Abstract
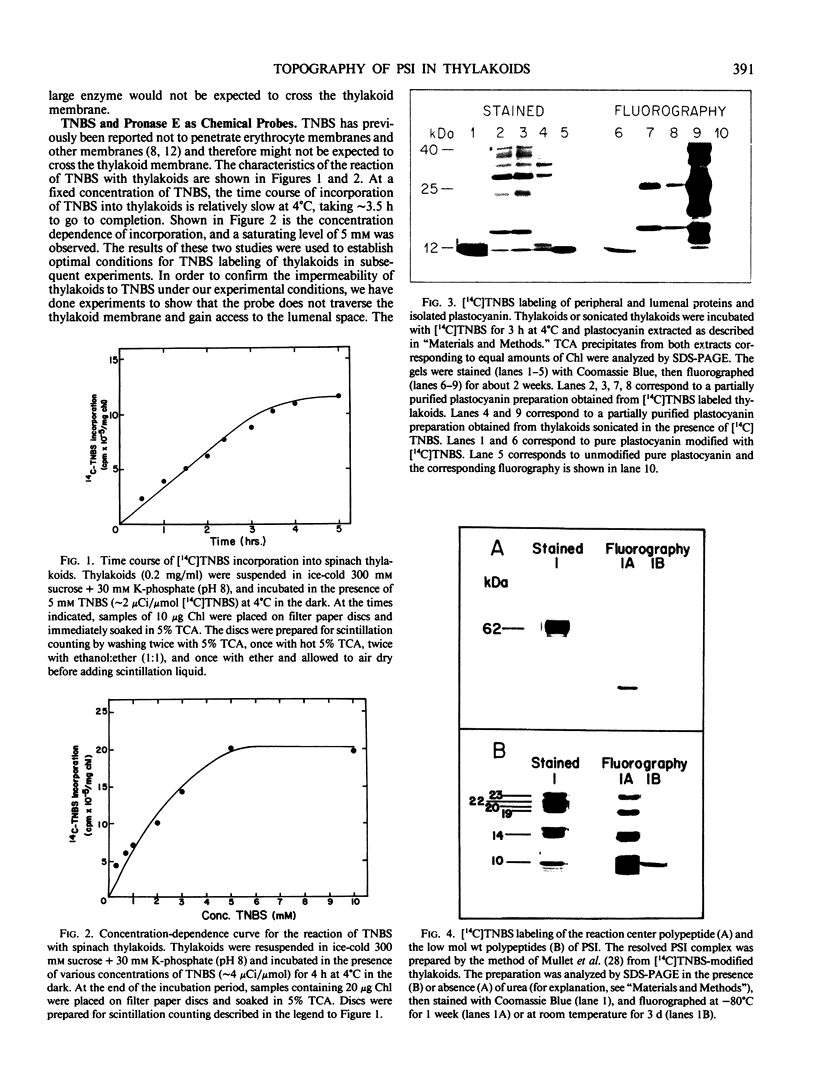
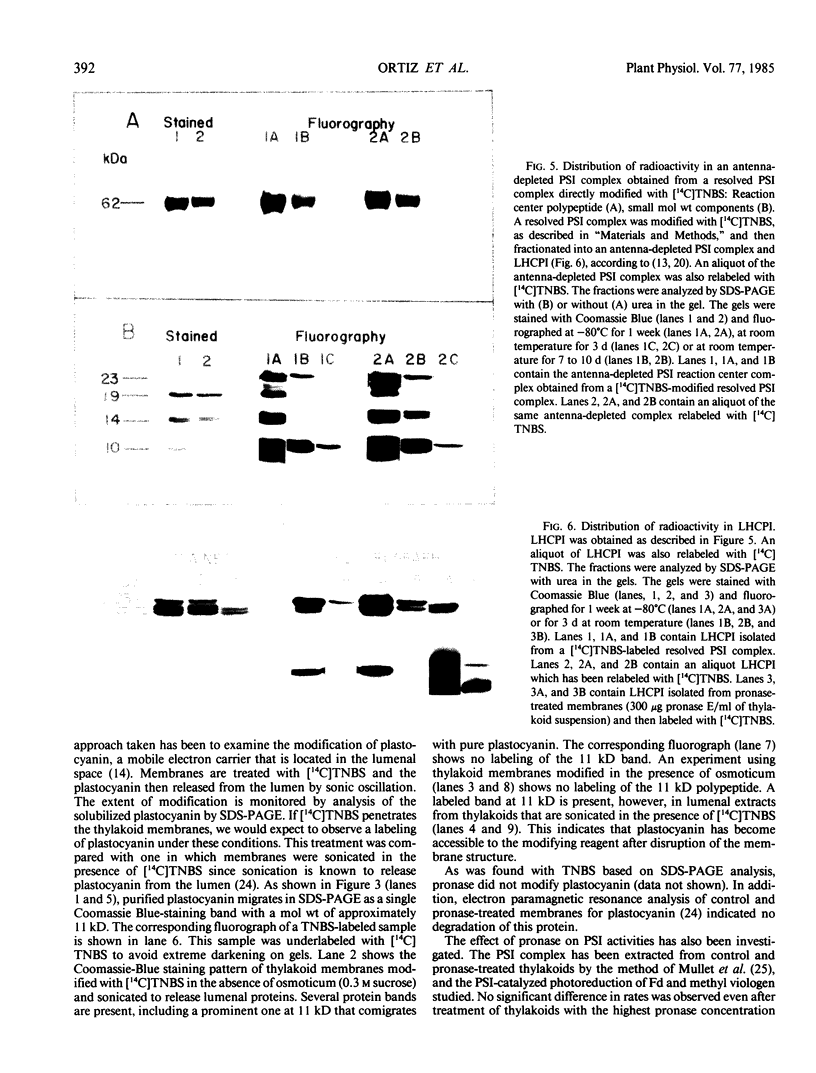
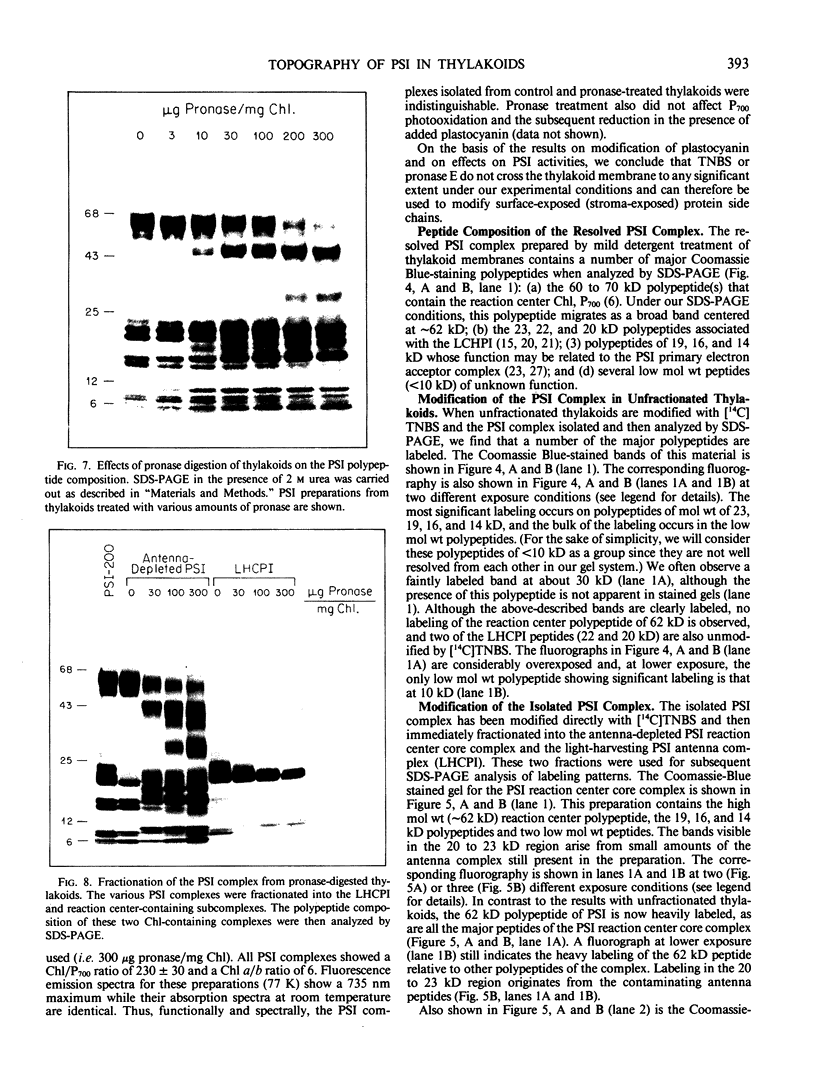
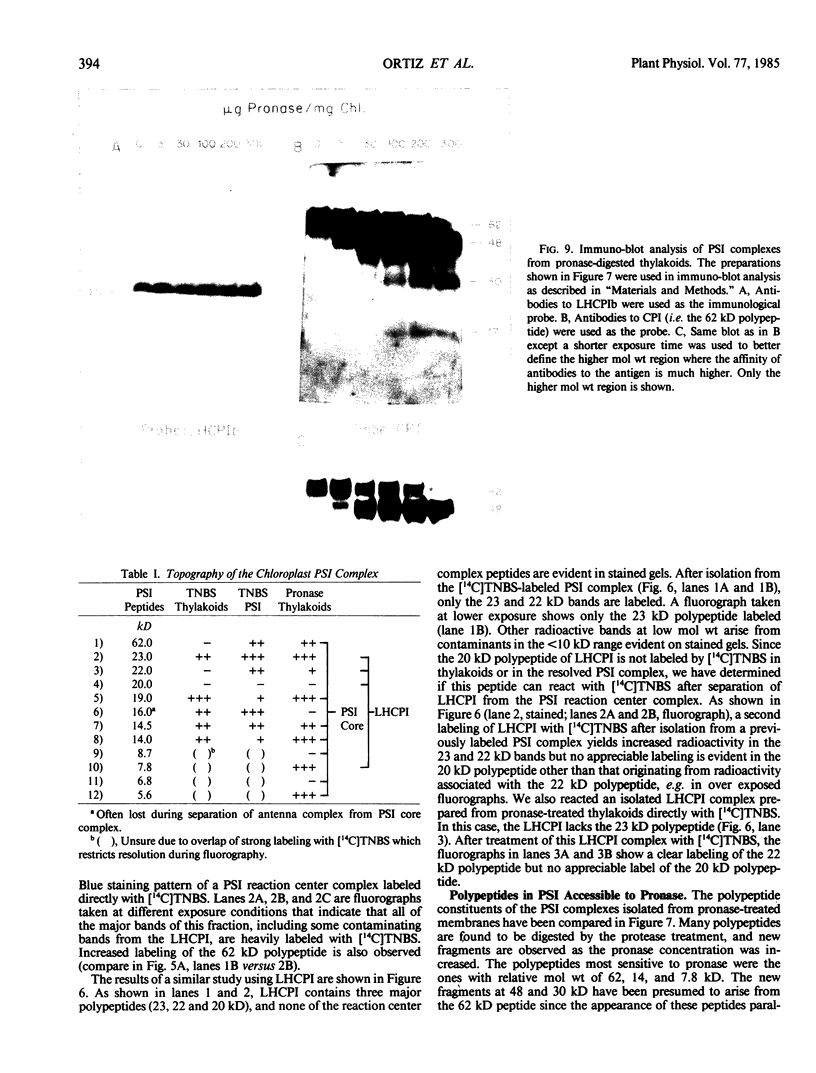
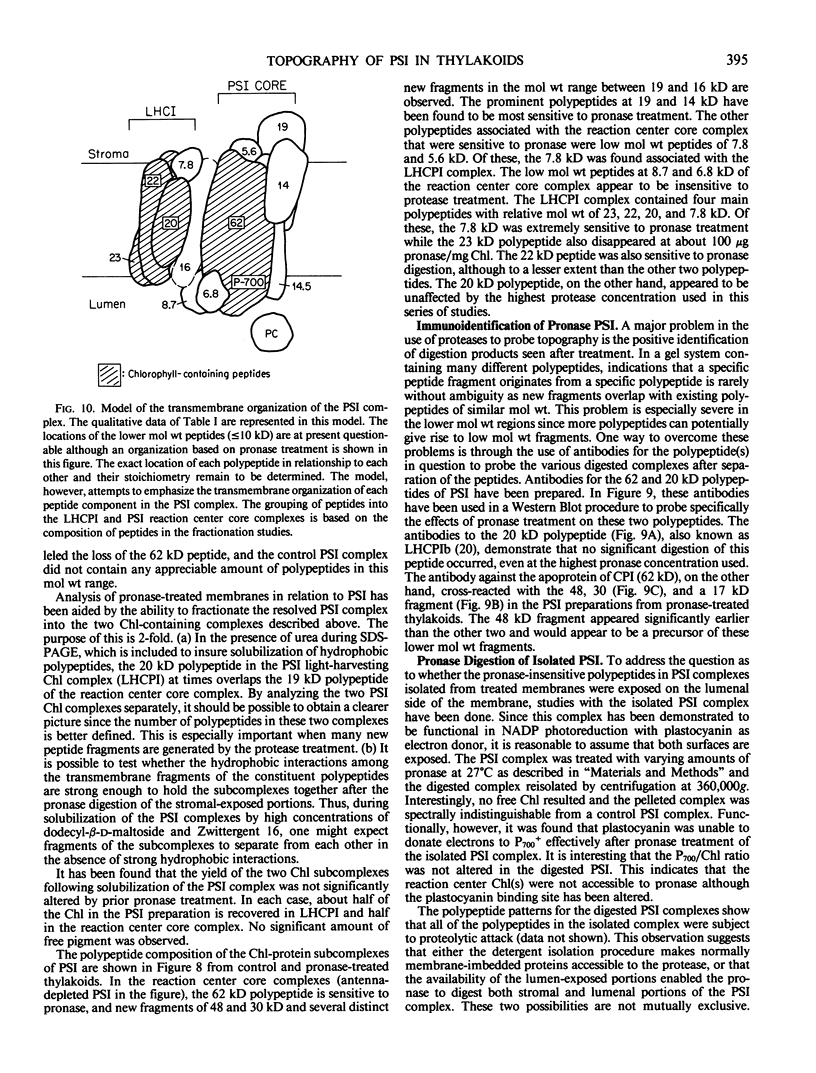
The transverse heterogeneity of the polypeptides associated with the Photosystem I (PSI) complex in spinach thylakoid membranes and in a highly resolved PSI preparation has been studied using the impermeant chemical modifier, 2,4,6-trinitrobenzenesulfonate (TNBS) and the proteolytic enzyme, Pronase E. The present study has shown that the PSI reaction center polypeptide of ∼62 kilodaltons and the 22 and 20 kilodalton polypeptides of the PSI light-harvesting chlorophyll protein (LHCPI) complex are not labeled by [14C]TNBS in unfractionated thylakoids. On the other hand, the 23 kilodalton polypeptide of the PSI LHCP and the 19 and 14 kilodalton polypeptides associated with the PSI primary electron acceptor complex are readily labeled by [14C]TNBS and are exposed to the stromal side of the thylakoid. Differences and similarities in the labeling of polypeptides associated with the PSI complex in thylakoids and in the isolated PSI complex are also noted. Treatment of thylakoids