Abstract
The COG Diversity and Health Disparities Committee’s mission is to guarantee the highest standard of care for children and AYA with cancer regardless of ethnic, racial, gender, or socioeconomic background. We strive to identify and address issues of disparity within the existing scientific structure of COG and to support research across COG to improve survival by ensuring equitable access to COG-sponsored clinical trials. We are committed to advance COG-led research identifying mechanistic drivers of disparities and, concurrently, evaluating interventions to alleviate disparities in the COG trial setting.
As trials identify the most promising therapies, diverse representation is critical to ensure that findings are relevant to everyone. Factors impacting clinical trial participation among vulnerable populations are complex, consisting of barriers at societal, systems, and individual levels. Recent efforts by investigators within DHDC demonstrated that trial-embedded collection of family-reported sociodemographic data and SDoH is feasible and acceptable in the context of COG.
Diversity in the pediatric oncology workforce is essential and one potential approach to improving representation on clinical trials. To support and retain diverse oncology providers and researchers, a MYIA was created to facilitate opportunities for graduating trainees and YIs with an interest in childhood cancer disparities research within COG.
Although there are challenges to achieve the DHDC’s priorities, only through collaboration and support for this work we will be able to elucidate mechanisms underlying inferior survival outcomes for historically marginalized children and AYA, and more importantly, implement interventional investigation to improve outcomes.
Background
Ensuring equitable access and outcomes on clinical trials to address stark disparities within pediatric oncology is an essential component of the work of Children’s Oncology Group (COG). The COG Diversity and Health Disparities Committee (DHDC) mission is to guarantee the highest standard of care for children and adolescents and young adults (AYA) regardless of ethnic, racial, gender, or socioeconomic background. We strive to identify and address issues of disparity within the existing scientific structure of COG. The DHDC supports research across COG to improve survival by ensuring equitable access to COG-sponsored clinical trials, advancing COG-led research identifying mechanistic drivers of disparities and, concurrently, evaluating interventions to alleviate disparities in the COG trial-setting.
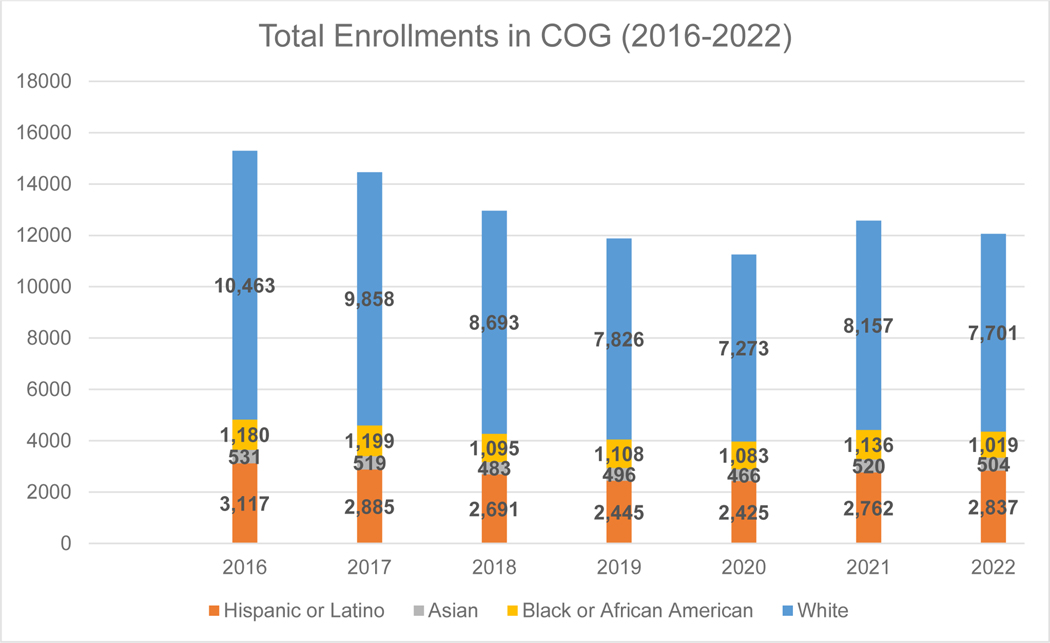
Underserved populations are defined across a variety of dimensions including race and ethnicity, socioeconomic status (SES), and geography. Data from the 2020 census in the United States (US) reported a more diverse population than ever before with minoritized ethnic and racial groups accounting for an increasingly large share.1 Together, the more than 200 COG member institutions treat the vast majority of children with cancer in the US, many of whom are enrolled in clinical trials (Figure 1).
Figure 1:
Total COG Enrollments 2016 – 2022 by Race and Ethnicity in the US
Figure 1 shows overall enrollment of patients by race and ethnicity on COG studies over the last seven years (ranging from ~11,000–15,000 per year including ~7,000 patients enrolled on the registry study, Project Every Child) in the US. During this period, the percentage of minoritized patients enrolled is trending up from 31.6% in 2016 to 36.1% in 2022.
As trials identify the most promising therapies, diverse representation is critical to ensure that findings are relevant to everyone. Underrepresentation of vulnerable patients compromises generalizability of trial results, may lead to incorrect estimates of disease-free survival and treatment efficacy, and may further exacerbate health disparities. Factors impacting clinical trial participation among vulnerable populations are complex, consisting of barriers at societal, systems, and individual levels. Historical data from previous pediatric cooperative groups suggest minoritized populations are proportionally represented among those enrolled on clinical trials.2–4 However, several studies suggest lower enrollment among Hispanic5,6 and Black patients,6 AYA,5–7 uninsured patients,7 and those who live farther from tertiary care centers.8 Even in recent studies, results remain mixed depending on the methodology employed.9,10 Multicenter data to ascertain whether socially vulnerable families are less likely to be offered or are less likely to agree to participate in COG clinical trials are lacking.
