Abstract
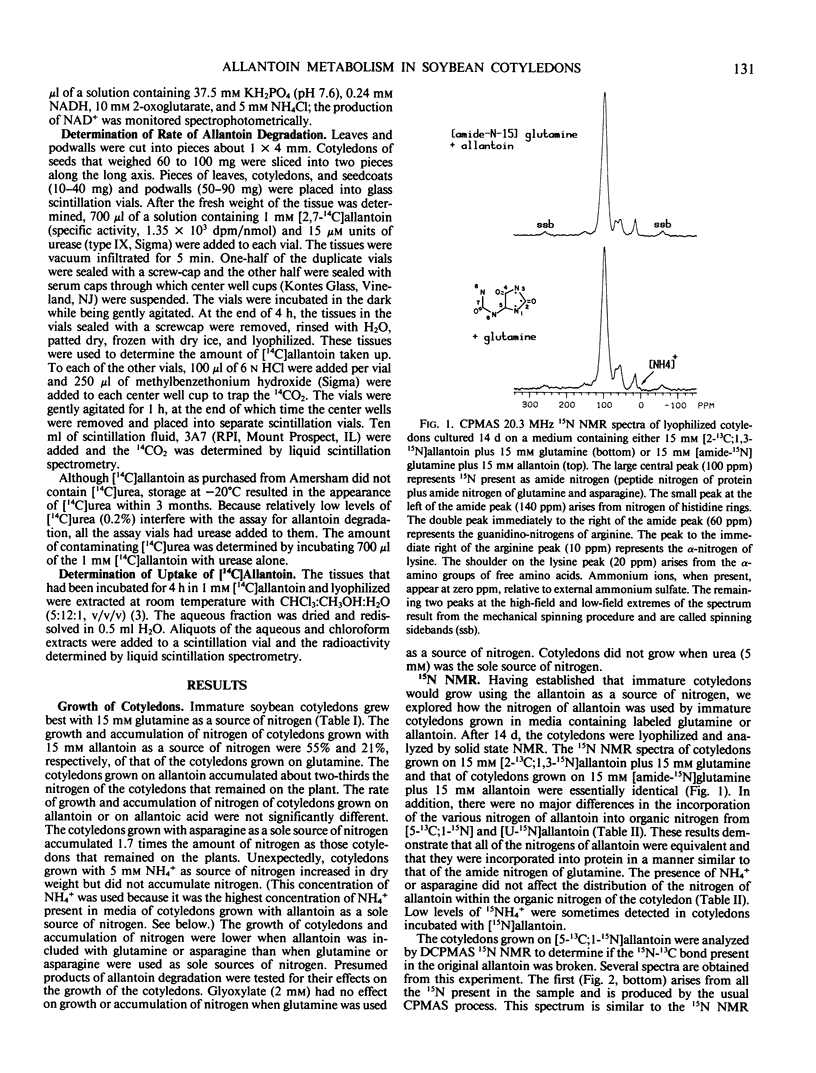
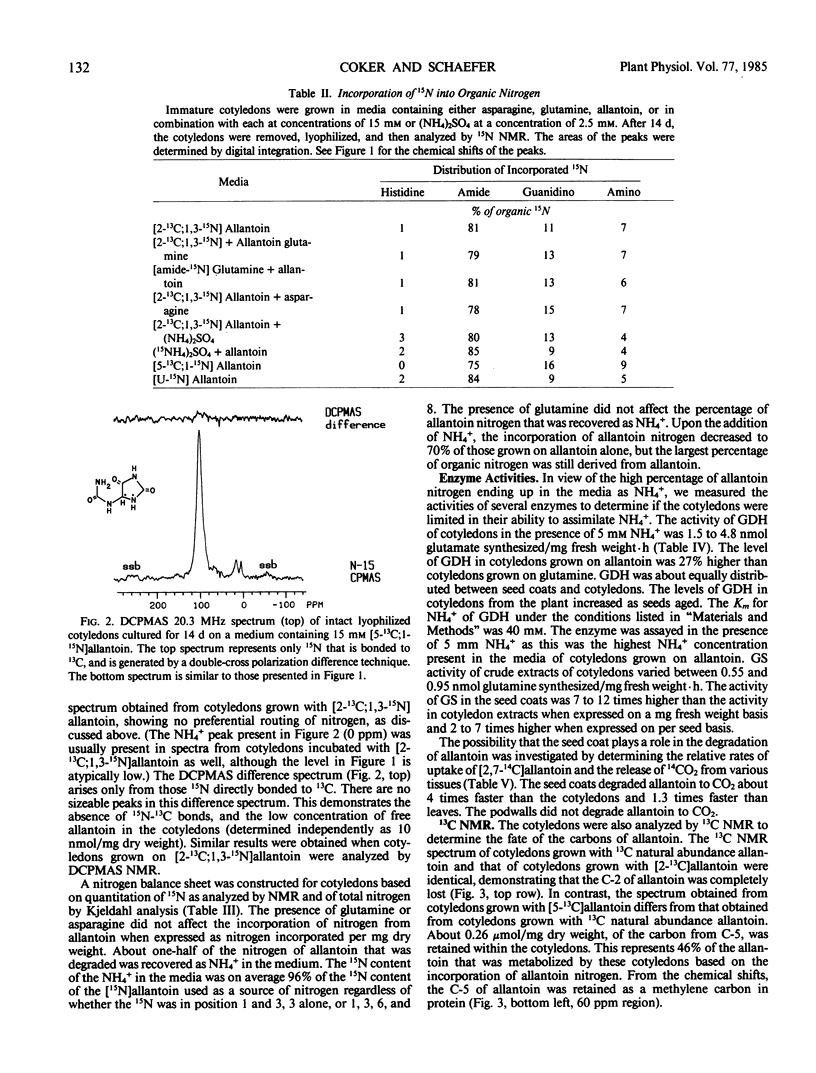
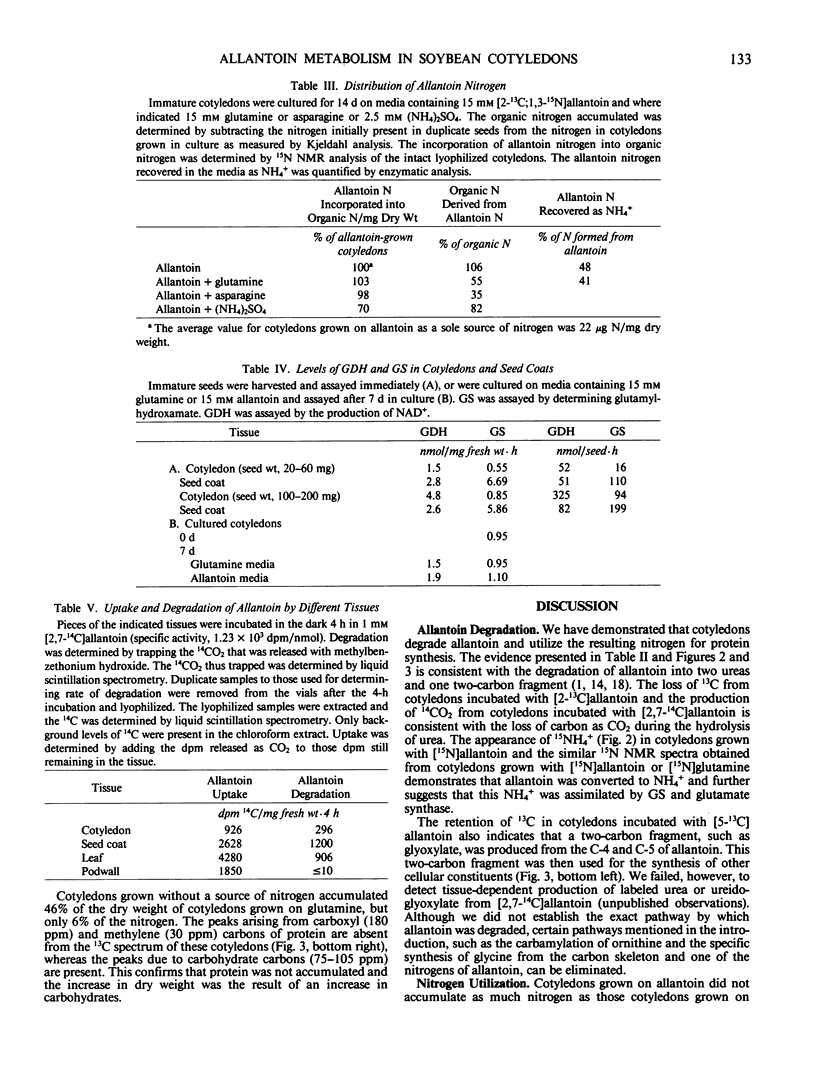
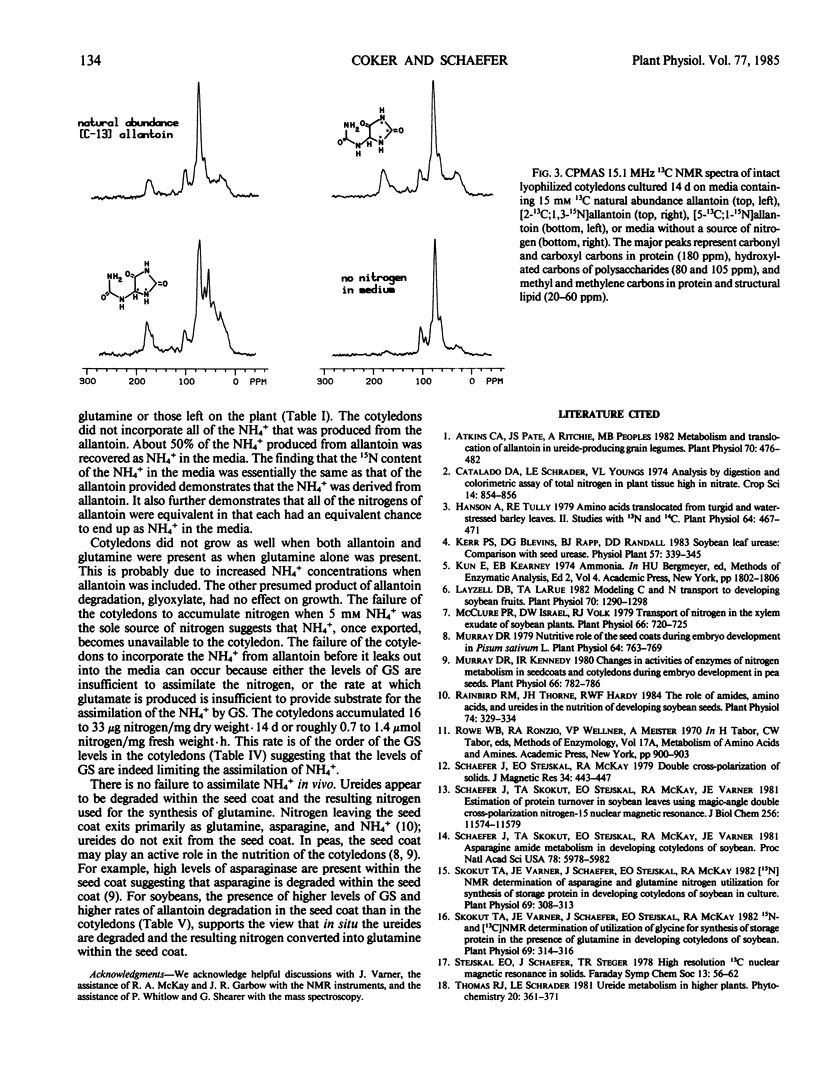
The metabolism of allantoin by immature cotyledons of soybean (Glycine max L. cv Elf) grown in culture was investigated using solid state 13C and 15N nuclear magnetic resonance. All of the nitrogens of allantoin were incorporated into protein in a manner similar to that of each other and to the amide nitrogen of glutamine. The C-2 of allantoin was not incorporated into cellular material; presumably it was lost as CO2. About 50% of the C-5 of allantoin was incorporated into cellular material as a methylene carbon; the other 50% was presumably also lost as CO2. The 13C-15N bonds of [5-13C;1-15N] and [2-13C;1,3-15N]allantoin were broken prior to the incorporation of the nitrogens into protein. These data are consistent with allantoin's degradation to two molecules of urea and one two-carbon fragment. Cotyledons grown on allantoin