Abstract
Treatment with rapamycin, an inhibitor of the mechanistic Target Of Rapamycin Complex One (mTORC1) protein kinase, has been repeatedly demonstrated to extend lifespan and prevent or delay age-related diseases in diverse model systems. Concerns over the risk of potentially serious side effects in humans, including immunosuppression and metabolic disruptions, have cautiously limited the translation of rapamycin and its analogs as a treatment for aging associated conditions. During the last decade, we and others have developed a working model that suggests that while inhibition of mTORC1 promotes healthy aging, many of the negative side effects of rapamycin are associated with “off-target” inhibition of a second mTOR complex, mTORC2. Differences in the kinetics and molecular mechanisms by which rapamycin inhibits mTORC1 and mTORC2 suggest that a therapeutic window for rapamycin could be exploited using intermittent dosing schedules or alternative rapalogs that may enable more selective inhibition of mTORC1. However, the optimal dosing schedules and the long-term efficacy of such interventions in humans are unknown. Here, we highlight ongoing or upcoming clinical trials that will address outstanding questions regarding the safety, pharmacokinetics, pharmacodynamics, and efficacy of rapamycin and rapalogs on several clinically oriented outcomes. Results from these early phase studies will help guide the design of phase 3 clinical trials to determine whether rapamycin can be used safely to inhibit mTORC1 for the treatment and prevention of age-related diseases in humans.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00935-x.
Keywords: Aging, mTOR, Sirolimus, Everolimus, Metabolism, Muscle
Introduction
The world is experiencing an unprecedented increase in the number of aged individuals [1]. The number of people over the age of 65 is expected to reach 2 billion by the year 2050 [2]. Age is a significant risk factor for most major causes of morbidity and mortality in the USA, including type 2 diabetes (T2D), cardiovascular disease (CVD), frailty, and dementia (Fig. 1). The financial impact of caring for elderly individuals is daunting with Medicare expenditures expected to top $1 trillion this year [4]. The high comorbidity of age-related diseases in the elderly individuals limits the benefit that can be obtained by targeting any one chronic condition individually [5]. Therefore, it is important to test geroprotective interventions that have shown efficacy in preclinical models to determine if these agents can simultaneously delay or even prevent multiple age-related conditions in humans.
Fig. 1.
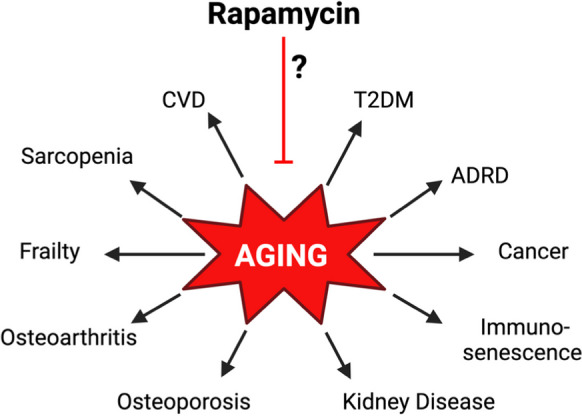
Age is one of the greatest risk factors for nearly every chronic condition. Rapamycin is the most repeatable and effective pharmacological approaches to extend lifespan and delay or treat many age-related pathologies in diverse model systems. A critical translational gap in knowledge is whether rapamycin or rapamycin analogs (rapalogs) can safely maintain or improve healthy aging in humans. CVD, cardiovascular disease; T2DM, type 2 diabetes mellitus; ADRD, Alzheimer’s disease and related dementias. Figure adapted from Kaeberlein et al. [3]
The evolutionarily conserved mTOR protein kinase is a key regulator of growth, metabolism, and aging. Genetic inhibition of mTOR complex 1 (mTORC1) signaling extends the lifespan in yeast, worms, flies, and mice [6–9]. mTORC1 inhibition by rapamycin is one of the most repeatedly and rigorously tested pharmacological approaches to extend lifespan in diverse model systems and across multiple independent laboratories. In addition to mTORC1 inhibition, rapamycin has significant impact on several proposed biomarkers and fundamental mechanisms of the biology of aging, such as increased markers of autophagy, suppression of the senescent-associated secretory phenotype (SASP), regulation of nuclear transcription factors, selective protein translation, and altering epigenetic patterns in rodents [10–14]. The goal of this review is to (1) briefly showcase how mTORC1 inhibition by rapamycin and rapamycin analogs (rapalogs) can extend healthspan by maintaining, improving, or restoring select indices of physiological function in pre-clinical and some human studies (Table 1); (2) discuss potential risks and key knowledge gaps regarding the clinical use of rapamycin for geroprotection; and (3) highlight several ongoing or upcoming clinical trials that will begin to test if the benefits of rapamycin in pre-clinical models can be safely translated to humans. It is important to note that this is not an endorsement for or against the use of mTOR inhibitors, but rather a compendium of upcoming clinical trials leveraging the last two decades of pre-clinical data to help advance our understanding of the impact of rapamycin on human healthspan.
Table 1.
