Abstract
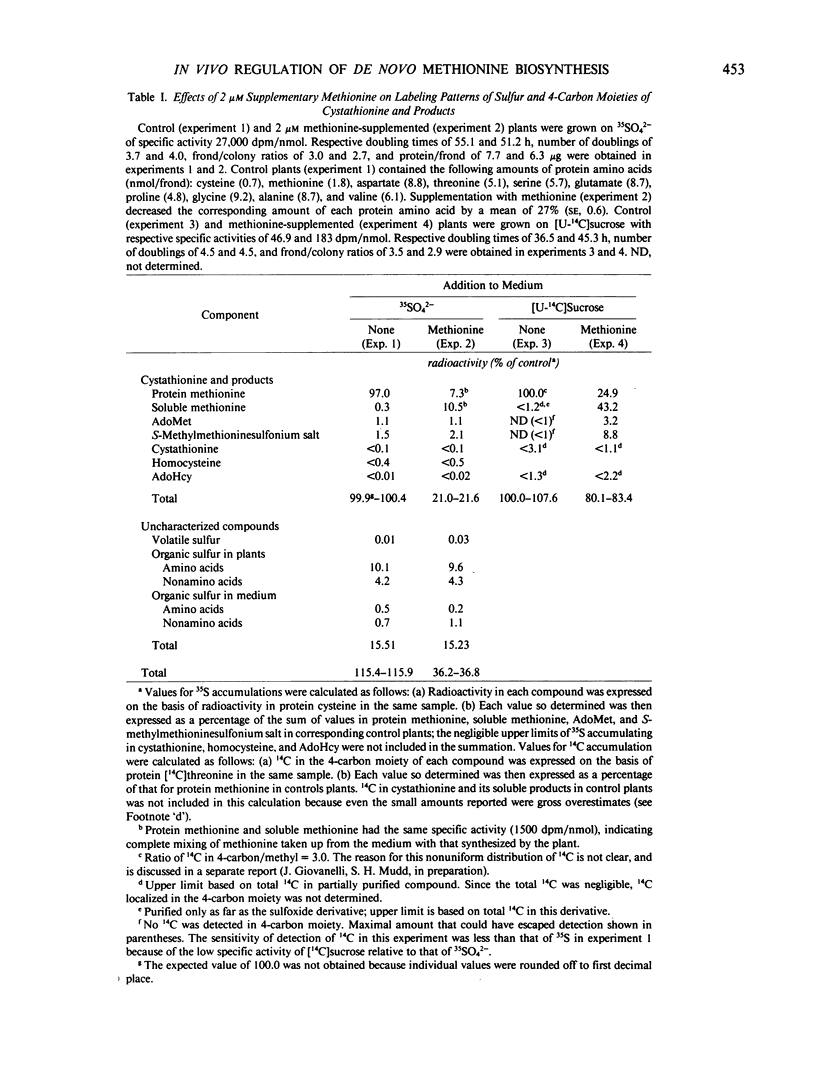
Administration of methionine to growing Lemna had essentially no effect on accumulation of sulfate sulfur in protein cysteine, but decreased accumulation into cystathionine and its products (homocysteine, methionine, S-methylmethioninesulfonium salt, S-adenosylmethionine, and S-adenosylhomocysteine) to as low as 21% that of control plants, suggesting that methionine regulates its own de novo synthesis at cystathionine synthesis. Methionine caused only a slight reduction (to 80% that of control plants) in the accumulation of sucrose carbon into the 4-carbon moieties of cystathionine and products. This observation was puzzling since cystathionine synthesis proceeds by incorporation of equivalent amounts of sulfur (from cysteine) and 4-carbon moieties (from O-phosphohomoserine). The apparent inconsistency was resolved by the demonstration in Lemna (Giovanelli, Datko, Mudd, Thompson 1983 Plant Physiol 71: 319-326) that de novo synthesis of the methionine 4-carbon moiety occurs not only via the established transsulfuration route from O-phosphohomoserine, but also via the ribose moiety of 5′-methylthioadenosine. It is now clear that the more accurate assessment of the flux of sulfur (and 4-carbon moieties) through transsulfuration is provided by the amount of 35S from 35SO42− that accumulates in cystathionine and its products, rather than by the corresponding measurements with 14C. These studies therefore unequivocally demonstrate in higher plants that methionine does indeed feedback regulate it own de novo synthesis in vivo, and that cystathionine synthesis is a locus for this regulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright S. W., Miflin B. J., Rognes S. E. Threonine accumulation in the seeds of a barley mutant with an altered aspartate kinase. Biochem Genet. 1982 Apr;20(3-4):229–243. doi: 10.1007/BF00484421. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datka A. H., Mudd S. H., Giovanelli J. Homocysteine biosynthesis in green plants: studies of the homocysteine-forming sulfhydrylase. J Biol Chem. 1977 May 25;252(10):3436–3445. [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Methionine biosynthesis in lemna: inhibitor studies. Plant Physiol. 1982 May;69(5):1070–1076. doi: 10.1104/pp.69.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H. Responses of Sulfur-Containing Compounds in Lemna paucicostata Hegelm. 6746 to Changes in Availability of Sulfur Sources. Plant Physiol. 1984 Jun;75(2):474–479. doi: 10.1104/pp.75.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall D. K. The biosynthesis of protein amino acids in plant tissue culture I. Isotope competition experiments using glucose-U-C14 and the protein amino acids. Plant Physiol. 1965 Sep;40(5):891–897. doi: 10.1104/pp.40.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Datko A. H., Mudd S. H., Thompson G. A. In vivo metabolism of 5'-methylthioadenosine in lemna. Plant Physiol. 1983 Feb;71(2):319–326. doi: 10.1104/pp.71.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homoserine esterification in green plants. Plant Physiol. 1974 Nov;54(5):725–736. doi: 10.1104/pp.54.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Transsulfuration in higher plants. Partial purification and properties of beta-cystathionase of spinach. Biochim Biophys Acta. 1971 Mar 10;227(3):654–670. doi: 10.1016/0005-2744(71)90015-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Veluthambi K., Thompson G. A., Mudd S. H., Datko A. H. Threonine Synthase of Lemna paucicostata Hegelm. 6746. Plant Physiol. 1984 Oct;76(2):285–292. doi: 10.1104/pp.76.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R. A., Das P. K., Widholm J. M. Characterization of cultured tobacco cell lines resistant to ethionine, a methionine analog. Plant Physiol. 1984 Mar;74(3):640–644. doi: 10.1104/pp.74.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. T., Thompson J. F. Threonine synthetase from higher plants: stimulation by S-adenosylmethionine and inhibition by cysteine. Biochem Biophys Res Commun. 1976 Jul 26;71(2):684–691. doi: 10.1016/0006-291x(76)90842-1. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Sloger M., Owens L. D. Control of Free Methionine Production in Wild Type and Ethionine-resistant Mutants of Chlorella sorokiniana. Plant Physiol. 1974 Mar;53(3):469–473. doi: 10.1104/pp.53.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H. Methionine Synthesis in Lemna: Inhibition of Cystathionine gamma-Synthase by Propargylglycine. Plant Physiol. 1982 Nov;70(5):1347–1352. doi: 10.1104/pp.70.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]