At the system level, geographic access to sites where trials are available and insurance barriers may disproportionately impact minoritized or low-income patients. At the individual level, cultural norms, health beliefs, and communication barriers contribute. Discordance in attitudes and beliefs about trial enrollment between patients and providers has been documented.11 One known barrier to facilitating discussions regarding clinical trials is provider-participant language discordance. Logistical considerations associated with Non-English language preference (NELP)12 create barriers to enrollment. At one large, academic institution, only 65% of medical oncology providers and research staff correctly understood the process to discuss, consent, and enroll patients with NELP onto clinical trials.13
Diversity in the pediatric oncology workforce is essential and one potential approach to improving representation on clinical trials. The number of pediatricians from populations characterized as underrepresented in medicine (URM) is not growing at the same pace14 as the US minoritized populations requiring their care. Several studies demonstrate that racial and ethnic concordance between patients and physicians is associated with better communication, greater patient trust, and higher levels of patient satisfaction, all of which may contribute to better health outcomes.15–17
Data collected by American Society of Pediatric Hematology Oncology (ASPHO) and American Board of Pediatrics (ABP) regarding the pediatric hematology-oncology (PHO) workforce demonstrate that Hispanic/Latinx (7%), African American/Black (3.1%) and American Indigenous, Native Alaskan and Hawaiian (<1%) trainees remain markedly underrepresented.18 Eighty percent of the PHO workforce practices in academic centers19 leading to geographic disparities in physician-to-patient ratios in rural compared to metropolitan areas.
Among those patients who enroll on clinical trials for neuroblastoma,20 central nervous system tumors,21,22 leukemia,23,24 and lymphoma25,28, Black and Hispanic race and ethnicity, public insurance25 and high neighborhood poverty20 are each associated with worse survival outcomes. Black, Hispanic and Asian patients as well as those from ZIP codes with lower median household income or lower education also have substantially higher rates of loss to follow up relative to their White counterparts and those from higher SES ZIP codes.26 Proposed hypotheses for cancer disparities include individual, socioeconomic, and environmental factors, known as social determinants of health (SDoH), such as poor living conditions, unstable housing, and lack of access to quality education, transportation, or healthcare.27
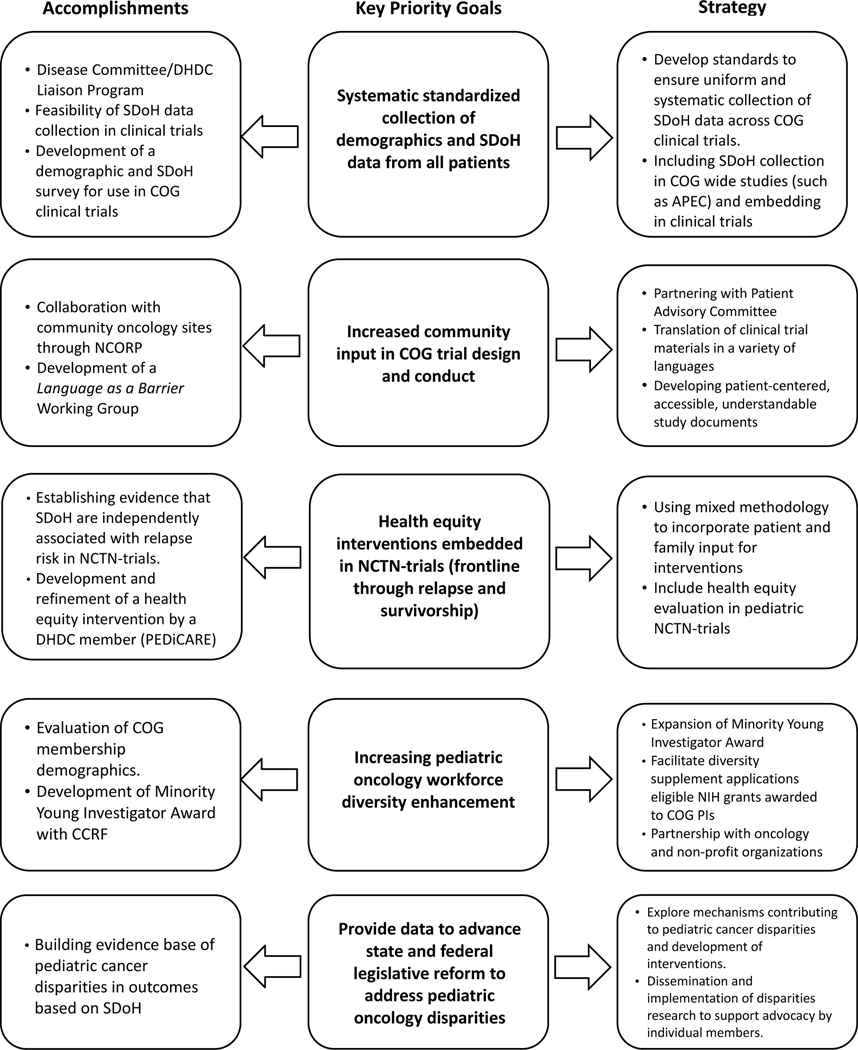
Strategic DHDC priorities to advance health equity in pediatric cancer over the next 5 years include the following (and are summarized in Figure 2):
Figure 2:
DHDC Accomplishments, Related Key Priority Goals and Strategies for Next Steps
Systematic standardized collection of demographics and SDoH data from all patients
Community engagement in COG trial design and conduct
Health equity interventions embedded in NCTN-trials
Other areas that are critical to achieving these priorities:
Pediatric oncology workforce diversity
Engagement with policy/legislative reform to address pediatric oncology disparities
Key Achievements
The COG DHDC has begun to address disparities in trial access and outcomes via a multi-level approach including building retrospective evidence of outcome disparities, establishment of a disease committee/DHDC liaison program, proactive collaboration with NCI Community Oncology Research Program (NCORP), demonstration of the feasibility of SDoH data collection in clinical trials, evaluation of COG membership diversity, and young investigator (YI) engagement.
Building Retrospective Evidence Base.