Summary of completed rapamycin clinical trials*
| Population | mTOR inhibitor dosing regimen | Duration | Study design | Primary endpoint | Primary endpoint met? | Reference |
|---|---|---|---|---|---|---|
| 11 men (28 ± 1 years) | Rapamycin, 6 mg single dose | Once | Randomized, double-blinded, placebo controlled, cross-over design | Increase in peripheral insulin sensitivity during insulin clamp plus AA infusion | Yes | Krebs et al. 2007 [15] |
| 15 men (29 ± 2 years) | Rapamycin 12 mg (12 × 1 mg tablets) single dose 2h before resistance exercise (RE) | Once | Acute bout of RE ± rapamycin | Difference in post-RE mixed-muscle protein synthesis rates | Yes | Drummond et al. 2009 [16] |
| 16 men (26 ± 1 years) | Rapamycin 16 mg (16 × 1 mg tablets) single dose 1h before blood flow restricted resistance exercise (RE) | Once | Acute bout of blood flow restriction RE ± rapamycin | Difference in post-RE mixed-muscle protein synthesis rates | Yes | Gunderman 2014 [17] |
| 218 adults age ≥ 65 years |
Everolimus 0.5 mg daily Everolimus 5 mg weekly Everolimus 20 mg weekly Placebo |
6 weeks | Phase 2, randomized, single observer blinded, placebo-controlled | Improvement in influenza vaccination response | Yes for 0.5 mg daily and 5 mg weekly | Mannick et al. 2014 [18] |
| 264 adults age ≥ 65 years |
Everolimus 0.1 mg daily Everolimus 0.5 mg daily BEZ235 10 mg daily BEZ235 10 mg+0.1 mg everolimus daily Placebo |
6 weeks | Phase 2a, randomized, double-blinded, placebo-controlled | Improvement in influenza vaccination response | Yes for BEZ235 10 mg + 0.1 mg everolimus daily | Mannick et al. 2018 [19] |
| 25 healthy adults 70–95 years |
Rapamycin 1 mg Placebo |
8 weeks | Phase 2, Randomized, double-blinded, placebo-controlled | Immunological response | No | Kraig et al., 2018 [20] |
| 36 adults with aging skin > 40 years |
Topical rapamycin (10 μM) Topical placebo |
8 months | Phase 2, randomized, placebo-controlled | Decreased p16-positive senescent cells in skin | Yes | Chung et al. 2019 [21] |
| 1024 adults age ≥ 65 years |
RTB101 10 mg daily Placebo |
16 weeks | Phase 3, randomized, placebo-controlled | Decrease in clinically symptomatic respiratory illness | No | Mannick et al. 2021 [22] |
*Studies are presented in chronological order from the publication date. AA, amino acid
Impact of rapamycin on select physiological outcomes in pre-clinical and clinical studies
Metabolic function
The incidence of metabolic dysfunction, such as insulin resistance, prediabetes, and overt T2D, increases with age and further increases the risk of nearly every age-related condition including heart disease, frailty, cerebrovascular disease, and dementia [23–27]. The nutrient overload model of insulin resistance posits that insulin resistance results, in part, from constitutive activation of mTORC1 leading to S6K1 and Grb10 mediated feedback inhibition on insulin receptor substrate 1/2 (IRS-1/2) [28–30]. Consistent with this model, (1) mice lacking S6K1, a downstream target of mTOR, are protected from age and diet-induced insulin resistance [29, 31] and (2) rapamycin given intermittently or at a low dose improves metabolic health and insulin sensitivity in mouse models of obesity [32–34]. In healthy young men, a single dose of rapamycin (6 mg) attenuated mTORC1 during an amino acid infusion and increased peripheral insulin sensitivity by nearly 20% [15]. Conversely, long-term treatment with rapamycin doses ≥2 mg/kg/day in mice disrupts a second mTOR complex (mTORC2) and leads to insulin resistance [7, 35, 36] as evident by increased fasting insulin (104 vs. 86 pmol/L), elevated basal endogenous glucose production (EGP: 30.4 vs. 16.1 mg/kg/min), impaired suppression of EGP during a hyperinsulinemic-euglycemic clamp (39% vs. 67%), and glucose intolerance (39711 vs. 23075 AUC) in rapamycin treated versus vehicle controls [7]. We have also shown that hyperglycemia induced by 12 weeks of dietary rapamycin (14ppm) was correlated with greater osteoarthritis severity in guinea pigs prone to idiopathic osteoarthritis [37] suggesting that metabolic disruptions by long-term daily rapamycin may exacerbate some geriatric conditions in animal models.
Cognitive function
Decreased cerebral blood flow (CBF) occurs during normal aging and deficits in CBF are worsened by vascular risk factors like impaired glucose homeostasis and insulin resistance [24, 38, 39]. The decline in CBF that accompanies age and insulin resistance is associated with memory dysfunction before the development of overt diseases like T2D and Alzheimer’s [24, 25]. Therefore, therapies that can slow the biological rate of aging and age-related conditions may provide an effective strategy to mitigate the age-related risk of dementia. Rapamycin can cross the blood-brain barrier and mTOR attenuation by rapamycin prevents and reverses cognitive and cerebrovascular dysfunction in multiple models of Alzheimer’s disease as well as high-fat diet–induced vascular cognitive impairment [40–42]. In C57BL/6 mice, dietary rapamycin (14ppm) enhances cognitive function by ~25–100%, depending on the outcome, in young adult mice and blocks age-associated cognitive decline in older mice (12 and 25 months old) [40]. Additionally, 15 months of dietary rapamycin (14ppm) improves spatial learning and memory in 34-month-old F344BNF1 rats by ~50% [43]. In aging animals, improvements in cognitive performance by mTOR inhibition are linked to restoring cerebrovascular blood flow to adult animals [43].
Cardiac function
With age, there is an increase in cardiac stiffness and a decrease in ventricular volume that are associated with increased risk for heart failure and CVD [44]. Rapamycin reverses pre-existing age-dependent cardiac hypertrophy and diastolic dysfunction in mice [45, 46] and the effects of rapamycin on diastolic function, hypertrophy, and myocardial stiffness persist even following cessation of treatment [47]. Similarly, 10 weeks of rapamycin treatment (0.05–0.1 mg/kg 3×/week) increased diastolic and systolic cardiac function compared to placebo in a small cohort of dogs, with the greatest improvements in those dogs with lowest baseline scores [48].
Physical function
Skeletal muscle health and cardiorespiratory fitness (CRF) play a critical role in maintaining mobility, whole-body metabolism, and survival [49–53]. Age-related loss of skeletal muscle mass and CRF increase the risk of disability, loss of independence, and mortality [53–56]. Higher skeletal muscle function and CRF are associated with protection from multi-morbidity and mortality [57–60]. In animal models and humans, there is evidence of an age-related increase in skeletal muscle mTOR signaling [61–67] and an impaired muscle protein synthetic response to acute anabolic stimuli [68]. Genetic activation of mTORC1 by knockout of the upstream inhibitor TSC1 contributes to muscle loss [69, 70]. Correcting hyperactive skeletal muscle mTORC1 signaling in aged rats by low-dose treatment with the rapalog everolimus (equivalent to 0.5 mg/day in humans) and dietary rapamycin in old mice partially or completely prevents age-related muscle atrophy [61, 65]. Furthermore, 3 months of high-dose dietary rapamycin (126ppm) slowed the age-related decline in physical function in older mice as evident by greater forelimb grip strength and longer rotarod run time in rapamycin treated versus control mice even 3 months after stopping treatment [71]. These findings, like that of cardiac function, suggest that the benefits on physical function in older mice may persist even after ending rapamycin treatment. However, a single dose of rapamycin (12 or 16 mg) attenuated the acute increase in muscle protein synthesis rates after resistance exercise in young men [16, 17], which is one process involved in muscle growth. Therefore, it remains unclear if attenuation of mTOR signaling by rapamycin with or without exercise may positively or negatively impact skeletal muscle and physical function in older adults.
