Abstract
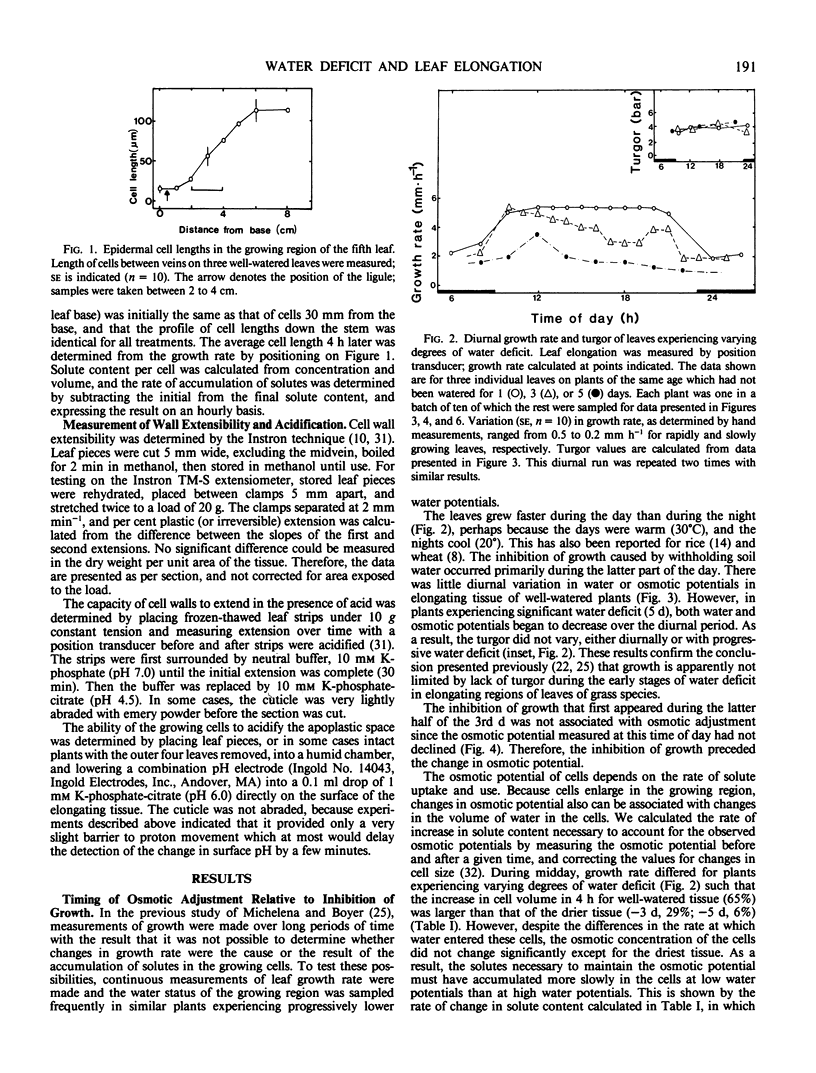
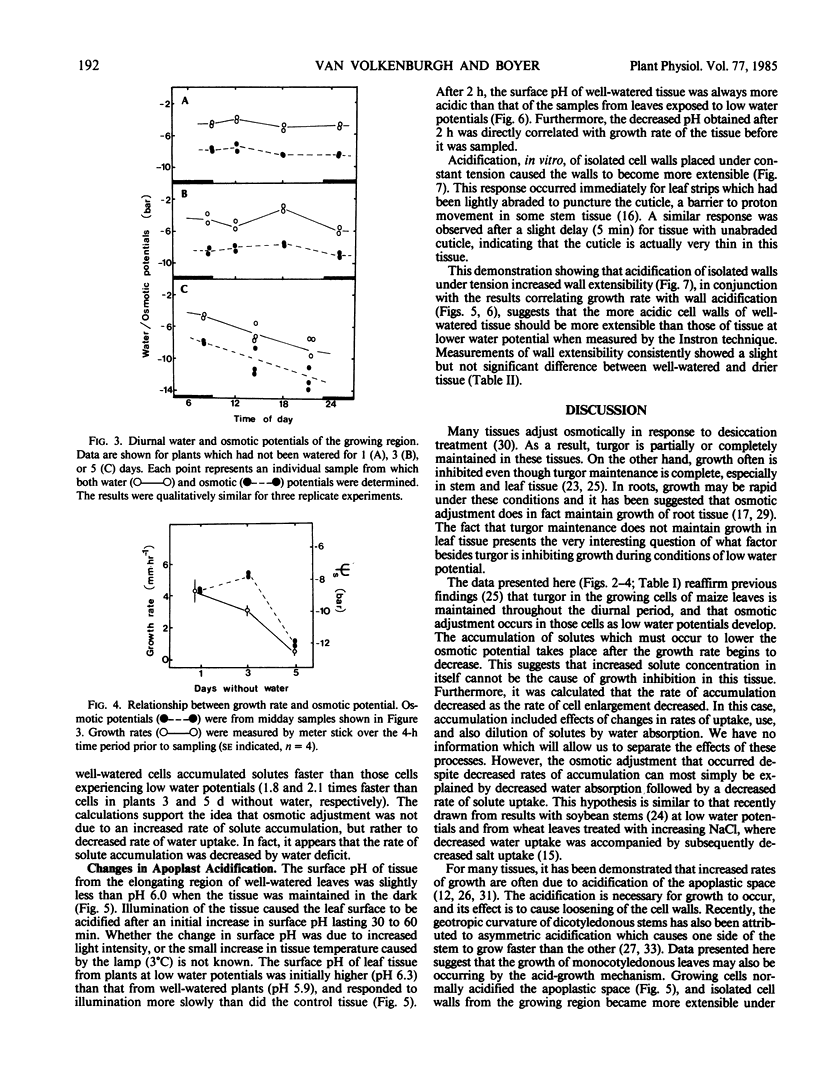
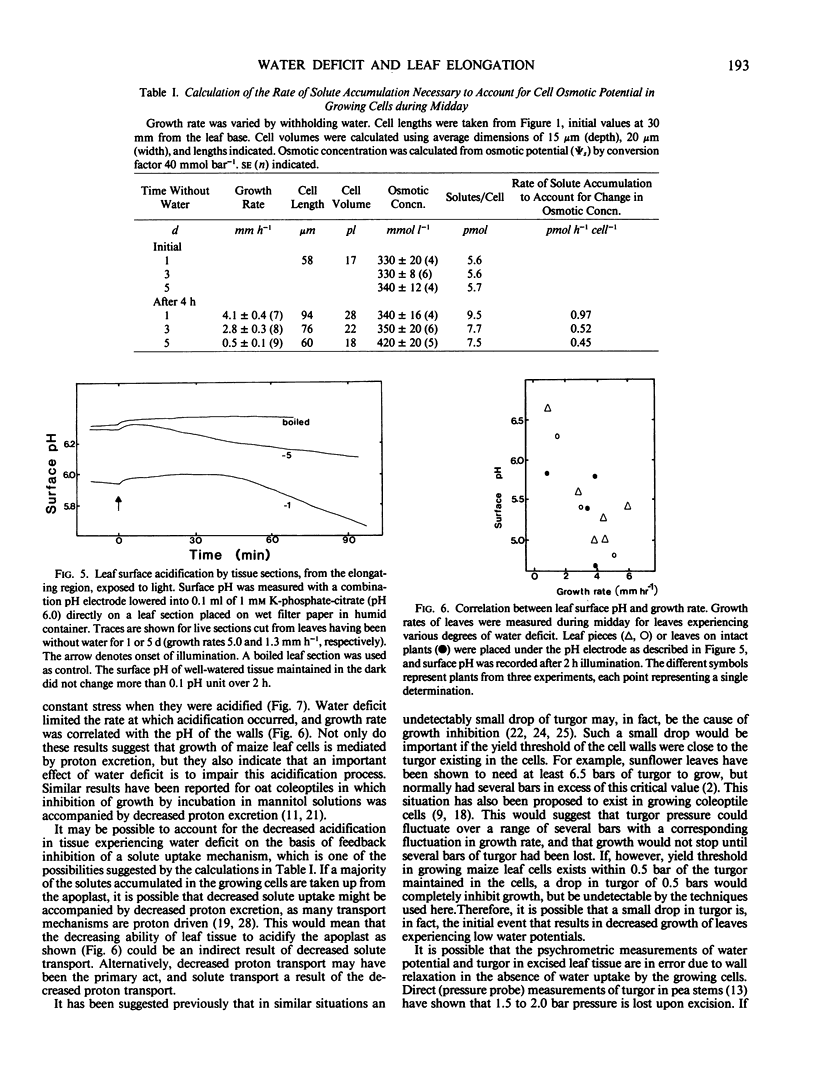
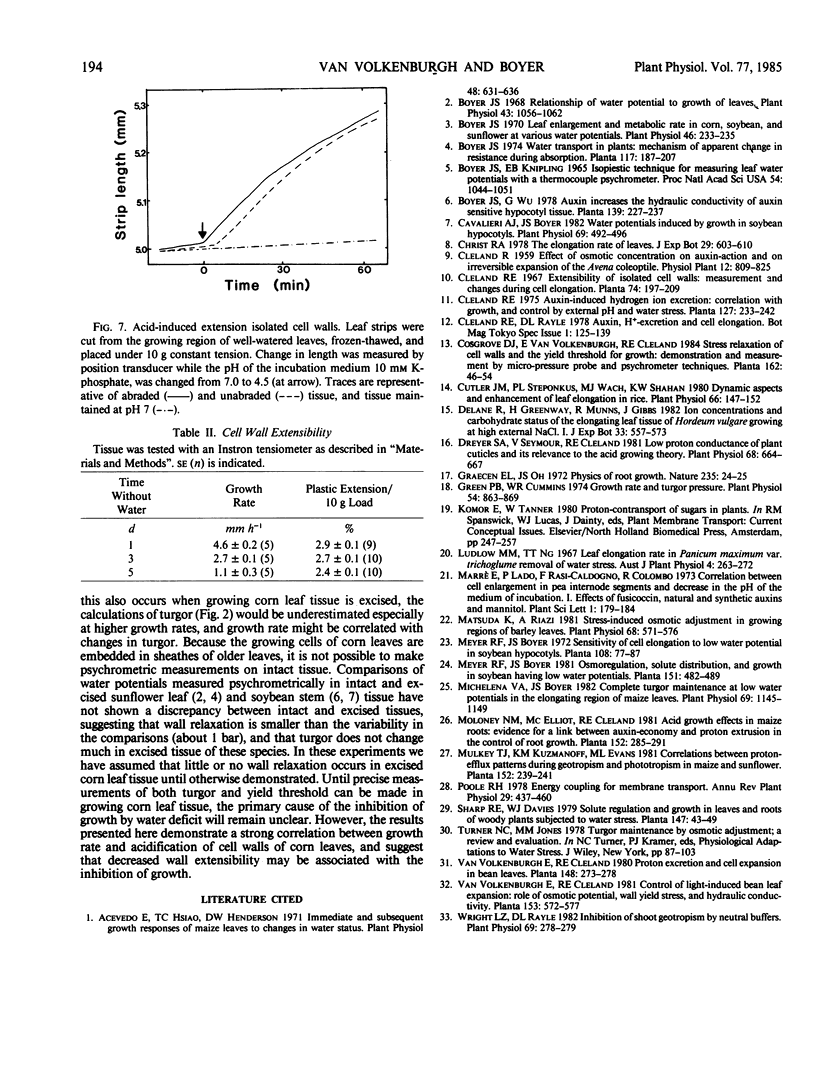
The growth rate of maize leaves has been investigated for plants grown in pots in controlled conditions and subjected to slow drying over a period of days. The elongation of leaves experiencing water deficit was inhibited primaily during the afternoon. No decrease in the turgor of the growing cells could be detected at that time. Solute concentration in the growing cells increased in tissue experiencing water deficit, but this was shown to occur after the growth rate had fallen. Calculations of the rate of solute accumulation necessary to maintain these concentrations indicated that the rate was less in slowly growing than in rapidly growing cells. The growing tissue of well-watered leaves excreted protons into the apoplastic space, but this acidification decreased in tissue exposed to water deficit. The pH of