with pronase had no effect on the organization of the polypeptides in the LHCPI or the reaction center core complex, as manifested by the separation of these two subcomplexes from pronase-treated membranes. The 62, 19, and 14 kilodalton polypeptides associated with the reaction center core complex and the 23 and 22 kilodalton polypeptides associated with LHCPI are sensitive to pronase treatment while the 20 kilodalton polypeptide of LHCPI was inaccessible to the protease. The proteolysis of the 62 kilodalton polypeptide generated first a single immunodetectable fragment at about 48 kilodaltons, and further proteolytic digestion generated two other fragments at 30 and 17 kilodaltons respectively. These results are discussed in relation to the organization of the PSI complex in spinach thylakoids. A model for the transmembrane topography of the polypeptide constituents of PSI has been developed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulaev N. G., Feigina M. Y., Kiselev A. V., Ovchinnikov Y. A., Drachev L. A., Kaulen A. D., Khitrina L. V., Skulachev V. P. Products of limited proteolysis of bacteriorhodopsin generate a membrane potential. FEBS Lett. 1978 Jun 15;90(2):190–194. doi: 10.1016/0014-5793(78)80366-4. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. P-700 content and polypeptide profile of chlorophyll-protein complexes of spinach and barley thylakoids. Biochim Biophys Acta. 1980 Jun 10;591(1):113–126. doi: 10.1016/0005-2728(80)90225-x. [DOI] [PubMed] [Google Scholar]
- Andersson B., Anderson J. M., Ryrie I. J. Transbilayer organization of the chlorophyll-proteins of spinach thylakoids. Eur J Biochem. 1982 Apr 1;123(2):465–472. doi: 10.1111/j.1432-1033.1982.tb19790.x. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball M. B., Bell R. L., Capaldi R. A. Trypsin cleavage of ubiquinone-cytochrome c reductase (complex III). FEBS Lett. 1977 Nov 1;83(1):99–102. doi: 10.1016/0014-5793(77)80650-9. [DOI] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Purification and properties of the photosystem I reaction center from chloroplasts. J Biol Chem. 1975 Apr 25;250(8):2783–2788. [PubMed] [Google Scholar]
- Bonsall R. W., Hunt S. Reactivity of the human erythrocyte membrane to sodium trinitrobenzenesulphonate. Biochim Biophys Acta. 1971 Oct 12;249(1):281–284. doi: 10.1016/0005-2736(71)90105-2. [DOI] [PubMed] [Google Scholar]