as a source of nitrogen accumulated 21% of the nitrogen of cotyledons grown on glutamine. Only 50% of the nitrogen of the degraded allantoin was incorporated into the cotyledon as organic nitrogen; the other 50% was recovered as NH4+ in the media in which the cotyledons had been grown. The latter results suggests that the lower accumulation of nitrogen by cotyledons grown on allantoin was in part due to failure to assimilate NH4+ produced from allantoin. The seed coats had a higher activity of glutamine synthetase and a higher rate of allantoin degradation than cotyledons indicating that seed coats play an important role in the assimilation and degradation of allantoin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Ritchie A., Peoples M. B. Metabolism and translocation of allantoin in ureide-producing grain legumes. Plant Physiol. 1982 Aug;70(2):476–482. doi: 10.1104/pp.70.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Tully R. E. Amino Acids Translocated from Turgid and Water-stressed Barley Leaves : II. Studies with N and C. Plant Physiol. 1979 Sep;64(3):467–471. doi: 10.1104/pp.64.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W., Volk R. J. Evaluation of the Relative Ureide Content of Xylem Sap as an Indicator of N(2) Fixation in Soybeans: GREENHOUSE STUDIES. Plant Physiol. 1980 Oct;66(4):720–725. doi: 10.1104/pp.66.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. R. Changes in Activities of Enzymes of Nitrogen Metabolism in Seedcoats and Cotyledons during Embryo Development in Pea Seeds. Plant Physiol. 1980 Oct;66(4):782–786. doi: 10.1104/pp.66.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. R. Nutritive Role of the Seedcoats during Embryo Development in Pisum sativum L. Plant Physiol. 1979 Nov;64(5):763–769. doi: 10.1104/pp.64.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Skokut T. A., Stejskal E. O., McKay R. A., Varner J. E. Asparagine amide metabolism in developing cotyledons of soybean. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5978–5982. doi: 10.1073/pnas.78.10.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J., Skokut T. A., Stejskal E. O., McKay R. A., Varner J. E. Estimation of protein turnover in soybean leaves using magic angle double cross-polarization nitrogen 15 nuclear magnetic resonance. J Biol Chem. 1981 Nov 25;256(22):11574–11579. [PubMed] [Google Scholar]
- Skokut T. A., Varner J. E., Schaefer J., Stejskal E. O., McKay R. A. N- and [C]NMR determination of utilization of glycine for synthesis of storage protein in the presence of glutamine in developing cotyledons of soybean. Plant Physiol. 1982 Feb;69(2):314–316. doi: 10.1104/pp.69.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokut T. A., Varner J. E., Schaefer J., Stejskal E. O., McKay R. A. [N]NMR determination of asparagine and glutamine nitrogen utilization for synthesis of storage protein in developing cotyledons of soybean in culture. Plant Physiol. 1982 Feb;69(2):308–313. doi: 10.1104/pp.69.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]


