SUMMARY
One of the main goals of the 2016 Global Health Sector Strategy on viral hepatitis is the elimination of hepatitis C virus (HCV) as a public health problem by 2030, defined as an 80% reduction in incidence and 65% reduction in mortality relative to 2015. Although monitoring HCV incidence is key to validating HCV elimination, it can be resource-intensive for countries to measure this metric using the gold-standard method, which involves prospective HCV re-testing of people at risk. Additionally, few countries have collected quality data in 2015 to document an 80% decrease by 2030. In this paper, we first review different methods by which HCV incidence can be monitored and discuss their resource implications and applicability to various populations. Second, using mathematical models developed for various global settings, we assess whether trends in HCV chronic or antibody prevalence or scale-up levels for HCV testing, treatment and preventative interventions can be used as reliable alternative indicators to validate the HCV incidence target. Third, we discuss the advantages and disadvantages of an absolute HCV incidence target and suggest a suitable threshold. We then propose three options that countries can use to validate the HCV incidence target, depending on the available surveillance infrastructure.
INTRODUCTION
In 2016, the World Health Assembly adopted the Global Health Sector Strategy (GHSS) on viral hepatitis[1]. One of the main goals of the strategy is the elimination of chronic hepatitis C virus (HCV) infection as a public health problem by 2030, defined as an 80% reduction in incidence and 65% reduction in mortality relative to 2015[1]. These targets were based on mathematical modelling conducted by our groups, which suggested that achievable scale-up of HCV treatment and preventative interventions could deliver this level of impact over 15-years[2, 3]. While progress has been slow and uneven across countries, as of 2019, 9.4 million people had received HCV treatment compared to 5 million in 2015[4]. Some champion countries have made considerable strides and have requested guidance from the World Health Organization (WHO) on the process and criteria needed to validate that they have reached the elimination targets[5]. In this context and considering the substantial country-level variation in the level of HCV burden, epidemic dynamics and resources, having a choice of options to validate the HCV targets is important.
Although HCV incidence is one of the key indicators for validating elimination of HCV infection as a public health problem, it can be difficult to measure reliably. The gold-standard method involves ascertaining new HCV cases prospectively among individuals at risk of infection who are followed over time. However, this approach is not efficient for measuring rare outcomes like population-level HCV incidence, as it can require large sample sizes. Alternative methods for monitoring HCV incidence, some of which build on methods developed to estimate HIV incidence[6–8], leverage cross-sectional or routinely-collected data and have been adopted in some settings[9–13]. Some are more feasible in certain countries than others, depending on the available surveillance infrastructure and the populations needing to be monitored, which can include the general population, sub-populations at high risk of infection (e.g., people who inject drugs (PWID), men who have sex with men (MSM)) or both. Yet, their strengths, limitations and applicability to the validation of the HCV incidence target have not been previously reviewed.
Even with a choice of methods, some countries will still find it logistically difficult to undertake large-scale studies to accurately monitor HCV incidence at the national level. The extent to which other HCV-specific indicators, which may be more easily measured, could be used as reliable alternatives to validate whether the HCV incidence target has been achieved has not been previously explored. Mathematical modelling conducted by our groups has been used to project future trends in chronic HCV prevalence and incidence following the scale-up of HCV treatment, and in some cases, HCV preventative measures, in a broad range of settings globally[14–27]. These models can be extended to examine how well trends in chronic HCV prevalence and coverage levels for programmatic indicators of HCV testing, treatment and preventative interventions track HCV incidence.
In addition to the challenges of measuring HCV incidence, few countries have collected 2015—typically referred to as “baseline”—incidence[28], which is required to document a relative decrease by 2030. Having the option to validate HCV elimination against an universal threshold to be met by all countries in 2030 (herein, referred to as an absolute incidence target), would obviate the need for baseline data.
In this Health Policy paper, we (i) review methods by which HCV incidence can be monitored and discuss their applicability in different contexts, (ii) assess the extent to which certain HCV-specific indicators track HCV incidence using mathematical modelling, and (iii) discuss the advantages and disadvantages of an absolute HCV incidence target compared to the current relative target, and suggest a suitable threshold. We then recommend several options that countries could use to validate the HCV incidence target. These recommendations have informed the interim framework recently developed by WHO[29, 30], which provides guidance on country validation of elimination of chronic HCV and hepatitis B virus infection as public health problems.
METHODS TO MONITOR HCV INCIDENCE
HCV incidence can be monitored using direct approaches through measuring new HCV infections (methods 1–3) or estimating recent infections (method 4), or indirectly through estimates derived from HCV antibody prevalence (methods 5–6). One additional method, which is based on surveillance of acute HCV infection (method 7), can help infer trends in incidence over time but can rarely be used to estimate HCV incidence. Below, we present an overview of each method, highlighting examples of previous applications and their potential for the monitoring of HCV incidence. The strengths and limitations associated with each method are presented in Table 1.
Table 1:
Strengths and limitations of methods to monitor hepatitis C virus (HCV) incidence
| METHOD | STRENGTHS | LIMITATIONS |
|---|---|---|
| Measurement based on prospective HCV re-testing of people at risk | • Can be used to estimate primary HCV infection or HCV reinfection • Systematic data collection procedures can be implemented to maximise data quality and participant retention in follow-up • Offers scope for adopting different sampling methods to recruit a nationally representative sample |
• Not an efficient study design for rare outcomes: sample size requirements could be very high if incidence is low • May not well represent true HCV incidence due to losses to follow-up and changes in behaviour due to risk reduction counselling • Expensive if HCV RNA testing is performed to capture reinfections • Resource intensive given the need to track individuals over time |
| Measurement based on retrospectively-collected HCV re-testing data | • Can be used to estimate primary HCV infection or HCV reinfection • Is an efficient study design for rare outcomes like HCV acquisition because it is relatively easy and inexpensive to obtain large sample sizes by capitalising on existing data |
• Limited application, since few population groups receive routine HCV testing • May not well represent true HCV incidence because there is no standardised data collection protocol (e.g., HCV testing interval may be too wide and variable) and participants receive risk reduction counselling • May not be generalizable to target population since method is based on convenience sample of individuals in contact with healthcare services and re-attending for care |
| Measurement based on a linked repeated cross-sectional study | • Can be used to estimate primary HCV infection or HCV reinfection • Can capitalise on cross-sectional surveys, which may be already ongoing or are generally easier to implement relative to cohort studies • Offers scope for adopting different sampling methods to recruit a nationally representative sample |
• May not well represent true incidence if participants captured in multiple survey rounds are systematically different to those who are not • Not an efficient study design for rare outcomes like HCV acquisition: sample size requirements could be very high if incidence is low • May lead to statistically imprecise estimates if too few participants are linked over time • Can be difficult to implement if survey participation is kept anonymous although methods can be used to link participants even with anonymised data • Expensive if HCV RNA testing is performed to capture reinfections |
| Estimation based on tests for recent HCV infection* | • Faster because a single sample derived from one survey is needed • Limitations typically related to longitudinal follow-up (i.e., bias resulting from participant attrition and changes in behaviour due to risk reduction counselling) are circumvented • Can capitalise on cross-sectional surveys, which may be already ongoing or are generally easier to implement • Offers scope for adopting different sampling methods to have a nationally representative sample |
• Only been used to estimate primary HCV incidence; unclear whether the antibody-avidity assay can be adapted to capture HCV reinfection • Unless assay is adequately calibrated to target population, it may over-estimate true primary HCV incidence due to potential for misclassification of non-recent infection as recent • Requires large sample sizes because the average duration of the recent infection state is short—not feasible if incidence is low and, for the viraemic pre-seroconversion assay, if the antibody prevalence is too high • Expensive because it requires HCV RNA testing • Requires elaborate laboratory infrastructure (at least for the antibody-avidity assay) |
| Estimation based on HCV antibody prevalence and duration of risk behaviour | • Lower cost and faster because a one-time measurement and a single test (HCV antibody) is needed • Limitations relating to longitudinal follow-up (i.e., losses to follow-up, changes in behaviour due to risk reduction counselling) are circumvented • Offers scope for adopting different sampling methods to have a nationally representative sample • Can capitalise on cross-sectional surveys, which may be already ongoing or are generally easier to implement |
• Cannot be used to estimate overall incidence, as these methods typically focus on people with recent onset of risk behaviour (e.g., people who have recently started injecting), who often have a higher risk of HCV infection than everyone else • Multiple simplifying assumptions are made, which could lead to biased estimates if they do not hold (e.g., HCV acquisition should not have occurred prior to initiation of injecting, HCV acquisition occurred at the midpoint between the date of initiation of injecting and date of the survey, HCV incidence has remained stable over time) • Use of self-reported data to define duration of risk behaviour could introduce errors, leading to a mis-estimation of incidence • Restricting the sample to individuals with a recent onset of risk behaviour can considerably reduce the statistical precision of estimates |
| Estimation based on serial measurements of HCV antibody prevalence | • Limitations relating to longitudinal follow-up (i.e., losses to follow-up, changes in behaviour due to risk reduction counselling) are circumvented • Offers scope for adopting different sampling methods to have a nationally representative sample • Can capitalise on cross-sectional surveys, which may be already ongoing (e.g., for HIV surveillance) or are generally easier to implement |
• Requires two consecutive survey measurements of HCV antibody prevalence to estimate HCV incidence and ≥three time points to monitor trends, thus could be expensive • Other relevant data (e.g., HCV-related mortality, level of in- and out-migration by HCV status) may not be available or be of poor quality which could introduce bias and/or uncertainty in the estimation • Large-scale studies can only be conducted once every couple of years, therefore, this method may not capture current trends in HCV incidence • Assumes a similar recruitment scheme and participant response by age groups between survey rounds • Requires large sample sizes • Requires additional data to capture HCV reinfection (e.g., HCV RNA, number of people who were treated and cured for different age groups) |
| Estimation of relative HCV incidence trends based on surveillance of acute HCV infection | • Relatively easy and inexpensive to implement on a large scale by capitalising on existing data | • Applicability of this method is limited since not many countries perform serological testing to distinguish between acute HCV and other types of viral hepatitis • It does not provide an estimate of HCV incidence because few participants with acute HCV seek testing, case ascertainment is prone to misclassification, and clinician reporting and data capture may be incomplete • Trends over time could mis-estimate trends in HCV incidence if the case definition of acute HCV, testing patterns or the reporting system have changed over time |
Strengths and limitations apply to both assays classified as tests of recent infection (i.e., viraemic pre-seroconversion assay and antibody avidity assay), unless indicated otherwise