The importance of clinical trial data in understanding what drives disparities across populations is highlighted by a series of secondary analyses that report disparate outcomes among patients enrolled on COG trials. Among patients enrolled on AHOD0431, AHOD0031, AHOD0831 for the treatment of newly diagnosed Hodgkin lymphoma, we identified significantly inferior overall survival (OS) among Black and Hispanic compared to White patients.28 Similarly among patients enrolled on ANBL0032 and ANBL09P1, we evaluated the association between poverty (defined by public insurance) and survival for children with high-risk neuroblastoma treated in COG immunotherapy trials and demonstrated significantly inferior event-free survival (EFS) and OS compared with poverty-unexposed children. Disparities among Black, Hispanic, and US publicly insured children with acute B lymphoblastic leukemia were demonstrated in the context of eight contemporary COG clinical trials.24 A sampling of the breadth of completed retrospective diversity and health disparities studies is reflected in Table 1. That minoritized and socioeconomically vulnerable children with cancer are more likely than their White counterparts to relapse and die even when receiving uniform cancer therapy in clinical trials highlights the stark reality that enrollment in clinical trials is not sufficient to ameliorate disparities in pediatric oncology.
Table 1.
Published Studies Related to Diversity and Health Disparities Using COG Clinical Trial Data from 2013–2023
| Disease | Study | COG Study ID | Key Findings Related to Diversity and Health Disparities |
|---|---|---|---|
| ALL | Bhatia, 201446 | AALL03N1 | 6MP adherence during maintenance chemotherapy for ALL lower in Asian Americans and African Americans. |
| Gupta, 202247 | AALL0331, AALL0232, AALL0434 | Boys with inferior EFS and OS of ALL attributable to increased relapses (particularly CNS relapse). | |
| Gupta, 202324 | AALL033, AALL0932, AALL0232, AALL1131, AALL0434, AALL1231, AALL0631, AALL15P1 | Black and Hispanic children had inferior EFS in B-ALL. No racial or ethnic disparities in T-ALL. | |
| Wadhwa, 202348 | AALL03N1 | Poverty associated with greater hazard of relapse and non-adherence to 6MP during maintenance chemotherapy for ALL. | |
| CNS | Nooka, 201649 | Multiple COG and PBTC phase 1 clinical trials | Sex and racial/ethnic groups are mostly proportionally represented phase 1 brain tumor trials. Subgroups of Hispanic children are underrepresented. |
| HOD | Kahn, 201928 | AHOD0341, AHOD0031, AHOD0831 | EFS did not differ by race/ethnicity in HOD. Adjusted OS was significantly worse in non-White patients, driven by increased post-relapse mortality. |
| NBL | Bona, 202120 | ANBL0032, ANBL0931 | Poverty independently associated with increased risk of relapse and death in children with NBL. |
| OST | Ilcesan, 202250 | AOST0331 | No association of poverty, race, or ethnicity with EFS or OS in children and adolescents with OST. Black children had inferior post-relapse survival. |
| RET | Green, 201651 | ARET0332 | Advanced RET disease associated with non-private insurance, non-White race, and Hispanic ethnicity. |
| RMS | Munnikhuysen, 202352 | D9602, D9802, D9803, ARST0331, ARST0431, ARST0531, ARST08P1 | Black and Hispanic patients presented with higher risk features at diagnosis of RMS, no difference in EFS or OS. |
| Multiple | Bitterman, 202053 | Multiple solid tumor clinical trials | Black patients enrolled on COG solid tumor prospective clinical trials less likely to receive proton radiotherapy. No association with SES on proton radiotherapy. |
| Faulk, 20209 | Multiple | Across COG clinical trials racial and ethnic groups and county-level SES factors were represented proportionally in relation to SEER. AYA and younger patients with solid and CNS tumors were underrepresented. | |
| Brown, 202254 | Multiple | Childhood Cancer Research Network (registry) cases represent 36% of expected childhood cancers diagnosed 2008 to 2015. Enrollment ratios highest in males, non-Hispanic patients, and ages 1–4 years. | |
| Puthenpura, 202326 | Multiple | In patients enrolled in phase 2/3 and phase 3 COG clinical trials, highest rates of loss to follow-up in AYAs, racial and ethnic minority patients, and lower SES. |
Abbreviations: 6MP – 6-mercaptopurine, ALL – acute lymphoblastic leukemia, AYA – adolescent and young adult, CNS – Brain tumors, COG – Children’s Oncology Group, EFS – event free survival, HOD – Hodgkin lymphoma, NBL – neuroblastoma, OS – overall survival, OST – osteosarcoma, PBTC- Pediatric Brain Tumor Consortium, RET -retinoblastoma, RMS – rhabdomyosarcoma, SES – socioeconomic status
Committee Liaison Program.
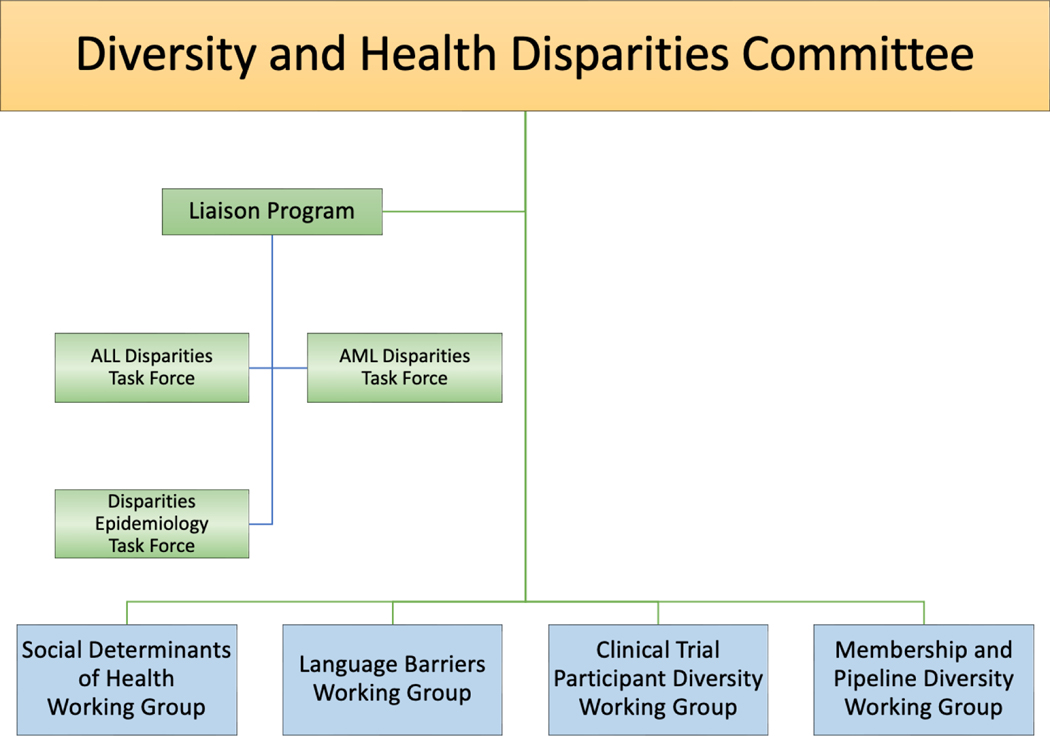
Modeled after several other committees that are cross-cutting and disease agnostic, we created a liaison program to foster bidirectional connections between the DHDC and other COG committees. The DHDC structure, task forces, and working groups are shown in Figure 3. Having a member from each COG committee who is actively focused on improving diversity and reducing disparities within that disease, domain, or discipline is vital to achieving our mission and expanding the DHDC’s impact. Not only do the liaisons serve as a point of contact for collaboration, but they also support the recruitment, mentorship, and retention of YIs interested in disparities research within their committees. In addition, our liaison to the YI committee has facilitated stronger involvement of YIs in our committee and through the YI committee’s mentor pairing program.29,30
Figure 3:
COG’s Diversity and Health Disparities Committee Structure
Collaboration within NCORP.
