despite some mixed results, the ULMF movement variables were generally associated with cognitive impairment and could aid in distinguishing cognitive impairment from healthy ageing.
Acknowledgements
The Wicking Dementia Research and Education Centre is supported by J.O. and J.R. Wicking Trust (Equity Trustees) and the University of Tasmania.
Appendix 1
Key search terms for PubMed Search performed on 14/03/2022
| Search | Query |
|---|---|
| #4 | ((#1) AND (#2)) AND (#3) |
| #3 | (Motor impairment[Title/Abstract]) OR (motor function[Title/Abstract])) OR (movement[Title/Abstract])) OR (motor test[Title/Abstract])) OR (motor dysfunction[Title/Abstract])) OR (Motor test[Title/Abstract]))) OR (fine motor test[Title/Abstract])) OR (motor performance[Title/Abstract])) OR (movement test[Title/Abstract])) OR (gross motor function[Title/Abstract])) OR (gross motor impairment[Title/Abstract])) OR (motor dysfunction[Title/Abstract])) OR (motor decline[Title/Abstract])) OR (finger tapping[Title/Abstract])) OR (apraxia[Title/Abstract])) OR (dyspraxia[Title/Abstract])) OR (dexterity[Title/Abstract])) OR (grasp*[Title/Abstract])) OR (grip*[Title/Abstract])) OR (tap*[Title/Abstract])) OR (hand tapping[Title/Abstract])) OR (keyboard tapping[Title/Abstract])) OR (holding[Title/Abstract])) OR (draw*[Title/Abstract])) OR (writ*[Title/Abstract])) OR (Purdue pegboard test[Title/Abstract]) |
| #2 |
Hand*[Title/Abstract] OR Forearm*[Title/Abstract] OR Finger*[Title/Abstract] OR Upper limb*[Title/Abstract] OR "Upper limb"[Title/Abstract] |
| #1 | Dementia*[Title/Abstract] OR "Cognitive impairment"[Title/Abstract] OR "Cognitive decline"[Title/Abstract] OR "Alzheimer’s Disease"[Title/Abstract] OR "Mild Cognitive Impairment"[Title/Abstract] OR Cognition*[Title/Abstract] OR "Cognitive domains"[Title/Abstract] |
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. KR is supported by a Research Training Program living allowance and University of Tasmania tuition fee scholarship. The Wicking Dementia Research and Education Centre is supported by the J.O. and J.R. Wicking Trust (Equity Trustees). The funding bodies have no direct role in the study design, data collection, analysis, and interpretation or manuscript preparation.
Declarations
Competing interests
The authors declare that they have no conflict of interest.
Standard of reporting: This review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses -extension for scoping reviews (PRISMA-ScR) guidelines.
Disclosure
Authors have no conflict of interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kivimaki Mika, Singh-Manoux A. Prevention of dementia by targeting risk factors. Lancet. 2018;391(10130):1574–1575. doi: 10.1016/S0140-6736(18)30578-6. [DOI] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Pieruccini-Faria F, Ismail Z, et al. CCCDTD5 recommendations on early non cognitive markers of dementia: A Canadian consensus. Alzheimers Dement (N Y) 2020;6(1):e12068. doi: 10.1002/trc2.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kueper JK, Speechley M, Lingum NR, et al. Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing. 2017;46(5):729–738. doi: 10.1093/ageing/afx084. [DOI] [PubMed] [Google Scholar]
- 4.Muller K, Frohlich S, Germano AMC, et al. Sensor-based systems for early detection of dementia (SENDA): a study protocol for a prospective cohort sequential study. BMC Neurol. 2020;20(1):84. doi: 10.1186/s12883-020-01666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buracchio TDH, Diane Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed RM, Paterson RW, Warren JD, et al. Biomarkers in dementia: clinical utility and new directions. J Neurol Neurosurg Psychiatry. 2014;85(12):1426–1434. doi: 10.1136/jnnp-2014-307662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review. JAMA. 2019;322(16):1589–1599. doi: 10.1001/jama.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belleville S, Fouquet C, Hudon C, et al. Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer's type dementia in older adults: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(4):328–353. doi: 10.1007/s11065-017-9361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JA, Verghese J. Chapter 22 - Gait and dementia. In: Dekosky ST, Asthana SS, editors. Handbook of Clinical Neurology. Elsevier; 2019. pp. 419–427. [DOI] [PubMed] [Google Scholar]
