Abstract
Limited research exists on the association between resting-state functional network connectivity in the brain and learning and memory processes in advanced age. This study examined within-network connectivity of cingulo-opercular (CON), frontoparietal control (FPCN), and default mode (DMN) networks, and verbal and visuospatial learning and memory in older adults. Across domains, we hypothesized that greater CON and FPCN connectivity would associate with better learning, and greater DMN connectivity would associate with better memory. A total of 330 healthy older adults (age range = 65–89) underwent resting-state fMRI and completed the Hopkins Verbal Learning Test–Revised (HVLT-R) and Brief Visuospatial Memory Test–Revised (BVMT-R) in a randomized clinical trial. Total and delayed recall scores were assessed from baseline data, and a learning ratio calculation was applied to participants’ scores. Average CON, FPCN, and DMN connectivity values were obtained with CONN Toolbox. Hierarchical regressions controlled for sex, race, ethnicity, years of education, and scanner site, as this was a multi-site study. Greater within-network CON connectivity was associated with better verbal learning (HVLT-R Total Recall, Learning Ratio), visuospatial learning (BVMT-R Total Recall), and visuospatial memory (BVMT-R Delayed Recall). Greater FPCN connectivity was associated with better visuospatial learning (BVMT-R Learning Ratio) but did not survive multiple comparison correction. DMN connectivity was not associated with these measures of learning and memory. CON may make small but unique contributions to learning and memory across domains, making it a valuable target in future longitudinal studies and interventions to attenuate memory decline. Further research is necessary to understand the role of FPCN in learning and memory.
Keywords: Resting-state networks, Functional magnetic resonance imaging, Healthy aging, Learning, Memory
Introduction
A significant shift in global age demographics is expected by the year 2050, by which time the percentage of individuals 65 years of age and older is projected to rise by 6% (~480 million people) [1]. Over time, the growing aging population will create healthcare and economic strain, with more older adults developing Alzheimer’s disease and related dementias (ADRDs) concurrently with limited governmental and caregiver resources. In both ADRD and healthy adult samples, age-related changes in learning and memory are of particular relevance to study. Regardless of cognitive health status, the ability to learn and retain information by encoding and retrieving new memories declines significantly with age. Consequently, such learning and memory impairments can interfere with the aging population’s capacity to independently perform activities of daily living, thus adding to the overall medical and financial burden [2–5].
Previous research suggests that aging is linked to declines in verbal and visuospatial learning and memory performance, with a few studies demonstrating steeper age-related decline in visuospatial memory than verbal memory [6–10]. Nonetheless, it is generally accepted that verbal and visuospatial learning and memory performance decline at comparable rates. Such findings also extend to pre-clinical and ADRD samples [11–13]. These age-related declines are essential to examine, as they interfere with overall cognitive functioning in older adults and may serve as a pathway for further cognitive impairment or dementia [14–16].
In this paper, the concept of learning is operationalized as (a) the total number of stimuli recalled over repeated trials (i.e., total recall), and/or (b) a calculated Learning Ratio (see the “Methods” section). Memory is operationalized as the total number of stimuli recalled after a delay period (i.e., delayed recall) [17, 18]. The Hopkins Verbal Learning Test–Revised (HVLT-R) and Brief Visuospatial Memory Test–Revised (BVMT-R) are neuropsychological tests that measure verbal and visuospatial learning and memory, respectively. Research exploring the relationship between age and performance on both tests showed mixed results. Regarding the HVLT-R, some studies of healthy older adults reported that increasing age is associated with worse total and delayed recall performance [19–23]. However, one study reported that advanced age does not significantly impact total and delayed recall scores, possibly because of inadequate demographic norm adjustment [24]. With the BVMT-R, researchers consistently found that older age is associated with worse total and delayed recall in large international samples [19, 20, 24–26].
Modern-day neuroimaging techniques serve to deepen our understanding of neurological changes associated with normal aging. Resting-state functional magnetic resonance imaging (rs-fMRI) serves as a measure of functional connectivity within and between brain networks. Functional connectivity from rs-fMRI is measured by evaluating the synchronicity of blood-oxygen-level-dependent (BOLD) fluctuations in remote, but functionally related brain regions while participants lie at rest. These resting-state networks are assumed to map the functional arrangement of brain networks, and greater within-network connectivity typically implies better network integrity [27–30]. While numerous resting-state networks have been discovered, those found to be active during higher-order cognitive processes, such as the cingulo-opercular network (CON), frontoparietal control network (FPCN), default mode network (DMN), and dorsal attention network (DAN), are often studied in the aging population. These networks, also known as higher-order resting-state networks, are important to study, given that their integrity generally declines with age, and such declines are associated with impaired learning and memory performance [27, 28, 31–36].
Considered “cognitive control networks,” CON and FPCN are involved in various aspects of the learning process. Specifically, CON may be necessary for detection of task-relevant information and maintenance of task rules, and FPCN may be important for goal-directed attention and adaptive feedback control [37–44]. Additionally, DMN has been extensively studied for over two decades, with findings suggesting that DMN assists with consolidation, storage, and retrieval of episodic memories [27, 31, 45–47]. Currently, the roles of higher-order resting-state networks such as CON, FPCN, and DMN in verbal and visuospatial learning and memory performance remain unclear, particularly in older adults. One recent study of healthy older adults found that greater within-network CON connectivity was associated with better performance on the NIH Toolbox Picture Sequence Memory task, a measure of nonverbal episodic memory [48]. In healthy older adults, greater within-network CON and FPCN connectivity have also been linked to higher scores on complex verbal working memory tasks, such as the Digit Span Backwards and WAIS-IV Letter-Number Sequencing tests [28, 49]. Several studies on older adults indicate that greater within-network DMN functional connectivity is associated with better verbal learning and memory performance [33, 36, 46]. More specifically, in one study, greater within-network DMN functional connectivity was associated with better delayed recall, but not immediate recall, on the Auditory Verbal Learning Test [46]. Less is known about how within-network DMN functional connectivity relates to visuospatial memory. Studies suggest that DAN functional connectivity declines more slowly with age, relative to other networks [35, 47]. Additionally, although DAN is important for visual distractor suppression and orientation of visual attention, it has not been found to directly impact verbal or visuospatial learning and memory performance [28, 33, 35, 45, 47, 48, 50]. Hence, in the analyses that follow, DAN was not included as a functional network of interest.