Immune function
Older adults are characterized by a decline in immune function that contributes to lower vaccine efficacy and increased vulnerability to infection. Age-related loss of immune function contributes to mortality linked to influenza and COVID-19 which disproportionately affects older persons. Just 6 weeks of treating older mice with rapamycin restored immune cell function and improved response to the influenza vaccine [72]. In older humans, a 6-week treatment with daily (0.5 mg/day) or intermittent (5 mg/week) everolimus rejuvenated aspects of the immune system and boosted the subsequent response to the influenza vaccination by >1.25-fold against two of three strains tested without metabolic side effects [18]. However, a higher weekly dose (20 mg/week) did not meet primary endpoints of increasing the serologic response to the influenza vaccine and led to more than double the number of adverse events compared to placebo and lower everolimus doses. While phase 2b and 3 clinical trials also showed that mTOR inhibitors increased antiviral gene expression in older patients, primary endpoints of decreasing laboratory confirmed or clinically symptomatic respiratory illness were not met [22]. The discrepancies between studies of meeting or not meeting primary endpoints are likely related to the pre-specified primary outcome. Although mTOR inhibitors appear to generally have a positive impact on immune function in older adults, additional work is needed to clarify the role of mTORC1 inhibition on immune function and vaccine efficacy in older adults.
Potential risks and unknowns
Despite the positive effects on lifespan and many indices of healthspan, mTOR inhibitor–based therapies for diseases of aging have not yet translated to clinical practice, largely due to a small number of human trials and potential safety concerns. Consistent with many animals studies, chronic treatment with rapamycin or rapamycin analogs (rapalogs) at the FDA-approved immunosuppressive doses (i.e., rapamycin 2–5 mg per day; everolimus up to 10 mg/day) to prevent organ transplant rejection or to treat some cancers are associated with deleterious metabolic consequences, including glucose intolerance (22% of patients), dyslipidemia (30–72% of patients), and an increased risk of developing new-onset diabetes (hazard ratio: 1.36 to 1.9) [73–78]. Furthermore, in a small study of healthy older adults, 8 weeks of daily rapamycin (1mg/day) tended to increase HbA1c, triglycerides, and VLDL [20] which challenges whether this represents an appropriate long-term dosing regimen. While these adverse side effects may be acceptable to patient populations, alternative dosing schedules are likely needed to minimize these adverse effects in older adults to achieve an acceptable risk to benefit ratio for geroprotection.
Even with the potential risks, unknown impact, and limited testing in humans, recent estimates suggest over 2000 people across the USA are currently taking rapamycin off label. Additionally, telemedicine services are beginning to launch that will increase accessibility to rapamycin. A recent observational study compared rapamycin users (n=333) to non-rapamycin users (n=172) [79]. Most users followed a weekly rapamycin dosing schedule, across a range of doses, for the purpose of “healthy longevity/anti-aging.” Mouth ulceration was the only self-reported adverse event (AE) that was greater in rapamycin vs. non-rapamycin users [79]. Interestingly, 50% of rapamycin users agreed that rapamycin improved their health while ~25–38% of rapamycin users felt younger, more confident, more energetic, and/or helped with other perceived health benefits. However, the remaining ~62–75% of rapamycin users did not perceive that rapamycin improved these domains [79]. Although difficult to assess without a double-blinded, placebo-controlled study design, rapamycin users did report less abdominal cramps and pain, signs of depression, muscle tightness, anxiety, and eye pain. No clinical laboratory results were evaluated to determine any potential differences in health between rapamycin versus non-rapamycin users.
Working model
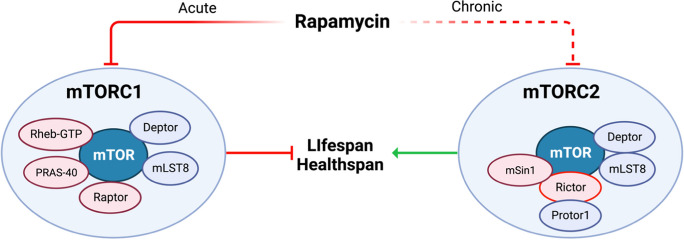
The mTOR kinase is the catalytic core of mTORC1 and mTORC2, each of which is composed of shared as well as unique protein subunits (Fig. 2), and which phosphorylate different substrates to regulate different metabolic processes. One of the major differences between mTORC1 and mTORC2 is that rapamycin acutely inhibits mTORC1 while chronic treatment with rapamycin also inhibits mTORC2 signaling in cultured cells and in mice [7, 80, 81]. Rapamycin inhibits mTORC1 by first forming a complex with FK506-binding protein 12 (FKBP12) which then binds to the FKBP12-rapamycin-binding domain of mTOR located on the surface of mTORC1 [82–84]. mTORC2 is not acutely sensitive to rapamycin because components of mTORC2, specifically Rictor and mSin1, hinder the binding of FKBP12-rapamycin to mTOR [85–87]. When high doses of rapamycin treatment are continued for a prolonged period of time, mTORC2 activity is decreased in most mouse tissues [7, 80, 81, 88] and this effect of rapamycin on mTORC2 is believed to be indirect, with rapamycin sequestering free mTOR and hindering the formation of new mTORC2 [80].
Fig. 2.
Rapamycin acutely and potently inhibits mTORC1 while prolonged exposure to high, daily doses of rapamycin can lead to off-target inhibition of mTORC2. We propose to test the model that inhibition of mTORC1 is geroprotective, while inhibition of mTORC2 mediates metabolic side effects of rapamycin. Rapalog strategies that safely exploit the potent geroprotective effects of mTORC1 inhibition may warrant further testing for the treatment and prevention of age-related diseases in larger phase 3 clinical trials
Using genetic models, inhibition of mTORC1 signaling alone extends lifespan and healthspan [7, 8] while tissue-specific and whole body genetic depletion of mTORC2 has negative effects on metabolic health, frailty, and survival in mice [89–93]. Similarly, genetic inhibition of mTORC2 activity in the heart impairs cardiac function in flies, while genetically increasing mTORC2 activity preserves cardiac function with aging and extends the lifespan of flies [94, 95]. These data support a model shown in Fig. 2 in which rapalog-mediated inhibition of mTORC1 is geroprotective, while the “off-target” inhibition of mTORC2 may be responsible for many negative effects of rapamycin. Therefore, to enhance translation of mTOR inhibitor-based therapies from pre-clinical models to human clinical trials and clinical practice, rapalog dosing strategies that preferentially inhibit mTORC1 rather than mTORC2 should be tested to potentially capitalize on healthspan extension while minimizing adverse side effects.
Collectively, these data indicate an urgent need to build on the exciting pre-clinical and clinical work that has already been completed to determine if rapamycin and rapalogs can be used safely and efficaciously for geroprotection in humans. There is currently a lack of pharmacokinetic and pharmacodynamic (PK/PD) data in healthy older adults. Therefore, it remains unknown what dose or dosing schedule of rapamycin or rapalogs minimizes undesirable side effects in older adults and whether rapamycin can have a beneficial impact on proposed biomarkers of aging and human healthspan as it does in pre-clinical models. We also do not understand if the dose of rapamycin for geroprotection will differ between sex, the age-related condition(s), or impacted tissue(s). Furthermore, we do not know how rapamycin will interact with healthy lifestyle practices such as exercise and diet. To address this need for additional information, a number of new clinical trials at the University of Wisconsin-Madison and around the world have begun or will begin in the near future to better study the effects of rapamycin and its analogs on age-related conditions (Table 2). The studies that are summarized within this review were identified by using search criteria rapamycin and mTOR on clinicaltrials.gov and through recent funding announcements. Studies were not included that focused on transplant or cancer patients. These relatively small trials will be signal-generating and will be used to inform on future, well-powered phase 3 clinical trials that will be needed for more definitive assessments of the therapeutic potential of rapalogs for human aging-related conditions.