DHDC collaborates closely with the NCORP, an NCI-funded national network that aims to increase the reach of cancer research studies to individuals in their own communities.31,32 NCORP sites provide access to cancer clinical trials and care delivery research at community-based institutions and institutions with patient populations that include sizeable historically marginalized populations. NCORP is committed to integrating health disparities research questions across all studies in the network. Increasingly, cross-discipline collaborations between the DHD, NCORP, CCDR, AYA, CCL, and LTE committees are leading to efforts to assess inequities in cancer clinical research recruitment, enrollment, care delivery, and long-term follow-up.
SDoH Data Collection.
Historically NCTN trials have not systematically collected patient or family-reported sociodemographic data (such self-reported race or ethnicity) or SDoH. In the last decade only 63% of oncology drug trials even reported race—a proxy for exposure to adverse SDoH due to centuries of structural racism.33 Standard data elements collected in NCTN trials include institution-reported patient race and ethnicity, insurance, and ZIP code. These data elements have been utilized to conduct retrospective analyses to describe racial, ethnic, and socioeconomic disparities in the context of clinical trial–delivered care.10,20,24,28 However, they are inadequate to conduct scientific inquiry to address disparities, namely mechanistic and interventional investigations. Trial-embedded collection of robust and modifiable SDoH data to facilitate investigation of mechanisms underlying inferior outcomes and to identify patients for whom targeted interventions are warranted is essential to improve outcomes, which is the primary mission of the COG and the NCTN. Current and planned studies collecting SDoH data within COG clinical trials are included in Table 2.
Table 2.
Current and Planned Studies Related to Health Disparities Within COG Clinical Trials from 2013 to 2023
| Current/Ongoing | |||
|---|---|---|---|
| COG Study ID | NCT ID | Years | Summary of aims/objectives related to Health Disparities |
| AALL1631 | NCT03007147 | 2017-Present | Measure adherence to oral chemotherapeutic agents and identify factors (including SDoH) associated with poor adherence during the maintenance phase in SR Ph+ ALL patients and after allogeneic HSCT in HR Ph+ ALL patients. |
| AALL1731 | NCT03914625 | 2019-Present | Compare change in neurocognitive functioning from baseline to end-of-therapy among patients with ALL between children from poor families (defined as presence of HMH) and non-poor families (absence of HMH). |
| AALL1732 | NCT03959085 | 2019-Present | Determine the impact of proposed adherence-enhancing interventions on adherence to oral mercaptopurine in patients with ALL. |
| AAML18P1 | NCT03817398 | 2019-Present | Describe clinical and demographic factors affecting the persistence of MMR and re-initiation of treatment after stopping TKI. |
| ACCL20N1CD | NCT04928599 | 2022-Present | Describe trajectories of financial distress for parents of children with newly diagnosed ALL. |
| ACNS2031 | NCT05382338 | 2022-Present | Evaluate HMH as a determinant of neurocognitive, quality of life, and psychosocial outcomes in patients with average-risk and low-risk medulloblastoma. |
| AHOD2131 | NCT05675410 | 2023-Present | Evaluate the association between self-reported race/ethnicity and social determinants of health. Evaluate the associations between race/ethnicity and post-progression/post-relapse overall survival. |
| ALTE03N1 | NCT00082745 | 2004-Present | Identify treatment-related and demographic risk factors to develop key adverse events through a direct comparison of the case-group and controls identified from the remaining patients with the same primary diagnosis. |
| ALTE05N1 | NCT00736749 | 2008-Present | Establish and maintain a systematic, cost-effective mechanism to facilitate and ensure timely and efficient collection of off-therapy, protocol-driven and demographics including SDoH data by member institutions to enhance the scope and quality of COG health-related outcomes research. |
| ANBL1531 | NCT03126916 | 2018-Present | Determine the association between HMH and clinical outcomes and 131I-MIBG receipt. |
| ASCT2031 | NCT05457556 | 2022-Present | Describe and compare outcomes by race/ethnicity, area-based SES, SDoH and transcriptional correlate adversity. Compare HRQoL outcomes between White patients and racial/ethnic minority patients receiving haploHCT. |
| Studies in development | |||
| Incorporation of a Health Equity Intervention in a Neuroblastoma Clinical Trial | Explore the impact of a Health Equity Intervention on clinical outcomes in children with neuroblastoma. | ||
| Systematic Collection of Social Determinants of Health within Clinical Trials | Embed systematic data collection of demographics and social determinants of health within COG Clinical Trials | ||
Abbreviations: ALL – acute lymphoblastic leukemia, haploHCT – haploid hematopoietic cell transplant, HMH – Household Material Hardships, HR – high risk, HRQoL – Health related Quality of Life, HSCT – hematopoietic stem cell transplantation, MIBG - meta-iodobenzylguanidine, MMR- major molecular response, NCT ID – ClinicalTrials.gov identifier, Ph+ - Philadelphia positive, SES – socioeconomic status, SR – standard risk, TKI – Tyrosine Kinase Inhibitor
Recent efforts by investigators within DHDC demonstrated that trial-embedded collection of family-reported sociodemographic data and SDoH is feasible and acceptable in the context of COG. The most recent COG frontline trial for high-risk neuroblastoma, ANBL1531 (NCT03126916), included an opt-in, correlative aim to collect SDoH via paper/pencil surveys available in English, Spanish or French. Surveys have been self-completed or read-aloud with interpreters in seven languages across 101 COG sites at a median of 11 days post-enrollment, demonstrating site-level engagement and feasibility. To minimize site burden, survey data are submitted to a central disparities study team for data cleaning and entry into an electronic database. Over the first three years, 360 (87%) families opted-in and 88% of those who opted in completed SDoH surveys.34 Data missingness for the primary exposure of interest, household material hardship (HMH), was 0.8%, demonstrating the acceptability to families of sharing this information. Taken together, these data support the feasibility and acceptability of collecting essential family reported SDoH data as an embedded component of groupwide COG trials.
