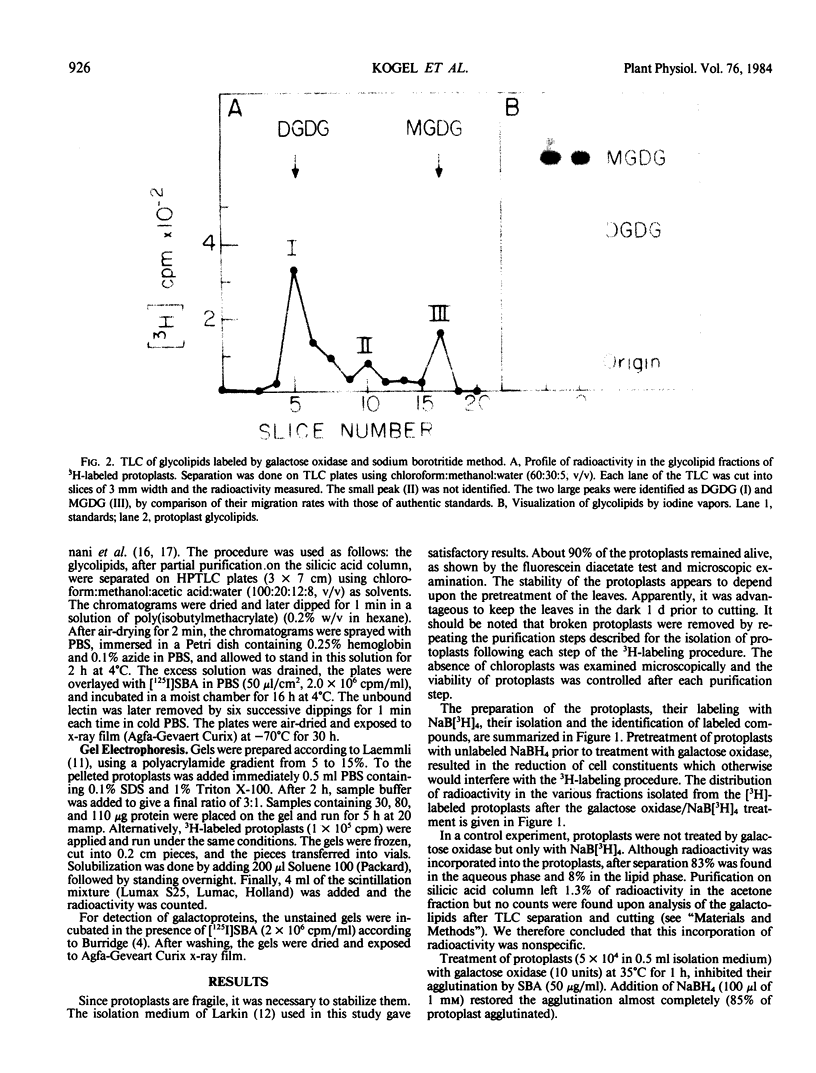
Abstract
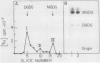
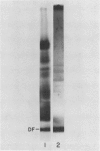
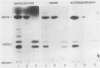
Soybean agglutinin, a lectin specific for N-acetyl-d-galactosamine and d-galactose, was previously shown to agglutinate wheat leaf protoplasts (Larkin 1978 Plant Physiol 61: 626-629). We investigated the receptors for soybean agglutinin on the plasma membrane of these protoplasts. After treatment of the protoplasts with galactose oxidase, they were no longer agglutinated by the lectin, whereas upon reduction of the galactose oxidase-treated protoplasts with sodium borohydride the susceptibility to agglutination was restored. Analysis of the glycolipids of protoplasts surface labeled by the galactose oxidase-borotritide method, revealed that the radioactivity was mainly present in monogalactosyldiglyceride and digalactosyldiglyceride. The same galactolipids were identified as the only receptors for soybean agglutinin by direct binding of the 125I-labeled lectin to a thin layer chromatogram of the glycolipids of wheat leaf protoplasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., AMARAL D., ASENSIO C., HORECKER B. L. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962 Sep;237:2736–2743. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Janero D. R., Barrnett R. Cellular and thylakoid-membrane glycolipids of Chlamydomonas reinhardtii 137+. J Lipid Res. 1981 Sep;22(7):1119–1125. [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. The sphingolipid composition of bovine kidney cortex, medulla and papilla. Biochim Biophys Acta. 1973 Sep 25;316(3):317–335. doi: 10.1016/0005-2760(73)90072-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkin P. J. Plant protoplast agglutination by lectins. Plant Physiol. 1978 Apr;61(4):626–629. doi: 10.1104/pp.61.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Jaffe C. L., Sharon N. Variations in the susceptibility of erythrocyte membrane glycolipids to galactose oxidase. FEBS Lett. 1982 Oct 4;147(1):59–63. doi: 10.1016/0014-5793(82)81011-9. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Momoi T., Tokunaga T., Nagai Y. Specific interaction of peanut agglutinin with the glycolipid asialo GM1. FEBS Lett. 1982 May 3;141(1):6–10. doi: 10.1016/0014-5793(82)80003-3. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Comparative biochemistry of plant glycoproteins. Biochem Soc Trans. 1979 Aug;7(4):783–799. doi: 10.1042/bst0070783. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]