Table 2.
Summary of ongoing or upcoming rapamycin clinical trials*
| Population | mTOR inhibitor dosing regimen | Duration | Study design | Primary endpoint | Status | Study name and/or NCT# |
|---|---|---|---|---|---|---|
| 100 adults (30–90 yrs old) taking rapamycin off-label | Rapamycin or everolimus, varied doses | Single time point | Cross-sectional | Lower HOMA-IR in rapamycin treated vs. non-treatment control | Ongoing |
RAP PROTECT Communication with Dr. Dudley Lamming |
| 10 young men (22–35 yrs old) |
Rapamycin 16 mg, single dose Placebo |
Once | Non-randomized, double-blinded, placebo controlled, cross-over design | Post-exercise, insulin stimulated muscle glucose uptake | Ongoing | NCT05233722 Communication with Jørgen Wojtaszewski |
| 35 residents ≥ 65 years of age in nursing homes with COVID |
RTB101 10 mg daily Placebo |
4 weeks | Randomized, double-blinded, placebo-controlled | Percentage of people who develop laboratory confirmed COVID-19 | Pending |
Communication with Dr. Joan Mannick |
| 72 adults (55–80 yrs old) |
Rapamycin 5, 10, or 15 mg weekly Everolimus 5, 10, or 15 mg weekly |
6 weeks | Allocated, open label, dose finding trial | Dose limited toxicities to determine RP2D |
Active, Not yet recruiting |
RAP PAC Dr. Adam Konopka |
| 50 patients with periodontitis |
Rapamycin 5 mg weekly Placebo |
8 weeks | Randomized, placebo-controlled | Clinical attachment loss | Not active | Communication with Dr. Jonathan An |
| 50 women with premature ovarian failure |
Rapamycin 5 mg weekly Placebo |
12 weeks | Randomized, placebo-controlled | Ovarian reserve | Ongoing |
VIBRANT Communication with Drs. Samuel Williams and Sajad Zalzala |
| 16 males age ≥ 65 years |
Rapamycin 1 mg daily Placebo |
16 weeks | Randomized, single-blinded, placebo-controlled, unilateral resistance exercise training vs. sedentary contralateral leg | Change in muscle mass | Ongoing | NCT05414292 |
| 72 adults with insulin resistance (55–80 yrs old) |
Everolimus 0.5 mg daily Everolimus 5 mg weekly Placebo |
24 weeks | Randomized, double-blinded, placebo-controlled | Change in peripheral insulin sensitivity | Ongoing |
EVERLAST Dr. Adam Konopka |
| 150 adults ( ≥ 50 yrs old) |
Rapamycin 5 mg weekly Rapamycin 10 mg weekly Placebo |
12 months | Prospective, randomized, placebo-controlled | Change in visceral fat | Ongoing |
PEARL NCT04488601 Communication with Dr. Sajad Zalzala |
| 40 patients with MCI/early AD |
Rapamycin 1 mg daily Placebo |
12 months | Randomized, double-blinded, placebo-controlled | Number of adverse events | Ongoing |
REACH Communication, Dr. Mitzi Gonzales |
*Studies are presented as observational study first, followed by studies with increasing rapamycin treatment duration
Observational and clinical trials testing mTOR inhibitors for geroprotection at the University of Wisconsin-Madison
The Evaluate the safety and efficacy of RAPamycin as a geroPROTECTor (RAP-PROTECT) study, led by Dr. Dudley Lamming and colleagues, is an ongoing study examining some of the growing number of individuals who are taking rapamycin off-label under the belief that it will extend their lifespan. This study is actively recruiting approximately 100 subjects taking or planning to take rapamycin or rapamycin analogs from across the contiguous United States to examine how clinical labs, the blood/plasma transcriptome, metabolome, and lipidome, and the blood methylation clock compare in rapamycin users vs. matched control subjects. The primary outcome of this cross-sectional trial is that rapamycin users will have a lower HOMA-IR than controls. While these data will expand on the insight from a recent observational study [79], there may be some difficulties in interpreting the data in the absence of longitudinal data from each subject, blood samples prior to rapamycin initiation, and double-blinded placebo-control as well as the diverse dosing regimens. Despite these limitations, this cross-sectional comparison will begin to reveal if there are differences in rapamycin users versus non-users in clinical laboratory results as well as molecular and metabolic signatures associated with aging. Therefore, this study may be informative to the geroscience community and those interested in potentially enrolling in one of the physician-supervised clinical trials described within this review.
The Rapalog Pharmacology (RAP PAC) Trial led by Dr. Adam Konopka and colleagues at the University of Wisconsin-Madison will identify a recommended phase 2 weekly dose (RP2D) for the mTOR inhibitors rapamycin and everolimus by performing a phase I, dose finding trial in healthy older men and women (n=18 per drug per sex, 55–80years). For each mTOR inhibitor, we propose to test up to 3 weekly dose levels (5, 10, 15 mg/week) for 6 weeks. RAP PAC will evaluate safety, pharmacokinetics (PK), pharmacodynamics (PD), and mTORC1/2 inhibition. The occurrence of dose limited toxicities (DLTs), defined as ≥grade 2 AE using the Common Terminology Criteria for Adverse Events (CTCAE), will serve as the primary endpoint. We will determine AEs by using a 20-point questionnaire to query about common mTOR inhibitor AEs, review participant diaries, perform clinical bloodwork, and if needed, complete a physical exam. Secondary and exploratory outcomes include investigating the impact of mTOR inhibitors on whole-body glucose metabolism and insulin sensitivity via a 75-g oral glucose tolerance test (OGTT) and 7–10 days of continuous glucose monitoring before and at the end of the 6-week treatment. mTOR signaling will be determined by conventional immunoblotting and immunoprecipitation as well as novel approaches to identify a molecular signature that distinguishes mTORC1 versus mTORC1/2 signaling by integrating transcriptomics, metabolomics, and lipidomics. Therefore, this study will combine comprehensive molecular, pharmacologic, and metabolic approaches to evaluate PK/PD in humans and identify dosing regimens that safely inhibit mTORC1 and intervene on the biology and metabolism of aging. A detailed review of the clinical trial protocol and experimental design can be found in the Supplement.