To our knowledge, no studies have specifically investigated the role of higher-order resting-state networks on verbal and visuospatial learning and memory in a large sample of older adults. The primary aim of this analysis was to determine whether within-network resting-state functional connectivity of CON, FPCN, or DMN contributes to verbal and visuospatial learning and memory functioning in later life. We hypothesized that greater within-network CON and FPCN functional connectivity would be associated with better verbal and visuospatial learning, as reflected in higher HVLT-R and BVMT-R Total Recall scores and Learning Ratio (see the “Methods” section). We also predicted that greater within-network DMN functional connectivity would be associated with better verbal and visuospatial memory, as reflected in higher HVLT-R and BVMT-R Delayed Recall scores. Our objective was to help bridge the gap between functional connectivity of higher-order resting-state networks and performance in verbal and visuospatial learning and memory in the aging population.
Methods
Participants
Baseline data were collected for participants in the Augmenting Cognitive Training in Older Adults (ACT; R01AG054077) study. ACT is a phase III, double-blind, randomized, sham-controlled clinical trial funded by the National Institute on Aging. Our sample comprised 330 healthy older adults, 65 to 89 years old. ACT participants were recruited across two sites: the University of Florida and the University of Arizona (Table 1).
Table 1.
Sample demographics and covariates
| University of Florida (n = 222) | University of Arizona (n = 108) |
Combined (n = 330) |
|
|---|---|---|---|
| Age M (SD), range | 71.70 (5.54), 65–89 | 71.48 (4.32), 65–84 | 71.63 (5.17), 65–89 |
| Sex (male:female), % female | 87:135, 60.8% | 32:76, 70.4% | 119:211, 63.9% |
| Race (White:non-White), % White | 196:26, 88.3% | 94:14, 87.0% | 290:40, 87.9% |
| Ethnicity (Hispanic:non-Hispanic), % Hispanic | 5:217, 2.3% | 16:92, 14.8% | 21:309, 6.4% |
| Years of education M (SD), range | 16.27 (2.53), 11–22 | 16.36 (2.08), 12–20 | 16.30 (2.39), 11–22 |
M mean, SD standard deviation
Complete inclusion and exclusion criteria were outlined in the seminal discussion paper for the ACT study [51]. To summarize, eligible participants were right-handed older adults, 65 to 89 years old. Participants did not report a clinical history or current diagnosis of any neurological disorders (e.g., dementia, traumatic brain injury); major psychiatric disorders (e.g., schizophrenia); or chronic illnesses (e.g., cancer). Individuals did not have any medical contraindications to transcranial direct current stimulation and MRI (e.g., medication, implants). Additionally, all participants who scored at least 1.5 standard deviations below the mean in at least one cognitive domain based on the age, sex, and education normative data from the National Alzheimer’s Coordinating Center Uniform Data Set, Version 3 (NACC UDS3), were excluded prior to randomization, because of potential evidence of mild cognitive impairment [52]. All eligible participants provided written informed consent that was approved by the Institutional Review Boards at the University of Florida and the University of Arizona.
A final dataset of 330 subjects was selected from the original dataset of 384 participants; 54 were excluded for the following reasons: Thirty-eight had missing resting-state functional connectivity data; 9 were scanned at baseline with a 20-channel coil instead of a 32- or 64-channel coil at the Universities of Arizona and Florida, respectively; and 6 had more than 40 of 120 (i.e., 1/3) invalid rs-fMRI scan volumes, indicating a high level of in-scanner motion. One case was removed due to being an outlier CON functional connectivity value (greater or less than 3 times the interquartile range).
Measures
Hopkins Verbal Learning Test–Revised
The HVLT-R is a brief word list-learning, recall, and recognition task designed to measure verbal learning and memory abilities in both cognitively healthy and cognitively impaired adults [53]. Although originally normed on a disproportionately White sample and correcting only for age, more recent research has shown much promise in expanding norm correction to other highly relevant demographic variables, such as gender identity, ethnic and racial identity, years of education, and non-English speakers [19–21, 23, 24, 54]. Additionally, the HVLT-R has demonstrated good to high sensitivity, specificity, construct validity, reliability, and dementia classification accuracy across a variety of populations and its six alternate forms [55–58].
At baseline, participants were randomized to receive forms 1, 2, or 3 of the HVLT-R. Each form began with a similar learning trial. Participants listened to a trained researcher read a list of 12 words, one word every 2 s. Words on each HVLT-R form are organized into three semantic categories (four words per category). Once all 12 words were vocalized, participants were asked to freely recall as many words as possible. The same list-learning/free recall sequence was repeated for an additional two trials. The HVLT-R Total Recall score was calculated by summing the number of correct words recalled across all three learning trials (possible score range = 0 to 36). Participants were not informed that they would be required to recall these words at a later time. A delay period of 20 to 25 min followed the third learning trial and consisted of only nonverbal tasks to prevent verbal interference (i.e., trials A and B and paper-and-pencil self-report questionnaires). After the delay period, participants were asked to freely recall as many words from the list as possible, without experimenter repetition. The HVLT-R Delayed Recall score was calculated by summing the number of words correctly recalled after the delay period (possible score range = 0 to 12). The HVLT-R Learning Ratio was calculated for each participant based on the following formula: [59, 60]. That is, learning ratio is the proportion of a participant’s learned information across trials to their remaining learning capacity after the first trial.
Brief Visuospatial Memory Test–Revised
The BVMT-R combines visual and spatial learning and memory by asking participants to learn six unique designs presented in an array (i.e., the visual component), as well as each design’s particular location on the stimulus page (i.e., the spatial component) [61]. Similar to the HVLT-R, the BVMT-R was initially normed using a majority White sample, with newer research aimed at norm correction for sex, years of education, and non-English speakers [19, 20, 24, 25], as well as confirming high validity and reliability across its six alternate forms [26, 61]. At baseline, participants were randomized to receive forms 1, 2, or 3 of the BVMT-R. The BVMT-R mirrors the HVLT-R with three learning trials. Each trial involves presentation of the stimulus page for 10 s, immediately followed by redrawing the figures in their correct locations on a blank piece of paper. A drawing receives 2 points (full credit) for accurate design and placement on the page; 1 point (partial credit) for either accurate design or placement; and 0 points (no credit) for no attempt to draw. The BVMT-R Total Recall score was calculated by summing the number of points awarded across all three learning trials (possible score range = 0 to 36). Participants were informed that they might be required to recall these designs and their spatial locations later. A delay of 25 min occurred after the third learning trial and consisted of only verbal tasks to prevent nonverbal interference (i.e., Paced Auditory Serial Addition Test, Controlled Oral Word Association Test). After the delay, participants were asked to freely recall as many designs and their locations as possible, without viewing the designs again. The BVMT-R Delayed Recall score was calculated by summing the number of points awarded for drawing accuracy and placement after the delay (possible score range = 0 to 12). The BVMT-R Learning Ratio was calculated using the same equation as for HVLT-R Learning Ratio, presented above.