- 11.Allali G, Annweiler C, Blumen HM, et al. Gait phenotype from mild cognitive impairment to moderate dementia: results from the GOOD initiative. Eur J Neurol. 2016;23(3):527–541. doi: 10.1111/ene.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahureksa L, Najafi B, Saleh A, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology. 2017;63(1):67–83. doi: 10.1159/000445831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beauchet O, Allali G, Sekhon H, et al. Guidelines for assessment of gait and reference values for spatiotemporal gait parameters in older adults: the Biomathics and Canadian gait consortiums initiative. Front Hum Neurosci. 2017;11:353. doi: 10.3389/fnhum.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert Briggs SPK, O’Neill D. Drug treatments in Alzheimer's disease. Clin Med. 2016;16(3):247–53. doi: 10.7861/clinmedicine.16-3-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherder E, Dekker W, Eggermont L. Higher-level hand motor function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life–a mini-review. Gerontology. 2008;54(6):333–341. doi: 10.1159/000168203. [DOI] [PubMed] [Google Scholar]
- 17.Carment L, Abdellatif A, Lafuente-Lafuente C, et al. Manual dexterity and aging: A pilot study disentangling sensorimotor from cognitive decline. Front Neurol. 2018;9:910. doi: 10.3389/fneur.2018.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesseberg K, Tangen GG, Pripp AH, et al. Associations between cognition and hand function in older people diagnosed with mild cognitive impairment or dementia. Dement Geriatr Cogn Dis Extra. 2020;10(3):195–204. doi: 10.1159/000510382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darweesh SK, Wolters FJ, Hofman A, et al. Simple test of manual dexterity can help to identify persons at high risk for neurodegenerative diseases in the community. J Gerontol A Biol Sci Med Sci. 2017;72(1):75–81. doi: 10.1093/gerona/glw122. [DOI] [PubMed] [Google Scholar]
- 20.Williams S, Zhao Z, Hafeez A, et al. The discerning eye of computer vision: Can it measure Parkinson's finger tap bradykinesia? J Neurol Sci. 2020;416:117003. doi: 10.1016/j.jns.2020.117003. [DOI] [PubMed] [Google Scholar]
- 21.PRISMA. PRISMA for Scoping Reviews. https://prisma-statement.org/Extensions/ScopingReviews. Accessed date 2018.
- 22.Peters MDJ GC, McInerney P, Munn Z, Tricco AC, Khalil, H. JBI Manual for Evidence Synthesis. https://synthesismanual.jbi.global. Accessed Date 2020.
- 23.Rudd KD, Lawler K, Callisaya ML, Alty J. Methods used to investigate the association of cognitive impairment with upper limb motor function in adults: a scoping review protocol. Figshare; 2022. 10.6084/m9.figshare.19406981.v1
- 24.Ott BR, Ellias SA, Lannon MC. Quantitative assessment of movement in Alzheimer's disease. J Geriatr Psychiatry Neurol. 1995;8(1):71–75. [PubMed] [Google Scholar]
- 25.Welch LW, Cunningham AT, Eckardt MJ, et al. Fine motor speed deficits in alcoholic Korsakoff's syndrome. Alcoholism: Clin Exp Res. 1997;21(1):134–138. [PubMed] [Google Scholar]
- 26.Camicioli R, Howieson D, Oken B, et al. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496–1498. doi: 10.1212/WNL.50.5.1496. [DOI] [PubMed] [Google Scholar]
- 27.Goldman WP, Baty JD, Buckles VD, et al. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55(5):674–680. doi: 10.1001/archneur.55.5.674. [DOI] [PubMed] [Google Scholar]
- 28.Willis L, Behrens M, Mack W, et al. Ideomotor apraxia in early Alzheimer's disease: time and accuracy measures. Brain Cogn. 1998;38(2):220–233. doi: 10.1006/brcg.1998.1029. [DOI] [PubMed] [Google Scholar]
- 29.Goldman W, Baty J, Buckles V, et al. Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology. 1999;53(5):956–956. doi: 10.1212/WNL.53.5.956. [DOI] [PubMed] [Google Scholar]
- 30.Schröter A, Mergl R, Bürger K, et al. Kinematic analysis of handwriting movements in patients with Alzheimer’s disease, mild cognitive impairment, depression and healthy subjects. Dement Geriatr Cogn Disord. 2003;15(3):132–142. doi: 10.1159/000068484. [DOI] [PubMed] [Google Scholar]
