Abstract
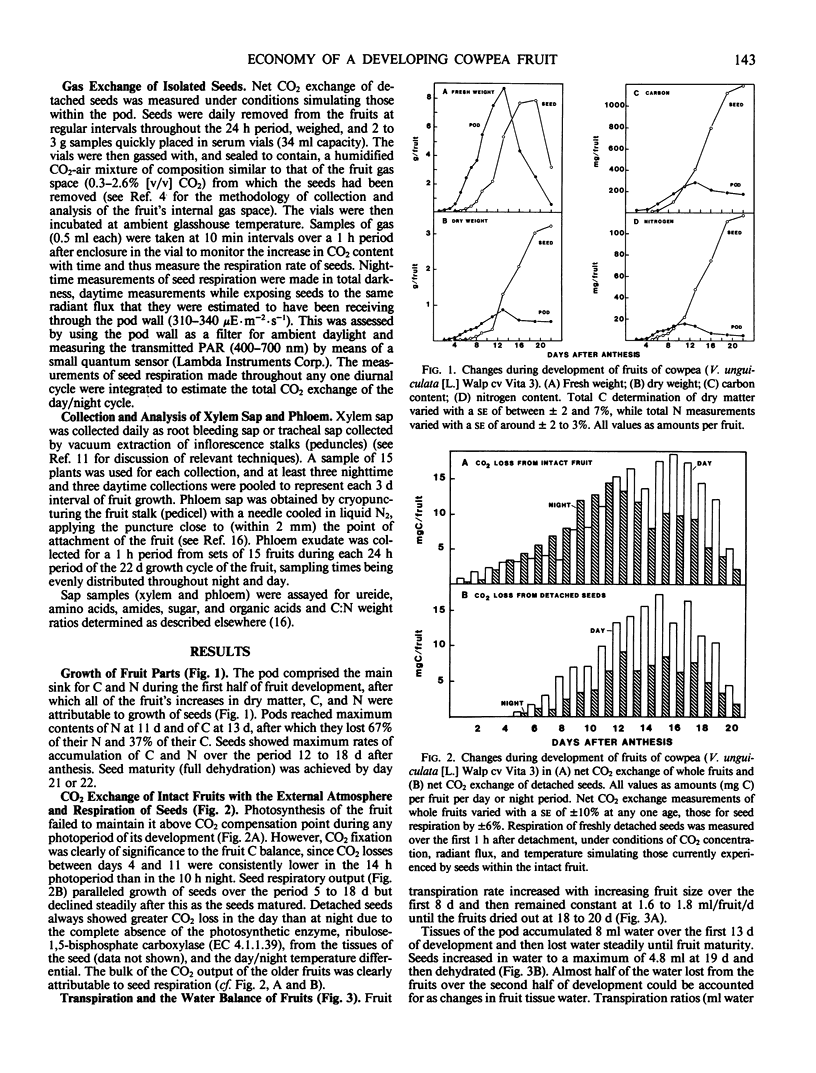
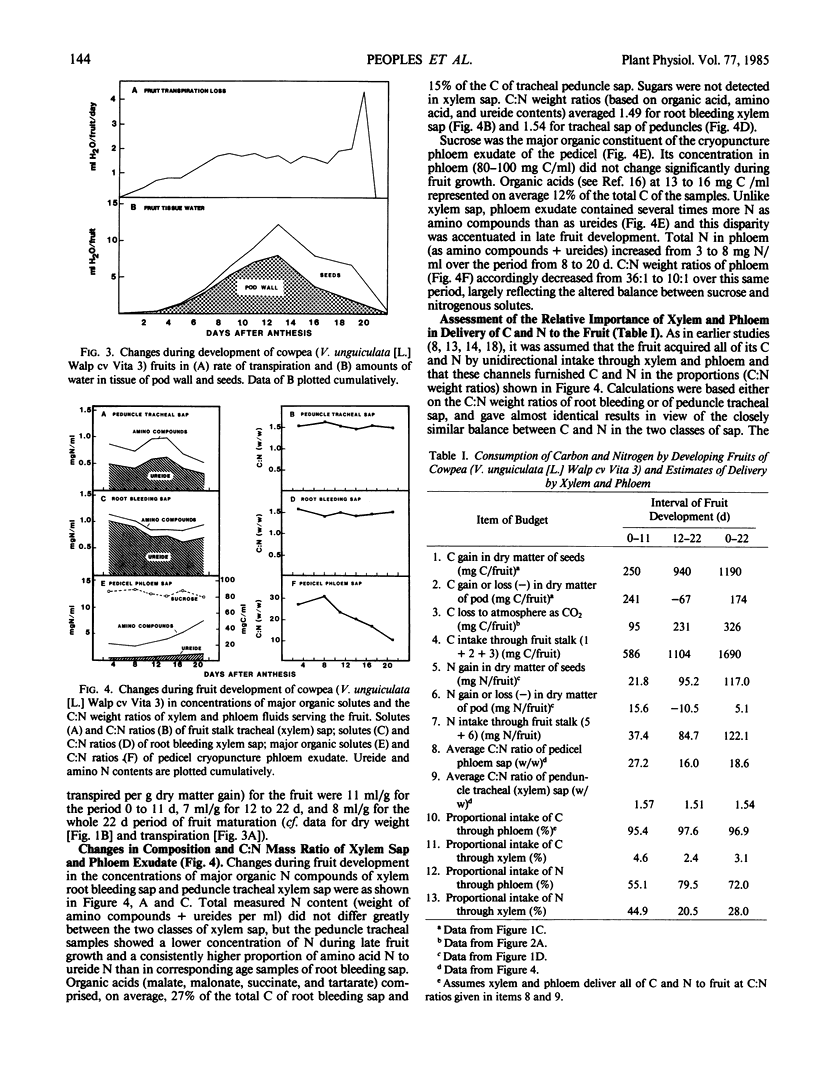
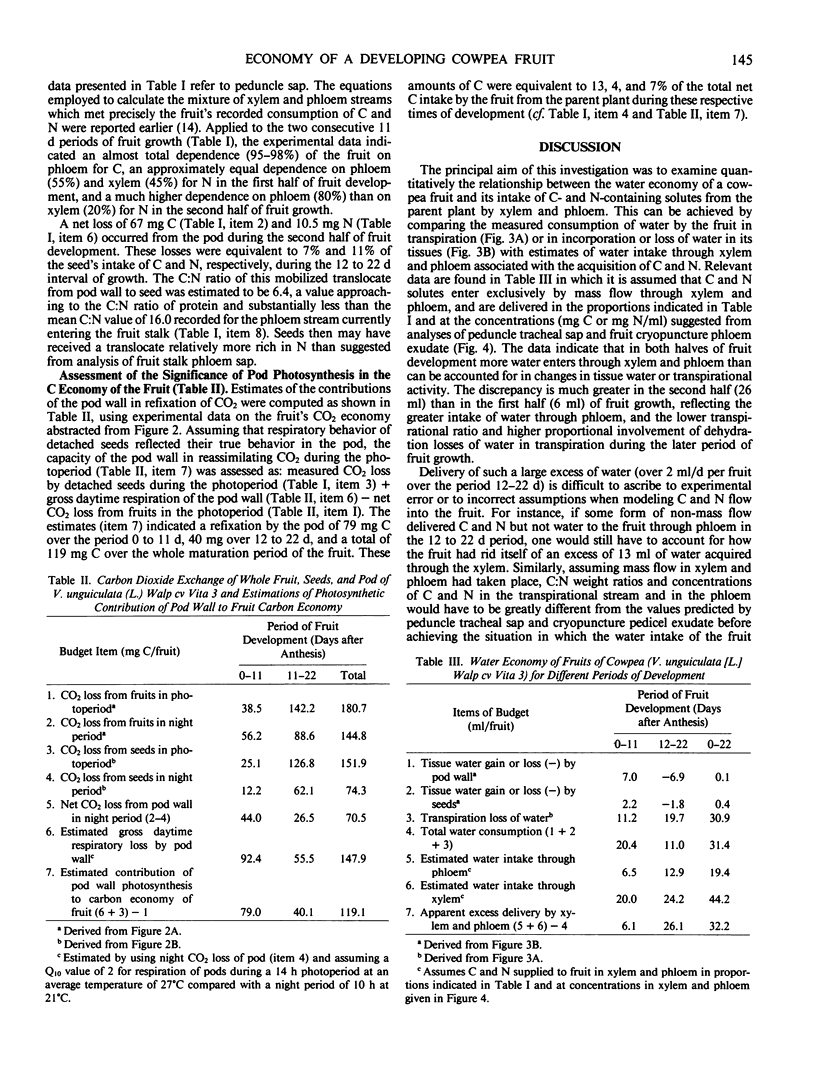
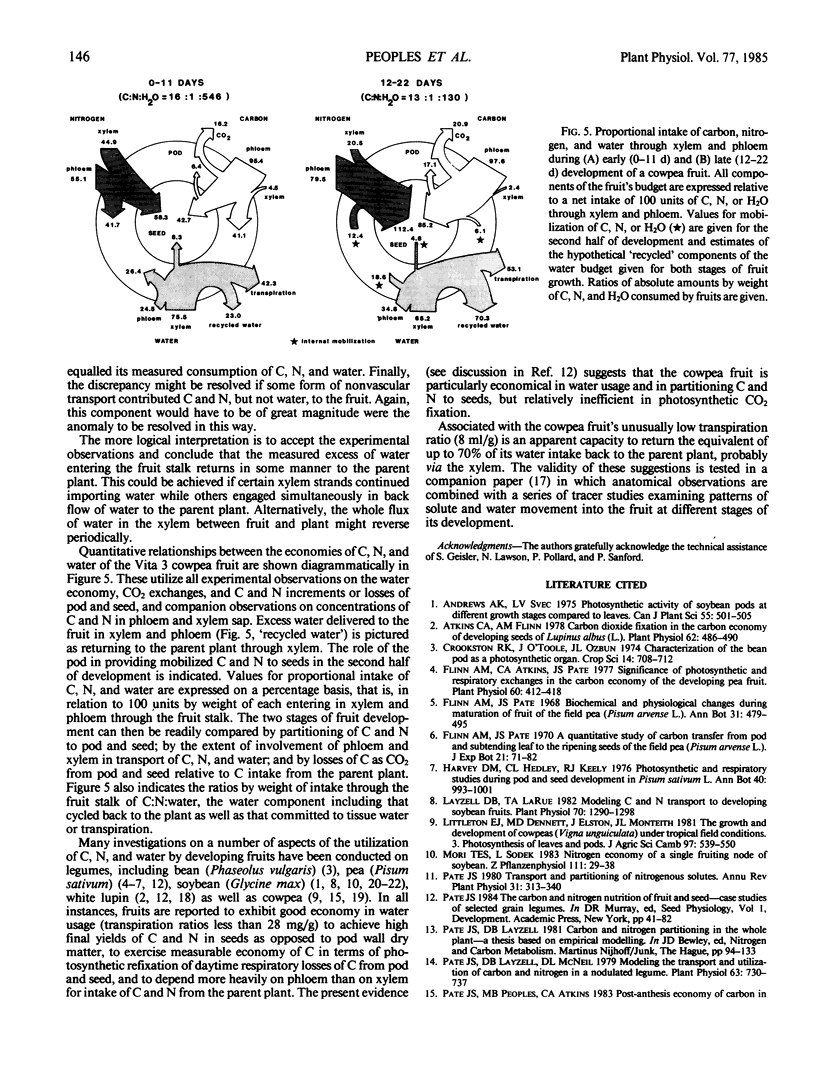
The nutritional economy of the fruit of cowpea (Vigna unguiculata (L.) Walp cv Vita 3) was assessed quantitatively from intake and utilization of carbon, nitrogen, and water. Fruits failed to make net gains of CO2 from the atmosphere during daytime, although pod photosynthesis did play a role in the fruit's carbon economy by refixing a proportion of the fruit's respired CO2. Of every 100 units by weight of carbon entering the fruit, 70.4 were finally incorporated into seeds, 10.3 remained as nonmobilizable material in pod walls, and the remaining 19.3 were lost in fruit respiration. Phloem supplied 97% of the fruit's carbon and 72% of its nitrogen. The xylem contribution of nitrogen occurred mainly in early growth. Ninety-six% of the fruit's