Imaging acquisition
The MRI data were collected using a 3-T Siemens MAGNETOM Prisma scanner with a 64-channel head/neck coil at the McKnight Brain Institute at the University of Florida. A 3-T Siemens MAGNETOM Skyra scanner with a 32-channel head coil (Siemens Healthcare, Erlangen, Germany) was used at the Evelyn F. McKnight Brain Institute at the University of Arizona. Scanner type was included as a covariate in our regression analyses to control for possible differences in neuroimaging acquisition and quality across test sites. Scanner type was synonymous with scanner site and thus only included as a single covariate in analyses. Participants underwent the same scanning procedures at both study sites, such as wearing foam padding to minimize head motion and earplugs to prevent hearing injuries from scanner noise. A 3-min T1-weighted 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) structural scan was acquired (TR = 1800 ms; TE = 2.26 ms; flip angle = 8°; voxel size = 1.0 × 1.0 × 1.0 mm3, 176 slices; FOV = 256 × 256 mm). Resting-state data were acquired from a 6-min scan. Participants were told to keep their eyes open, let their mind wander, and gaze at a centrally placed in-scanner fixation cross during the BOLD protocol (number of volumes = 120; repetition time [TR] = 3000 ms; echo time [TE] = 30 ms; flip angle = 70°; voxel size = 3.0 × 3.0 × 3.0 mm3, 44 slices; field of view [FOV] = 240 × 240 mm). Individuals who had 40 or fewer invalid rs-fMRI scan volumes (e.g., due to head motion) were included in our statistical analyses (Table 1).
Preprocessing and analyses of rs-fMRI
Structural and functional MRI images were preprocessed and analyzed through the open-source functional connectivity toolbox, CONN, in MATLAB R2019b [62, 63], and the neuroimaging software, SPM 12 [64]. The default CONN preprocessing pipeline was as follows: functional realignment and unwarping; slice timing correction; direct segmentation, co-registration, and normalization to MNI space; and smoothing with an 8-mm Gaussian filter. Physiological noise correction was performed using aCompCor to improve the quality of our functional connectivity results [65]. Temporal band-pass filtering was applied to isolate the 0.008 to 0.09 Hz range, as our goal with rs-fMRI was to analyze low-frequency fluctuations while improving the signal-to-noise ratio [66, 67]. Outlier scans due to head movement (i.e., invalid scans) were identified using the Artifact Rejection Toolbox set at the 97th percentile (https://www.nitrc.org/projects/artifact_detect). Invalid scans were defined as a BOLD signal deviation of ± 5 standard deviations from the mean global signal or framewise displacement of at least 0.9 mm and were scrubbed during the denoising process. Average within-network functional connectivity values for CON, FPCN, and DMN were extracted using the previously identified Yeo network regions of interest [30].
Statistical analyses
Demographic, HVLT-R performance, and BVMT-R performance data were managed and extracted from the web-based data collection software, REDCap, at the University of Florida [68, 69]. All statistical analyses were conducted using R version 4.3.0 and RStudio. To examine the relationships among resting-state functional connectivity of three higher-order cognitive networks (CON, FPCN, and DMN) and verbal and visuospatial learning and memory functioning, six hierarchical regressions were executed. Regression assumptions (e.g., absence of multicollinearity, normally distributed residuals) were confirmed before performing the analyses. The first block of each hierarchical regression controlled for sex, race, ethnicity, years of education, and scanner site. Because the sample was disproportionately White, race was binarized into “White” and “non-White” to meet regression assumptions. The second block of each hierarchical regression included the mean within-network resting-state functional connectivity values for CON, FPCN, and DMN. This provides an analytic method for evaluating the unique contribution of the three functional networks of interest relative to the selected dependent variables. The dependent variable for each regression was one of the six HVLT-R and BVMT-R performance measures obtained, three for each neuropsychological test (Table 2).
Table 2.
HVLT-R and BVMT-R performance
| University of Florida (n = 222) |
University of Arizona (n = 108) |
Combined (n = 330) |
|
|---|---|---|---|
| HVLT-R Total Recall M (SD), range | 24.88 (4.51), 13–34 | 26.40 (4.34), 16–36 | 25.38 (4.50), 13–36 |
| HVLT-R Learning Ratio M (SD), range | 0.63 (0.27), 0–1 | 0.68 (0.27), 0–1 | 0.65 (0.27), 0–1 |
| HVLT-R Delayed Recall M (SD), range | 9.21 (2.12), 3–12 | 9.83 (1.91), 5–12 | 9.42 (2.07), 3–12 |
| BVMT-R Total Recall M (SD), range | 21.63 (6.40), 1–36 | 20.98 (5.69), 7–33 | 21.42 (6.17), 1–36 |
| BVMT-R Learning Ratio M (SD), range | 0.57 (0.26), 0–1 | 0.64 (0.24), 0–1 | 0.59 (0.26), 0–1 |
| BVMT-R Delayed Recall M (SD), range | 8.73 (2.41), 1–12 | 9.03 (1.96), 3–12 | 8.83 (2.27), 1–12 |
M mean, SD standard deviation
Results
HVLT-R performance and within-network resting-state functional connectivity
The six hierarchical regressions that examine the relationships among raw resting-state functional connectivity values of CON, FPCN, and DMN with HVLT-R performance (total recall, learning ratio, and delayed recall), controlling for demographic and experimental covariates, are shown in Table 3. Figure 1 a–c serve as a representation of significant findings from the parent models by depicting the raw relationships among CON functional connectivity and HVLT-R Total Recall, Learning Ratio, and Delayed Recall performance scores. All analyses were corrected at a false discovery rate (FDR) of p=0.05 to adjust for the six multiple comparisons.
Table 3.