1. Measurement based on prospective HCV re-testing of people at risk.
This method, considered the gold-standard for measuring incidence, consists of ascertaining new HCV infection cases among susceptible (i.e., HCV antibody or HCV RNA negative) individuals who are followed prospectively and receive repeat HCV testing at regular intervals. Although it has been one of the most widely used methods, prospective cohort studies have generally been established for local research purposes rather than for nationwide surveillance. These have been conducted among high-risk groups, such as PWID[31, 32], MSM[33, 34] and prisoners[35, 36], in large urban cities of high-income countries, such as the US, Canada and the UK. In the general population, Egypt is one of the few countries that implemented prospective cohort studies to measure HCV incidence[37]. A recent study used a large community-based test-and-treat programme across 73 villages in the Nile Delta region to assess impact on HCV incidence by re-testing individuals who initially tested negative for HCV antibody infection[5]. This approach now forms the basis of a national assessment of HCV incidence in Egypt. Since developing a nationwide prospective cohort study for the sole purpose of measuring HCV incidence is resource-intensive, and so not a realistic option for monitoring levels in the general population, the approach undertaken in Egypt may represent a practical alternative[5]. As countries expand HCV testing and treatment programs, they could develop an embedded HCV surveillance system, whereby individuals who initially test HCV antibody negative are tracked and re-tested.
2. Measurement based on retrospectively-collected HCV re-testing data.
This method consists of using routinely-collected health data to ascertain new HCV infection cases among susceptible individuals who receive multiple HCV tests over time as part of routine care. Aside from a few studies conducted in PWID[38, 39] and blood donors[40, 41], these cohorts have typically been used to measure HCV incidence among HIV-positive, and increasingly, HIV-negative MSM in care, since they are one of the few populations receiving routine HCV testing[33, 34, 42, 43]. As with prospective cohorts, these studies have been carried out primarily in high-income settings. Recently, this method was adopted to assess progress towards HCV elimination among HIV-positive MSM in two cities in the UK[42]. Routine health data could also be used to monitor HCV incidence as part of a prospective study, as done in Netherlands[44], which offers scope for standardising testing procedures and data collection across individuals, thereby minimising some of the limitations associated with retrospectively-collected data. Although this method can be easily expanded to other populations (e.g., PWID attending a harm reduction programme, individuals on prison entry), it is dependent on availability of regular routine testing and data collection in defined cohorts and could frequently be biased towards individuals engaged in care.
3. Measurement based on linked repeated cross-sectional surveys.
In contrast to cohort studies, repeat cross-sectional surveys recruit a new sample of participants with each round. Yet, if some participants appear in multiple rounds and individual-level data can be linked over time, then these surveys can be used to measure HCV incidence as for a cohort study. This method has been used to estimate HCV incidence primarily among PWID in settings like Canada[45], Australia[10], and Greece (ARISTOTLE cohort—unpublished data). While HCV antibody or HCV RNA data collected through repeated cross-sectional surveys can be easily leveraged to estimate HCV incidence, in settings or populations with low baseline incidence or large populations, very large sample sizes would be required, as a low proportion of individuals typically participate in multiple survey rounds. This method is, therefore, likely to be primarily applicable to populations at high risk of infection (e.g., PWID, MSM).
4. Estimation based on tests for recent HCV infection.
This method, which builds on approaches developed in the HIV field[7, 8], consists of estimating recent HCV infection through using a combination of measurable biomarkers, derived from a single sample, which change in a predictable manner following infection[46]. Two assays have been proposed. The first one is based on detecting the RNA-only phase of HCV infection, when acutely-infected individuals have high levels of HCV plasma viremia prior to antibody seroconversion[47]. The second consists of estimating an avidity index based on the binding capacity of HCV antibody to HCV antigen[46]. Several studies, conducted in various settings, have demonstrated the utility of these assays—when used separately or in combination—for the estimation of HCV incidence among PWID[9, 47–51]. Although tests for recent infection have been used to estimate HCV incidence in other groups, like blood donors and military veterans[47, 51], the applicability of these assays to populations with low HCV incidence is limited. Given that the duration for remaining in a recent infection state is brief (~51–75 and ~60–180 days for the first and second approach, respectively[9, 47]), very large sample sizes are required to estimate HCV incidence with precision especially as countries approach GHSS targets[49]. Although such approaches represent an attractive option for populations who have high HCV incidence because it obviates the need for longitudinal follow-up, they have not yet gained traction for monitoring HCV incidence. With both approaches, there is variability and uncertainty in the duration of the recent infection state and in the probability of misclassifying non-recent infection as recent across distinct populations, which can lead to biased incidence estimates[9, 47–51]. To minimise this bias, both assays require calibration to the study population, which ideally would use a panel of HCV-infected individuals with a known seroconversion date[47–49]. To enable application of this method at scale, the applicability of different assays in distinct populations and epidemiological contexts needs to be evaluated.
5. Estimation based on HCV antibody prevalence and duration of risk behaviour.
Three relatively similar approaches have been used to estimate HCV incidence based on HCV antibody prevalence specifically among PWID. With all three, the duration of injection drug use was used to infer time-at-risk. The first approach consists of using HCV antibody prevalence among PWID who have initiated injection drug use recently (e.g., past two-five years, herein referred to as recent PWID) as a crude proxy of cumulative HCV incidence in recent years. The second approach involves estimating HCV incidence among recent PWID by assuming the date of HCV seroconversion to have occurred at the midpoint between the date of initiation of injecting and the survey date. The third approach consists of estimating the force of infection—which is closely related to and can be expressed as the HCV incidence rate—using various parametric or non-parametric methods. The first approach has been used to monitor HCV incidence trends over time among PWID in several countries in Europe by national and international public health agencies, such as Public Health England[52] and the European Monitoring Centre for Drugs and Drug Addiction[53]. The other two have been used in the US[11], Kenya[54] and in several countries across Europe, such as the UK and Russia[13, 55, 56]. As with method 4, this method consists of using data collected cross-sectionally, though it only requires HCV antibody prevalence and not HCV RNA, which makes it easier to implement. The same approaches can be used to estimate HCV incidence among MSM and other groups using the date of first risk behaviour, as has been done for HIV[57].
