Abstract
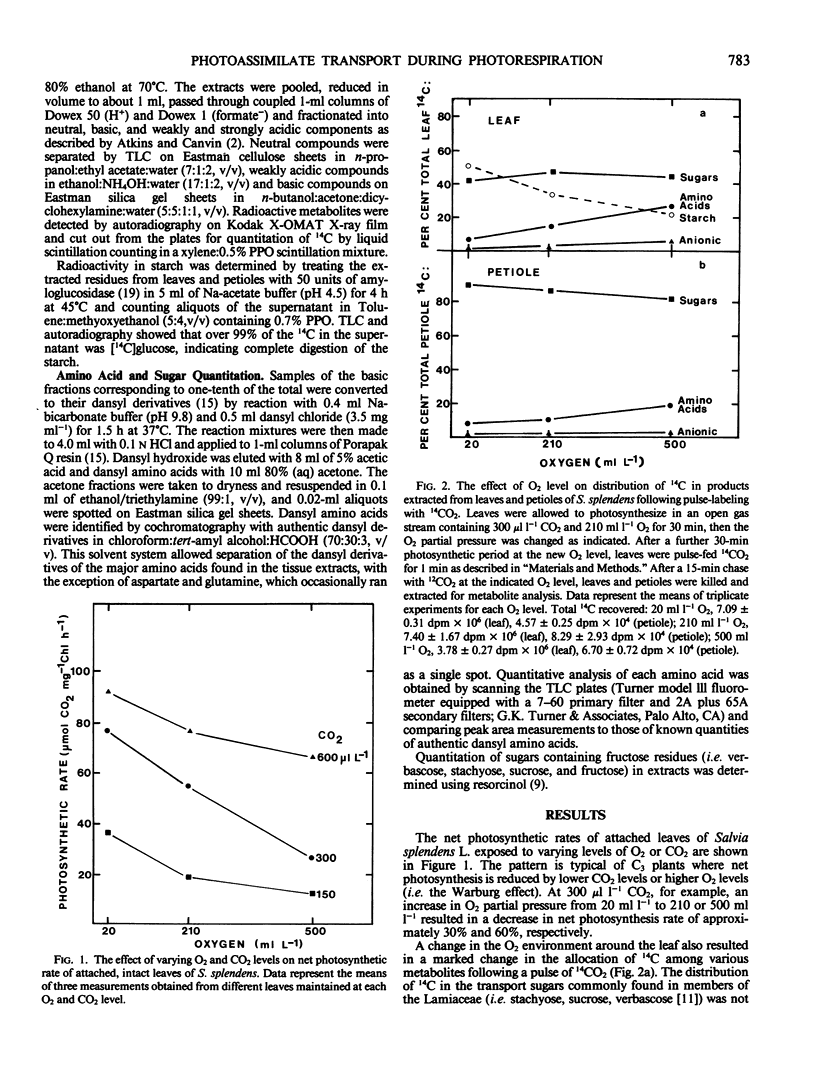
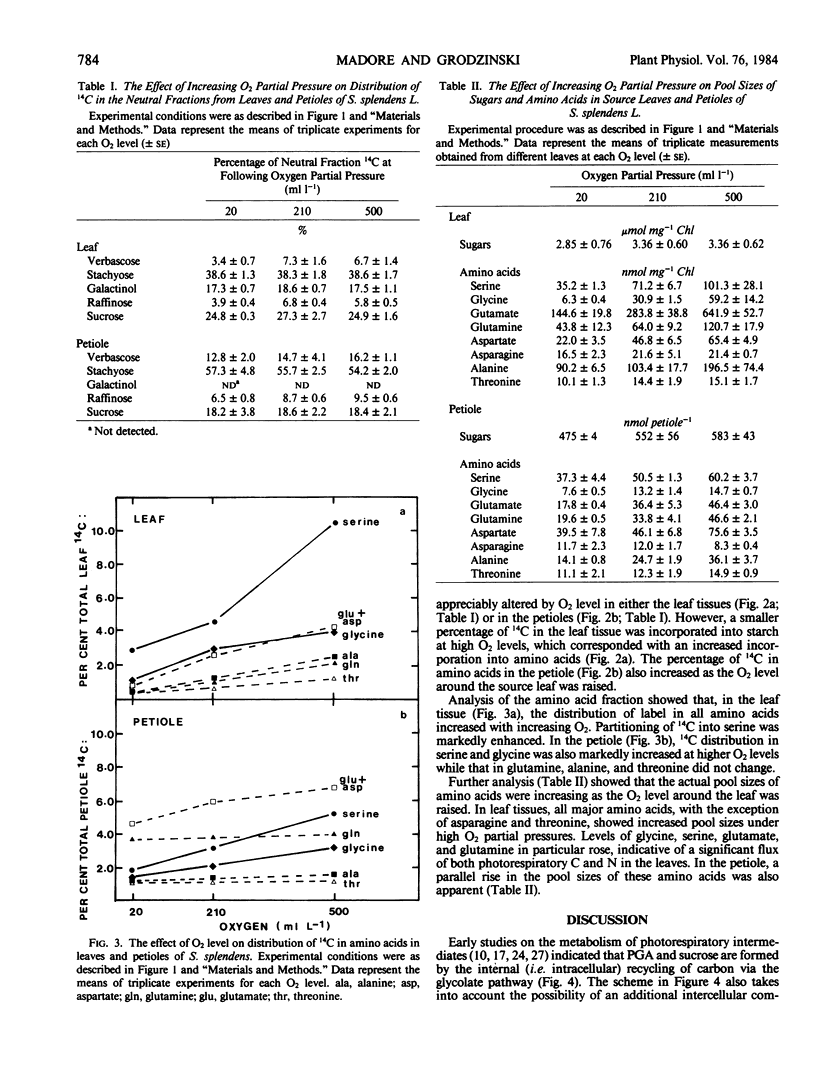
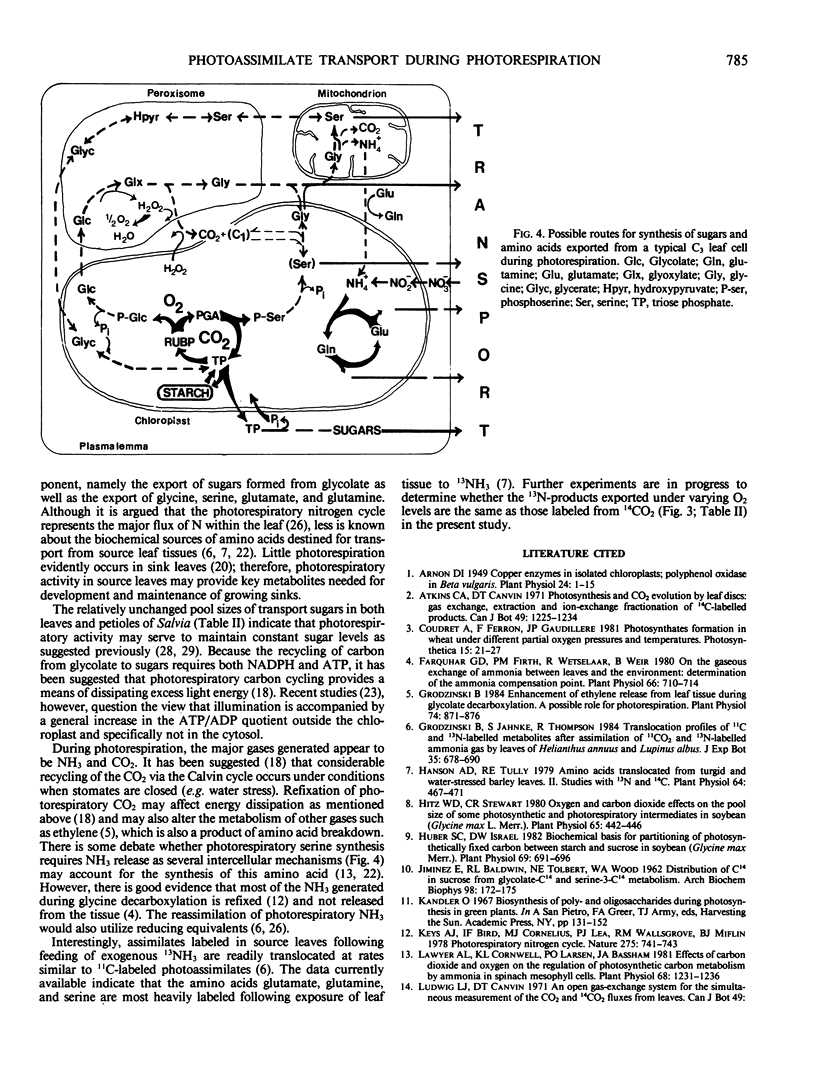
Partitioning and transport of recently fixed photosynthate was examined following 14CO2 pulse-labeling of intact, attached leaves of Salvia splendens L. maintained in an atmosphere of 300 microliters per liter CO2 and 20, 210, or 500 milliliters per liter O2. Under conditions of increasing O2 (210, 500 milliliters per liter), a smaller percentage of the recently fixed 14C in the leaf was allocated to starch, whereas a greater percentage of the fixed 14C appeared in amino acids, particularly serine. The increase in 14C in amino acids was reflected in material exported from source leaves. A higher percentage of 14C in serine, glycine, and glutamate was recovered in petiole extracts when source leaves were maintained under elevated O2 levels. Although pool sizes of these amino acids were increased in both the leaves and petioles with increasing photorespiratory activity, no significant changes in either 14C distribution or concentration of transport sugars (i.e. stachyose, sucrose, verbascose) were observed. The data indicate that, in addition to being recycled intracellularly into Calvin cycle intermediates, amino acids produced during photorespiration may also serve as transport metabolites, allowing the mobilization of both carbon