The Everolimus Aging Study (EVERLAST) led by Dr. Adam Konopka and colleagues at the University of Wisconsin-Madison is an ongoing phase 2 trial of 72 insulin-resistant, older adults (55–80 years old) who are at increased risk of multiple aged-related conditions, including type 2 diabetes, cardiovascular disease, frailty, and dementia [23, 24, 96]. In this randomized, double-blinded study, subjects will receive either 0.5 mg/day everolimus, 5 mg/week everolimus, or placebo-control for approximately 24 weeks. Everolimus tablets and placebo will be over-encapsulated to be indistinguishable from each other. To determine if everolimus can change molecular and physiological aging toward that of young healthy individuals, an additional group of 14 subjects between 18 and 35 years of age will serve as a young healthy reference group to complete baseline testing only (no intervention). The primary endpoint is the change in peripheral insulin sensitivity determined using a dual isotope oral glucose tolerance test (OGTT). Safety and incidence of treatment-associated adverse events will be determined from participant diaries, questionnaires, and changes in baseline blood chemistry, cell count, lipids, glucose, and insulin. Secondary and other pre-determined exploratory end points include additional indices of metabolic function (glucose tolerance, glycemic variability, hepatic insulin sensitivity), cognitive function (micro- and macro-vessel cerebral blood flow, learning, memory), cardiac function (fractional shortening, E/A ratio, ejection fraction, etc.), and physical function (VO2max, maximal knee extensor power and strength, body composition). To comprehensively examine the molecular target specificity and the impact on mechanisms of aging by everolimus, EVERLAST will evaluate mTORC1 and mTORC2 signaling, assess mitochondrial bioenergetics, and perform multi-omics (epigenomics, transcriptomics, proteomics, lipidomics, and metabolomics) in muscle biopsy and/or blood samples. EVERLAST will also explore the role of everolimus on senescent-associated secretory phenotype (SASP), DNA methylation clocks, and other proposed biomarkers of aging in saliva, urine, blood, and/or skeletal muscle. A detailed review of the clinical trial protocol and experimental design can be found in the online Supplement.
At this time, one EVERLAST ancillary study application is under review and another ancillary study led by Dr. Alexey Terskikh and colleagues at Sanford Burnham Prebys Medical Discovery Institute is funded. The funded study will perform microscopic imaging of epigenetic age (miEpiAge) to determine how the epigenetic landscape and heterogeneity in peripheral mononuclear blood cells (PMBCs) and skeletal muscle biopsy samples correlate to metabolic, cognitive, cardiac, and physical function with or without mTOR inhibition by everolimus. miEpiAge is a novel technique rooted in the analysis of epigenome topography at the single cell level to quantitate change in chromatin landscape. Therefore, this ancillary study will capture patterns of nuclear staining of epigenetic marks (e.g., acetylated and methylated histones) and employ automated microscopy and machine learning to determine a multiparametric signature of the cellular state.
Clinical trials of mTOR inhibitors for aging-related conditions in humans
Metabolism and body composition
The mTOR as Mediator of Exercise-induced Insulin Sensitivity Study led by Dr. Jørgen FP Wojtaszewski and colleagues in Denmark will evaluate the role of mTOR signaling on insulin sensitivity and muscle protein synthesis after a single exercise bout. This is a non-randomized, double-blinded, cross-over study where young men (22–35 years old) will perform two identical visits separated by at least 14 days that only differ by subjects taking either oral rapamycin (16 mg) or placebo 2 h before completing 1 h of one-legged kicking exercise (80–100% of maximal work). The combination of skeletal muscle biopsies, stable isotopes, femoral arterial and venous blood samples, and blood flow measurements will allow the measurement of post-exercise skeletal muscle glucose uptake and protein synthesis rates with and without insulin stimulation. The primary outcome is insulin stimulated skeletal muscle glucose uptake during recovery from exercise. Secondary outcomes include insulin stimulated skeletal muscle protein synthesis during recovery from exercise and phosphoproteomics to identify the intracellular signaling network regulating muscle glucose uptake and protein synthesis. Although this study is restricted to young men, these findings could provide the framework to understand how rapamycin may impact the acute benefits of exercise on substrate metabolism in both older men and women.
A relatively small trial in the UK, led by Drs. Philip Atherton and Lynne Cox, is aiming to determine how rapamycin affects skeletal muscle and immune function in men between the ages of 50 and 90 years. A total of 16 subjects will be randomized to either 1 mg/day rapamycin or placebo for 16 weeks. Subjects will complete a 2-week rapamycin lead-in before engaging in 14 weeks of unilateral, leg extension resistance exercise (3×/week at 75% of 1 repetition maximum) while the contralateral leg will remain sedentary. Although there may be some crossover effects between the trained and sedentary legs, this trial may be able to determine both the independent and combined effects of rapamycin and resistance exercise on study endpoints. The primary outcomes are the change from baseline in skeletal muscle mass assessed by MRI and ultrasound of the thigh muscles after 5, 8, and 16 weeks. Secondary outcomes include change in skeletal muscle strength (1-RM), power (counter jump movement), and physical function (short-performance physical battery). The combination of muscle biopsy samples and the use of the stable isotope deuterium oxide will be used to measure cumulative skeletal muscle protein synthesis rates. Blood samples taken will be used to assess immune cell senescence and inflammation.
The Participatory Evaluation of Aging with Rapamycin for Longevity Study (PEARL), led by Dr. James Watson of the University of California, Los Angeles and Dr. Sajad Zalzala of AgelessRx, is now underway. A total of 150 subjects located throughout the USA aged 50–85 have been randomized to receive either 5 mg or 10 mg of rapamycin once per week or a placebo-control. The primary outcome of the 12-month-long trial will be changes in visceral fat as estimated by dual-energy X-ray absorptiometry (DXA). Secondary outcomes will include bone density, fat-free mass, changes in blood glucose, HbA1c, and electrolytes, and effects on liver and renal function, as well as adverse events. Outcomes relevant to molecular aging may also include microbiome analysis, DNA methylation clock, and PhenoAge score. A potential limitation is the standardization between DXA scanners located across the country; however, participants will have pre- and post-measurements conducted on the same DXA machine.