6. Estimation based on serial measurements of HCV antibody prevalence.
Referred to as the “demographic method”, this method was originally developed and used to estimate population-level HIV incidence[6, 58]. Although this method has not yet been applied to estimate HCV incidence, the underlying principle would be similar. Age-stratified HCV antibody prevalence data, collected at two consecutive time points, could be used to estimate a measure of cumulative HCV incidence for the inter-survey period, after accounting for HCV-related mortality and potentially other demographic changes (e.g., in- and out-migration by HCV status). HCV RNA could also be used instead of HCV antibody, but this would require additional data for the inter-survey period, such as the number of people treated for different age groups. The feasibility and reliability of this method in estimating HCV incidence needs to be examined, as it could be an attractive option, particularly for countries which have implemented population-based surveys with measurement of HCV antibody or HCV RNA. So far, two countries – Egypt[59] and Liberia – have integrated testing for HCV antibody and HCV RNA within their Demographic Health Survey (DHS), whereas others, like Cameroon[60], Burkina Faso[61] and the Democratic Republic of the Congo[62], have tested retrospectively dried blood spot specimens collected through these same surveys. Similarly, Rwanda[63], Nigeria[64] and Tanzania[65] have integrated HCV antibody or HCV RNA testing within their Population-based HIV Impact Assessment surveys (PHIA).
7. Estimation of relative HCV incidence trends based on surveillance of acute HCV infection.
This method consists of using the number of reported cases of acute HCV infection, based on routine notification by clinicians, laboratories or sentinel surveillance, to infer trends in HCV incidence. It has been used in several settings, including the US[12], several countries in Europe and across the European Union[66, 67], Egypt[68] and Taiwan[69]. Given the considerable level of under-reporting of acute HCV infection in most settings, it is unlikely that HCV incidence can be estimated unless this fraction can be corrected for, as done in the US[70]. However, if case definitions, testing and reporting patterns have remained consistent over time, capturing a constant fraction of acute HCV cases may still be useful for monitoring trends over time, although such circumstances are unlikely in most settings, especially if HCV testing is scaledup. Therefore, this method may have limited utility for the monitoring of HCV incidence on its own, particularly given that many countries do not even have a national surveillance system for acute HCV[71].
Key considerations:
Method 1 is the preferred option for monitoring HCV incidence because it offers a direct measurement of incidence rather than an estimate and offers scope for adopting strategies to minimise bias (e.g., systematic re-testing at regular intervals). However, one of the main challenges associated with this method is that it is resource-intensive and will become difficult to implement and sustain as incidence falls even in populations with higher baseline HCV incidence, such as PWID and MSM. Furthermore, with all methods, it can be challenging to recruit a nationally representative sample particularly for populations like PWID, since there is no sampling frame. Sampling techniques developed specifically for these groups (e.g., respondent-driven sampling) should be prioritised if possible, whereas for methods that rely on existing or routine data (e.g., 2 and 7), findings may have to be triangulated with those derived through other methods. Lastly, methods 4–6 can only capture primary HCV infection and will become unreliable for an overall assessment of HCV incidence as reinfection increases following the scale-up of HCV treatment, unless they can be revised and adapted (e.g., use HCV RNA instead of HCV antibody for method 6). While measuring primary HCV infection may be sufficient to monitor incidence in the general population, it will be important to also track HCV reinfection in high-risk groups.
ALTERNATIVE INDICATORS FOR VALIDATING THE HCV INCIDENCE TARGET
We explored four indicators that could be used to infer whether a setting has reached the HCV incidence target. The modelling methods that informed this assessment are summarised in Panel 1 and described more fully in the supplementary materials. Findings are summarised in Table 2, where we also outline the minimum data needs and possible data sources for the two indicators found to be useful for the validation of the HCV incidence target.
Panel 1: Summary of modelling methods
We used projections from 17 dynamic HCV transmission models[14–27] to examine whether four different indicators could be used to validate the relative HCV incidence target, which was defined as an 80% or 90% decrease in HCV incidence since baseline, depending on the study. We also considered if an absolute prevalence indicator could be used to validate an absolute incidence target. We firstly used up to 17 models (number used depends on the indicator) to examine the relationship between HCV incidence and (i) chronic HCV prevalence, (ii) HCV antibody prevalence, (iii) scale-up level of HCV treatment and (iv) scale-up level of HCV screening, each for when the HCV incidence target has only been achieved through scaling-up HCV treatment. Second, and based on up to 10 models that also included HCV preventative interventions, we examined these same relationships when the HCV incidence target has been achieved through scaling-up both HCV treatment and HCV preventative interventions. This second step was done to assess whether the scale-up of HCV preventative interventions moderates the relationship between the four indicators and HCV incidence based on HCV treatment scale-up only.
Models were conducted among PWID[17, 18, 21, 23, 27] (n=9), MSM[19] (n=1) and the general population[14, 15, 20, 22, 24–26] (n=7) with all models simulating the prevention benefits of HCV treatment in those populations. The general population models also included PWID or a generic ‘high-risk’ sub-group and accounted for their contribution to HCV transmission, with all models assuming universal HCV treatment access for all modelled sub-groups. Modelled settings included: stable or expanding populations; stable or changing HCV epidemics; and settings with different baseline burdens of chronic HCV. The elimination time frame also had a variable starting point, ranging from 2015 to 2021, although the endpoint was consistently 2030. Given these differences across models, we were able to speculate on the influence of different factors on the relationship between each indicator and HCV incidence. We were only able to conduct stratified analyses for the effect of preventative interventions on chronic prevalence because otherwise the number of models was too small.
Panel 2: Advantages and disadvantages of an absolute compared to a relative HCV incidence target
| Advantages | Disadvantages |
|---|---|
| • Obviates the need to collect HCV incidence data at baseline • Benefits countries which already have low baseline HCV incidence, as it prevents need for further reduction • Directs global efforts towards countries with high baseline HCV incidence, and thus, higher need for intervention • Sets a universal threshold below which the rate of HCV transmission can be considered negligible, independent of setting |
• Penalises countries with high baseline HCV incidence, as these would need to achieve greater reductions than if a relative target was used • Does not provide information on the past trajectory of HCV incidence |
Panel 3: Populations to be monitored to validate the HCV incidence target
Given that population-based studies are unlikely to capture representative samples of people who inject drugs (PWID), men who have sex with men (MSM), homeless individuals, prisoners and other populations at high risk of HCV acquisition, dedicated resources are needed to estimate HCV incidence in these groups. In some countries, most new HCV infection cases are concentrated in these groups, making them the primary target group for monitoring HCV incidence and for achieving HCV elimination. In these settings, general population-level incidence data may not be necessary. PWID are one of the most highly affected groups by infection with HCV and, according to modelling analyses conducted by our group[25], contribute 43% of all new HCV transmission globally. Furthermore, in 88% of countries globally, ≥20% of new HCV infection cases occur among PWID (Supplementary Table 3), suggesting that HCV incidence in this group should be monitored in nearly all settings in order to document the incidence elimination target, unless supportive data indicates otherwise. Overall, the distribution of HCV transmission across different populations in a country is expected to vary, and so it is crucial that all countries have a good understanding of which populations are contributing sufficiently to HCV transmission to ensure that they are captured in the monitoring and validation process.