and nitrogen from the leaf under conditions of limited photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G. D., Firth P. M., Wetselaar R., Weir B. On the Gaseous Exchange of Ammonia between Leaves and the Environment: Determination of the Ammonia Compensation Point. Plant Physiol. 1980 Oct;66(4):710–714. doi: 10.1104/pp.66.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B. Enhancement of Ethylene Release from Leaf Tissue during Glycolate Decarboxylation : A Possible Role for Photorespiration. Plant Physiol. 1984 Apr;74(4):871–876. doi: 10.1104/pp.74.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Tully R. E. Amino Acids Translocated from Turgid and Water-stressed Barley Leaves : II. Studies with N and C. Plant Physiol. 1979 Sep;64(3):467–471. doi: 10.1104/pp.64.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz W. D., Stewart C. R. Oxygen and Carbon Dioxide Effects on the Pool Size of Some Photosynthetic and Photorespiratory Intermediates in Soybean (Glycine max [L.] Merr.). Plant Physiol. 1980 Mar;65(3):442–446. doi: 10.1104/pp.65.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIMENEZ E., BALDWIN R. L., TOLBERT N. E., WOOD W. A. Distribution of C14 in sucrose from glycolate-C14 and serine 3-C14 metabolism. Arch Biochem Biophys. 1962 Jul;98:172–175. doi: 10.1016/0003-9861(62)90163-7. [DOI] [PubMed] [Google Scholar]