Specific geriatric conditions
The Rapamycin – Effects on Alzheimer’s and Cognitive Health (REACH) trial led by Drs. Mitzi Gonzales, Sudha Seshardi, and collaborators at the University of Texas San Antonio Health Sciences Center is now recruiting subjects to explore the possibility that rapamycin could be used as an intervention in Alzheimer’s disease (AD). This trial is based on the extensive pre-clinical evidence that orally administered rapamycin attenuates cerebral mTOR signaling, restores cerebral blood flow, reduces amyloid beta (Aβ) and tau accumulation, and ameliorates cognitive deficits in AD and aged rodent models [42, 97, 98]. In this trial, 40 subjects with amnestic mild cognitive impairment (aMCI) or early-stage AD will be randomized to receive 1 mg/day of rapamycin or placebo for 12 months, followed by a 6-month observational period. The safety, efficacy, and feasibility of rapamycin treatment for aMCI/AD will be determined by tracking compliance, adverse events, rapamycin levels within the CSF, and changes in cognition. Molecular effects of the treatment on CSF levels of Aβ and tau will also be determined, and neuroimaging will be performed to examine how the treatment impacts AD biomarkers, including blood flow, glucose metabolism, neurovascular coupling, and cerebral volumetry.
Based on the lessons learned from previous phase 2b and phase 3 trials (Table 1) [19, 22], a pilot trial was recently completed to test an endpoint that assessed severity rather than incidence of respiratory tract infections (RTIs) caused by a specific virus (COVID-19) in nursing home patients. Specifically, the trial assessed whether 10mg daily dose of the mTOR catalytic inhibitor BEZ235 decreased the incidence of severe COVID-19 in residents of nursing homes experiencing a COVID-19 outbreak. Results of the clinical trial are pending.
The Validating Benefits of Rapamycin for Reproductive Aging Treatment (VIBRANT) trial led by Drs. Samuel Williams and Yousin Suh at Columbia University will explore the benefits of rapamycin in women undergoing early ovarian failure. Fifty subjects are being actively recruited and will be randomized to either 5 mg of rapamycin once weekly or placebo for 3 months, and then followed for 9 months. This trial is based in part on studies in rodents showing that short-term treatment with rapamycin can increase ovarian lifespan [99, 100]. The primary endpoint of this study will be ovarian reserve determined by transvaginal ultrasound, with secondary endpoints related to concentration of reproductive hormones.
An upcoming clinical trial led by Dr. Jonathan An at the University of Washington is aiming to start recruitment later in 2023 to study the effects of rapamycin on periodontal disease. This trial is based on pre-clinical work in mice showing that rapamycin can partially restore oral health in aged mice [101, 102]. Approximately 50 subjects aged 50 and up will be recruited and randomized to receive either rapamycin (most likely 5–6 mg per week) or placebo for 8 weeks. The primary endpoint of the study will be clinical attachment loss, an established clinical readout for the progression of periodontal disease.
Conclusions
Overall, there is significant interest by the scientific community and greater public to determine if rapamycin can safely and effectively extend healthy longevity in humans like it does in multiple model systems. The a priori goal of this review was to highlight ongoing and upcoming clinical trials testing the safety and utility of rapamycin to intervene in fundamental mechanisms of aging with the goal of slowing, improving, or restoring the age-related decline in physiological function. At the completion of these trials, it is expected that the geroscience community will identify dosing strategies that minimize adverse events for older adults and improve our understanding of whether rapamycin can improve key predictors of clinical outcomes in aging humans either at risk for or with overt, pre-existing geriatric conditions. Positive or null findings will be equally informative on the experimental design of future clinical trials in terms of dosing regimens, pre-specified primary endpoints, candidate subject populations, and statistical approaches. Furthermore, there is a need for the ongoing mTOR inhibitor trials to make data publicly available so that datasets can be combined for further exploratory analyses on dose, safety, aging biomarkers, and overlapping outcomes. Many of these studies have just recently started or will begin enrollment within the next 2 years. Therefore, study details were not always widely available for each protocol. For example, it was unclear whether some studies had a matching placebo and/or if studies were double or single-blinded. Therefore, the evaluation of rigor was not completely possible but, inherently, each of the studies mentioned within this review, including our own, will have strengths and limitations. Additionally, while there is growing infatuation for the potential of using rapamycin for geroprotection, there are currently no human data to suggest that long-term use of mTOR inhibitors can safely extend healthy longevity in humans and underscore the importance of the emerging clinical trials discussed within this review to close this translational knowledge gap.
Supplementary information
Acknowledgements
We appreciate Drs. Jonathan An, Philip Atherton, Mitzi Gonzales, Joan Mannick, Mandeep Singh, Samuel Williams, Sajad Zalzala and Jørgen Wojtaszewski for sharing information about their studies. We thank Jacob Rome, JD for his assistance in navigating the submission process for FDA Investigational New Drug Applications (161611 and 166577). For EVERLAST, we thank Dr. Lindsay Clark for her input on cognitive assessments, Dr. Adrian Vella for assistance with the design and calculations to determine glucose kinetics during the dual isotope oral glucose tolerance test, Dr. Scott Bauer for the suggestion to include sex-specific lower urinary tract symptoms (LUTS) questionnaires, and Mihaela Teodorescu for sleep quality questionnaires. We also thank the faculty and staff within the Clinical Research Unit and the Pharmaceutical Research Center for initiating EVERLAST and RAP PAC which are supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. EVERLAST is supported by NIH/NIA U01-AG076941 (to ARK), EVERLAST Biomarker Study is supported by the Impetus Longevity Grant by the Norn Group (to ARK), miEpiAge is supported by R21-AG083782 (to AT), RAP PAC is supported by U01-AG081482 (to ARK and DWL), and RAP-PROTECT is supported by the Impetus Longevity Grant by the Norn Group (to DWL). The Konopka Laboratory is also supported in part by NIH/NIA R21-AG067464 and New Investigator Award from AFAR/Hevolution. The Lamming laboratory is also supported in part by the NIH/NIA (AG056771, AG062328, and AG061635) and the NIH/NIDDK (DK125859 and DK133479). Both Konopka and Lamming Laboratories are also supported by startup and other funds from the University of Wisconsin-Madison School of Medicine and Public Health and Department of Medicine. This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital.