Table 2:
Summary of using alternative indicators for monitoring decreases in hepatitis C virus (HCV) incidence
| Alternative indicator | Relationship with HCV incidence | Factors that can affect relationship with HCV incidence | Minimum country-level data needed and their data sources (only included for useable indicators 1 and 3) |
|---|---|---|---|
| 1. Trends in chronic HCV prevalence | Tracks trends in HCV incidence well in different settings and populations | • Prevention intervention scale-up and other factors directly affecting HCV incidence • Population heterogeneity in risk and targeting of HCV treatment Note: numerous other factors did not affect the relationship* |
Trends in chronic HCV prevalence at baseline and endpoint of HCV elimination initiative for all population groups contributing significantly to HCV transmission Data Sources Gold standard for general population is population-based surveys such as the Demographic Health Survey (DHS) or the HIV Impact Assessment surveys (PHIA). Other routine testing data, such as among pregnant women or blood donors, may be useable but likely to have biases. Gold standard for populations at high risk of infection (e.g., PWID, MSM, prisoners) are national bio-behavioural surveys (already done for HIV in many settings). Routine testing within services or institutions such as harm reduction programs, sexual health clinics or prisons may be useable but likely to have biases. |
|
| |||
| 2. Trends in HCV antibody prevalence | Does not track HCV incidence well: relationship is highly variable, even among young or recent PWID | • Prevention intervention scale-up • Population turnover • Population heterogeneity in risk and targeting of HCV treatment |
N/A |
|
| |||
| 3. Scale-up levels of HCV interventions | Tracks trends in HCV incidence well but no universal target can be set; country-specific modelling is needed | • Prevention intervention scale-up • Population growth • Underlying epidemic dynamics • Elimination time frame • Population heterogeneity in risk and targeting of HCV treatment |
The following data are needed for all population groups that are contributing significantly to HCV transmission: • Baseline chronic or antibody HCV prevalence and historic trends in prevalence Data Sources: similar as for #1 • Scale-up levels of HCV treatment and HCV preventative interventions Data Sources: administrative data (e.g., health records, prescription registries), programmatic data (e.g., data collected as part of harm reduction programs or community clinics) and self-reported data captured through population-based surveys • The effect of HCV prevention interventions in reducing HCV risk Data Sources: country-specific studies or systematic reviews, such as for estimating the effectiveness of OST and NSP in PWID[82]; evidence is limited for community-level interventions and so may need to rely on indirect data or conservatively discount their effect altogether • Population size for different risk groups Data Sources: country-specific size estimation studies • Rate of population growth Data Sources: projections by international agencies |
|
| |||
| 4. Scale-up levels of HCV testing | Does not track HCV incidence well: relationship is highly variable | • Prevention intervention scale-up • Population sub-groups that are tested and retested • Downstream cascade of care (e.g., referral for care, uptake of HCV treatment) |
N/A |
Abbreviations: N/A = not applicable; NSP = needle and syringe program; OST = opioid substitution treatment
The models considering the chronic HCV prevalence indicator also varied in terms of their baseline prevalence of chronic HCV infection, risk group modelled, underlying dynamics of the epidemic (stable or increasing), levels of population growth (stable or growing), and time period over which elimination was achieved. None of these factors seemed to affect the relationship between chronic prevalence and incidence.
1. Trends in population-level chronic HCV prevalence.
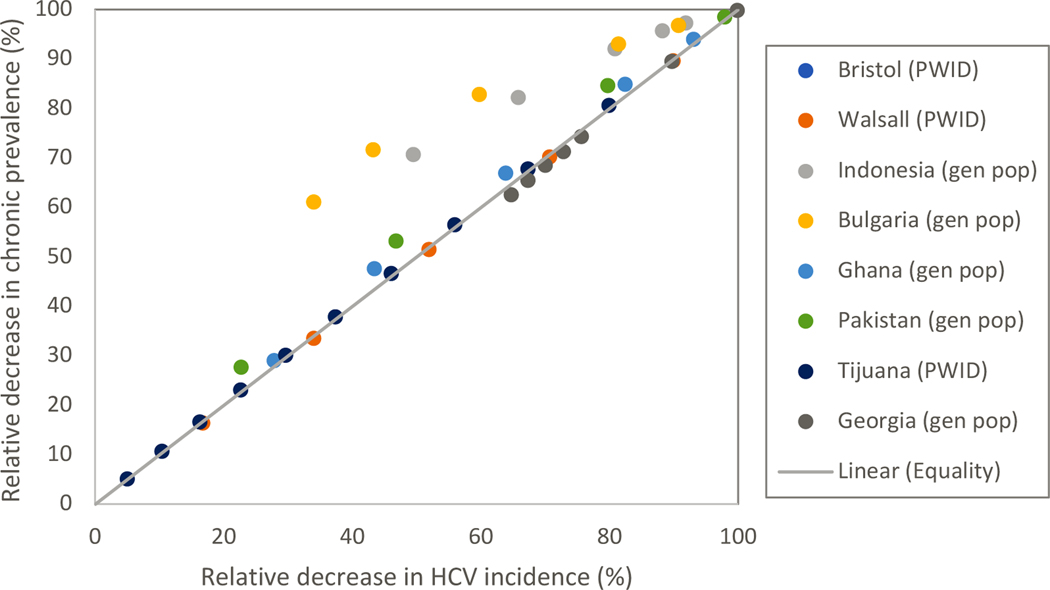
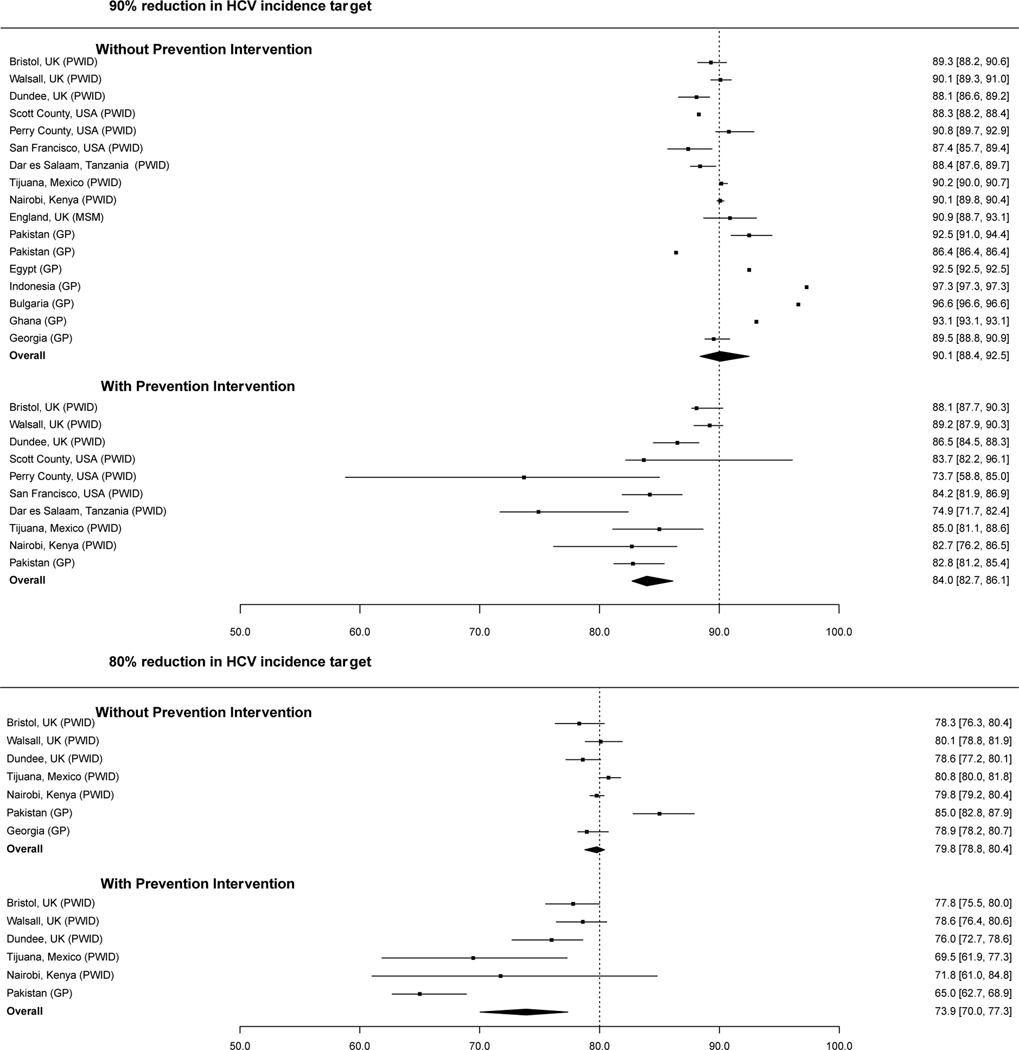
Our modelling work suggests that, in a context where HCV elimination initiatives mainly consist of scaling-up HCV treatment and not HCV preventative interventions, changes in chronic HCV prevalence over time will closely track changes in HCV incidence (Figure 1a). We found that overall, when HCV incidence has decreased by 80% or 90% relative to baseline levels, the corresponding median decrease in chronic HCV prevalence is estimated at 79.8% (interquartile range (IQR): 78.8–80.4; Figure 1b) and 90.1% (IQR: 88.4–92.5; Figure 1b), respectively. The only exception seems to be in general population settings where PWID contribute substantially to HCV transmission (e.g., Indonesia, Bulgaria) but are not more likely to receive HCV treatment. In these contexts, the overall decrease in chronic prevalence may be greater than the corresponding decrease in HCV incidence. To achieve an equivalent decrease in incidence and prevalence, a greater rate of HCV treatment is needed in PWID than in the general population, because of their higher level of reinfection.