Two-step hierarchical regressions evaluating the unique relationships of within-network CON, FPCN, and DMN functional connectivity with verbal (HVLT-R) and visuospatial (BVMT-R) learning and memory performance
| Block 1 | Block 2 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Race | Ethnicity | Education | Scanner Site | CON | FPCN | DMN | |||||||||||||||||
| β | t | f2 | β | t | f2 | β | t | f2 | β | t | f2 | β | t | f2 | β | t | f2 | β | t | f2 | β | t | f2 | |
| HVLT-R Total Recall | 0.24 | 4.72*** | 0.06 | −0.11 | −2.00† | 0.01 | −0.03 | −0.55 | 0.00 | 0.20 | 3.87*** | 0.04 | 0.11 | 2.12† | 0.01 | 0.17 | 2.91* | 0.02 | −0.01 | −0.25 | 0.00 | −0.07 | −1.22 | 0.00 |
| HVLT-R Learning Ratio | 0.13 | 2.43* | 0.02 | −0.15 | −2.60† | 0.02 | −0.09 | −1.52 | 0.01 | 0.11 | 2.00† | 0.01 | 0.04 | 0.68 | 0.00 | 0.17 | 2.71* | 0.02 | 0.00 | 0.04 | 0.00 | −0.11 | −1.73 | 0.01 |
| HVLT-R Delayed Recall | 0.26 | 5.08*** | 0.07 | −0.12 | −−2.24† | 0.01 | −0.05 | −0.95 | 0.00 | 0.17 | 3.12** | 0.03 | 0.09 | 1.71 | 0.01 | 0.11 | 1.92 | 0.01 | 0.05 | 0.87 | 0.00 | −0.09 | −1.46 | 0.01 |
| BVMT-R Total Recall | 0.06 | 1.06 | 0.00 | −0.06 | −1.01 | 0.00 | 0.02 | 0.40 | 0.00 | 0.11 | 1.89 | 0.01 | −0.06 | −1.15 | 0.00 | 0.15 | 2.37* | 0.02 | 0.08 | 1.31 | 0.01 | −0.02 | −0.31 | 0.00 |
| BVMT-R Learning Ratio | 0.01 | 0.17 | 0.00 | −0.06 | −1.04 | 0.00 | −0.04 | −0.62 | 0.00 | 0.02 | 0.27 | 0.00 | 0.10 | 1.73 | 0.01 | 0.11 | 1.80 | 0.01 | 0.13 | 2.06† | 0.01 | −0.08 | −1.36 | 0.01 |
| BVMT-R Delayed Recall | 0.02 | 0.28 | 0.00 | −0.09 | −1.55 | 0.01 | −0.01 | −0.23 | 0.00 | 0.09 | 1.68 | 0.01 | 0.04 | 0.71 | 0.00 | 0.16 | 2.57* | 0.02 | 0.10 | 1.57 | 0.01 | −0.04 | −0.72 | 0.00 |
†p-uncorrected < 0.05; *pFDR < 0.05; **pFDR < 0.01; ***pFDR < 0.001
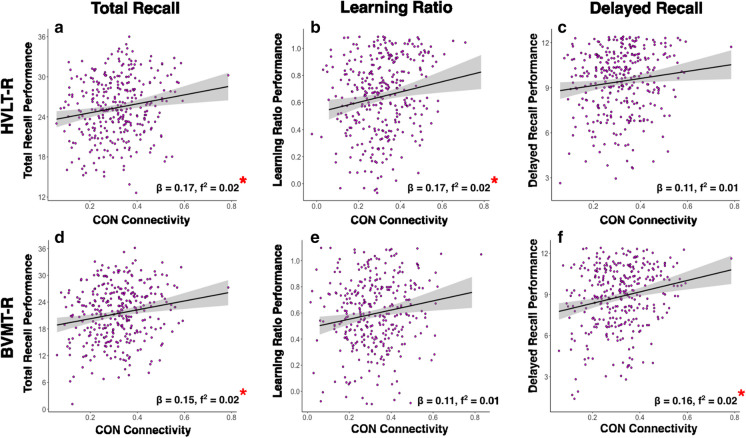
Fig. 1.
These correlation plots are depictions of the relationships found between CON connectivity and learning/memory performance, according to the parent regression models. Plots depict the raw correlations between CON connectivity and a–c HVLT-R performance and d–f BVMT-R performance, without controlling for covariates. For reference, beta and f2-values from the parent models are presented with each plot *pFDR < 0.05
The overall model for HVLT-R Total Recall explained significantly more than zero variance (R2 = 0.16, F[8,321] = 7.57, pFDR < 0.001). Of the three higher-order cognitive networks, CON was the only network significantly associated with HVLT-R Total Recall performance (β = 0.17, pFDR = 0.021; f2 = 0.02), such that greater resting-state functional connectivity in CON was uniquely associated with higher HVLT-R Total Recall scores. The overall model for HVLT-R Learning Ratio was significant (R2 = 0.09, F[8,321] = 3.81, pFDR = 0.003). Only CON was significantly associated with HVLT-R Learning Ratio (β = 0.17, pFDR = 0.021; f2 = 0.02), such that greater resting-state functional connectivity in CON was uniquely associated with higher HVLT-R Learning Ratio values. The overall model for HVLT-R Delayed Recall was significant (R2 = 0.15, F[8,321] = 6.78, pFDR < 0.001), yet none of the three higher-order cognitive networks were significantly associated with HVLT-R Delayed Recall performance. Notably, the unique relationship between CON and HVLT-R Delayed Recall did not reach significance (β = 0.11, pFDR > 0.05; f2 = 0.01). Significant covariates are identified in Table 3. We calculated Cook’s D, a metric of leverage statistics, to see if any data points significantly impacted the slope of the regression line on functional connectivity and HVLT performance. We found two significant Cook D values, one for high HVLT performance and one for low HVLT performance. However, removal of these two high leverage values did not alter the findings, and thus, we kept these data points in.
BVMT-R performance and within-network resting-state functional connectivity
The six hierarchical regressions that examine the relationships among raw resting-state functional connectivity values of CON (Fig. 1), FPCN (Fig. 2), and DMN (Fig. 3) and BVMT-R performance (total recall, learning ratio, and delayed recall), controlling for demographic and experimental covariates, are shown in Table 3. The raw relationships among CON functional connectivity and BVMT-R Total Recall, Learning Ratio, and Delayed Recall performance scores are shown in Fig. 1 d–f. Similarly, the raw relationships among FPCN functional connectivity and BVMT-R Total Recall, Learning Ratio, and Delayed Recall performance scores are shown in Fig. 2 d–f.
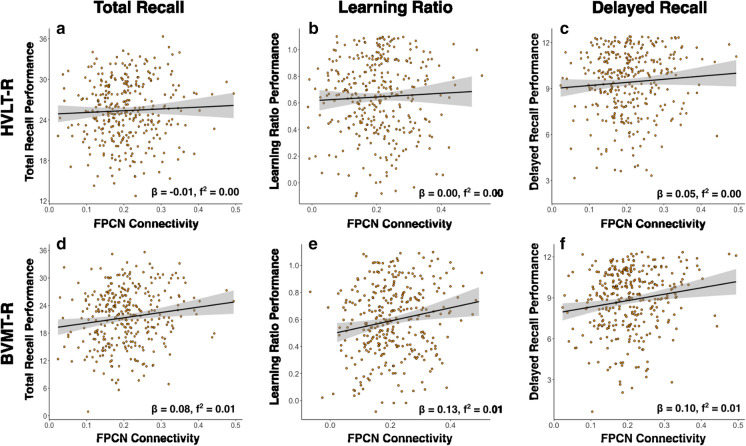
Fig. 2.