Work is underway to improve collection of self-reported race and ethnicity, income, education, insurance, and HMH across COG studies. A group of experienced pediatric and AYA health services researchers convened to compile a package of basic, essential sociodemographic and SDoH questions for use in COG trials. The purpose was to create a tool to clearly capture socio-contextual factors driving observed associations between race, ethnicity, insurance, and survival. The questionnaire includes the following elements: age, sex, race, ethnicity, languages spoken at home, health insurance, parent education level, material hardship, household income, number of people in the household, and home address. Several members of the COG Patient Advocacy Committee (PAC) reviewed the survey to assess its acceptability. Collection of these data will allow for studies evaluating how differences in demographics, social factors, disease and host biology, and up-front and post-relapse therapy impact pediatric cancer outcomes and will inform interventions to optimize care for diverse populations going forward.
Language as a Barrier to Trial Access.
The resources available to COG institutions recruiting patients with NELP are unknown. We created a Language as a Barrier Working Group to evaluate major institutional-level and systems-level barriers to informed consent conferences with families with NELP. In 2022, we conducted a 20-item cross-sectional survey study of COG Principal Investigators (PIs) to identify common challenges that may impede recruitment and consent of NELP participants and assess resources to support study document translation, interpreter services, and inclusive study approaches. These findings will inform future strategies to reduce barriers to recruitment of NELP participants onto COG clinical trials.
Assessment of Membership Diversity.
In the fall of 2022, we undertook a systematic assessment of the diversity of the existing membership. In collaboration with the COG Membership Committee, we developed a brief survey to gather demographic information about members of COG that was distributed to the more than 10,000 members. The analysis and interpretation of these data are ongoing and will be instrumental in developing COG diversity, equity and inclusion (DEI) initiatives.
Support and Retention of Diverse Oncology Providers and Researchers.
The retention of minoritized members serves a three-fold purpose: 1) ameliorates underrepresentation in our current pediatric oncology workforce, 2) improves the quality of disparities research when diverse perspectives are included among the research team, 3) improves minoritized patient outcomes through direct patient care and interventions developed based on research findings. Through a collaboration between DHDC and Children’s Cancer Research Foundation (CCRF), starting in 2021, a Minority Young Investigator Award (MYIA) was created to facilitate opportunities for graduating trainees and YIs with an interest in childhood cancer disparities research to develop and complete a research study within COG. This award allows awardees to gain experience with the process of research using national COG data and to form collaborative relationships with established COG investigators. We hope that this award will serve as a foundation for the development of careers that focus on disparities-oriented research and helps to address the leaky pipeline of underrepresented minoritized practitioners within pediatric oncology.
Current Challenge
Current barriers to achievement of the DHDC’s priorities include the need to align funding priorities with health equity research. The pace of advancement has been hindered by operational restrictions and lack of alignment between stated overarching goals and concrete support in the form of resources. The pervasive disparities in cancer outcomes even among clinical trial participants necessitate further attention and response within leading organizations. COG institutions’ longstanding commitment to enrolling children on frontline NCTN trials reflects the reality that the conduct of statistically powered clinical investigation is impossible outside the cooperative group setting. For this reason, inclusion of SDoH data collection and SDoH-targeted interventions within COG trials is essential to high-quality, meaningful health equity research. Only through concerted effort and intentional support for this work will we be able to elucidate mechanisms underlying inferior survival outcomes for historically marginalized children and AYA, and more importantly, implement interventional investigation to improve outcomes.
Strategic Plan
1. Systematic standardized collection of demographics and SDoH data
Biologic and social determinants both contribute to cancer outcomes and, by extension, to outcome disparities. To understand their relative contributions, multi-dimensional data from prospective cohorts are needed. We propose that collecting SDoH data from patients enrolled on COG studies should be a consistent component of all clinical trials for two reasons. First, poverty, HMH (including food, energy, and housing insecurity), structural racism, discrimination, and environmental exposures influence not only families’ interactions with the healthcare system, but also their epigenetic profile, which is increasingly recognized as an important contributor to health disparities. Without these SDoH data, key factors impacting outcomes may be overlooked. Second, as we develop interventions to improve DEI in clinical trials, mechanisms to measure their impact and efficacy are essential. Standards must be developed to ensure uniform and systematic collection of SDoH data across all clinical trials. For COG sites, guidance around how these data should be collected, how frequently they should be reviewed, and how findings can be expeditiously translated into targeted interventions are critical.
2. Community engagement in COG trial design and conduct
Participant-informed research methods are the most effective way to develop appropriate, effective, and sustainable interventions for specific populations.35 For vulnerable pediatric and AYA patients with cancer including the patient and parent/caregiver voice from the outset of research inception is imperative. In partnership with PAC, we aim to find approaches to reflect the perspectives of caregivers from minoritized backgrounds, low SES status, NELP parents, single parents, and AYA patients themselves to ensure patient and parent experiences are reflective of the diversity of the population COG serves.