Figure 1a:
Correlation between relative decrease in chronic HCV prevalence and HCV incidence over the course of the HCV elimination initiative for different scale-up levels in HCV treatment without concurrent scale-up in HCV preventative interventions “Gen pop” denotes results from a model of HCV transmission in the general population; PWID denotes results from a model of HCV transmission among PWID
Figure 1b:
Estimated decrease (%) in chronic HCV prevalence when an 80 or 90% decrease in HCV incidence is achieved from scaling-up HCV treatment with or without the concurrent scale-up of HCV preventative interventions. Overall estimates reflect the median and interquartile range of the individual point estimates. GP denotes results from a model of HCV transmission in the general population; PWID denotes results from a model of HCV transmission among PWID; and MSM denotes results from a model of HCV transmission among MSM. In the scenarios including preventative interventions, the level of scale-up is given in Supplementary Table 1.
We also found that the relationship between chronic HCV prevalence and HCV incidence holds reasonably well if HCV elimination initiatives also include scale-up of HCV preventative interventions as part of a combined elimination approach, although in this case, a smaller decrease in chronic HCV prevalence relative to baseline (i.e., median: 73.9%; IQR: 70.0–77.3%) can equate to an 80% decrease in HCV incidence (Figure 1b). Since the moderating effect of HCV preventative interventions on this relationship is modest (Supplementary Figure 1), and in practice will be difficult to quantify, we suggest that countries should always aim for an 80% reduction in chronic prevalence. As with HCV preventative intervention, other factors that directly affect incidence (e.g., unstable housing, incarceration, prescription opioid injecting[12, 72, 73]) and that change substantially over the course of the elimination initiative could also influence the relationship between chronic HCV prevalence and HCV incidence and should be considered. Conversely, such things as the dynamics of the epidemic, population growth and time period of the elimination initiative appear to have little impact on the relationship with chronic HCV prevalence. As described in the next section, while these factors influence the level of HCV treatment scale-up needed to decrease chronic HCV prevalence to a specific threshold, if chronic prevalence has decreased by 80%, we still expect HCV incidence to have decreased by a similar amount.
Australia and the UK are currently using similar methods to evaluate the impact of HCV elimination initiatives among PWID: observed decreases in chronic HCV prevalence paired with measures of the scale-up in HCV treatment[74, 75] are being used with modelling to estimate the likely decrease in HCV incidence. Similar methods are also being used in more disseminated epidemics like Georgia, where a national survey undertaken in 2015 is being repeated in 2021 to estimate the decrease in chronic prevalence resulting from their national scale-up in treatment[76]. Our modelling for this setting[16] suggests that the observed decrease in chronic prevalence is likely to track closely the unobserved decrease in HCV incidence (Figure 1b).
2. Scale-up levels of HCV interventions.
Our modelling suggests that the level of scale-up in HCV preventative and treatment interventions can be used to track changes in HCV incidence, although there is no universal target for these programmatic metrics that equates to an 80% reduction in HCV incidence. Indeed, our modelling suggests the required level of treatment will generally be above the baseline number of individuals chronically infected but will vary by setting, depending on several factors (Supplementary Figure 3). For example, projections suggest that the required level of treatment will decrease with the concurrent scale-up in preventative interventions or if the time period of the elimination initiative is shortened, while it will increase if there is population growth, or the epidemic is expanding. For example, the expected treatment coverage (i.e., overall percentage of baseline number of infections that are treated) was found to decrease by about 12–13% for each 10% relative decrease in incidence achieved by the scale-up of preventative interventions rather than treatment (Supplementary Figure 4). The precise effect of other factors is less clear, and so country-specific modelling will be required for validating specific countries. This method has been used to undertake an interim impact analysis of the Georgia HCV elimination initiative[16], where data on the historical HCV epidemic and the ongoing scale-up in HCV treatment and preventative interventions (e.g., needle and syringe programs, opioid agonist treatment) were used to estimate the interim decrease in HCV incidence. The prevalence survey planned for 2021 will be used to test the modelled impact projections.
3. Other indicators.
We also considered the utility of other markers—trends in HCV antibody prevalence and scale-up of HCV testing—and they were not found to be reliable indicators of associated changes in HCV incidence. Indeed, when HCV incidence was reduced by 80% or 90%, the corresponding decrease in HCV antibody prevalence was found to be highly variable across settings and consistently much smaller (Supplementary Figure 2). Even among young or recent PWID, for whom we would expect a stronger correlation, the decrease in antibody prevalence that corresponds to an 80 or 90% decrease in incidence appears highly variable. This poor correlation is due to HCV treatment not having a direct effect on antibody prevalence, which will only decrease as a result of reductions in the numbers of new primary infections or increases in the number of people leaving the population due to death or ceasing injecting drug use. Similarly, although few models (n=2) were available to inform this assessment, our modelling suggests that the impact of HCV testing scale-up on HCV incidence would be highly variable depending on who is tested, referral rates for treatment, levels of re-testing and treatment uptake.
Key considerations:
The relationships found between these four indicators and HCV incidence should be considered in the context of several limitations. First, given that this assessment is based on secondary analyses, there are variations across mathematical models with respect to the underlying structure, parameterisation and calibration, and the impact of these differences on findings is difficult to predict. However, the consistent relationships observed between some indicators and HCV incidence, despite these differences, strengthens our confidence in these results. Second, there were few models that considered certain indicators (e.g., HCV testing) and specific risk groups (e.g., MSM) or that evaluated the effect of HCV prevention strategies in general population settings. Further modelling work is needed to consider these aspects. Finally, it is very important that our model-based findings are supported by empirical data. This step is essential for determining whether the assumptions underlying our mechanistic models, which link scale-up in HCV interventions to changes in HCV prevalence and incidence, are accurate. A recent study conducted in an Australian prison setting lends some support to our findings, as our modelled projections of HCV incidence following the scale-up of HCV treatment were found to be very similar to observed data[77].
Aside from these limitations, the reliability of any alternative indicator in estimating HCV incidence will closely depend on the quality of data used and its representativeness for the target population. Ongoing population-based prevalence surveys like the DHS and PHIA offer a platform for including HCV testing to measure chronic HCV infection. These surveys are one of the best options to capture nationally representative estimates, yet are expensive and therefore conducted infrequently, and also liable to bias due to certain groups being systematically excluded (e.g., prisoners, mobile and homeless individuals for household surveys).[78] Conversely, routine testing data such as among women attending antenatal clinics or blood donors could provide easy and ready access to a cross-section of the population, yet the estimates obtained might not be representative of the whole population and may not be available on a national scale[78]. The challenge of achieving representativeness when measuring chronic HCV prevalence in high-risk populations like PWID and MSM is amplified, given that there is no sampling frame. For these groups, more complex sampling designs, such as those adopted in bio-behavioural surveys can help minimise bias[79] and these surveys represent a good option for monitoring chronic HCV prevalence. Otherwise, scale-up levels of HCV interventions are often derived through administrative and programmatic data and, as with other indicators, these can be limited by sparse geographic coverage, systematic under-representation of certain groups and, because they are not generally collected for research purposes, could be incomplete, missing, or poorly archived. Given that different data sources have different weaknesses, combining multiple sources, if available, can strengthen the robustness of estimates.