These correlation plots are depictions of the relationships found between FPCN connectivity and learning/memory performance, according to the parent regression models. Plots depict the raw correlations between FPCN connectivity and a–c HVLT-R performance and d–f BVMT-R performance, without controlling for covariates. For reference, beta and f2-values from the parent models are presented with each plot *pFDR < 0.05
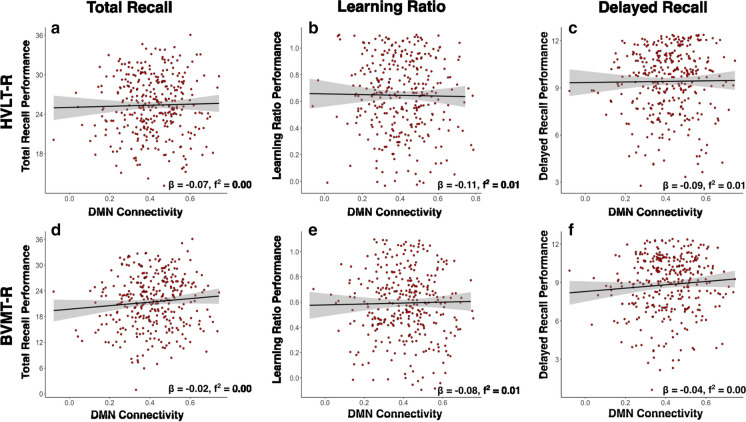
Fig. 3.
These correlation plots are depictions of the relationships found between DMN connectivity and learning/memory performance, according to the parent regression models. Plots depict the raw correlations between DMN connectivity and a–c HVLT-R performance and d–f BVMT-R performance, without controlling for covariates. For reference, beta and f2-values from the parent models are presented with each plot *pFDR < 0.05
The overall model for BVMT-R Total Recall explained significantly more than zero variance (R2 = 0.06, F[8,321] = 2.70, pFDR = 0.001). Of the three higher-order cognitive networks, CON was the only network significantly associated with BVMT-R Total Recall performance (β = 0.15, pFDR = 0.027; f2 = 0.02), such that greater resting-state functional connectivity in CON was associated with higher BVMT-R Total Recall scores. The overall model for BVMT-R Learning Ratio was significant (R2 = 0.05, F[8,321] = 2.22, pFDR = 0.006). Only FPCN was significantly associated with BVMT-R Learning Ratio such that greater resting-state functional connectivity in FPCN was uniquely associated with higher BVMT-R Learning Ratio values; however, this finding did not survive multiple comparisons corrections (β = 0.13, pFDR = 0.243; f2 = 0.01) The relationship between CON and BVMT-R Learning Ratio did not reach significance (β = 0.11, p = 0.073; f2 = 0.01). The overall model for BVMT-R Delayed Recall was significant (R2 = 0.07, F[8,321] = 3.01, pFDR < 0.001). Only CON was significantly associated with BVMT-R Delayed Recall performance (β = 0.16, p = 0.021; f2 = 0.02), such that greater resting-state functional connectivity in CON was uniquely associated with higher BVMT-R Delayed Recall scores. Significant covariates are identified in Table 3. We calculated Cook’s D to assess data points that may have impacted the slope of the regression line on functional connectivity and BVMT performance. We found no significant Cook D values for BVMT performance.
Exploratory analysis: the effects of age on HVLT-R and BMVT-R performance and functional connectivity
Given the impact of age on functional connectivity, we ran an exploratory analysis to assess how age may impact the relationship between HVLT and BVMT performance and functional connectivity. To our surprise, age was not associated with any of the HVLT or BVMT performance metrics (all p-uncorrected values > 0.05). However, there was a trending relationship between age and HVLT delayed recall score with younger age equating to better performance (p-uncorrected=0.06). There were no sex by age interactions for any of the HVLT and BVMT performance metrics. Even after controlling for age, the relationship between CON and HVLT Total Recall (p-uncorrected= 0.023) and CON and HVLT Learning Ratio (p-uncorrected= 0.032) remained, although this did not survive FDR corrections (both pFDR values > 0.05)
Discussion
The current study investigated the association between higher-order resting-state functional connectivity and HVLT-R and BVMT-R performance in a large older adult sample. Specifically, the association of within-network resting-state CON, FPCN, and DMN functional connectivity with HVLT-R and BVMT-R Total Recall, calculated Learning Ratio, and Delayed Recall was explored. Greater CON connectivity was significantly associated with better HVLT-R and BVMT-R Total Recall, HVLT-R Learning Ratio, and BVMT-R Delayed Recall performance. Furthermore, greater FPCN connectivity was significantly associated with higher BVMT-R Learning Ratio. HVLT-R Delayed Recall performance was not associated with functional connectivity in any network, and DMN connectivity was not associated with verbal or visuospatial learning or memory performance. Notably, significant associations generally had small effect sizes. This suggests that while relationships exist between these networks and performance, the unique contribution of within-network functional connectivity to verbal and visuospatial learning and memory is overall quite small.
As hypothesized, greater resting-state CON functional connectivity predicted higher scores in measures of verbal and visuospatial learning: HVLT-R Total Recall and Learning Ratio, as well as BVMT-R Total Recall. Greater connectivity within CON was also associated with better visuospatial memory, or BVMT-R Delayed Recall, performance. This pattern of results suggests that CON may have distinct contributions across visual and verbal domains. That is, in the verbal domain, CON may be involved in learning, but not memory, while CON may play a role in both visuospatial learning and memory processes. These findings also reinforce prior literature that used a subset of data from the same trial and found that greater CON connectivity was associated with better verbal and nonverbal memory performance on subtasks of the NIH Toolbox Cognitive Battery [48, 49, 70]. Additionally, CON and its neuroanatomical hubs (e.g., anterior insula/operculum, anterior cingulate cortex) may support verbal and visuospatial learning performance through their roles in attending to and maintaining stimuli across trials [38–41]. CON may be a therapy target in longitudinal age-related learning and memory studies and in future non-invasive interventions to attenuate later-life decline.