- Burckhardt J., Telford J., Birnstiel M. L. Detection of labelled RNA species by contact hybridization. Nucleic Acids Res. 1979 Jul 11;6(9):2963–2971. doi: 10.1093/nar/6.9.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. P., Staehelin L. A. Proteolysis of chloroplast thylakoid membranes. I. Selective degradation of thylakoid pigment--protein complexes at the outer membrane surface. Arch Biochem Biophys. 1980 Apr 1;200(2):364–373. doi: 10.1016/0003-9861(80)90366-5. [DOI] [PubMed] [Google Scholar]
- Drozdovskaya N. R., Kozlov I. A., Milgrom YaM, Tsybovski I. S. The membrane in submitochondrial particles protects F1-ATPase from trinitrobenzolsulphonate and dinitrofluorobenzole. FEBS Lett. 1982 Dec 27;150(2):385–389. doi: 10.1016/0014-5793(82)80773-4. [DOI] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Lam E., Malkin R. Reconstruction of the chloroplast noncyclic electron transport pathway from water to NADP with three integral protein complexes. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5494–5498. doi: 10.1073/pnas.79.18.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Oritz W., Mayfield S., Malkin R. Isolation and Characterization of a Light-Harvesting Chlorophyll a/b Protein Complex Associated with Photosystem I. Plant Physiol. 1984 Mar;74(3):650–655. doi: 10.1104/pp.74.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Light-induced changes of bound chloroplast plastocyanin as studied by EPR spectroscopy: the role of plastocyanin in noncyclic photosynthetic electron transport. Biochim Biophys Acta. 1973 Jan 18;292(1):169–185. doi: 10.1016/0005-2728(73)90261-2. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Burke J. J., Arntzen C. J. Evidence for the role of surface-exposed segments of the light-harvesting complex in cation-mediated control of chloroplast structure and function. Arch Biochem Biophys. 1979 Jul;195(2):546–557. doi: 10.1016/0003-9861(79)90381-3. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Katoh S. Functional subunit structure of photosystem 1 reaction center in Synechococcus sp. Arch Biochem Biophys. 1982 Nov;219(1):219–227. doi: 10.1016/0003-9861(82)90152-7. [DOI] [PubMed] [Google Scholar]
- Vierling E., Alberte R. S. P(700) Chlorophyll a-Protein : Purification, Characterization, and Antibody Preparation. Plant Physiol. 1983 Jul;72(3):625–633. doi: 10.1104/pp.72.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]