The incorporation of evidence-based and innovative approaches focused on recruitment of understudied populations at the time of study design is essential to maximize minoritized patient trial enrollment.36–39 Ensuring that survey instruments are available in the primary language of participants will improve participation among NELP patients. We have established a precedent for translating study documents for NELP families, and more importantly, following a rigorous methodology to culturally adapt and validate study measures.40 This process requires investigators’ time and dedicated funding to establish this approach as the standard for COG studies moving forward. Patient-facing documents should be patient-centered, understandable, and accessible. Modes of information sharing, such as infographics or interactive visualizations, may be effective to increase engagement with historically underserved populations, including those with low health literacy.41,42 The barriers to participation become increasingly complex as therapies become less widely distributed, such as for novel agents in the early phase clinical trial setting as well as highly technical and innovative therapies such as MIBG, immunotherapy, and cellular therapy. To identify true disparities, it is necessary to evaluate study participation in the context of broader pediatric oncology populations treated at COG institutions as well as those treated outside of COG institutions.
3. Health equity interventions embedded in NCTN trials
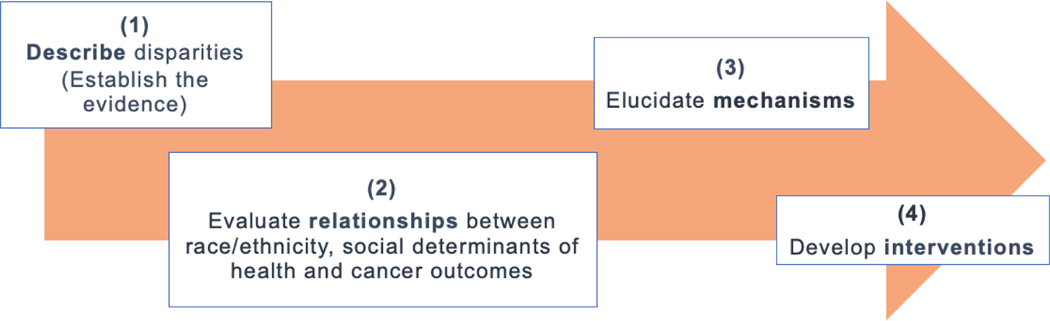
Given robust evidence that SDoH are independently associated with relapse in COG trials,10, 20,24,28 evaluation of trial-embedded investigations targeting SDoH exposures is the logical next step to advance childhood cancer outcomes (Figure 4). This approach should be modeled directly on successful approaches to biological risk—prospectively measuring risk factors in clinical trials to identify children who may benefit from targeted intervention, investigating mechanisms by which these factors confer excess risk, and evaluating approaches to treat them—beginning with readily available non-specific approaches, and eventually introducing targeted approaches to mitigate the excess risk these factors confer. This approach to biologic risk has been highly successful and can be directly applied to SDoH in the trial context.
Figure 4:
Approach to identifying and addressing health equity targets in pediatric oncology
One drug will not cure all cancers and most cancers require multi-modal therapy; SDoH are no different. Reducing SDoH-associated relapse in NCTN trials will require a portfolio of multi-level health equity interventions—alone and in combination—to address modifiable SDoH. Health equity interventions should be developed and tested following the model of successful early phase oncology consortia. Health equity intervention development benefits from organized multi-disciplinary teams—including oncologists, nurses, social workers, patients, caregivers, community-organizations, economists, and policymakers among others.
These interventions can first be pre-tested and refined using patient and family input, and then evaluated for feasibility prior to large-scale randomized controlled trials of efficacy. Just like drugs, these interventions will require the equivalent of early phase trials to determine feasibility of interventions prior to efficacy evaluation. A recent example of this approach included the mixed-methods development and refinement of a scalable intervention targeting food and transportation insecurity with the provision of in-kind resources called PediCARE.43 The feasibility of administering the PediCARE intervention was subsequently demonstrated in a randomized, multicenter context, supporting a proposed randomized efficacy trial among children with high-risk neuroblastoma.44 This approach to pilot testing will facilitate the rapid development of an intervention portfolio for efficacy evaluations across pediatric cancer types.
The evaluation of this pipeline of similar interventions requires commitment and funding by NCI to support health equity intervention evaluation in pediatric NCTN-trials, a methodological step aligned with the 2023 NCI National Cancer Plan to support the science necessary to improve health equity in cancer.45
4. Pediatric oncology workforce diversity
Building on the early success of the CCRF MYIA, we are determined to further expand opportunities across the spectrum of trainees preparing for a career in pediatric oncology. First and foremost, we seek to ensure the sustainability and ideally expansion of the MYIA. Secondly, we hope to leverage the portfolio of NIH-funded projects within COG to facilitate applications for diversity supplements to R01s already awarded to COG investigators by raising awareness among both PIs and YIs and emphasizing the mutual benefits of the program. In the meantime, our committee is actively pursuing a diversity supplement to the research base UG1 grant. Thirdly, partnering with disease-specific societies and non-profit organizations for whom diversity is a priority may allow us to link the tremendous opportunities within COG to funding for training and mentorship. A prerequisite for expanding the pipeline of underrepresented trainees is ensuring that mentors are available who have the capability and commitment to guide promising YIs. Ensuring that mentors understand DEI principles will allow them to support trainees more effectively in academic medicine.
5. Engagement with policy/legislative reform to address pediatric oncology disparities
Policy-level advocacy is needed to address root causes of adverse SDoH and to sustain novel healthcare delivery interventions effective in reducing relapse and death in childhood cancer. A deeper understanding of the mechanisms that contribute to disparities in clinical trial access and outcomes, including structural factors, will help build the evidence base needed for interventions that are effective at ameliorating these barriers. Since COG is a National Institute of Health grant recipient, it cannot engage in policy advocacy. However, its individual members can advocate at the policy level for interventions that have been scientifically proven. Advocacy from pediatric oncologists and patient advocates for policies that support the development, evaluation, and sustainability of interventions targeting SDoH is needed to continue supporting and funding data-driven research addressing barriers to healthcare. It is our greatest hope that the rigorous research that comes out of COG will contribute to our knowledge base and support the development of more just and equitable legislative policies at the state and federal levels including evidence-based reforms to Medicaid, private insurance regulations, and safety net programs.