ABSOLUTE HCV INCIDENCE TARGET
An absolute HCV incidence target carries several advantages and disadvantages compared to the current relative target (Panel 2). Importantly, it obviates the need for baseline incidence data, which is a challenge faced by most countries[28]. To ensure HCV elimination is achieved in all groups who are contributing sufficiently to HCV transmission, separate absolute targets are needed for the general population and for specific high-risk groups, particularly PWID. Since empirical data on HCV incidence are scarce, targets would have to be derived from modelled estimates[1, 25, 80, 81], which are uncertain. For the general population, assuming an 80% reduction relative to the WHO 2015 global estimate[1], the threshold that countries would need to meet would be about 5 per 100,000 person-years. Based on our modelling of 88 countries[25], 1% of countries would not have to decrease incidence to meet this threshold, whereas 10%, 46% and 44% of countries would have to decrease incidence by <50%, 50–80%, >80%, respectively (Supplementary Table 3). For PWID, based on a single study that has estimated the global HCV incidence in this group[25], the equivalent threshold would be about 2 per 100 person-years (Supplementary Table 3).
Using the same mathematical models as before, we examined whether an absolute estimate of chronic HCV prevalence in 2030 would track the absolute HCV incidence target, and so could serve as a reliable alternative indicator. Our results suggests that no universal prevalence target exists either for the general population or PWID (Supplementary material).
PROPOSED PROCESS FOR VALIDATING THE HCV INCIDENCE TARGET
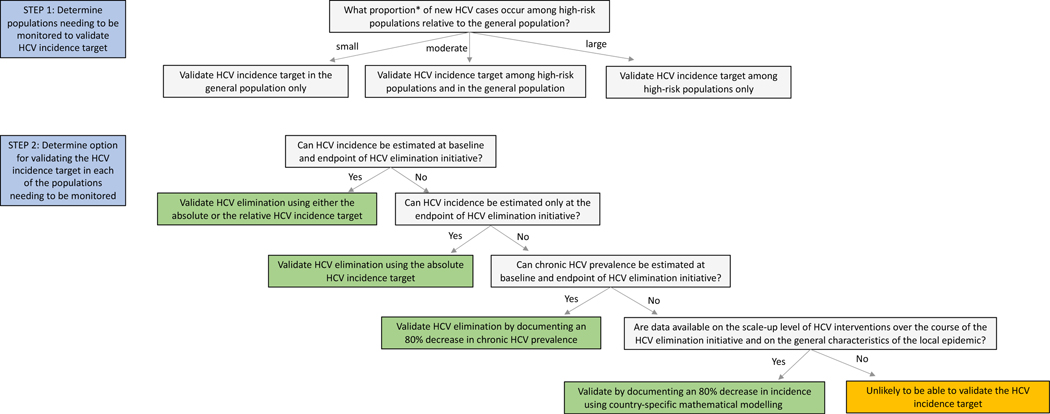
The populations that need to be monitored in different countries to validate the HCV incidence target will vary depending on their level of contribution to HCV transmission. It is therefore essential that the first step be an assessment of these contributions to ensure that all populations who contribute sufficiently are reached by HCV elimination initiatives and are included in the validation process (Panel 3). Based on the different methods and indicators presented, we propose three options that countries could use to validate the incidence target in each of these populations (Figure 2).
Figure 2:
Proposed process to validate the hepatitis C virus (HCV) incidence target *If a high-risk population (e.g., people who inject drugs) contributes ≥20% of ongoing incident HCV infections, then it should definitely be monitored because reaching an 80% decrease in country-level incidence would be impossible without decreasing incidence in that group. However, even if a high-risk population contributes ≥10% and <20%, then it should still probably be monitored because decreasing incidence in that group could still be important for reaching the HCV incidence target.
The first option is to monitor HCV incidence and to validate the estimate(s) against either an absolute incidence threshold or the original GHSS relative target, depending on the available data and epidemiological context. Countries can select any of the seven methods for monitoring HCV incidence. However, given that some are highly likely to produce biased estimates or may not be useful for capturing HCV reinfection when treatment is scaled-up, these should be generally triangulated with findings derived from other methods. Method 1 is the preferred option, however this is likely to be resource-intensive, and therefore not feasible for many countries.
Second, if HCV incidence cannot be monitored reliably but trends in chronic HCV prevalence can be, at least at the beginning and end of the elimination initiative, these estimates could be used as alternative indicators to validate the HCV incidence target. If chronic HCV prevalence has decreased by 80%, it can be generally assumed that HCV incidence has also decreased by about 80%. Since chronic HCV prevalence is generally more easily measured than HCV incidence, this option represents a simpler alternative.
Third, if neither HCV incidence nor trends in chronic HCV prevalence can be measured reliably, data on the levels of HCV treatment and preventative interventions scale-up could be used to model their expected impact on incidence. The data demands associated with this option are greater compared to the second option and country-specific modelling is needed. In addition to data on the level of HCV interventions scale-up, information is also needed on HCV prevalence (chronic or antibody) at baseline and other characteristics of the local HCV epidemic and population (e.g., stability of the HCV epidemic, main risk groups accounting for HCV transmission, population growth). This option presents an alternative for countries that generally have robust data on HCV elimination initiatives but limited data on HCV incidence and chronic prevalence.
We suggest that it will not be possible to validate whether a country has achieved the HCV incidence target if they do not have quality data to inform one of these three options in all populations contributing sufficiently to HCV transmission. Given the large time lag (i.e., 20–30 years) between HCV acquisition and symptomatic infection, other indicators, like the number of hospital admissions related to HCV, liver cancer or liver transplantations attributable to HCV, cannot be used for validating the HCV incidence target. Although options one and two only require data on HCV incidence and chronic HCV prevalence, respectively, information on the level of HCV prevention and treatment scale-up are still important for contextualising the observed decreases over time or, if an absolute incidence target is used, the single low estimate. Aside from the scale-up of HCV interventions, many other factors can be implicated in decreasing incidence, such as changes in risk behaviour or in injection drug use initiation and cessation patterns. Understanding the reasons behind the observed decreases is important to preventing a resurgence in HCV infection and this process can be supported through mathematical modelling. Most of our recommendations regarding the process to validate the HCV incidence target have been included in the interim guidance framework for country validation of viral hepatitis elimination recently published by WHO[29]. The feasibility of the various elimination criteria proposed in the guidance document will be evaluated as part of a series of country pilots conducted across the WHO regions[29].
CONCLUSION
There are different methods to monitor HCV incidence that countries can use to validate the HCV incidence target, depending on the resources available and the populations needing to be monitored. In settings where HCV incidence cannot be monitored, our work suggests that monitoring trends in chronic HCV prevalence or trends in HCV preventative and treatment interventions linked with mathematical modelling could serve as reliable alternatives to determining whether the HCV incidence target has been achieved. As the 2030 deadline for eliminating HCV infection as a public health problem approaches, these options can afford countries some of the flexibility needed to adopt methods and indicators for validating the HCV incidence target that are best suited to their context.
Supplementary Material
Acknowledgement:
This work was commissioned and funded by the World Health Organization (WHO). The Global HIV, Hepatitis and Sexually Transmitted Infections Programmes at WHO had an active role in the design, conduct, and analysis of the study, interpretation of the data, and writing of the report. Copyright in the original work on which this article is based belongs to WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the WHO. AA is supported through postdoctoral fellowships from the Canadian Institutes of Health Research, Fonds de la Recherche en Santé du Québec and Canadian Network on Hepatitis C. LKM acknowledges support from the National Institute of Drug Abuse (NIDA), National Institutes of Health (NIH) (grant number T32 DA023356). LJA and HHA acknowledge support by the National Priorities Research Program (NPRP) [grant number 12S-0216-190094] from the Qatar National Research Fund (a member of Qatar Foundation). NKM, PV, HF, JS and AGL acknowledge support from National Institute of Allergy and Infectious Diseases (NIAID)/NIDA (R01 AI147490) and NIDA (NIDA grant number R01 DA033679, R01 DA037773, R21 DA046809, R21 DA047902 and R01 DA047952). NKM is also funded by the UCSD Center for AIDS Research (P30 AI036214). PV, AGL, JS and MH received funding from the Health Protection Unit for Evaluation of Interventions and Behavioural Science funded by the UK National Institute for Health Research. AA and PV had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Declaration of interests: PV and JGW have received unrestricted research grants from Gilead, outside the submitted work. NM has received unrestricted research grants from Gilead and Merk outside the submitted work. MH has received speaker fees honoraria in last five years from Gilead and MSD. HF has received an honorarium from MSD unrelated to this research. All other authors have no disclosures.