- 31.Amieva H, Letenneur L, Dartigues JF, et al. Annual rate and predictors of conversion to dementia in subjects presenting mild cognitive impairment criteria defined according to a population-based study. Dement Geriatr Cogn Disord. 2004;18(1):87–93. doi: 10.1159/000077815. [DOI] [PubMed] [Google Scholar]
- 32.Muhlack S, Przuntek H, Müller T. Transdermal Rivastigmine treatment does not worsen impaired performance of complex motions in patients with Alzheimer’s disease. Pharmacopsychiatry. 2006;39(1):16–19. doi: 10.1055/s-2006-931473. [DOI] [PubMed] [Google Scholar]
- 33.Bramell-Risberg E, Jarnlo G-B, Elmståhl S. Slowing of alternating forearm movements is associated with cognitive impairment in community-dwelling older people. Dement Geriatr Cogn Disord. 2010;29(5):457–466. doi: 10.1159/000305093. [DOI] [PubMed] [Google Scholar]
- 34.Ameli M, Kemper F, Sarfeld A-S, et al. Arbitrary visuo-motor mapping during object manipulation in mild cognitive impairment and Alzheimer's disease: A pilot study. Clin Neurol Neurosurg. 2011;113(6):453–458. doi: 10.1016/j.clineuro.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Rousseaux M, Rénier J, Anicet L, et al. Gesture comprehension, knowledge and production in Alzheimer’s disease. Eur J Neurol. 2012;19(7):1037–44. 10.1111/j.1468-1331.2012.03674.x. [DOI] [PubMed]
- 36.Rabinowitz I, Lavner Y. Association between finger tapping, attention, memory, and cognitive diagnosis in elderly patients. Percept Mot Skills. 2014;119(1):259–278. doi: 10.2466/10.22.PMS.119c12z3. [DOI] [PubMed] [Google Scholar]
- 37.Henley SMD, Downey LE, Nicholas JM, et al. Degradation of cognitive timing mechanisms in behavioural variant frontotemporal dementia. Neuropsychologia. 2014;65:88–101. doi: 10.1016/j.neuropsychologia.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnen A, Tokaj A, Kirschner A, Wiendl H, Lueg G, Duning T, Lohmann H. Apraxia profile differentiates behavioural variant frontotemporal from Alzheimer's dementia in mild disease stages. J Neurol Neurosurg Psychiatry. 2015;86(7):809–15. 10.1136/jnnp-2014-308773. [DOI] [PubMed]
- 39.Ward M, Cecato JF, Aprahamian I, et al. Assessment for apraxia in Mild Cognitive Impairment and Alzheimer's disease. Dement Neuropsychol. 2015;9:71–75. doi: 10.1590/S1980-57642015DN91000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagahama Y, Okina T, Suzuki N. Impaired imitation of gestures in mild dementia: comparison of dementia with Lewy bodies, Alzheimer's disease and vascular dementia. J Neurol Neurosurg Psychiatry. 2015;86(11):1248. doi: 10.1136/jnnp-2014-309436. [DOI] [PubMed] [Google Scholar]
- 41.Lin YC, Hsu WC, Wu CK, et al. Comparison of motor performance of upper and lower extremities in dual-task tests in patients with mild Alzheimer's dementia. Aging Clin Exp Res. 2016;28(3):491–496. doi: 10.1007/s40520-015-0441-1. [DOI] [PubMed] [Google Scholar]
- 42.Toosizadeh N, Najafi B, Reiman EM, et al. Upper-extremity dual-task function: an innovative method to assess cognitive impairment in older adults. Front Aging Neurosci. 2016;8:167. doi: 10.3389/fnagi.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1–7. doi: 10.1016/j.gaitpost.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Souza CP, Oliveira GN, Foss MP, et al. The interlocking finger test in patients with Parkinson’s disease and healthy subjects. J Clin Neurosci. 2016;29:145–148. doi: 10.1016/j.jocn.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Dahdal P, Meyer A, Chaturvedi M, et al. Fine motor function skills in patients with Parkinson disease with and without mild cognitive impairment. Dement Geriatr Cogn Disord. 2016;42(3–4):127–134. doi: 10.1159/000448751. [DOI] [PubMed] [Google Scholar]
- 46.Kay CD, Seidenberg M, Durgerian S, et al. Motor timing intraindividual variability in amnestic mild cognitive impairment and cognitively intact elders at genetic risk for Alzheimer’s disease. J Clin Exp Neuropsychol. 2017;39(9):866–875. doi: 10.1080/13803395.2016.1273321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartoli E, Caso F, Magnani G, et al. Low-cost robotic assessment of Visuo-motor deficits in Alzheimer's disease. IEEE Trans Neural Syst Rehabil Eng. 2017;25(7):852–860. doi: 10.1109/TNSRE.2017.2708715. [DOI] [PubMed] [Google Scholar]