Funding support:
Grant support from the National Institute of Health, U10CA180886, U10CA180899, U10CA098543, U10CA098413, UG1CA189955. Justine Kahn and Lena Winestone’s effort is partially supported by funding from the Robert A. Winn Diversity in Clinical Trials Award.
Abbreviations:
- COG
Children’s Oncology Group
- DHDC
Diversity & Health Disparities Committee
- AYA
Adolescents and Young Adults
- SES
Socioeconomic Status
- US
United States
- NELP
Non-English Language Preference
- URM
Underrepresented in Medicine
- ASPHO
American Society of Pediatric Hematology Oncology
- ABP
American Board of Pediatrics
- PHO
Pediatric Hematology-Oncology
- SDoH
Social Determinants of Health
- NCORP
NCI Community Oncology Research Program
- YI
Young Investigator
- OS
Overall Survival
- EFS
Event-free Survival
- CCDR
Cancer Care Delivery Research
- CCL
Cancer Control
- LTE
Late Effects
- NCTN
NCI’s National Clinical Trials Network
- PIs
Principal Investigators
- DEI
Diversity, Equity and Inclusion
- CCRF
Children’s Cancer Research Foundation
- MYIA
Minority Young Investigator Award
- PAC
Patient Advocacy Committee
References
- 1.2020 Census Demographic Data. United States Census Bureau. https://www.census.gov/programs-surveys/decennial-census/decade/2020/2020-census-data.html]. Published 2021. Accessed2023.
- 2.Krailo MD, Bernstein L, Sullivan-Halley J, Hammond GD. Patterns of enrollment on cooperative group studies. An analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer. 1993;71(10 Suppl):3325–3330. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer WA, Tejeda HA, Murphy SB, Brawley OW, Smith MA, Ungerleider RS. Equal participation of minority patients in U.S. national pediatric cancer clinical trials. J Pediatr Hematol Oncol. 1997;19(5):423–427. [DOI] [PubMed] [Google Scholar]
- 4.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88(12):812–816. [DOI] [PubMed] [Google Scholar]
- 5.Aristizabal P, Singer J, Cooper R, et al. Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Pediatr Blood Cancer. 2015;62(8):1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund MJ, Eliason MT, Haight AE, Ward KC, Young JL, Pentz RD. Racial/ethnic diversity in children’s oncology clinical trials: ten years later. Cancer. 2009;115(16):3808–3816. [DOI] [PubMed] [Google Scholar]
- 7.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pole JD, Barber R, Bergeron RE, et al. Most children with cancer are not enrolled on a clinical trial in Canada: a population-based study. BMC Cancer. 2017;17(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faulk KE, Anderson-Mellies A, Cockburn M, Green AL. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004–2015. PLoS One. 2020;15(4):e0230824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winestone LE, Getz KD, Rao P, et al. Disparities in pediatric acute myeloid leukemia (AML) clinical trial enrollment. Leuk Lymphoma. 2019;60(9):2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillyer GC, Beauchemin M, Hershman DL, et al. Discordant attitudes and beliefs about cancer clinical trial participation between physicians, research staff, and cancer patients. Clin Trials. 2020;17(2):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega P, Shin TM, Martínez GA. Rethinking the Term “Limited English Proficiency” to Improve Language-Appropriate Healthcare for All. Journal of Immigrant and Minority Health. 2022;24(3):799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staples JN, Lester J, Li A, et al. Language as a barrier to cancer clinical trial accrual: assessing consenting team knowledge and practices for cancer clinical trial consent among low English fluency patients. Applied Cancer Research. 2018;38(1):14. [Google Scholar]
- 14.Turner AL, Gregg CJ, Leslie LK. Race and Ethnicity of Pediatric Trainees and the Board-Certified Pediatric Workforce. Pediatrics. 2022;150(3). [DOI] [PubMed] [Google Scholar]
- 15.Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians’ implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsan M, Garrick O, Graziani G. Does Diversity Matter for Health? Experimental Evidence from Oakland. American Economic Review. 2019;109(12):4071–4111. [Google Scholar]
- 17.Greenwood BN, Hardeman RR, Huang L, Sojourner A. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020;117(35):21194–21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latest Race and Ethnicity Data for Pediatricians and Pediatric Trainees. The American Board of Pediatrics. https://www.abp.org/content/latest-race-ethnicity-data-pediatricians-pediatric-trainee. Published 2023. Accessed2023.
- 19.Hastings C, Borinstein SC, Bergsagel DJ, et al. The American Society of Pediatric Hematology Oncology workforce, productivity, and fellowship assessment: Current state of the workforce. Pediatr Blood Cancer. 2023;70(5):e30221. [DOI] [PubMed] [Google Scholar]
- 20.Bona K, Li Y, Winestone LE, et al. Poverty and Targeted Immunotherapy: Survival in Children’s Oncology Group Clinical Trials for High-Risk Neuroblastoma. J Natl Cancer Inst. 2021;113(3):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell HK, Morris M, Ellis L, Abrahao R, Bonaventure A. Racial/ethnic and socioeconomic survival disparities for children and adolescents with central nervous system tumours in the United States, 2000–2015. Cancer Epidemiol. 2020;64:101644. [DOI] [PubMed] [Google Scholar]
- 22.Haizel-Cobbina J, Spector LG, Moertel C, Parsons HM. Racial and ethnic disparities in survival of children with brain and central nervous tumors in the United States. Pediatr Blood Cancer. 2021;68(1):e28738. [DOI] [PubMed] [Google Scholar]
- 23.Kahn JM, Cole PD, Blonquist TM, et al. An investigation of toxicities and survival in Hispanic children and adolescents with ALL: Results from the Dana-Farber Cancer Institute ALL Consortium protocol 05–001. Pediatr Blood Cancer. 2018;65(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Dai Y, Chen Z, et al. Racial and ethnic disparities in childhood and young adult acute lymphocytic leukaemia: secondary analyses of eight Children’s Oncology Group cohort trials. Lancet Haematol. 2023;10(2):e129–e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn JM, Zhang X, Kahn AR, et al. Racial Disparities in Children, Adolescents, and Young Adults with Hodgkin Lymphoma Enrolled in the New York State Medicaid Program. J Adolesc Young Adult Oncol. 2022;11(4):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puthenpura V, Ji L, Xu X, et al. Loss to follow-up of minorities, adolescents, and young adults on clinical trials: A report from the Children’s Oncology Group. Cancer. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji X, Sohn H, Sil S, Castellino S. Moving Beyond Patient-Level Drivers of Racial/Ethnic Disparities in Childhood Cancer. Cancer Epidemiol Biomarkers Prev 2022;31:1154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn JM, Kelly KM, Pei Q, et al. Survival by Race and Ethnicity in Pediatric and Adolescent Patients with Hodgkin Lymphoma: A Children’s Oncology Group Study. J Clin Oncol. 2019;37(32):3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esbenshade AJ, Pierson CR, Thompson AL, et al. Long-term evidence that a pediatric oncology mentorship program for young investigators is feasible and beneficial in the cooperative group setting: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2018;65(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy AS, Pyke-Grimm KA, Lee DA, et al. Mentoring in pediatric oncology: a report from the Children’s Oncology Group Young Investigator Committee. J Pediatr Hematol Oncol. 2013;35(6):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCI Community Oncology Research Program (NCORP) National Cancer Institute. https://ncorp.cancer.gov/about/. Accessed2023.