the apoplastic space correlated with the growth rate of the tissue. In vitro acidification of isolated, frozen-thawed tissue, maintained under constant tension, increased wall extensibility. The results suggest that one role of proton excretion may be to promote wall-loosening events necessary for cell enlargement, and that inhibition of this process may have reduced growth rate in leaves exposed to water deficit.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970 Aug;46(2):233–235. doi: 10.1104/pp.46.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Van Volkenburgh E., Cleland R. E. Stress relaxation of cell walls and the yield threshold for growth: demonstration and measurement by micro-pressure probe and psychrometer techniques. Planta. 1984;162(1):46–54. doi: 10.1007/BF00397420. [DOI] [PubMed] [Google Scholar]
- Cutler J. M., Steponkus P. L., Wach M. J., Shahan K. W. Dynamic aspects and enhancement of leaf elongation in rice. Plant Physiol. 1980 Jul;66(1):147–152. doi: 10.1104/pp.66.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer S. A., Seymour V., Cleland R. E. Low proton conductance of plant cuticles and its relevance to the Acid-growth theory. Plant Physiol. 1981 Sep;68(3):664–667. doi: 10.1104/pp.68.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greacen E. L., Oh J. S. Physics of root growth. Nat New Biol. 1972 Jan 5;235(53):24–25. doi: 10.1038/newbio235024a0. [DOI] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Riazi A. Stress-induced osmotic adjustment in growing regions of barley leaves. Plant Physiol. 1981 Sep;68(3):571–576. doi: 10.1104/pp.68.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena V. A., Boyer J. S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982 May;69(5):1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Wright L. Z., Rayle D. L. Inhibition of shoot geotropism by neutral buffers. Plant Physiol. 1982 Jan;69(1):278–279. doi: 10.1104/pp.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]