Likewise, these results support the hypothesis that greater resting-state FPCN functional connectivity is associated with better BVMT-R Learning Ratio. Based on the posited roles of FPCN in cognition, resting-state connectivity of this network is also expected to be associated with the other three learning measures: HVLT-R and BVMT-R Total Recall, and HVLT-R Learning Ratio [37–44]. While there is a paucity of research that directly investigates the role of FPCN in visuospatial learning, previous work theorizes that FPCN is involved in pertinent visuospatial processes. For example, a recent rs-fMRI study found that greater FPCN connectivity was associated with better performance on the Posit Science Double Decision task, which measures visual speed of processing and divided attention [45]. Additionally, in a study of the oldest-old, with older adults between the ages of 95 and 103, greater FPCN connectivity predicted visuospatial ability improvements on the Addenbrooke Cognitive Examination–Revised [71]. Also, FPCN is theorized to adaptively interact with and modulate other higher-order resting-state networks (e.g., CON, DMN, DAN) to facilitate goal-directed behavior and support cognitive functioning as humans age [28, 29, 47, 72]. Such goals may potentially require encoding and consolidating novel stimuli (e.g., geometric designs). For instance, this literature proposes that FPCN upregulates DAN (task-positive) and downregulates DMN (task-negative) during externally oriented tasks, with an opposite effect during internally focused tasks. Future research should continue to examine the role of FPCN connectivity in learning and memory abilities. To this end, follow-up studies should also investigate verbal and visuospatial memory trajectories in the context of within- and between-network functional connectivity, as some research has suggested a steeper age-related decline in visuospatial memory compared to verbal memory [6–10].
To our surprise, DMN functional connectivity was not significantly associated with HVLT-R or BVMT-R performance. This lack of DMN findings might partly be explained by the sample studied: relatively healthy older adults who were on normal cognitive aging trajectories. The relationship between DMN connectivity and cognition in healthy older adults has been mixed in the literature, with some research suggesting only small age-related declines [27]. However, a disruption of DMN connectivity has been frequently identified in samples of older adults with mild cognitive impairment or Alzheimer’s disease [32, 73–77]. Hence, conducting a similar study in a cognitively impaired population may elucidate the role of DMN in verbal and visuospatial learning and memory as Alzheimer’s disease pathology progresses.
Lastly, we found significant relationships between sex and scores on HVLT, but, not on metrics of the BVMT. Specifically, female sex was associated with higher scores on all three of the HLVT metrics (total recall, learning ratio, and delayed recall), even after controlling for multiple comparisons. Although it is widely found that women outperform men in episodic and verbal memory in late life, the reason for this phenomenon is unknown. One plausible hypothesis for this is that women may have greater cognitive reserve than men. A large epidemiological study by Levine and colleagues found that women had higher initial scores across most cognitive domains, compared to men, and these findings persisted into later life [78]. Thus, future research should examine the impact of cognitive reserve on cognition, as well as ways to maintain levels of cognitive reserve throughout the aging process.
Limitations
While this study offers novel findings on relationships among resting-state functional connectivity and learning and memory performance in older adults, a few methodological limitations should be addressed in further work. First, these results necessitate a greater focus on increasing sample heterogeneity. Approximately 15% of our sample identified as members of one or more racial/ethnic minority groups. In comparison, a community survey in 2020 indicated that about 1 in 4 Americans ages 65 years or older identifies as a member of one or more racial/ethnic minority groups. The study sample was also generally more educated compared to the US population, with greater than two-thirds (69%) of participants completing a bachelor’s degree or higher (i.e., 16 or more years of education). As of 2020, only one-third of older Americans met these same criteria [79]. Future research efforts should strive to promote and maintain a more inclusive study environment for underrepresented groups [80, 81]. Also, this study used only the baseline data from a clinical trial and therefore were cross-sectional analyses. To determine cognitive trajectories of within-network connectivity and HVLT-R/BVMT-R performance, and to infer causality, follow-up studies should analyze longitudinal trends. Adding a young adult comparison group to future studies may provide insight into age-related changes in higher-order resting-state functional connectivity and verbal and visuospatial learning and memory abilities.
Future directions
One potential extension of our research is to analyze between-network connectivity of higher-order resting-state networks such as CON, FPCN, and DMN. Various studies suggest not only age-related changes in connectivity among these three networks, but also that such modulations across time may relate to cognitive performance in memory, processing speed, and executive functioning [28, 34, 47, 50, 82]. Future research should expand on these findings by conducting seed-based functional connectivity analyses on brain regions involved in memory processes, such as the hippocampus [83, 84]. Related evidence in volumetric brain research has associated greater bilateral hippocampal volume with better HVLT-R and BVMT-R learning and memory performance in older adults [84, 85]. Lastly, using this same dataset, we can explore relationships among other collected neural markers (e.g., neurotransmitter concentrations via proton magnetic resonance spectroscopy) and HVLT-R/BVMT-R performance to strengthen our findings.
Conclusion
This study adds a novel perspective to the extensive body of learning and memory literature by investigating the association between higher-order resting-state networks and HVLT-R and BVMT-R performance in healthy older adults. Of the three networks studied, CON demonstrated a small but significant relationship to both verbal and visuospatial components of learning and memory—more specifically, in HVLT-R and BVMT-R Total Recall, HVLT-R Learning Ratio, and BVMT-R Delayed Recall. In contrast, FPCN connectivity was significantly associated with only BVMT-R Learning Ratio. DMN did not have a significant relationship with the verbal and visuospatial domains, and none of the networks evidenced a relationship with HVLT-R Delayed Recall. Hence, the strength of within-network CON connectivity appears to contribute broadly to verbal and visuospatial learning and memory function in nonpathological older adults, while FPCN connectivity appears to play a more limited role. These networks may serve as potential intervention targets or markers of functional network improvement in future trials seeking to remediate age-related declines in learning and memory.
Acknowledgements
We would like to thank all the research participants at the McKnight Brain Institutes of the Universities of Florida and Arizona, who generously volunteered their time and effort to help make this manuscript possible. We are also grateful to all research team members for their contributions to this project.
Funding
This study received support from the National Institute on Aging (NIA R01AG054077, NIA K01AG050707, NIA P30AG019610), the State of Arizona and Arizona Department of Health Services (ADHS), and the McKnight Brain Research Foundation.
Data availability
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board (DSMB) in the context of a phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).
Declarations
Disclosures
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations Department of Economic and Social Affairs, Population Division. World population prospects 2022: summary of results. [online] 2022. Available at: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf. Accessed 9/1/23.