REFERENCES
- 1.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: World Health Organization, 2016. Available at: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06eng.pdf?ua=1. Accessed August 2017. [Google Scholar]
- 2.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013; 58(5): 1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57 Suppl 2: S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva, Switzerland: World Health Organization, 2021. Available at: https://www.who.int/publications/i/item/9789240027077. Accessed June 2021. [Google Scholar]
- 5.Shiha G, Soliman R, Mikhail NNH, Easterbrook P. Reduced incidence of hepatitis C in 9 villages in rural Egypt: Progress towards national elimination goals. J Hepatol 2021; 74(2): 303–11. [DOI] [PubMed] [Google Scholar]
- 6.Hallett TB. Estimating the HIV incidence rate: recent and future developments. Current opinion in HIV and AIDS 2011; 6(2): 102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastro TD, Kim AA, Hallett T, et al. Estimating HIV Incidence in Populations Using Tests for Recent Infection: Issues, Challenges and the Way Forward. J HIV AIDS Surveill Epidemiol 2010; 2(1): 1–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Busch MP, Pilcher CD, Mastro TD, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 2010; 24(18): 2763–71. [DOI] [PubMed] [Google Scholar]
- 9.Hope VD, Harris RJ, Vickerman P, et al. A comparison of two biological markers of recent hepatitis C virus (HCV) infection: implications for the monitoring of interventions and strategies to reduce HCV transmission among people who inject drugs. Euro Surveill 2018; 23(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iversen J, Wand H, Topp L, Kaldor J, Maher L. Reduction in HCV incidence among injection drug users attending needle and syringe programs in Australia: a linkage study. Am J Public Health 2013; 103(8): 1436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan AE, Des Jarlais DC, Arasteh K, McKnight C, Nash D, Perlman DC. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend 2015; 152: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health 2018; 108(2): 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton AJ, Hope VD, Mathei C, et al. A comparison between the force of infection estimates for blood-borne viruses in injecting drug user populations across the European Union: a modelling study. J Viral Hepat 2008; 15(11): 809–16. [DOI] [PubMed] [Google Scholar]
- 14.Ayoub HH, Abu-Raddad LJ. Treatment as prevention for hepatitis C virus in Pakistan: mathematical modelling projections. BMJ Open 2019; 9(5): e026600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim AG, Walker JG, Mafirakureva N, et al. Effects and cost of different strategies to eliminate hepatitis C virus transmission in Pakistan: a modelling analysis. Lancet Glob Health 2020; 8(3): e440–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker JG, Kuchuloria T, Sergeenko D, et al. Interim effect evaluation of the hepatitis C elimination programme in Georgia: a modelling study. Lancet Glob Health 2020; 8(2): e244–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser H, Vellozzi C, Hoerger TJ, et al. Scaling Up Hepatitis C Prevention and Treatment Interventions for Achieving Elimination in the United States: A Rural and Urban Comparison. Am J Epidemiol 2019; 188(8): 1539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez LK, Cepeda JA, Borquez A, et al. Is hepatitis C virus (HCV) elimination achievable among people who inject drugs in Tijuana, Mexico? A modeling analysis. Int J Drug Policy 2021; 88: 102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macgregor L, Desai M, Martin NK, et al. Scaling up screening and treatment for elimination of hepatitis C among men who have sex with men in the era of HIV pre-exposure prophylaxis. EClinicalMedicine 2020; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trickey A, Hiebert L, Perfect C, et al. Hepatitis C virus elimination in Indonesia: Epidemiological, cost and cost-effectiveness modelling to advance advocacy and strategic planning. Liver Int 2020; 40(2): 286–97. [DOI] [PubMed] [Google Scholar]
- 21.Ward Z, Platt L, Sweeney S, et al. Impact of current and scaled-up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings-what is required to achieve the WHO’s HCV elimination targets? Addiction 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayoub HH, Abu-Raddad LJ. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: A case for treatment as prevention. Journal of Viral Hepatitis 2017; 24(6): 486–95. [DOI] [PubMed] [Google Scholar]
- 23.Stone J, Fraser H, Walker JG, et al. Modelling the Impact of Prevention and Treatment Interventions on HIV and Hepatitis C Virus Transmission Among People Who Inject Drugs in Kenya. medRxiv 2021: 2021.02.02.21251008. [Google Scholar]
- 24.Trickey A, Fraser H, Lim AG, et al. Modelling the potential prevention benefits of a treat-all hepatitis C treatment strategy at global, regional and country levels: A modelling study. J Viral Hepat 2019; 26(12): 1388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trickey A, Fraser H, Lim AG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol 2019; 4(6): 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim AG, Qureshi H, Mahmood H, et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol 2018; 47(2): 550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser H, Zibbell J, Hoerger T, et al. Scaling-up HCV prevention and treatment interventions in rural United States-model projections for tackling an increasing epidemic. Addiction 2018; 113(1): 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S, Harmanci H, Hutin Y, et al. Global progress on the elimination of viral hepatitis as a major public health threat: An analysis of WHO Member State responses 2017. JHEP Rep 2019; 1(2): 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Interim guidance for country validation of viral hepatitis elimination. Geneva, Switzerland: World Health Organization, 2021. Available at: https://www.who.int/publications/i/item/9789240028395. Accessed June 2021. [Google Scholar]
- 30.Easterbrook P, Luhmann N, Newman M, Walsh N, Lesi O, Doherty M. New WHO guidance for country validation of viral hepatitis B and C elimination [In Press]. Lancet Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 31.Morris MD, Shiboski S, Bruneau J, et al. Geographic Differences in Temporal Incidence Trends of Hepatitis C Virus Infection Among People Who Inject Drugs: The InC3 Collaboration. Clin Infect Dis 2017; 64(7): 860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiessing L, Ferri M, Grady B, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014; 9(7): e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghisla V, Scherrer AU, Nicca D, Braun DL, Fehr JS. Incidence of hepatitis C in HIV positive and negative men who have sex with men 2000–2016: a systematic review and meta-analysis. Infection 2017; 45(3): 309–21. [DOI] [PubMed] [Google Scholar]
- 34.Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6(1): 39–56. [DOI] [PubMed] [Google Scholar]
- 35.Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology 2013; 58(4): 1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciani F, Bretana NA, Teutsch S, et al. A prospective study of hepatitis C incidence in Australian prisoners. Addiction 2014; 109(10): 1695–706. [DOI] [PubMed] [Google Scholar]
- 37.Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep 2018; 8(1): 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micallef JM, Macdonald V, Jauncey M, et al. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat 2007; 14(6): 413–8. [DOI] [PubMed] [Google Scholar]
- 39.Zou X, Ling L, Zhang L. Trends and risk factors for HIV, HCV and syphilis seroconversion among drug users in a methadone maintenance treatment programme in China: a 7-year retrospective cohort study. BMJ open 2015; 5(8): e008162-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida S, Satake M, Kurisu A, et al. Incidence rates of hepatitis C virus infection among blood donors in Japan: a nationwide retrospective cohort study. Transfusion 2018; 58(12): 2880–5. [DOI] [PubMed] [Google Scholar]
- 41.Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010; 50(7): 1495–504. [DOI] [PubMed] [Google Scholar]
- 42.Garvey LJ, Cooke GS, Smith C, et al. Decline in Hepatitis C Virus (HCV) Incidence in Men Who Have Sex With Men Living With Human Immunodeficiency Virus: Progress to HCV Microelimination in the United Kingdom? Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradat P, Huleux T, Raffi F, et al. Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS 2018; 32(8): 1077–82. [DOI] [PubMed] [Google Scholar]
- 44.Boerekamps A, van den Berk GE, Lauw FN, et al. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immunodeficiency Virus-Positive Men Who Have Sex With Men After Unrestricted Access to HCV Therapy. Clin Infect Dis 2018; 66(9): 1360–5. [DOI] [PubMed] [Google Scholar]