- 48.Sanin GN, Benke T. Bimanual gesture imitation in Alzheimer's disease. J Alzheimers Dis. 2017;57(1):53–59. doi: 10.3233/jad-160680. [DOI] [PubMed] [Google Scholar]
- 49.Garre-Olmo J, Faúndez-Zanuy M, López-de-Ipiña K, et al. Kinematic and pressure features of handwriting and drawing: preliminary results between patients with mild cognitive impairment, Alzheimer disease and healthy controls. Curr Alzheimer Res. 2017;14(9):960–968. doi: 10.2174/1567205014666170309120708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzumura S, Osawa A, Maeda N, et al. Differences among patients with Alzheimer's disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr Gerontol Int. 2018;18(6):907–914. doi: 10.1111/ggi.13277. [DOI] [PubMed] [Google Scholar]
- 51.Roalf DR, Rupert P, Mechanic-Hamilton D, et al. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer's disease, and Parkinson's disease. J Neurol. 2018;265(6):1365–1375. doi: 10.1007/s00415-018-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta A, Murthy P, Rao S. Brief screening for cognitive impairment in addictive disorders. Indian J Psychiatry. 2018;60(Suppl 4). [DOI] [PMC free article] [PubMed]
- 53.Rycroft SS, Quach LT, Ward RE, et al. The relationship between cognitive impairment and upper extremity function in older primary care patients. J Gerontol A Biol Sci Med Sci. 2018;74(4):568–574. doi: 10.1093/gerona/gly246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulde P, Leippold K, Armstrong A, Kohl S, Grimmer T, Diehl-Schmid J, Hermsdörfer J. An explorative note on apraxia tests. Front Neurol. 2018;8(9). 10.3389/fneur.2018.00660. [DOI] [PMC free article] [PubMed]
- 55.Jeppesen Kragh F, Bruun M, Budtz-Jørgensen E, et al. Quantitative measurements of motor function in Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies: a proof-of-concept study. Dement Geriatr Cogn Disord. 2018;46(3–4):168–179. doi: 10.1159/000492860. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Zhao Y, Shen C, Lei L, Dong J, Zou D, Zou J, Wang M. Can long-term regular practice of physical exercises including taichi improve finger tapping of patients presenting with mild cognitive impairment? Front Physiol. 2018;28(9). 10.3389/fphys.2018.01396. [DOI] [PMC free article] [PubMed]
- 57.Fadda L, Corona F, Floris G, et al. Upper limb movements in dementia with Lewy body: a quantitative analysis. Exp Brain Res. 2019;237(8):2105–2110. doi: 10.1007/s00221-019-05575-2. [DOI] [PubMed] [Google Scholar]
- 58.Toosizadeh N, Ehsani H, Wendel C, et al. Screening older adults for amnestic mild cognitive impairment and early-stage Alzheimer's disease using upper-extremity dual-tasking. Sci Rep. 2019;9(1):10911. doi: 10.1038/s41598-019-46925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mollica MA, Tort-Merino A, Navarra J, et al. Early detection of subtle motor dysfunction in cognitively normal subjects with amyloid-beta positivity. Cortex. 2019;121:117–124. doi: 10.1016/j.cortex.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 60.Tomita Y, Tanaka S, Takahashi S, et al. Detecting cognitive decline in community-dwelling older adults using simple cognitive and motor performance tests. Geriatr Gerontol Int. 2020;20(3):212–217. doi: 10.1111/ggi.13863. [DOI] [PubMed] [Google Scholar]
- 61.Bologna M, Guerra A, Colella D, et al. Bradykinesia in Alzheimer's disease and its neurophysiological substrates. Clin Neurophysiol. 2020;131(4):850–858. doi: 10.1016/j.clinph.2019.12.413. [DOI] [PubMed] [Google Scholar]
- 62.Liou W-C, Chan L, Hong C-T, et al. Hand fine motor skill disability correlates with dementia severity. Arch Gerontol Geriatr. 2020;90:104168. doi: 10.1016/j.archger.2020.104168. [DOI] [PubMed] [Google Scholar]