- 2.Farias ST, Harrell E, Neumann C, Houtz A. The relationship between neuropsychological performance and daily functioning in individuals with Alzheimer’s disease: ecological validity of neuropsychological tests. Arch Clin Neuropsychol. 2003;18:655–672. doi: 10.1093/arclin/18.6.655. [DOI] [PubMed] [Google Scholar]
- 3.Hall JR, Vo HT, Johnson LA, Barber RC, O’Bryant SE. The link between cognitive measures and ADLs and IADL functioning in mild Alzheimer’s: what has gender got to do with it? Int J Alzheimers Dis. 2011;2011:276734. doi: 10.4061/2011/276734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 5.Woods SP, Weinborn M, Velnoweth A, Rooney A, Bucks RS. Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. J Int Neuropsychol Soc. 2012;18:134–138. doi: 10.1017/S1355617711001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bopp KL, Verhaeghen P. Age-related differences in control processes in verbal and visuospatial working memory: storage, transformation, supervision, and coordination. J Gerontol B Psychol Sci Soc Sci. 2007;62:P239–P246. doi: 10.1093/geronb/62.5.P239. [DOI] [PubMed] [Google Scholar]
- 7.Janssen SMJ, Kristo G, Rouw R, Murre JMJ. The relation between verbal and visuospatial memory and autobiographical memory. Conscious Cogn. 2015;31:12–23. doi: 10.1016/j.concog.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Murre JMJ, Janssen SMJ, Rouw R, Meeter M. The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychol. 2013;142:96–107. doi: 10.1016/j.actpsy.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- 10.Reichert JL, Kober SE, Witte M, Neuper C, Wood G. Age-related effects on verbal and visuospatial memory are mediated by theta and alpha II rhythms. Int J Psychophysiol. 2016;99:67–78. doi: 10.1016/j.ijpsycho.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Cronin-Golomb A. Visuospatial function in Alzheimer’s disease and related disorders. In: Budson AE, Kowall NW, editors. The Handbook of Alzheimer’s Disease and Other Dementias. 1. Chichester, West Sussex, UK: Wiley-Blackwell; 2011. pp. 457–482. [Google Scholar]
- 12.Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22:729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, et al. Decline in verbal memory during preclinical Alzheimer’s disease: examination of the effect of APOE genotype. J Int Neuropsychol Soc. 2002;8:943–955. doi: 10.1017/S1355617702870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis D, Bendayan R, Muniz Terrera G, Hardy R, Richards M, Kuh D. Decline in search speed and verbal memory over 26 years of midlife in a British birth cohort. NED. 2017;49:121–128. doi: 10.1159/000481136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muffato V, Miola L, Pazzaglia F, Meneghetti C. Map learning in aging individuals: the role of cognitive functioning and visuospatial factors. Brain Sci. 2021;11:1033. doi: 10.3390/brainsci11081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wearn AR, Saunders-Jennings E, Nurdal V, Hadley E, Knight MJ, Newson M, et al. Accelerated long-term forgetting in healthy older adults predicts cognitive decline over 1 year. Alzheimer’s Res Ther. 2020;12:119. doi: 10.1186/s13195-020-00693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casaletto KB, Marx G, Dutt S, Neuhaus J, Saloner R, Kritikos L, et al. Is “learning” episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia. 2017;102:19–28. doi: 10.1016/j.neuropsychologia.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. SLEEP. 2013;36:203–220. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Díaz-Santos M, Suárez PA, Marquine MJ, Umlauf A, Rivera Mindt M, Artiola i Fortuny L, et al. Updated demographically adjusted norms for the Brief Visuospatial Memory Test-Revised and Hopkins Verbal Learning Test-Revised in Spanish-speakers from the U.S.-Mexico border region: The NP-NUMBRS project. Clin Neuropsychol. 2021;35:374–395. doi: 10.1080/13854046.2020.1861329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duff K. Demographically corrected normative data for the Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised in an elderly sample. Appl Neuropsychol. 2016;23:179–185. doi: 10.1080/23279095.2015.1030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan J, Woods RL, Murray AM, Shah RC, Britt CJ, Reid CM, et al. Normative performance of older individuals on the Hopkins Verbal Learning Test-Revised (HVLT-R) according to ethno-racial group, gender, age and education level. Clin Neuropsychol. 2021;35:1174–1190. doi: 10.1080/13854046.2020.1730444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderploeg RD, Schinka JA, Jones T, Small BJ, Borenstein Graves A, Mortimer JA. Elderly norms for the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol. 2000;14:318–324. doi: 10.1076/1385-4046(200008)14:3;1-P;FT318. [DOI] [PubMed] [Google Scholar]
- 23.Vicente SG, Ramos-Usuga D, Barbosa F, Gaspar N, Dores AR, Rivera D, et al. Regression-based norms for the Hopkins Verbal Learning Test-Revised and the Rey–Osterrieth Complex Figure in a Portuguese adult population. Arch Clin Neuropsychol. 2021;36:587–596. doi: 10.1093/arclin/acaa087. [DOI] [PubMed] [Google Scholar]
- 24.Cherner M, Suarez P, Lazzaretto D, Fortuny L, Mindt M, Dawes S, et al. Demographically corrected norms for the Brief Visuospatial Memory Test-Revised and Hopkins Verbal Learning Test-Revised in monolingual Spanish speakers from the U.S.–Mexico border region. Arch Clin Neuropsychol. 2007;22:343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale SD, Baxter L, Connor DJ, Herring A, Comer J. Sex differences on the Rey Auditory Verbal Learning Test and the Brief Visuospatial Memory Test–Revised in the elderly: normative data in 172 participants. J Clin Exp Neuropsychol. 2007;29:561–567. doi: 10.1080/13803390600864760. [DOI] [PubMed] [Google Scholar]
- 26.Kane KD, Yochim BP. Construct validity and extended normative data for older adults for the Brief Visuospatial Memory Test, Revised. Am J Alzheimers Dis Other Demen. 2014;29:601–606. doi: 10.1177/1533317514524812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Shaw EE, Schultz AP, Sperling RA, Hedden T. Functional connectivity in multiple cortical networks is associated with performance across cognitive domains in older adults. Brain Connect. 2015;5:505–516. doi: 10.1089/brain.2014.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 32.Damoiseaux JS. Effects of aging on functional and structural brain connectivity. NeuroImage. 2017;160:32–40. doi: 10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- 33.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102:345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 35.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA [Internet]. 2014;111 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed]
- 36.He J, Carmichael O, Fletcher E, Singh B, Iosif A-M, Martinez O, et al. Influence of functional connectivity and structural MRI measures on episodic memory. Neurobiol Aging. 2012;33:2612–2620. doi: 10.1016/j.neurobiolaging.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci USA [Internet]. 2018;115 10.1073/pnas.1715766115. [DOI] [PMC free article] [PubMed]
- 38.Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. TiCS. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han SW, Eaton HP, Marois R. Functional fractionation of the cingulo-opercular network: alerting insula and updating cingulate. Cereb Cortex. 2019;29:2624–2638. doi: 10.1093/cercor/bhy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 2014;26:551–568. doi: 10.1162/jocn_a_00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardcastle C, Hausman HK, Kraft JN, Albizu A, Evangelista ND, Boutzoukas EM, et al. Higher-order resting state network association with the useful field of view task in older adults. GeroScience. 2022;44:131–145. doi: 10.1007/s11357-021-00441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huo L, Li R, Wang P, Zheng Z, Li J. The default mode network supports episodic memory in cognitively unimpaired elderly individuals: different contributions to immediate recall and delayed recall. Front Aging Neurosci. 2018;10:6. doi: 10.3389/fnagi.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016;41:159–172. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Hausman HK, O’Shea A, Kraft JN, Boutzoukas EM, Evangelista ND, Van Etten EJ, et al. The role of resting-state network functional connectivity in cognitive aging. Front Aging Neurosci. 2020;12:177. doi: 10.3389/fnagi.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hausman HK, Hardcastle C, Albizu A, Kraft JN, Evangelista ND, Boutzoukas EM, et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. GeroScience. 2022;44:847–866. doi: 10.1007/s11357-021-00503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, et al. Early age-related functional connectivity decline in high-order cognitive networks. Front Aging Neurosci [Internet]. 2017;8 10.3389/fnagi.2016.00330/full. [DOI] [PMC free article] [PubMed]
- 51.Woods AJ, Cohen R, Marsiske M, Alexander GE, Czaja SJ, Wu S. Augmenting cognitive training in older adults (the ACT study): design and methods of a phase III tDCS and cognitive training trial. Contemp Clin Trials. 2018;65:19–32. doi: 10.1016/j.cct.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Means and standard deviations for the UDS3 neuropsychological battery in cognitively normal participants [Internet]. NACC; 2020. Available at: https://files.alz.washington.edu/documentation/uds3-means.pdf. Accessed 9/1/2023.