nitrogen was incorporated into seeds, approximately 10% of this mobilized from the senescing pod. The mean transpiration ratio of the fruit was very low—8 milliliters water transpired per gram dry matter accumulated. Models of carbon, nitrogen, and water flow were constructed for the two consecutive 11 day periods of fruit development, and indicated a considerably greater entry of water through xylem and phloem than could be accounted for in changes in fruit tissue water and transpiration loss. This discrepancy was greater in the second half of fruit growth and was interpreted as evidence that a significant fraction of the water entering the fruit through phloem cycled back to the parent plant via the xylem.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A. Carbon Dioxide Fixation in the Carbon Economy of Developing Seeds of Lupinus albus (L.). Plant Physiol. 1978 Oct;62(4):486–490. doi: 10.1104/pp.62.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn A. M., Atkins C. A., Pate J. S. Significance of photosynthetic and respiratory exchanges in the carbon economy of the developing pea fruit. Plant Physiol. 1977 Sep;60(3):412–418. doi: 10.1104/pp.60.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., McNeil D. L. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume. Plant Physiol. 1979 Apr;63(4):730–737. doi: 10.1104/pp.63.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Peoples M. B., Atkins C. A. Spontaneous Phloem bleeding from cryopunctured fruits of a ureide-producing legume. Plant Physiol. 1984 Mar;74(3):499–505. doi: 10.1104/pp.74.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Peoples M. B., van Bel A. J., Kuo J., Atkins C. A. Diurnal water balance of the cowpea fruit. Plant Physiol. 1985 Jan;77(1):148–156. doi: 10.1104/pp.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]