Consortium authors
EVERLAST Investigators
Amanjot K. Yadev1, Rebecca C. Marrah1, Brittany A. Grasso1, Sara Decker2, Samantha Pabich3, Didier Mandelbrot4, Thomas R. Wallhaus5, Oliver Wieben6, Fay Osman7, Richard J. Chappell8, Irene M. Ong8, Reid S. Alisch9, Judith A. Simcox11, Christian J. Elliehausen1, Dennis M. Minton1, Michaela E. Trautman1, Alma Spahic6, Barbara B. Bendlin1, Sanjay Asthana1, Alexey Terskikh12
RAP PAC Investigators
Brittany A. Grasso1, Rebecca C. Marrah1, Sara Decker2, Neetika Garg4, Yeonhee Park8, Sin Yin Lim10, Judith A. Simcox11, Cara L. Green3, Isaac Grunow3
1Division of Geriatrics, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
2Clinical Research Unit, University of Wisconsin Health System, Madison, WI, USA
3Division of Endocrinology, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
4Division of Nephrology, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
5Division of Cardiovascular Medicine, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
6Department of Medical Physics and Radiology, University of Wisconsin-Madison, Madison, WI, USA
7Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA
8Biostatistics and Medical Informatics, University of Wisconsin-Madison, Madison, WI, USA
9 Department of Neurological Surgery, University of Wisconsin-Madison, Madison, WI, USA
10School of Pharmacy, University of Wisconsin-Madison, Madison, WI, USA
11Department of Biochemistry, University of Wisconsin-Madison, Madison, WI, USA
12Development, Aging, and Regeneration Program, Sanford Burnham Prebys, San Diego, CA, USA
Declarations
Conflict of interest
DWL has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. Aeovian Pharmaceutical mTOR inhibitors will not be used in the clinical trials discussed within this manuscript.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the US Government.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adam R. Konopka, Email: akonopka@medicine.wisc.edu
RAP PAC Investigators:
Brittany A. Grasso, Rebecca C. Marrah, Sara Decker, Neetika Garg, Yeonhee Park, Sin Yin Lim, Judith A. Simcox, Cara L. Green, and Isaac Grunow
EVERLAST Investigators:
Amanjot K. Yadev, Rebecca C. Marrah, Brittany A. Grasso, Sara Decker, Samantha Pabich, Didier Mandelbrot, Thomas R. Wallhaus, Oliver Wieben, Fay Osman, Richard J. Chappell, Irene M. Ong, Reid S. Alisch, Judith A. Simcox, Christian J. Elliehausen, Dennis M. Minton, Michaela E. Trautman, Alma Spahic, Barbara B. Bendlin, Sanjay Asthana, and Alexey Terskikh
References
- 1.Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. The Journals of Gerontology: Series A. 2014;69(Suppl_1):S1–S3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ageing in the twenty-first century. Accessed May 10, 2021. /publications/ageing-twenty-first-century
- 3.Kaeberlein M. The biology of aging: citizen scientists and their pets as a bridge between research on model organisms and human subjects. Vet Pathol. 2016;53(2):291–298. doi: 10.1177/0300985815591082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr P. The boomer challenge. As baby boomers age, they will strain the system’s ability to care for them. Trustee. 2014;67(2):13–16. [PubMed] [Google Scholar]
- 5.Olshansky SJ. Pursuing longevity: delay vs elimination of degenerative diseases. Am J Public Health. 1985;75(7):754–757. doi: 10.2105/ajph.75.7.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selman C, Tullet JMA, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 10.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy BK, Lamming DW. The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab. 2016;23(6):990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannick JB, Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nat Aging. 2023;3(6):642–660. doi: 10.1038/s43587-023-00416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole JJ, Robertson NA, Rather MI, et al. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. 2017;18:58. doi: 10.1186/s13059-017-1185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Yu Z, Sunchu B, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16(3):564–574. doi: 10.1111/acel.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs M, Brunmair B, Brehm A, et al. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56(6):1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 16.Drummond MJ, Fry CS, Glynn EL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gundermann DM, Walker DK, Reidy PT, et al. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. American Journal of Physiology - Endocrinology and Metabolism. 2014;306(10):E1198. doi: 10.1152/ajpendo.00600.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 19.Mannick JB, Morris M, Hockey HUP, et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Science Translational Medicine. 2018;10(449):eaaq1564. doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- 20.Kraig E, Linehan LA, Liang H, et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol. 2018;105:53–69. doi: 10.1016/j.exger.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung CL, Lawrence I, Hoffman M, et al. Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. GeroScience. 2019;41(6):861–869. doi: 10.1007/s11357-019-00113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannick JB, Teo G, Bernardo P, et al. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: phase 2b and phase 3 randomised trials. The Lancet Healthy Longevity. 2021;2(5):e250–e262. doi: 10.1016/S2666-7568(21)00062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. The Journal of Clinical Endocrinology & Metabolism. 2001;86(8):3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 24.Hoscheidt SM, Kellawan JM, Berman SE, et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J Cereb Blood Flow Metab. 2017;37(6):2249–2261. doi: 10.1177/0271678X16663214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willette AA, Xu G, Johnson SC, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36(2):443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barzilay JI, Cotsonis GA, Walston J, et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged ≥70 years. Diabetes Care. 2009;32(4):736–738. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaslavsky O, Walker RL, Crane PK, Gray SL, Larson EB. Glucose levels and risk of frailty. The Journals of Gerontology: Series A. 2016;71(9):1223–1229. doi: 10.1093/gerona/glw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu PP, Kang SA, Rameseder J, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay F, Brûlé S, Hee Um S, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104(35):14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166(2):213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 32.Deepa SS, Walsh ME, Hamilton RT, et al. Rapamycin modulates markers of mitochondrial biogenesis and fatty acid oxidation in the adipose tissue of db/db mice. J Biochem Pharmacol Res. 2013;1(2):114–123. [PMC free article] [PubMed] [Google Scholar]
- 33.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014;13(4):616–622. doi: 10.1111/acel.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Ye S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biology International. 2018;42(10):1282–1291. doi: 10.1002/cbin.11015. [DOI] [PubMed] [Google Scholar]
- 35.Lamming DW, Ye L, Astle M, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12(4):712–718. doi: 10.1111/acel.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreiber KH, Arriola Apelo SI, Yu D, et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun. 2019;10(1):3194. doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minton DM, Elliehausen CJ, Javors MA, Santangelo KS, Konopka AR. Rapamycin-induced hyperglycemia is associated with exacerbated age-related osteoarthritis. Arthritis Research & Therapy. 2021;23(1):253. doi: 10.1186/s13075-021-02637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birdsill AC, Carlsson CM, Willette AA, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity (Silver Spring). 2013;21(7):1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A, Tosetti M. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging. 2007;25(4):696–702. doi: 10.1002/jmri.20839. [DOI] [PubMed] [Google Scholar]