- 53.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 54.Shi J, Tian J, Wei M, Miao Y, Wang Y. The utility of the Hopkins Verbal Learning Test (Chinese version) for screening dementia and mild cognitive impairment in a Chinese population. BMC Neurol. 2012;12:136. doi: 10.1186/1471-2377-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neil-Pirozzi TM, Goldstein R, Strangman GE, Glenn MB. Test–re-test reliability of the Hopkins Verbal Learning Test-Revised in individuals with traumatic brain injury. Brain Inj. 2012;26:1425–1430. doi: 10.3109/02699052.2012.694561. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro AM, Benedict RHB, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test – Revised. Clin Neuropsychol. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 57.Stewart NJ. The Hopkins Verbal Learning Test had high sensitivity and good specificity for detecting mild dementia in older people. Evid Based Nurs. 2001;4:24–24. doi: 10.1136/ebn.4.1.24. [DOI] [Google Scholar]
- 58.Woods S, Scott J, Dawson M, Morgan E, Carey C, Heaton R, et al. Construct validity of Hopkins Verbal Learning Test—Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20:1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Hammers DB, Suhrie K, Dixon A, Gradwohl BD, Duff K, Spencer RJ. Validation of HVLT-R, BVMT-R, and RBANS learning slope scores along the Alzheimer’s continuum. Arch Clin Neuropsychol. 2022;37:78–90. doi: 10.1093/arclin/acab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spencer RJ, Gradwohl BD, Williams TF, Kordovski VM, Hammers DB. Developing learning slope scores for the repeatable battery for the assessment of neuropsychological status. Appl Neuropsychol. 2020;29:1–7. doi: 10.1080/23279095.2020.1791870. [DOI] [PubMed] [Google Scholar]
- 61.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8:145. doi: 10.1037/1040-3590.8.2.145. [DOI] [Google Scholar]
- 62.Nieto-Castanon A. Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN. Boston: Hilbert Press; 2020.
- 63.Whitfield-Gabrieli S, Nieto-Castanon A. CONN : A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 64.Friston KJ. editor. Statistical parametric mapping: the analysis of functional brain images. 1st ed. Amsterdam. Boston: Elsevier/Academic Press; 2007. [Google Scholar]
- 65.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He L, Hu D, Wan M, Wen Y. Measuring temporal dynamics of resting-state fMRI data. Biomed Mater Eng. 2014;24:939–945. doi: 10.3233/BME-130888. [DOI] [PubMed] [Google Scholar]
- 67.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 68.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang J, Liu T, Crawford JD, Kochan NA, Brodaty H, Sachdev PS, et al. Stronger bilateral functional connectivity of the frontoparietal control network in near-centenarians and centenarians without dementia. NeuroImage. 2020;215:116855. doi: 10.1016/j.neuroimage.2020.116855. [DOI] [PubMed] [Google Scholar]
- 72.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, et al. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 74.Cao Y, Yang H, Zhou Z, Cheng Z, Zhao X. Abnormal default-mode network homogeneity in patients with mild cognitive impairment in Chinese communities. Front Neurol. 2021;11:569806. doi: 10.3389/fneur.2020.569806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33:828.e19–828.e30. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grieder M, Wang DJJ, Dierks T, Wahlund L-O, Jann K. Default mode network complexity and cognitive decline in mild Alzheimer’s disease. Front Neurosci. 2018;12:770. doi: 10.3389/fnins.2018.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levine DA, Gross AL, Briceño EM, Tilton N, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Hingtgen S, Elkind MSV, Manly JJ, Gottesman RF, Gaskin DJ, Sidney S, Sacco RL, Tom SE, Wright CB, Yaffe K, Galecki AT. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4(2):e210169. doi: 10.1001/jamanetworkopen.2021.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Administration for Community Living. 2020 Profile of older Americans [Internet]. 2021. Available at: https://acl.gov/sites/default/files/aging%20and%20Disability%20In%20America/2020Profileolderamericans.final_.pdf. Accessed 9/1/23.
- 80.Brewster P, Barnes L, Haan M, Johnson JK, Manly JJ, Nápoles AM, et al. Progress and future challenges in aging and diversity research in the United States. Alzheimers Dement. 2019;15:995–1003. doi: 10.1016/j.jalz.2018.07.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilmore-Bykovskyi A, Croff R, Glover CM, Jackson JD, Resendez J, Perez A, et al. Traversing the aging research and health equity divide: toward intersectional frameworks of research justice and participation. Meeks S, editor. Gerontologist. 2022;62:711–720. [DOI] [PMC free article] [PubMed]
- 82.Onoda K, Ishihara M, Yamaguchi S. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 2012;24:2186–2198. doi: 10.1162/jocn_a_00269. [DOI] [PubMed] [Google Scholar]
- 83.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 84.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 85.Bonner-Jackson A, Mahmoud S, Miller J, Banks SJ. Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimer’s Res Ther. 2015;7:61. doi: 10.1186/s13195-015-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board (DSMB) in the context of a phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).