- 63.San Martín-Valenzuela C, Borras-Barrachina A, Gallego JJ, Urios A, Mestre-Salvador V, Correa-Ghisays P, Ballester MP, Escudero-García D, Tosca J, Montón C, Ríos MP. Motor and cognitive performance in patients with liver cirrhosis with minimal hepatic encephalopathy. J Clin Med. 2020;9(7). 10.3390/2Fjcm9072154. [DOI] [PMC free article] [PubMed]
- 64.Ntracha A, Iakovakis D, Hadjidimitriou S, et al. Detection of mild cognitive impairment through natural language and touchscreen typing processing. Front Digit Health. 2020;2:567158. doi: 10.3389/fdgth.2020.567158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ehsani H, Parvaneh S, Mohler J, et al. Can motor function uncertainty and local instability within upper-extremity dual-tasking predict amnestic mild cognitive impairment and early-stage Alzheimer's disease? Comput Biol Med. 2020;120:103705. doi: 10.1016/j.compbiomed.2020.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Chen Y, Yu H, et al. What can “drag & drop” tell? Detecting mild cognitive impairment by hand motor function assessment under dual-task paradigm. Int J Hum Comput Stud. 2021;145:102547. doi: 10.1016/j.ijhcs.2020.102547. [DOI] [Google Scholar]
- 67.Paixão C, Tavares A, Marques A. Respiratory function and upper extremity functional activity performance in people with dementia: a shout for attention. J Aging Phys Act. 2021;29(1):89–98. doi: 10.1123/japa.2020-0005. [DOI] [PubMed] [Google Scholar]
- 68.Mancioppi G, Fiorini L, Rovini E, et al. Innovative motor and cognitive dual-task approaches combining upper and lower limbs may improve dementia early detection. Sci Rep. 2021;11(1):7449. doi: 10.1038/s41598-021-86579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagahama Y, Okina T, Suzuki N. Neuropsychological basis of impaired gesture imitations in patients with Alzheimer's disease and dementia with Lewy bodies. Int J Geriatr Psychiatry. 2021;37(1). 10.1002/gps.5622. [DOI] [PubMed]
- 70.Uwa-Agbonikhena IF, Gryb VA, Gerasymchuk VR. Associations between the upper extremity function and cognition in post-stroke patients. Wiad Lek. 2021;74(8):1917–1920. doi: 10.36740/WLek202108124. [DOI] [PubMed] [Google Scholar]
- 71.Beeri MS, Leurgans SE, Bennett DA, et al. Diverse motor performances are related to incident cognitive impairment in community-dwelling older adults. Front Aging Neurosci. 2021;13. 10.3389/fnagi.2021.717139. [DOI] [PMC free article] [PubMed]
- 72.Zhao L, Liu G, Zhang L, et al. Long-term physical exercise improves finger tapping of patients with Alzheimer's disease. Curr Alzheimer Res. 2021;18(14):1077–1086. doi: 10.2174/1567205018666211215150157. [DOI] [PubMed] [Google Scholar]
- 73.Suzumura S, Kanada Y, Osawa A, et al. Assessment of finger motor function that reflects the severity of cognitive function. Fujita Med J. 2021;7(4):122–129. doi: 10.20407/fmj.2020-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colella D, Guerra A, Paparella G, et al. Motor dysfunction in mild cognitive impairment as tested by kinematic analysis and transcranial magnetic stimulation. Clin Neurophysiol. 2021;132(2):315–322. doi: 10.1016/j.clinph.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 75.Cosgrove J, Hinder MR, St George RJ, et al. Significant cognitive decline in Parkinson's disease exacerbates the reliance on visual feedback during upper limb reaches. Neuropsychologia. 2021;157:107885. doi: 10.1016/j.neuropsychologia.2021.107885. [DOI] [PubMed] [Google Scholar]
- 76.Davoudi A, Dion C, Amini S, et al. Classifying non-dementia and Alzheimer's disease/vascular dementia patients using kinematic, time-based, and visuospatial parameters: the digital clock drawing test. J Alzheimers Dis. 2021;82(1):47–57. doi: 10.3233/jad-201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kutz DF, Fröhlich S, Rudisch J, et al. Finger tapping as a biomarker to classify cognitive status in 80+-Year-Olds. J Personalized Med. 2022;12(2):286. doi: 10.3390/jpm12020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt N, Strohmaier T, Witt K. A modified version of the interlocking finger test as a bedside screening test for visuospatial deficits and dementia in Parkinson's disease. Brain Behav. 2022;12(4):e2516. doi: 10.1002/brb3.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]