- 40.Halloran J, Hussong SA, Burbank R, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahrling JB, Lin AL, DeRosa N, et al. mTOR drives cerebral blood flow and memory deficits in LDLR−/− mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab. 2018;38(1):58–74. doi: 10.1177/0271678X17705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin AL, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33(9):1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skike CEV, Lin AL, Burbank RR, et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020;19(1):e13057. doi: 10.1111/acel.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai X, Hummel SL, Salazar JB, Taffet GE, Zieman S, Schwartz JB. Cardiovascular physiology in the older adults. J Geriatr Cardiol. 2015;12(3):196–201. doi: 10.11909/j.issn.1671-5411.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai DF, Karunadharma PP, Chiao YA, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13(3):529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flynn JM, O’Leary MN, Zambataro CA, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12(5):851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quarles E, Basisty N, Chiao YA, et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell. 2020;19(2):e13086. doi: 10.1111/acel.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urfer SR, Kaeberlein TL, Mailheau S, et al. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39(2):117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izumiya Y, Hopkins T, Morris C, et al. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7(2):159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seguin R, Nelson ME. The benefits of strength training for older adults. Am J Prev Med. 2003;25(3 Suppl 2):141–149. doi: 10.1016/s0749-3797(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 51.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42(2):53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 54.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 55.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 56.Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128(2 Suppl):351S–355S. doi: 10.1093/jn/128.2.351S. [DOI] [PubMed] [Google Scholar]
- 57.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.1989.03430170057028. [DOI] [PubMed] [Google Scholar]
- 58.Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. doi: 10.1001/jama.1995.03520380029031. [DOI] [PubMed] [Google Scholar]
- 59.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 60.Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL, Kaminsky LA. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol. 2018;72(19):2283–2292. doi: 10.1016/j.jacc.2018.08.2166. [DOI] [PubMed] [Google Scholar]
- 61.Ham DJ, Börsch A, Lin S, et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat Commun. 2020;11(1):4510. doi: 10.1038/s41467-020-18140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baar EL, Carbajal KA, Ong IM, Lamming DW. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell. 2016;15(1):155–166. doi: 10.1111/acel.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markofski MM, Dickinson JM, Drummond MJ, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 2015;65:1–7. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang H, Inoki K, Brooks SV, et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell. 2019;18(3):e12943. doi: 10.1111/acel.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joseph GA, Wang SX, Jacobs CE, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol. 2019;39(19) 10.1128/MCB.00141-19. [DOI] [PMC free article] [PubMed]
- 66.Barns M, Gondro C, Tellam RL, Radley-Crabb HG, Grounds MD, Shavlakadze T. Molecular analyses provide insight into mechanisms underlying sarcopenia and myofibre denervation in old skeletal muscles of mice. Int J Biochem Cell Biol. 2014;53:174–185. doi: 10.1016/j.biocel.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 67.White Z, White RB, McMahon C, Grounds MD, Shavlakadze T. High mTORC1 signaling is maintained, while protein degradation pathways are perturbed in old murine skeletal muscles in the fasted state. Int J Biochem Cell Biol. 2016;78:10–21. doi: 10.1016/j.biocel.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 68.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutrition & Metabolism. 2011;8(1):68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaiser MS, Milan G, Ham DJ, et al. Dual roles of mTORC1-dependent activation of the ubiquitin-proteasome system in muscle proteostasis. Commun Biol. 2022;5(1):1141. doi: 10.1038/s42003-022-04097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castets P, Lin S, Rion N, et al. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metabolism. 2013;17(5):731–744. doi: 10.1016/j.cmet.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Bitto A, Ito TK, Pineda VV, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trelinska J, Dachowska I, Kotulska K, Fendler W, Jozwiak S, Mlynarski W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. 2015;26(4):437–442. doi: 10.1097/cad.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 74.Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008;19(7):1411–1418. doi: 10.1681/ASN.2007111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krueger DA, Wilfong AA, Mays M, et al. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology. 2016;87(23):2408–2415. doi: 10.1212/WNL.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLoS One. 2017;12(8):e0180939. doi: 10.1371/journal.pone.0180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Firpi RJ, Tran TT, Flores P, et al. Sirolimus-induced hyperlipidaemia in liver transplant recipients is not dose-dependent. Aliment Pharmacol Ther. 2004;19(9):1033–1039. doi: 10.1111/j.1365-2036.2004.01923.x. [DOI] [PubMed] [Google Scholar]
- 78.Teutonico A, Schena PF, Di Paolo S. Glucose metabolism in renal transplant recipients: effect of calcineurin inhibitor withdrawal and conversion to sirolimus. Journal of the American Society of Nephrology. 2005;16(10):3128. doi: 10.1681/ASN.2005050487. [DOI] [PubMed] [Google Scholar]
- 79.Kaeberlein TL, Green AS, Haddad G, et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. GeroScience. 2023;16:1–12. doi: 10.1007/s11357-023-00818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 81.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2016;71(7):876–881. doi: 10.1093/gerona/glw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci U S A. 1995;92(11):4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38(5):768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang J, Choi J, Clardy J. Refined structure of the FKBP12–rapamycin–FRB ternary complex at 2.2 Å resolution. Acta Cryst D. 1999;55(4):736–744. doi: 10.1107/S0907444998014747. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Liu M, Tian Y, et al. Cryo-EM structure of human mTOR complex 2. Cell Res. 2018;28(5):518–528. doi: 10.1038/s41422-018-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scaiola A, Mangia F, Imseng S, et al. The 3.2-Å resolution structure of human mTORC2. Sci Adv. 2020;6(45):eabc1251. doi: 10.1126/sciadv.abc1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 88.Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell. 2015;14(2):265–273. doi: 10.1111/acel.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamming DW, Mihaylova MM, Katajisto P, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13(5):911–917. doi: 10.1111/acel.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamming DW, Demirkan G, Boylan JM, et al. Hepatic signaling by the mechanistic target of rapamycin complex 2 (mTORC2) FASEB J. 2014;28(1):300–315. doi: 10.1096/fj.13-237743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arriola Apelo SI, Lin A, Brinkman JA, et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. Elife. 2020;9:e56177. doi: 10.7554/eLife.56177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chellappa K, Brinkman JA, Mukherjee S, et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell. 2019;18(5):e13014. doi: 10.1111/acel.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu D, Tomasiewicz JL, Yang SE, et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep. 2019;29(1):236–248.e3. doi: 10.1016/j.celrep.2019.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang K, Kang P, Liu Y, et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy. 2020;16(10):1807–1822. doi: 10.1080/15548627.2019.1704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu P, Chang K, Requejo G, Bai H. mTORC2 protects the heart from high-fat diet-induced cardiomyopathy through mitochondrial fission in Drosophila. Front Cell Dev Biol. 2022;10:866210. doi: 10.3389/fcell.2022.866210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yi HS, Kim SY, Kim JT, et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death & Disease. 2019;10(3):1–15. doi: 10.1038/s41419-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caccamo A, Branca C, Talboom JS, et al. Reducing ribosomal protein S6 kinase 1 expression improves spatial memory and synaptic plasticity in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35(41):14042–14056. doi: 10.1523/JNEUROSCI.2781-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dou X, Sun Y, Li J, et al. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16(4):825–836. doi: 10.1111/acel.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang XM, Li L, Xu JJ, et al. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523(1):82–87. doi: 10.1016/j.gene.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 101.An JY, Quarles EK, Mekvanich S, et al. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017;39(4):457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.An JY, Kerns KA, Ouellette A, et al. Rapamycin rejuvenates oral health in aging mice. Elife. 2020;9:e54318. doi: 10.7554/eLife.54318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.