- 32.Children’s Oncology Group NCORP Committee https://cogmembers.org/Site/Disc/NCORP/default.aspx. Accessed2023.
- 33.Loree JM, Anand S, Dasari A, et al. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncology. 2019;5(10):e191870–e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones E NA, Winestone LE, Umaretiya PJ, Aziz-Bose R, Ilcisin LA, Greengard EG, DuBois SG, Bagatell R, and Bona K. Feasibility and acceptability of social determinants of health data collection in the context of a Children’s Oncology Group trial. American Society of Clinical Oncology (ASCO) Annual Meeting. 2023; Chicago, IL. [Google Scholar]
- 35.Taffere GR, Abebe HT, Zerihun Z, Mallen C, Price HP, Mulugeta A. Systematic review of community engagement approach in research: describing partnership approaches, challenges and benefits. Journal of Public Health. 2023. [Google Scholar]
- 36.Steffen AD, Kolonel LN, Nomura AM, Nagamine FS, Monroe KR, Wilkens LR. The Effect of Multiple Mailings on Recruitment: The Multiethnic Cohort. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(2):447–454. [DOI] [PubMed] [Google Scholar]
- 37.Sykes LL, Walker RL, Ngwakongnwi E, Quan H. A systematic literature review on response rates across racial and ethnic populations. Can J Public Health. 2010;101(3):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aristizabal P. Diverse populations and enrollment in pediatric cancer clinical trials: Challenges and opportunities. Pediatr Blood Cancer. 2020;67(11):e28296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satia JA, Galanko JA, Rimer BK. Methods and Strategies to Recruit African Americans into Cancer Prevention Surveillance Studies. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(3):718–721. [DOI] [PubMed] [Google Scholar]
- 40.Eremenco S, Pease S, Mann S, Berry P, on behalf of the PROCsPS. Patient-Reported Outcome (PRO) Consortium translation process: consensus development of updated best practices. Journal of Patient-Reported Outcomes. 2018;2(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcia A, Suero-Tejeda N, Bales ME, et al. Sometimes more is more: interative participatory design of infographics for engagement of community members with varying levels of health literacy. J Am Med Inform Assoc. 2016;23(1):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakken S, Arcia A, Woollen J. Promoting Latino Self-Management Through Use of Information Visualizations: A Case Study in New York City. Stud Health Technol Inform. 2020;269:153–160. [DOI] [PubMed] [Google Scholar]
- 43.Umaretiya PJ, Revette A, Seo A, et al. PediCARE: Development of a poverty-targeted intervention for pediatric cancer. Pediatr Blood Cancer. 2021;68(10):e29195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman H JE, Umaretiya PJ, Aziz-Bose R, Ilcisin LA, Zheng D, Wolfson JA, Wolfe J, and Bona K. Feasibility of the Poverty-Targeted Pediatric Cancer Resource Equity (PediCARE) intervention. Paper presented at: American Society of Clinical Oncology (ASCO) Annual Meeting.2023; Chicago, IL. [Google Scholar]
- 45.National Cancer Plan. https://nationalcancerplan.cancer.gov/about. Published 2023. Accessed2023.
- 46.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S, Teachey DT, Chen Z, et al. Sex-based disparities in outcome in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group report. Cancer. 2022;128(9):1863–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadhwa A, Chen Y, Hageman L, et al. Poverty and Relapse Risk in Children with Acute Lymphoblastic Leukemia: Children’s Oncology Group Study AALL03N1 Report. Blood. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nooka AK, Behera M, Lonial S, Dixon MD, Ramalingam SS, Pentz RD. Access to Children’s Oncology Group and Pediatric Brain Tumor Consortium phase 1 clinical trials: Racial/ethnic dissimilarities in participation. Cancer. 2016;122(20):3207–3214. [DOI] [PubMed] [Google Scholar]
- 50.Ilcisin LAS, Han R, Krailo MD, et al. Poverty, race, ethnicity, and survival among U.S. children with non-metastatic osteosarcoma treated on EURAMOS-1: A report from the Children’s Oncology Group. Journal of Clinical Oncology. 2022;40(16_suppl):10004–10004. [Google Scholar]
- 51.Green AL, Chintagumpala M, Krailo M, et al. Correlation of Insurance, Race, and Ethnicity with Pathologic Risk in a Controlled Retinoblastoma Cohort: A Children’s Oncology Group Study. Ophthalmology. 2016;123(8):1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munnikhuysen SR, Ekpo PA, Xue W, et al. Impact of race and ethnicity on presentation and outcomes of patients treated on rhabdomyosarcoma clinical trials: A report from the Children’s Oncology Group. Cancer Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitterman DS, Bona K, Laurie F, et al. Race Disparities in Proton Radiotherapy Use for Cancer Treatment in Patients Enrolled in Children’s Oncology Group Trials. JAMA Oncol. 2020;6(9):1465–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown AL, Sok P, Scheurer ME, et al. An updated assessment of 43,110 patients enrolled in the Childhood Cancer Research Network: A Children’s Oncology Group report. Cancer. 2022;128(14):2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]