- Lawyer A. L., Cornwell K. L., Larsen P. O., Bassham J. A. Effects of carbon dioxide and oxygen on the regulation of photosynthetic carbon metabolism by ammonia in spinach mesophyll cells. Plant Physiol. 1981 Dec;68(6):1231–1236. doi: 10.1104/pp.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Determination of specific radioactivity of plant amino acids using dansylation. Anal Biochem. 1978 Mar;85(1):71–78. doi: 10.1016/0003-2697(78)90275-0. [DOI] [PubMed] [Google Scholar]
- Miflin B. J., Marker A. F., Whittingham C. P. The metabolism of glycine and glycollate by pea leaves in relation to photosynthesis. Biochim Biophys Acta. 1966 Jun 8;120(2):266–273. doi: 10.1016/0926-6585(66)90346-3. [DOI] [PubMed] [Google Scholar]
- Salin M. L., Homann P. H. Changes of photorespiratory activity with leaf age. Plant Physiol. 1971 Aug;48(2):193–196. doi: 10.1104/pp.48.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Geiger D. R. Effects of light intensity and oxygen on photosynthesis and translocation in sugar beet. Plant Physiol. 1974 Oct;54(4):575–578. doi: 10.1104/pp.54.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingles R., Woodrow L., Grodzinski B. Effects of Glycolate Pathway Intermediates on Glycine Decarboxylation and Serine Synthesis in Pea (Pisum sativum L.). Plant Physiol. 1984 Mar;74(3):705–710. doi: 10.1104/pp.74.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]