- 45.Leclerc P, Roy É, Morissette C, Alary M, Parent R, Blouin K. Surveillance des maladies infectieuses chez les utilisateurs de drogues par injection – Épidémiologie du VIH de 1995 à 2016 – Épidémiologie du VHC de 2003 à 2016. Montréal, Quebec: Institut national de santé publique, 2018. Available at: https://www.inspq.qc.ca/sites/default/files/publications/2400_surveillance_maladies_infectieuses_utilisateurs_drogue_injection.pdf. Accessed June 2019. [Google Scholar]
- 46.Hope V, Kimber J, Vickerman P, Hickman M, Ncube F. Frequency, factors and costs associated with injection site infections: findings from a national multi-site survey of injecting drug users in England. BMC Infect Dis 2008; 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol 2008; 46(2): 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boon D, Bruce V, Patel EU, et al. Antibody avidity-based approach to estimate population-level incidence of hepatitis C. J Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel EU, Cox AL, Mehta SH, et al. Use of Hepatitis C Virus (HCV) Immunoglobulin G Antibody Avidity as a Biomarker to Estimate the Population-Level Incidence of HCV Infection. J Infect Dis 2016; 214(3): 344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platt L, Sweeney S, Ward Z, et al. Public Health Research. Assessing the impact and cost-effectiveness of needle and syringe provision and opioid substitution therapy on hepatitis C transmission among people who inject drugs in the UK: an analysis of pooled data sets and economic modelling. Southampton (UK): NIHR Journals Library; Copyright © Queen’s Printer and Controller of HMSO 2017. This work was produced by Platt et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK., 2017. [PubMed] [Google Scholar]
- 51.Tsertsvadze T, Sharvadze L, Chkhartishvili N, et al. The natural history of recent hepatitis C virus infection among blood donors and injection drug users in the country of Georgia. Virology Journal 2016; 13(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Public Health England. Hepatitis C in the UK 2019 Working to eliminate hepatitis C as a major public health threat. London, United Kingdom, 2019. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/831155/Hepatitis_C_in_the_UK_2019_report.pdf. Accessed May 2020. [Google Scholar]
- 53.European Monitoring Centre for Drugs and Drug Addiction. Hepatitis C among drug users in Europe: epidemiology, treatment and prevention, EMCDDA Insights 23. Luxembourg: Publications Office of the European Union, 2016. Available at: https://www.emcdda.europa.eu/system/files/publications/2953/TDXD16002ENN_final_web.pdf_en. Accessed April 2020. [Google Scholar]
- 54.Akiyama MJ, Cleland CM, Lizcano JA, Cherutich P, Kurth AE. Prevalence, estimated incidence, risk behaviours, and genotypic distribution of hepatitis C virus among people who inject drugs accessing harm-reduction services in Kenya: a retrospective cohort study. Lancet Infect Dis 2019; 19(11): 1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton AJ, Gay NJ, Edmunds WJ, Hope VD, Gill ON, Hickman M. Modelling the force of infection for hepatitis B and hepatitis C in injecting drug users in England and Wales. BMC Infect Dis 2006; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platt L, Sutton AJ, Vickerman P, et al. Measuring risk of HIV and HCV among injecting drug users in the Russian Federation. Eur J Public Health 2009; 19(4): 428–33. [DOI] [PubMed] [Google Scholar]
- 57.van Griensven F, Mock PA, Benjarattanaporn P, et al. Estimating recent HIV incidence among young men who have sex with men: Reinvigorating, validating and implementing Osmond’s algorithm for behavioral imputation. PloS one 2018; 13(10): e0204793-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hallett TB, Zaba B, Todd J, et al. Estimating incidence from prevalence in generalised HIV epidemics: methods and validation. PLoS Med 2008; 5(4): e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int 2017; 37(1): 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Njouom R, Siffert I, Texier G, et al. The burden of hepatitis C virus in Cameroon: Spatial epidemiology and historical perspective. J Viral Hepat 2018; 25(8): 959–68. [DOI] [PubMed] [Google Scholar]
- 61.Meda N, Tuaillon E, Kania D, et al. Hepatitis B and C virus seroprevalence, Burkina Faso: a cross-sectional study. Bull World Health Organ 2018; 96(11): 750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parr JB, Lodge EK, Holzmayer V, et al. An Efficient, Large-Scale Survey of Hepatitis C Viremia in the Democratic Republic of the Congo Using Dried Blood Spots. Clin Infect Dis 2018; 66(2): 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rwanda Biomedical Center (RBC). Rwanda Population-Based HIV Impact Assessment (RPHIA) 2018–2019: Final Report. Kigali:: RBC, September 2020. Available at: https://phia.icap.columbia.edu/wp-content/uploads/2020/11/RPHIA-FinalReport_Web.pdf. Accessed May 2021. [Google Scholar]
- 64.Federal Ministry of Health Nigeria. Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: Technical Report. Abuja, Nigeria, October 2019. Available at: http://ciheb.org/media/SOM/Microsites/CIHEB/documents/NAIIS-Report-2018.pdf. Accessed May 2021. [Google Scholar]
- 65.Tanzania Commission for AIDS (TACAIDS). Zanzibar AIDS Commission (ZAC). Tanzania HIV Impact Survey (THIS) 2016–2017: Final Report. Dar es Salaam, Tanzania, December 2018. Available at: https://phia.icap.columbia.edu/wpcontent/uploads/2019/06/FINAL_THIS-2016-2017_Final-Report__06.21.19_for-web_TS.pdf. Accessed May 2021. [Google Scholar]
- 66.Lazarus JV, Mozalevskis A, Safreed-Harmon K, Eramova I. Strengthening hepatitis B and C surveillance in Europe: results from the two global hepatitis policy surveys (2013 and 2014). Hepatol Med Policy 2016; 1: 3-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spada E, Mele A, Mariano A, Zuccaro O, Tosti ME. Risk factors for and incidence of acute hepatitis C after the achievement of blood supply safety in Italy: results from the national surveillance system. J Med Virol 2013; 85(3): 433–40. [DOI] [PubMed] [Google Scholar]
- 68.Talaat M, Afifi S, Reaves EJ, et al. Evidence of sustained reductions in the relative risk of acute hepatitis B and C virus infections, and the increasing burden of hepatitis a virus infection in Egypt: comparison of sentinel acute viral hepatitis surveillance results, 2001–17. BMC Infectious Diseases 2019; 19(1): 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo Y-C, Tsai M-S, Sun H-Y, Hung C-C, Chuang J-H. National Trend and Characteristics of Acute Hepatitis C among HIV-Infected Individuals: A Matched Case-Control Study-Taiwan, 2001–2014. PloS one 2015; 10(10): e0139687-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health 2014; 104(3): 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organization. Global policy report on the prevention and control of viral hepatitis in WHO member states. Geneva, Switzerland: World Health Organization, 2013. Available at: https://apps.who.int/iris/bitstream/handle/10665/85397/9789241564632_eng.pdf;jsessionid=4F5A5768E019660896F92FA75589471E?sequence=1. Accessed July 2020. [Google Scholar]
- 72.Arum C, Fraser H, Artenie AA, et al. Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. The Lancet Public health 2021; 6(5): e309–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18(12): 1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hickman M, Dillon JF, Elliott L, et al. Evaluating the population impact of hepatitis C direct acting antiviral treatment as prevention for people who inject drugs (EPIToPe) - a natural experiment (protocol). BMJ Open 2019; 9(9): e029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iversen J, Dore GJ, Catlett B, Cunningham P, Grebely J, Maher L. Association between rapid utilisation of direct hepatitis C antivirals and decline in the prevalence of viremia among people who inject drugs in Australia. J Hepatol 2019; 70(1): 33–9. [DOI] [PubMed] [Google Scholar]
- 76.Averhoff F, Lazarus JV, Sergeenko D, et al. Excellence in viral hepatitis elimination - Lessons from Georgia. J Hepatol 2019; 71(4): 645–7. [DOI] [PubMed] [Google Scholar]
- 77.Lim AG, Stone J, Hajarizadeh B, et al. Evaluating the prevention benefit of HCV treatment: Modelling the SToP-C treatment as prevention study in prisons. Hepatology 2021. [DOI] [PubMed] [Google Scholar]
- 78.UNAIDS/WHO Working Group on Global HIV/AIDS and STI surveillance. Guidelines for measuring national HIV prevalence in population-based surveys. Geneva, Switzerland: World Health Organization, 2005. Available at: Accessed August 2021. [Google Scholar]
- 79.WHO, CDC, UNAIDS, 360 F. Biobehavioral survey guidelines for populations at risk for HIV. Geneva: World Health Organization, 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/258924/9789241513012-eng.pdf?sequence=1. Accessed October 2nd, 2020. [Google Scholar]
- 80.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2(3): 161–76. [DOI] [PubMed] [Google Scholar]
- 81.Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet 2019; 393(10178): 1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2018; 113(3): 545–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.