Abstract
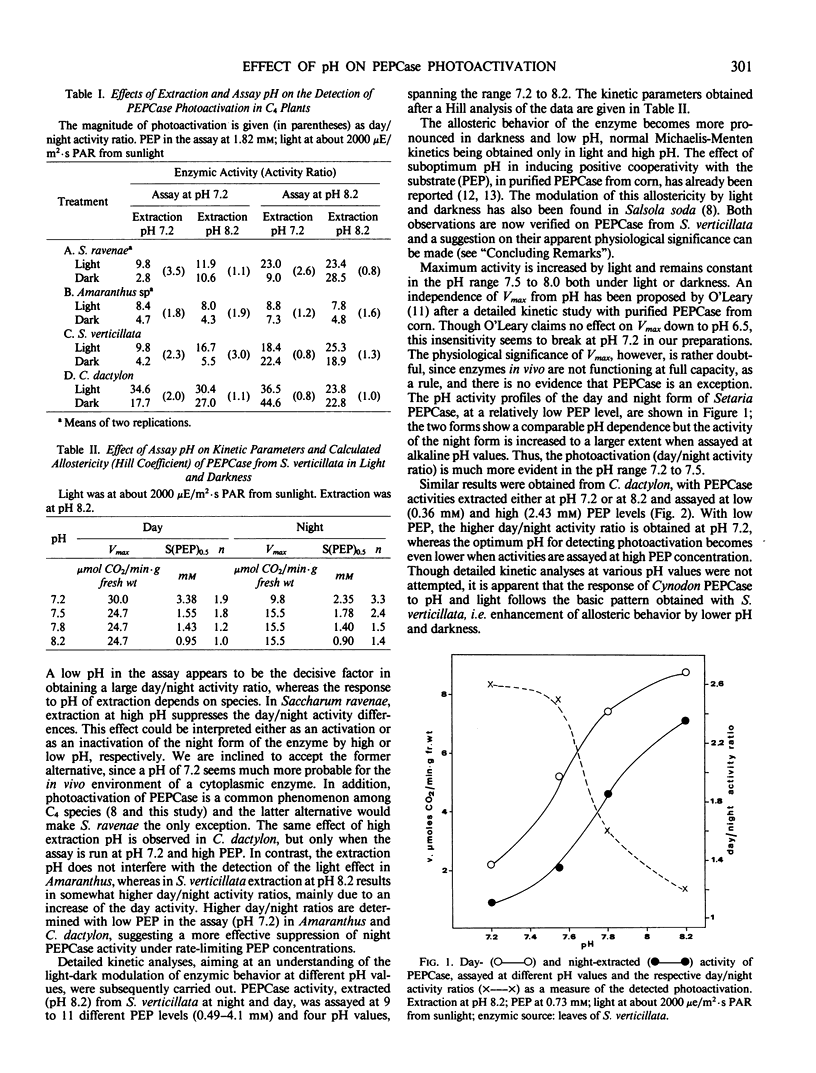
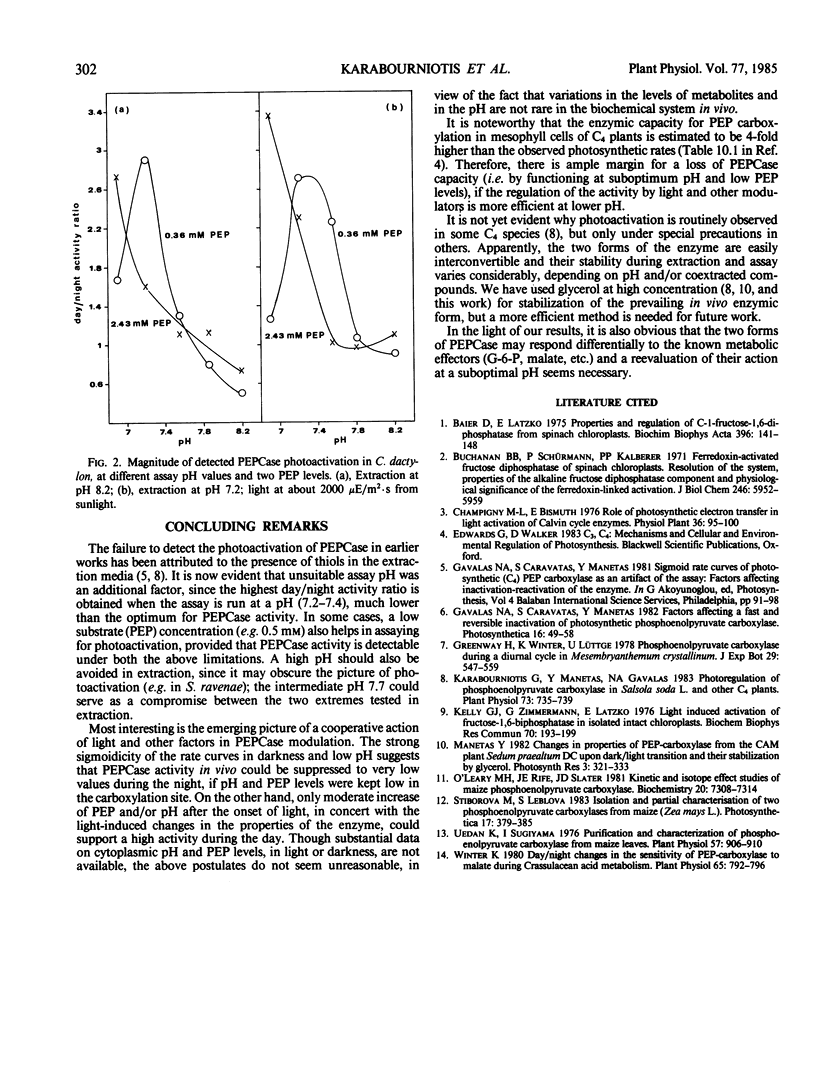
Photoactivation of phosphoenolpyruvate carboxylase in C4 plants is detected more efficiently when activity is assayed at suboptimum pH (e.g. 7.2); the magnitude of the light effect is often larger at low phosphoenolpyruvate concentration.
Darkness and low assay pH induce an allosteric behavior (positive cooperativity with phosphoenolpyruvate) which is relieved in light or by higher pH; thus, normal Michaelis-Menten kinetics are exhibited only when the enzyme is extracted during the day and assayed at pH 8.2.
Light activation, pH, and substrate level appear to be components of a regulatory device suppressing the activity in darkness and enhancing it under light.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Photoregulation of Phosphoenolpyruvate Carboxylase in Salsola soda L. and Other C(4) Plants. Plant Physiol. 1983 Nov;73(3):735–739. doi: 10.1104/pp.73.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Rife J. E., Slater J. D. Kinetic and isotope effect studies of maize phosphoenolpyruvate carboxylase. Biochemistry. 1981 Dec 8;20(25):7308–7314. doi: 10.1021/bi00528a040. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. Day/Night Changes in the Sensitivity of Phosphoenolpyruvate Carboxylase to Malate during Crassulacean Acid Metabolism. Plant Physiol. 1980 May;65(5):792–796. doi: 10.1104/pp.65.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]


